Abstract
Cross-sectional studies have found that an elevated ratio of arachidonic acid to omega-3 fatty acid is associated with depression, and controlled intervention studies have found that decreasing this ratio through administration of omega-3 fatty acids can alleviate depressive symptoms. Additionally, arachidonic acid and omega-3 fatty acids have opposing effects on inflammatory signaling. Exogenous administration of the inflammatory cytokine interferon-alpha (IFN-α) can trigger a depressive episode in a subset of vulnerable people, though associated risk factors remain poorly understood. Using a within-subject prospective design of 138 subjects, we examined whether baseline long-chain omega-3 (docosahexaenoic acid – DHA; eicosapentaenoic acid – EPA) and omega-6 (arachidonic acid – AA; di-homo-gamma-linolenic acid – DGLA) fatty acid status was associated with depression vulnerability in hepatitis C patients treated with IFN-α. Based on the literature, we had specific a priori interest in the AA/EPA+DHA ratio. Lower baseline DHA predicted depression incidence (p=0.04), as did elevated DGLA (p=0.02) and an elevated AA/EPA+DHA ratio (p=0.007). The AA/EPA+DHA ratio predicted depression even when controlling for other critical variables such as sleep quality and race. A higher AA/EPA+DHA ratio was positively associated with both increasing Montgomery-Asperg Depression Rating Scores over time (F=4.0; p<0.05) as well as interleukin-6 levels (F=107.4; p<0.05) but not C-reactive protein. Importantly, omega-3 and omega-6 fatty acid status was not associated with sustained viral response to IFN-α treatment. These prospective data support the role of fatty acid status in depression vulnerability and indicate a potential role for omega-3 fatty acids in the prevention of inflammation-induced depression.
Keywords: Omega-3 fatty acids, Inflammation, Arachidonic acid, Cytokine, Interleukin-6, C-reactive protein, Major depressive disorder
INTRODUCTION
Emerging evidence suggests that elevated systemic inflammation may contribute to the pathoetiology of major depressive disorder (MDD) (Dowlati et al., 2010; Howren et al., 2009; Raison et al., 2006; Zorrilla et al., 2001). Although there is accumulating evidence that a subset of MDD cases could be induced by inflammatory cytokines (Lotrich, 2012), most people are resilient to elevated inflammatory activity and do not develop MDD. For example, exogenous administration of inflammatory cytokines such as interferon-alpha (IFN-α) can trigger depression, but only in a subset (~30%) of patients (Capuron et al., 2002; Capuron and Miller, 2004; Lotrich et al., 2007; Musselman et al., 2001). While vulnerability factors for depression remain poorly understood, recent evidence has implicated interleukin-6 (Prather et al., 2009), poor sleep (Franzen et al., 2009), serotonin (Bull et al., 2008; Lotrich et al., 2009), and glucocorticoid resistance (Raison et al., 2008). Developing a better understanding of risk and resilience factors associated with inflammation-induced depression may provide novel targets for improving resilience.
Polyunsaturated fatty acids (PUFAs) play a critical influence in the regulation of inflammatory signaling and potentially vulnerability to MDD. The long-chain omega-6 fatty acid arachidonic acid (AA; 20:4n-6) is a substrate for the synthesis of prostacyclins, thromboxanes, and prostaglandins such as PGE2. PGE2 stimulates the synthesis of inflammatory cytokines (Portanova et al., 1998; Wang et al., 2010); and in turn prostaglandins may also be important in mediating the effect of peripheral inflammation on brain function. For example, inhibition of cyclooxygenase-2 (COX-2), the rate-limiting enzyme in the conversion of AA to PGE2, can attenuate lipopolysaccharide (LPS)-induced increases in extra-cellular hippocampal serotonin (Linthorst et al., 1996). Moreover, adjunctive treatment with celecoxib, a selective cyclooxygenase-2 (COX-2) inhibitor, was found to augment the therapeutic efficacy of fluoxetine in MDD patients (Akhondzadeh et al., 2009). In contrast, dietary dihomo-γ-linolenic acid (DGLA, 20:3n-6), an n-6 fatty acid precursor of AA, can be converted via COX-2 to PGE1 which has anti-inflammatory properties. Moreover, the long-chain omega-3 fatty acids eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3), and their COX and LOX metabolites (E- and D-series resolvins) have anti-inflammatory and inflammation resolving properties (Calder, 2008; Hong et al., 2003). Therefore, the balance between these different omega-3 and omega-6 fatty acids play a critical role in regulating inflammatory homeostasis.
Prior cross-sectional studies have repeatedly observed deficits in long-chain omega-3 fatty acids (EPA and/or DHA) but not AA in patients with MDD, as summarized in a recent meta-analysis (Lin et al., 2010). Accordingly, the AA/EPA+DHA ratio is elevated in MDD patients and may be positively associated with depression symptom severity across a variety of studies (Adams et al., 1996; Conklin et al., 2007; Frasur-Smith et al., 2004; Maes et al., 1996; Tiemeier et al., 2003). Independent meta-analyses of controlled intervention trials have found that chronic dietary EPA+DHA supplementation, resulting in a reduction in the AA/EPA+DHA ratio, is associated with significant reductions in depression symptom severity in MDD patients (Freeman et al., 2006; Lin and Su, 2007). Prior prospective studies have found that low erythrocyte DHA levels are associated with depression during IFN-α treatment (Su et al., 2010) as well as future suicidal attempts in medication-free MDD patients (Sublette et al., 2006). Importantly, rodent studies have found that dietary-induced deficiencies in omega-3 fatty acids, and elevations in the AA/EPA+DHA ratio, result in increased inflammatory cytokine production (Kozak et al., 1997; Mingam et al., 2008; Song et al., 2003) and associated changes in central serotonin turnover (Kodas et al., 2004; McNamara et al., 2010a). Moreover, omega-3 fatty acid deficiency has been found to up-regulate omega-6 fatty acid biosynthesis (Hofacer et al., 2011; Igarashi et al., 2007) as well as the expression of COX-2 in rat brain (Rao et al., 2007); and supplementation with omega-3 fatty acids can reverse some of the inflammatory and behavioral effects of IL-1 in rodent models (Song et al., 2004).
