Abstract
The conformational changes in myosin associated with ADP release and their influence on actin sliding velocity are not understood. Following actin binding, the myosin active site is in equilibrium between a closed and open ADP bound state, with the open state previously thought to favor ADP release and thus expected to be favored in faster myosins. However, our recent work with a variety of myosins suggests the opposite, that the open conformation is dominant in slower myosins, which have higher ADP affinities. To test if this correlation holds for fast myosin isoforms, we determined the relationships between conformational pocket dynamics, ADP affinity and velocity of four Drosophila myosins: indirect flight muscle (IFM) myosin (IFI), embryonic muscle myosin (EMB) and two IFI/EMB chimeras. Electron paramagnetic resonance (EPR) spectra of nucleotide-analog spin probes (SLADP) bound to IFI subfragment-1 (IFIS-1) in the absence of actin showed a high degree of immobilization, indicating a predominately closed nucleotide pocket. The A•M•SLADP spectra of all four myosins in fibers (actin bound) also indicated an equilibrium favoring the closed conformation with the closed state closing even further. However, the energetics of pocket closure did not correlate with Drosophila myosin actin velocity suggesting our previous model relating pocket dynamics to velocity does not hold for fast myosin isoforms. We conclude that for these fast myosins, and possibly other fast myosins, velocity is controlled by factors other than the ratio of open to closed nucleotide pocket conformation.
Keywords: myosin, muscle, Drosophila, sliding velocity, EPR, ADP
INTRODUCTION
Myosin-driven motility is the result of an orchestrated interaction between the motor, myosin (M), its energy source, ATP, and the polymer roadway upon which directed motion occurs, actin (A). ATP binds to myosin and its hydrolysis provides the chemical free energy to power motion. Following hydrolysis, M•ADP•Pi binds to actin. Pi and ADP are sequentially released from the A•M•ADP•Pi myosin cross-bridges, accompanied by a decrease in free energy of the actomyosin complex that is captured to produce force and motion (reviewed in Cooke 1990; Sweeney and Houdusse 2010). The sliding velocity of myosin varies approximately 1000-fold, from a lower limit of 50 nm/sec in human myosin-IXb (MYO9b) (Inoue et al. 2002; Post et al. 2002) to an upper limit of 60 μm/sec in Chara myosin XI (myoA) (Higashi-Fujime et al. 1995). The isoform specific differences that allow myosin to perform its multitude of different tasks in the cell, and the conformational changes in myosin that are associated with the conversion of chemical free energy into mechanical energy, remain unresolved.
EPR probes are valuable tools to monitor conformational changes in proteins. They can cleanly resolve multiple spectral components more easily than fluorescence spectroscopy. When bound to a protein, thermal agitation results in the EPR probe executing a random motion that is limited by the adjacent protein surface. In the presence of an external magnetic field, the motion gives rise to a magnetic resonance spectrum consisting of three lines, with spectral width inversely proportional to the magnitude of the spatial volume the probe can explore (reviewed in Griffith and Jost 1976).
Nucleotide-analog EPR probes are advantageous as they allow the experimenter to specifically target and monitor the nucleotide site of the myosin motor (reviewed in (Naber et al. 2011) and references therein). EPR spectroscopy of nucleotide-analog probes, with the spin probe attached to the 2′-carbon of the ribose, was initially used to monitor the conformation of the nucleotide site of fast and slow skeletal myosin (Naber et al. 2007). The EPR spectra indicated that in the absence of actin, the nucleotide pocket was in a closed conformation. When myosin bound actin an additional component from a more mobile EPR probe was present in the spectrum. This indicated an opening of the nucleotide pocket upon binding to actin. The terms open and closed used here refer to the conformation of the nucleotide pocket as sensed by the mobility of the spin probe attached to the ribose. Previous work has suggested that changes in mobility are likely due to changes in the conformation of the switch 1 region (Naber et al. 2007). The opening of the nucleotide pocket was not a complete transition as the closed conformation remained present, suggesting an equilibrium between the two conformations. Subsequent studies of Dictyostelium myosin II (Naber et al. 2010), myosin V (Purcell et al. 2011a,b), cardiac atrial and ventricular myosin, Drosophila IFM myosin (IFI), and smooth muscle myosin reached the same conclusion (Purcell et al. 2011a). An unexpected observation was that the more tightly ADP bound to the actomyosin complex for a specific isoform, the more the equilibrium between open and closed conformations of the nucleotide pocket for the isoform was shifted in favor of the open conformation of the nucleotide pocket. This appeared counterintuitive, as a closed conformation of the nucleotide pocket would be expected to enhance nucleotide-protein interactions, slowing ADP release and hence velocity as it has generally been thought that ADP release is rate limiting for most myosins (Siemankowski et al. 1985). Instead, we found an unanticipated correlation between increased closed conformation probability and increased velocity (Purcell et al. 2011a,b). This correlation led to a new model relating the energetics of the ADP bound states to filament sliding velocity and to mechanical efficiency.