In view of this evidence for a preliminary link between lower omega-3 fatty acid status and increased vulnerability to inflammation and depression, the present study prospectively investigated whether polyunsaturated fatty acid status was associated with an increased risk for developing depression in response to IFN-α treatment. We additionally examined whether the fatty acid profiles were associated with markers of inflammatory status, interleukin-6 (IL-6) and C-reactive protein (CRP), and whether fatty acid status influenced the ability of IFN-α to successfully resolve hepatitis C infection. Based on extant evidence reviewed above, our primary hypothesis was that low EPA and DHA levels at baseline, and specifically a higher AA/EPA+DHA ratio, would be associated with increased risk for developing depression during IFN-α treatment.
METHODS
Participants and depression assessment
138 adult subjects (between ages 18-80) were examined for plasma fatty acids levels prior to IFN-α therapy. Subjects had to be recommended by a hepatologist for treatment of HCV with IFN-α. Exclusion criteria were active mood, anxiety, psychotic, or drug/alcohol use disorders within 6 months prior to starting IFN-α treatment – using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I); known neurological disease; or taking corticosteroids, antidepressants, anticonvulsants, and/or antipsychotics (although they could be taking as-needed sleeping medications). An overlapping subset of these subjects were previously examined regarding the relationship between IL-6 and depression (Prather et al., 2009). The study was approved by the University of Pittsburgh Institutional Review Board.
Of these 138 subjects, 99 eventually started weekly injections of pegylated (PEG) IFN-α2 (PEG-IFN-α2a: 135 μg/week or PEG-IFN-α2b: 120 or 150 μg/week) augmented with oral ribavirin. Prior to initiating IFN-α therapy, and monthly for four months after therapy was initiated, depression was assessed using both subjective and objective measures including the Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979) and Beck Depression Inventory-II (BDI) as previously described (Franzen et al., 2009; Lotrich et al., 2009; Lotrich et al., 2007). Sleep quality was measured monthly using the Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 1989). Criteria for Major Depression (MDD; via an abbreviated SCID-I) were assessed at baseline, if BDI>15, within 48 hours of any request by either the treating heptalogist or subject, or minimally every two months. Participants who developed MDD during the course of treatment -- or where concerns about lethality arose -- were typically started on an antidepressant, though some did discontinue IFN-α treatment. Within sixteen weeks of IFN-α therapy, forty-one subjects required some type of psychiatric intervention for severe mood problem (such as suicidal ideation and/or MDD).
Phospholipid fatty acid extraction and gas chromatography
Plasma from whole blood was obtained from all subjects between 10AM and 4PM prior to initiating treatment for hepatitis C (HCV), and stored at -80°C until analysis. Folch reagent (2 mL Chloroform/Methanol 2:1) was added to 0.3ml of plasma to extract the lipid layer, dried under nitrogen, and reconstituted with chloroform (100 uL). The lipid extract was then transferred to a reversed-phase packed SPE column (Alltech, Nicholasville, KY) and washed with chloroform (10mL), to remove triglycerides, and then acetone (10mL) to remove the cholesteryl esters. Phospholipids were then eluted with methanol (20mL), and the combined methanol fractions evaporated. The sample was methylated using NaOH/MeOH (0.5 mL) and the derivatization was completed with BF3/MeOH followed by heating for 15 minutes at 85°C. To ensure total fatty acid methyl ester (FAME) extraction, NaCl (0.3 mL) was used before extraction with hexane. Sodium sulfate was added to the hexane layer to remove water, and the organic phase decanted and evaporated using nitrogen. Samples were then reconstituted with hexane (0.5 mL) and analyzed.
FAME’s were analyzed using an HP 6890/5973 gas chromatograph/mass selective detector (Agilent Technologies, Santa Clara, CA). The column used to separate FAME’s was an Agilent DB-FFAP 15m × 0.1 mm with 0.1 um of film thickness. Helium was used as carrier gas at a flow rate of 17.6 ml/min and a constant pressure of 53.8 psi. The initial temperature was set at 160°C and increased after injection of 1 ul of sample to 240°C at a rate of 15°C per minute. Once the temperature of 240°C was reached, it was maintained for 6 minutes for a total run time of 14.33 minutes. The transfer line was maintained at 280°C and the filament at 70Ev for EI. The data were evaluated using a TIC for compound identification and SCAN mode to measure relative percent of each fatty acid. Fatty acid identification was based on retention times of authenticated fatty acid methyl ester standards (GLC 473B) and controls (GLC 462 and GLC 463) to ensure reproducibility (NuCheck Prep, Elysian, MN). Data are expressed as weight percent of total fatty acid pool (mg fatty acid/100 mg fatty acids). Our primary measures of interest were the two long-chain omega-3 fatty acids, DHA (22:6n-3) and EPA (20:5n-3), the two long-chain omega-6fatty acids AA (20:4n-6) and DGLA (20:3n-6), and the ratio of AA to DHA+EPA.
IL-6 and CRP levels
IL-6 and CRP levels in serum samples, were determined using a high-sensitivity quantitative enzyme immunoassay (Diaclone, Besancon, France) as previously described (Prather et al., 2009). Samples were added to microplates coated with either IL-6 or CRP monoclonal antibodies, washed, biotinylated antibodies were added, and then samples were incubated with Streptavidin-bound horse radish peroxidase (HRP). All samples were measured in duplicate (450nm), and the average intra-assay and inter-assay coefficients of variation were below 5% and 10%, respectively. For the mixed-effect analyses, IL-6 levels were normalized using square-root transformation. Because serum was only obtained between 10AM and 4PM, we observed in prior studies that there was no relationship between IL-6 levels and time drawn (Prather et al., 2009).
Statistical Analyses
All statistics employed SPSS 18.0. For multivariate exams of depression incidence, Cox regression analyses were used. We used Kaplan-Meier with Mantel-Cox log rank comparisons to assess the incidence of categorical depression over time - using baseline measures of fatty acids that were dichotomized using the median value. Repeated-measure mixed-effect analyses, robust to randomly missing data, were used to compare changes over time. For these models, we first examined repeated covariance structures, selecting analyses which provided the smallest -2Log Likelihood (and typically the smallest AIC and BIC as well). Results are reported as mean +/- standard deviation, and in graphs are presented as mean +/- standard error of the mean.
RESULTS
The study sample (Table 1) was about 66% male and 83% European-American, primarily middle-aged, with low MADRS scores, but with some fatigue and poor sleep. This population on average had notably low omega-3 fatty acid levels (<1%), though fairly typical AA percentages (Table 1). Both IL-6 and CRP levels are indicated untransformed in this table. We used the cumulative illness rating scale-geriatric (CIRS-G) to quantify medical co-morbidity. All subjects have a minimum total score of 2, which is the minimum score for someone with HCV needing IFN-α. Subjects typically had less than two other co-morbid illnesses for a total CIRS-G score of about 4 (Table 1). For 41%, the only medical diagnosis was HCV. About 17% were also being treated for hypertension, 8% for diabetes, 3% for asthma, and 2% for hypercholesterolemia. About 56% smoked or chewed tobacco. Routine dietary assessments were not performed.
Table 1.
Subjects characteristics at baseline, prior to starting IFN-α, including Montgomery-Asperg Depression Rating Scale (MADRS), Beck Depression Inventory (BDI), Cumulative Illness Rating Scale- Geriatric (CIRS-G), interleukin- 6 (IL-6), C-reactiv protein (CRP), and the fatty acids docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), di-homo-gamma-linolenic acid (DGLA), and arachidonic acid (AA).
| Mean +/- SD | |
|---|---|
| Female | 34% |
| African-American | 17% |
| Age | 47.96 +/- 10.55 |
| Weight | 86.45 +/- 17.25 |
| MADRS | 3.38 +/- 4.21 |
| BDI | 8.2 +/- 7.2 |
| History of depression | 38.4% |
| CIRS-G total | 4.07 +/- 1.84 |
| CIRS-G number | 2.78 +/- 1.45 |
| CRP (untransformed) | 2.15 +/- 2.92 |
| IL-6 (untransformed) | 1.80 +/- 2.84 |
| DGLA | 1.93 +/- 0.67% |
| AA | 9.94 +/- 2.43% |
| EPA | 0.10 +/- 0.07% |
| DHA | 0.58 +/- 0.37% |
| AA/EPA+DHA ratio | 21.8 +/- 15.9 |
As noted in Table 2, both gender and age were associated with DHA fatty acids profiles (and ratios including DHA), but not the other fatty acids. For example, females had slightly lower AA/EPA+DHA ratios (18.7 +/- 11.8) than males (22.9 +/- 16.9). Racial self-identification was also an important variable. European-Americans had higher AA/EPA+DHA ratio (23.1 +/- 17.0) compared with African-Americans (14.6 +/- 7.0). Interestingly, weight was associated with none of the polyunsaturated fat percentages. We did not routinely document height, which would have allowed for calculation of BMI. In this cohort -- with ongoing liver inflammation because of chronic HCV infection, but specifically screened to not have active MDD -- none of the baseline fatty acids were correlated with baseline MADRS, BDI, PSQI, history of depression, or CRP levels (data not shown). However, AA was positively correlated with baseline IL-6 (square-root normalized) (R=0.21l p=0.024) and the AA/EPA+DHA ratio was positively correlated with baseline PSQI (R=0.25; p=0.013).
Table 2.
Linear correlations of demographics with baseline fatty acid profiles: omega-6 fatty acids, di-homo-gamma-linolineic acid (DGLA) and arachidonic acid (AA); and omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). For race, European ancestry = 1 and African ancestry = 2. For Gender, male = 1 and female = 2.
| Age | Weight | Race | Gender | |
|---|---|---|---|---|
| DGLA | B = 0.006 +/- 0.006 | B = 0.003 +/- 0.002 | B = -0.41 +/- 0.18 | B = 0.21 +/- 0.13 |
| p=0.32 | p=0.06 | p=0.02 | p=0.12 | |
| AA | B = -0.03 +/- 0.02 | B = 0.01 +/- 0.006 | B = 1.63 +/- 0.6 | B = -0.15 +/- 0.46 |
| p=0.15 | p=0.09 | p=0.008 | p=0.74 | |
| EPA | B = 0.001 +/- 0.001 | B = 0.00 +/- 0.00 | B = 0.003 +/- 0.022 | B = 0.004 +/- 0.016 |
| p=0.29 | p=0.26 | p=0.88 | p=0.82 | |
| DHA | B = 0.006 +/- 0.003 | B = 0.00 +/- 0.001 | B = 0.3 +/- 0.09 | B = 0.17 +/- 0.07 |
| p=0.04 | p=0.54 | p=0.001 | p=0.01 | |
| AA/EPA+DHA | B = -0.27 +/- 0.14 | B = -0.04 +/- 0.04 | B = -7.55 +/- 4.02 | B = -7.99+/- 3.07 |
| p=0.049 | p=0.26 | p=0.06 | p=0.01 |
In subsequent Cox Regression analyses to examine the incidence of depression during IFN–α treatment, age, race, and gender were therefore included as covariates. Lower baseline DHA predicted incidence of depression (B=-1.3 +/- 0.6; p=0.04), as did elevated DGLA (B=0.53 +/- 0.23; p=0.02), the ratio of DGLA+AA/DHA+EPA (B=0.013 +/- 0.006; p=0.04), and the specific hypothesis of an elevated AA/EPA+DHA ratio (B=1.22 +/- 0.45; P=0.007). However, EPA (B= -0.76 +/- 3.7; p=0.47), AA (B=0.12 +/- 0.08; p=0.14), and the AA/EPA ratio (B=0.002 +/- 0.002; p=0.35) were not significant predictors of depression. In these analyses, the only demographic covariate that continued to be significant was race. Of note however, the relationships between fatty acids and depression were similar for both people of European and African ancestry. For example, higher AA/EPA+DHA ratios were similarly associated with subsequent depression in both racial groups (Figure 1), although the sample size of African-Americans was too small to examine them separately.
Figure 1.

The ratio of AA/EPA+DHA at baseline is associated with subsequent major depression (MDD) development, regardless of race (F=4.0 p<0.05). Although African-Americans have lower AA/DHA+EPA ratios (F=11.0; p<0.001), there is no interaction between race and MDD on DHA levels (p=0.13).
Forward step-wise Cox regression was next employed, individually adding each of the fatty acid variables and covariates (including race and PSQI) to the model. Ultimately, in this forward step-wise model, only three of the factors continued to be significantly predictive of subsequent depression: PSQI (B = 0.12 +/- 0.04; p=0.006), race (B=1.5 +/- 0.5; p=0.006) and the AA/EPA+DHA ratio (B = 01.09 +/- 0.44; p=0.013). Likewise, employing a backward model -- in which all variables were each initially included in the model and then individually subtracted in a step-wise fashion -- PSQI (B = 0.13 +/- 0.04; p=0.003), race (B=1.9 +/- 0.6; p=0.002) and the AA/EPA+DHA ratio (B = 01.15 +/- 0.43; p=0.007) again continued to be the only three variables remaining that were predictive of depression. Thus, the AA/EPA+DHA ratio was the most informative summary of fatty acid status – and could predict subsequent depression in conjunction with both race and PSQI.
To help to justify the use of a median split in subsequent analyses, the role of the AA/EPA+DHA ratio in depression incidence was further examined using a median split in a Kaplan Meier survival analyses (Figure 2). Consistent with the results of the Cox analyses, individuals in the top median for AA/EPA+DHA ratios had a greater rate of developing depression (Mantel-Cox log rank X2 = 5.4; p=0.02). Of note, these findings were again similar in both African-Americans and European-Americans.
Figure 2.
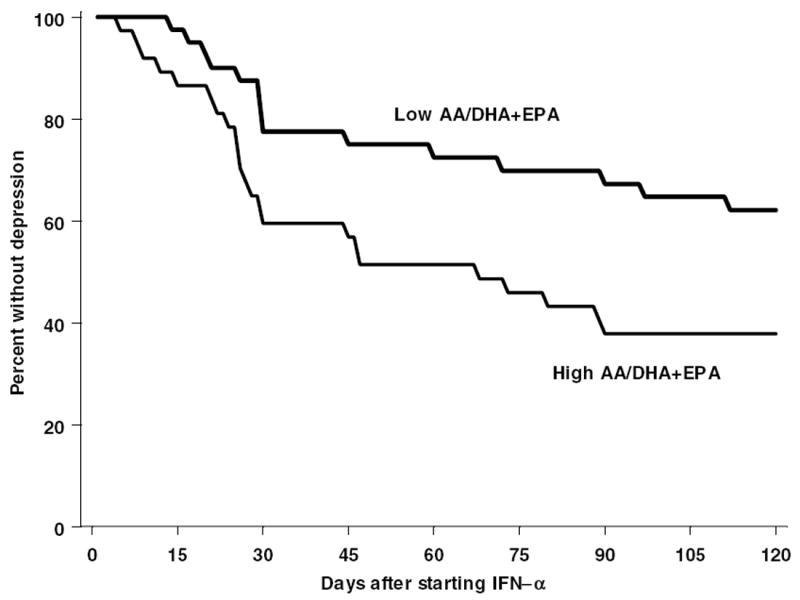
The ratio of AA to EPA and DHA at baseline (using a median split to define high and low) is associated with subsequent depression development after subjects initiate IFN-α therapy.
The median split of AA/EPA+DHA values was therefore next used in a mixed-effect repeated-measure analysis of MADRS depression scores over time (Figure 3). This fatty acid ratio predicted MADRS scores (F67.1,1= 4.0; p=0.049) as well as square-root normalized IL-6 levels (F65.5= 107.4; p=0.02) (Figure 4). However, the ratio AA/EPA+DHA did not have any consistent relationship with either BDI (p>0.2) or CRP (p>0.2). We also examined whether there was any relationship between fatty acid levels and subsequent sustained viral response to treatment (SVR). SVR was defined as undetectable hepatitis C for at least 3 months following the end of IFN-α treatment. As seen in Table 3, there were no fatty acid differences associated with subsequent SVR.
Figure 3.
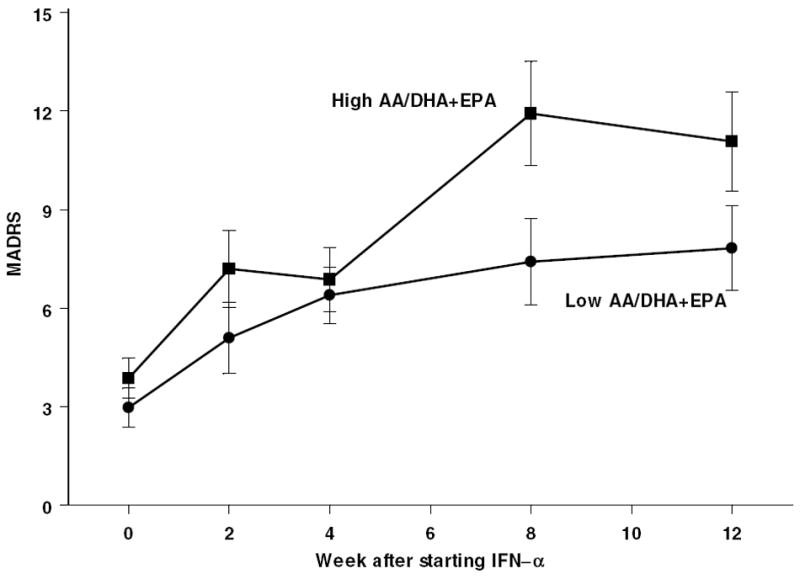
Montgomery-Asperg Depression Rating Scale scores (MADRS) increase during IFN-α treatment more in subjects with above median AA/EPA+DHA ratio.
Figure 4.
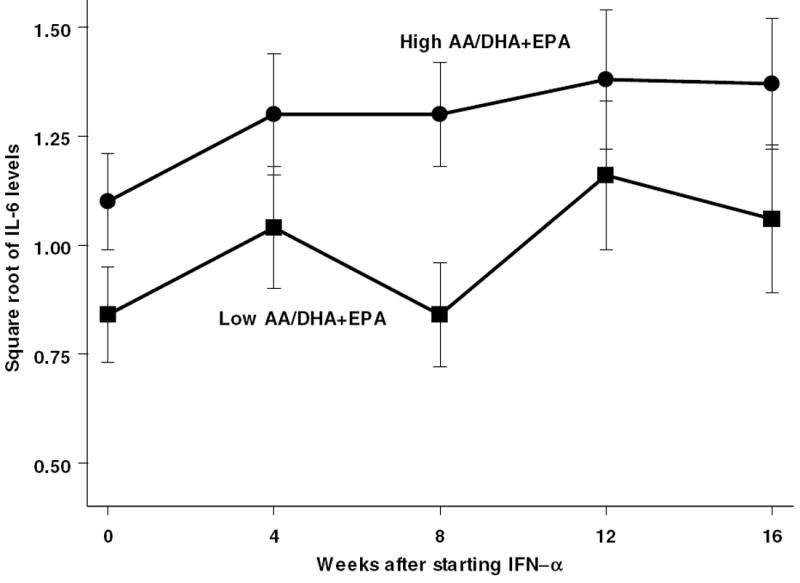
IL-6 scores are higher (square root transformed) throughout IFN-α treatment in subjects with above median AA/EPA+DHA ratio.
Table 3.
There are no differences in baseline fatty acid levels in people who have a sustained viral response (SVR), successfully clearing the hepatitis C viral infection after IFN-α treatment.
| +SVR | No SVR | |
|---|---|---|
| DGLA | 1.8 +/- 0.8 | 1.9 +/- 0.6 |
| AA | 9.6 +/- 2.3 | 9.6 +/- 2.3 |
| EPA | 0.09 +/- 0.06 | 0.11 +/- 0.07 |
| DHA | 0.49 +/- 0.33 | 0.54 +/- 0.39 |
| AA/DHA+EPA | 25.5 +/- 15.9 | 25.6 +/- 23.2 |
Discussion
These results provide prospective evidence that an elevated AA/EPA+DHA ratio is associated with increased vulnerability to developing depression in response to exposure to systemic inflammation. Additionally, the AA/EPA+DHA ratio was correlated with baseline sleep quality, and we have previously observed that poor sleep is a strong risk factor for depression during IFN-α therapy (Franzen et al., 2009; Prather et al., 2009). Notably therefore, the AA/EPA+DHA ratio continued to predict depression incidence even when including sleep in the model – suggesting that both are independent indicators of depression vulnerability. These results confirm and extend the findings of Su et al (Su et al., 2010), who found lower erythrocyte DHA levels were associated with depression during IFN-α treatment in 63 in Chinese subjects, and are consistent with accumulating evidence for the importance of omega-6/omega-3 ratios in chronic inflammatory diseases, including cardiovascular disease and MDD (Simopoulos, 2008).
Strengths of the study include the prospective assessment of depression development, the use of a standardized interview for diagnosis, and the confirmation of increased depression symptoms with an objective rating scale. Because fatty acid levels can be associated with race (Lemaitre et al., 2011; Sekikawa et al.; Sergeant et al., 2012), gender (Decsi and Kennedy, 2011) and age, another critical strength is that we also controlled for these potentially confounding variables. For enzymes in the fatty acid pathway, there are important genetic differences among those with Asian, African, and European ancestry (Lemaitre et al., 2011). A limitation of our study is that we did not have plasma or erythrocytes available from subjects after IFN-α was initiated, and cannot make any inferences regarding potential changes in fatty acid levels during therapy. Also, this is a unique population in several ways. That is, despite having active chronic HCV and despite over one-third having a past history of MDD, none of these subjects were depressed at the onset of IFN-α therapy. Low baseline depression and very low baseline omega-3 levels could be a potential explanation for our not finding any correlation between depression symptoms and fatty acid levels at baseline.
Omega-3 fatty acid supplements can affect cytokine synthesis (Vedin et al., 2008), and we have previously noted that elevated IL-6, and further ongoing increases in IL-6 during INF-α therapy, are predictive of increasing depression during IFN-α exposure (Prather et al., 2009). In the present study, we find that the AA/EPA+DHA ratio is associated with increased systemic IL-6 throughout therapy. This finding is also consistent with the observation that dietary-induced omega-3 fatty acid deficiency, and associated increase in the AA/EPA+DHA ratio, is associated with greater LPS-induced elevations in IL-6 in rodents (Mingam et al., 2008). Additionally, omega-3 fatty acids can modulate inflammation-induced transcription of TNF-α by inhibition of NF-kB (Novak et al., 2003), and we have previously found that genetic polymorphisms in TNF-α are associated with worsening psychiatric symptoms during IFN-α (Lotrich et al., 2010). Together, these and prior findings suggest that a higher AA/EPA+DHA ratio is associated with greater immune-inflammatory reactivity in response to a pro-inflammatory challenge, and that this greater reactivity is associated with increased vulnerability to developing depressive symptoms. In view of prior cross-sectional evidence that patients with MDD also exhibit a greater AA/EPA+DHA ratio (Adams et al., 1996; Conklin et al., 2007; Frasur-Smith et al., 2004; Maes et al., 1996; McNamara et al., 2010b; Tiemeier et al., 2003), this putative pathogenic mechanism may also be relevant to endogenous depression.
While the present prospective evidence is consistent with the hypothesis that an elevated AA/EPA+DHA ratio increases vulnerability to developing depression in response to exposure to systemic inflammation, definitive evaluation of this pathogenic mechanism will require evidence that decreasing the AA/EPA+DHA ratio is protective against the development of depression during IFN-α exposure. Prior controlled intervention trials have found that chronic dietary EPA+DHA supplementation, resulting in a reduction in the AA/EPA+DHA ratio, is associated with significant reductions in depression symptom severity in patients currently experiencing MDD (Freeman et al., 2006; Lin and Su, 2007). However, it is not currently known whether reducing the AA/EPA+DHA ratio can prevent the onset of depression (i.e., primary prevention). Related to this, although we found an association between baseline DGLA levels and depression symptoms, the role of DGLA is not clear. Adipose levels of DGLA correlate with depression symptoms in adolescents [Mamalakis et al., 2006]. But omega-3 supplementation could actually increase levels of DGLA [Cleland et al., 1990], possibly mitigating their benefit. Conversely, the PGE1 product of DGLA may be anti-inflammatory. Nonetheless, the present data suggest that this population (those receiving weekly injections of IFN-α) who exhibit extremely low EPA+DHA levels may be ideally suited to prospectively evaluate whether omega-3 supplementation can reduce depression risk. Moreover, omega-3 supplementation may even be protective against HCV-induced hepatosteatosis (Liu et al., 2010), and we demonstrate that omega-3 fatty acid status does not influence antiviral response. Thus this may be a safe approach to prevent depression in this population.
Highlights.
Elevated omega-6 fatty acids and lower omega-3 fatty acids were found to increase the risk for depression in people being treated with interferon-alpha.
Acknowledgments
Funding for this study was provided in part by NIMH grants R01 MH020950 (FEL) and MH083924 (RM). The NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The authors thank Mary Dinehart-Perry and Fermin Castro for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams PB, Lawson S, Sanigorski A, Sinclair AJ. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids. 1996;31:S157–S161. doi: 10.1007/BF02637069. [DOI] [PubMed] [Google Scholar]
- Bull SJ, Huezo-Diaz P, Binder EB, Cubells JF, Ranjith G, Maddock C, Miyazaki C, Alexander N, Hotopf M, Cleare AJ, Norris S, Cassidy E, Aitchison KJ, Miller AH, Pariante CM. Functional polymorphisms in the interleukin-6 and serotonin transporter genes, and depression and fatigue induced by interferon-a and ribavirin treatment. Mol Psychiatry. 2008;14:1095–1104. doi: 10.1038/mp.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Calder PC. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukotrienes and Essential Fatty Acids. 2008;79:101–108. doi: 10.1016/j.plefa.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacol. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry. 2004;56:819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Cleland LG, Gibson RA, Neumann M, French JK. The effect of dietary fish oil supplement upon the content of dihomo-gammalinolenic acid in human plasma phospholipids. Prostaglandins Leukotrienes & Essential Fatty Acids. 1990;40:9–12. doi: 10.1016/0952-3278(90)90108-w. [DOI] [PubMed] [Google Scholar]
- Conklin SM, Manuck SB, Yao JK, Flory JD, Hibbeln JR, Muldoon MF. High omega-6 and low omega-3 fatty acids are associated with depressive symptoms and neuroticism. Psychosomat Med. 2007;69:932–934. doi: 10.1097/PSY.0b013e31815aaa42. [DOI] [PubMed] [Google Scholar]
- Decsi T, Kennedy K. Sex-specific differences in essential fatty acid metabolism. Am J Clin Nutr. 2011;94:1914S–1919S. doi: 10.3945/ajcn.110.000893. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim E, Lanctot K. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Franzen PL, Buysse DJ, Rabinovitz M, Pollock BG, Lotrich FE. Poor sleep quality predicts onset of either major depression or subsyndromal depression with irritability during interferon-alpha treatment. Journal of Psychiatr Res. 2009;177:240–245. doi: 10.1016/j.psychres.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasur-Smith N, Lesperance F, Julien P. Major depression is associated with lower omega-3 fatty acid levels in patients with recent acute coronary syndromes. Biol Psychiatry. 2004;55:891–896. doi: 10.1016/j.biopsych.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry. 2006;67:1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- Hofacer R, Rider T, Jandacek R, Tso P, Magrisso IJ, Benoit SC, McNamara RK. Omega-3 fatty acid deficiency selectively up-regulates delta6-desaturase expression and activity indices in rat liver: Prevention by normalization of omega-3 fatty acid status. Nutr Res. 2011;31:715–722. doi: 10.1016/j.nutres.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Gronert K, Devchand P, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosomat Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI. Dietary n-3 PUFA deprivation for 15 weeks upregulates elongase and desaturase expression in rat liver but not brain. J Lipid Res. 2007;48:2463–2470. doi: 10.1194/jlr.M700315-JLR200. [DOI] [PubMed] [Google Scholar]
- Kodas E, Galineau L, Bodard S, Vancassel S, Guilloteau D, Besnard JC, Chalon S. Serotoninergic neurotransmission is affected by n-3 polyunsaterated fatty acids in the rat. J Neurochem. 2004;89:695–702. doi: 10.1111/j.1471-4159.2004.02401.x. [DOI] [PubMed] [Google Scholar]
- Kozak W, Soszynski D, Rudolph K, Conn DA, Kluger MJ. Dietary n-3 fatty acids differentially affect sickness behavior in mice during local and systemic inflammation. Am J Physiol. 1997;272:R1298–R1307. doi: 10.1152/ajpregu.1997.272.4.R1298. [DOI] [PubMed] [Google Scholar]
- Lemaitre RN, Tanaka T, Tang W, Manichaikul A, Foy M, Kabagambe EK, Nettleton JA, King IB, Weng L-C, Bhattacharya S, Bandinelli S, Bis JC, Rich SS, Jacobs DRJ, Cherubini A, et al. Genetic loci associated with plasma phospholipid n-3 fatty acids: a meta-analysis of genome-wide association studies from the CHARGE Consortium. PLoS Genetics. 2011;7:e1002193. doi: 10.1371/journal.pgen.1002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010;68:140–147. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68:1056–1061. doi: 10.4088/jcp.v68n0712. [DOI] [PubMed] [Google Scholar]
- Linthorst ACE, Flachskamm C, Holsboer F, Reul JMHM. Activation of serotonergic and noradrenergic neurotransmission in the rat hippocampus after peripheral administration of bacterial endotoxin: involvement of the cyclo-oxygenase pathway. Neurosci. 1996;72:989–997. doi: 10.1016/0306-4522(95)00604-4. [DOI] [PubMed] [Google Scholar]
- Liu Q, Bengmark S, Qu S. Nutrigenomics therapy of hepatisis C virus induced-hepatosteatosis. BMC Gastroenterol. 2010;10:49. doi: 10.1186/1471-230X-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotrich FE. Inflammatory cytokines, growth factors, and depression. Current Pharmaceutical Design. 2012;18 doi: 10.2174/138161212803523680. [DOI] [PubMed] [Google Scholar]
- Lotrich FE, Ferrell RE, Rabinovitz M, Pollock BG. Risk for depression during interferon-alpha treatment is affected by the serotonin transporter polymorphism. Biol Psychiatry. 2009;65:344–348. doi: 10.1016/j.biopsych.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotrich FE, Ferrell RF, Rabinovitz M, Pollock BG. Labile anger during interferon-alpha treatment is associated with a polymorphism in tumor necrosis factor-alpha. Clin Neuropharmacol. 2010;33:191–197. doi: 10.1097/WNF.0b013e3181de8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotrich FE, Rabinovitz F, Gironda P, Pollock BG. Depression following pegylated interferon-alpha: characteristics and vulnerability. J Psychosomat Res. 2007;63:131–135. doi: 10.1016/j.jpsychores.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Smith R, Christophe A, Cosyns P, Desnyder R, Meltzer H. Fatty acid composition in major depression: decreased omega 3 fractions in cholesteryl esters and increased C20:4 omega 6/C20:5 omega 3 ratio in cholesteryl esters and phospholipids. J Affect Dis. 1996;38:35–46. doi: 10.1016/0165-0327(95)00092-5. [DOI] [PubMed] [Google Scholar]
- Mamalakis G, Kiriakakis M, Tsibinos G, Hatzis C, Flouri S, Mantzoros C, Kafatos A. Depression and serum adiponectin and adipose omega-3 and omega-6 fatty acids in adolescents. Pharm Biochem & Behav. 2006;85:474–9. doi: 10.1016/j.pbb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Cole-Strauss A, Lipton JW. Omega-3 fatty acid deficiency increases constitutive pro-inflammatory cytokine production in rats: Relationshipwith central serotonin turnover. Prostaglandins Leukotrienes and Essential Fatty Acids. 2010a;83:185–191. doi: 10.1016/j.plefa.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, Pandey GN. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. J Affect Dis. 2010b;126:303–311. doi: 10.1016/j.jad.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingam R, Moranis A, Bluthe R-M, De Smedt-Peyrusse V, Kelley KW, Guesnet P, Lavialle M, Dantzer R, Laye S. Uncoupling of interleukin-6 from its signalling pathway by dietary n-3-polyunsaturated fatty acid deprivation alters sickness behaviour in mice. Eur J Neurosci. 2008;28:1877–1886. doi: 10.1111/j.1460-9568.2008.06470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, Greiner K, Nemeroff CB, Miller AH. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- Novak TE, Babcock TA, Jho DH, et al. NF-kB inhibition by omega-3 fatty acids modulates LPS-stimulated macrophage TNF-a transcription. Am J Physiol Cell Molec Physiol. 2003;284:L84–L89. doi: 10.1152/ajplung.00077.2002. [DOI] [PubMed] [Google Scholar]
- Portanova JP, Zhang Y, Anderson GD, Houser SD, Masferrer JL, Seibert K, Gregory SA, Isakson PC. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J Exp Med. 1998;184:883–891. doi: 10.1084/jem.184.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather AA, Rabinovitz M, Pollock BG, Lotrich FE. Cytokine-induced depression during IFN-α treatment: the role of IL-6 and sleep quality. Br Behav Immun. 2009;23:1109–1116. doi: 10.1016/j.bbi.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Woolwine BJ, Massung B, Vogt G, Miller AH. Interferon-[alpha] effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflammatory cytokines and behavior. Mol Psychiatry. 2010;15:535–547. doi: 10.1038/mp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao JS, Ertley RN, DeMar JC, Rapoport SI, Bazinet RP, Lee HJ. Dietary n-3 PUFA deprovation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol Psychiatry. 2007;12:151–157. doi: 10.1038/sj.mp.4001887. [DOI] [PubMed] [Google Scholar]
- Sekikawa A, Curb JD, Ueshima H, El-Saed A, Kadowaki T, Abbott RD, Evans RW, Rodriguez BL, Okamura T, Sutton-Tyrrell K, Nakamura Y, Masaki K, Edmundowicz D, Kashiwagi A, Willcox BJ, Takamiya T, Mitsunami K-i, Seto TB, Murata K, White RL. Marine-Derived n-3 Fatty Acids and Atherosclerosis in Japanese, Japanese-American, and White Men: A Cross-Sectional Study. J Am College Cardiol. 52:417–424. doi: 10.1016/j.jacc.2008.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant S, Hugenschmidt CE, Rudock ME, Ziegler JT, Ivester P, Ainsworth HC, Vaidya D, Case LD, Langefeld CD, Freedman BI, Bowden DW, Mathias RA, Chilton FH. Difference in arachidonic acid levels and fatty acid desaturase (FAD5) gene variants in African Americans and European Americans with diabetes or the metabolic syndrome. Br J Nutr. 2012;107:547–555. doi: 10.1017/S0007114511003230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos AP. The importance of the omega-6/omega-3 fatty acid raion in cardiovascular disease and other chronic diseases. Exp Biol Med. 2008;233:674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- Song C, Leonard BE, Horrobin DF. Dietary ethyl-eicosapentaenoic acid but not soybean oil reverses central interleukin-1-induced changes in behavior, coricosterone and immune responses in rats. Stress. 2004;7:43–54. doi: 10.1080/10253890410001667188. [DOI] [PubMed] [Google Scholar]
- Song C, Li X, Leonard BE, Horrobin DF. Effects of dietary n-3 or n-6 fatty acids on interleukin-1beta-induced anxiety, stress, and inflammatory responses in rats. J Lipid Res. 2003;44:1984–1991. doi: 10.1194/jlr.M300217-JLR200. [DOI] [PubMed] [Google Scholar]
- Su K-P, Huang S-Y, Peng C-Y, Lai H-C, Huang C-L, CHen Y-C, Aitchison KJ, Pariante CM. Phospholipase A2 and cycloxygenase 2 genes influence the risk of interferon-a-induced depression by regulating polyunsaturated faty acids levels. Biol Psychiatry. 2010;67:550–557. doi: 10.1016/j.biopsych.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA, Mann JJ. Omega-3 polyunsaturated essential fatty acid status as a predictor of future suicide risk. A J Psychiatry. 2006;163:1100–1102. doi: 10.1176/ajp.2006.163.6.1100. [DOI] [PubMed] [Google Scholar]
- Tiemeier H, van Tuijl HR, Hofman A, Kiliaan AJ, Breteler MM. Plasma fatty acid composition and depression are associated in the elderly: the Rotterdam study. Am J Clin Nutr. 2003;78:40–46. doi: 10.1093/ajcn/78.1.40. [DOI] [PubMed] [Google Scholar]
- Vedin I, Cederholm T, Levi YF, Basun H, Garlind A, Faxén Irving G, Jönhagen ME, Vessby B, Wahlund L-O, Palmblad J. Effects of docosahexaenoic acid–rich n-3 fatty acid supplementation on cytokine release from blood mononuclear leukocytes: the OmegAD study. Am J Clin Nutr. 2008;87:1616–1622. doi: 10.1093/ajcn/87.6.1616. [DOI] [PubMed] [Google Scholar]
- Wang P, Zhu F, Konstantopoulos K. Prostaglandin E2 induces interleukin-6 expression in human chondrocytes via cAMP/protein kinase A- and phosphatidylinositol 3-kinase-dependent NF-kappaB activation. Am J Physiol Cell Physiol. 2010;298:C1445–1456. doi: 10.1152/ajpcell.00508.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K. The relationship of depression and stressors to immunological assays: a meta-analytic review. Br Behav Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]