Drosophila myosin isoforms are ideal to test this unexpected observation between nucleotide-binding pocket conformation, velocity and ADP affinity as they are among the fastest myosin isoforms known. Previously, we only examined Drosophila indirect flight muscle (IFM) myosin (IFI). This myosin was the only one to show a predominately closed pocket (positive ΔG0) with ADP bound and when bound to actin. Here, we examine a second relatively fast myosin isoform, an embryonic Drosophila myosin isoform (EMB) (Swank et al. 2003), and two Drosophila myosin chimeras to better examine the relation of velocity and ΔG0 at the high end of the velocity scale. The chimeras were previously created by exchanging alternatively encoded exon regions from EMB into IFI (Swank et al. 2002; Miller et al. 2005). The pocket dynamics of the chimeric isoforms are particularly interesting to examine as their ADP affinities do not correlate with actin sliding velocity in the motility assay (Table 1) (Swank et al. 2002; Miller et al. 2005). This allows us to independently test if pocket conformation at the nucleotide site, as determined by EPR, correlates with ADP affinity or if pocket conformation correlates with velocity.
Table 1.
Values of Keq, corresponding values for ΔG0, sliding velocity, and dissociation constants for the Drosophila myosin isoforms examined. ΔG0 = −RTln(Keq)where Keq = [fraction of myosin showing the more mobile component of the EPR spectrum]/[fraction showing the more immobilized component of the EPR spectrum]. IFI, EMB, IFI-7a velocities are from (Miller et al. 2005). IFI-EC is unpublished data collected at the same time as data described in (Miller et al. 2005). Values for velocity are adjusted to 25°C assuming a Q10 of 2. KAD values measured from S-1 in solution are from (Miller et al. 2003; Miller et al. 2007).
| Myosin Isoform in IFM | ΔG0 (kJ/mol) Low temperature | ΔG0 (kJ/mol) High temperature | Keq (Low temperature), Percent Immobile | Keq (High temperature), Percent Immobile | KAD (μM) | Velocity (μm/s) |
|---|---|---|---|---|---|---|
| IFI | 4.2 (4°C) | 2.9 (23°C) | 0.16 (4°C), 86% | 0.31 (23°C), 76% | 409 | 7.0 |
| EMB | 4.2 (4°C) | 2.7 (22°C) | 0.16 (4°C), 86% | 0.33 (22°C), 75% | 587 | 4.9 |
| IFI-EC | 3.9 (2°C) | 2.7 (23°C) | 0.18 (2°C), 85% | 0.34 (23°C), 75% | 838 | 6.8 |
| IFI-7a | 3.1 (4°C) | 2.3 (23°C) | 0.26 (4°C), 79% | 0.39 (23°C), 72% | 239 | 7.6 |
Thus, we have a novel set of myosins with high actin sliding velocities coupled with a significant range of ADP off-rates with which to probe the relationships between myosin nucleotide pocket conformation, ADP biochemistry and sliding velocity. We found that the overwhelming majority (>75%) of the nucleotide pockets of the Drosophila isoforms are in the closed conformation in the A•M•D state despite their extremely rapid sliding velocities. The lack of a correlation between the energetics of pocket conformation and velocity within these Drosophila myosin isoforms suggests that our previous model does not hold for very fast myosin isoforms and possibly slower myosins. Our results support the hypothesis that a mechanism other than ADP affinity controls sliding velocity for very fast myosins.
METHODS
Spin labeling of proteins
Muscle fibers and myosin S-1 for the experiments were isolated from fly lines previously generated by P-element mediated transformation (Cripps and Bernstein 2000). IFI is myosin normally found in wild type IFM that has been transgenically expressed in a null IFM background, Mhc10 (Swank et al. 2000). IFI also serves as a positive control for the three other transgenic myosin fly lines. EMB is an embryonic myosin expressed in IFM fibers in the Mhc10 background (Wells et al. 1996). IFI-EC has the EMB version of the converter exchanged into IFI, and IFI-7a has the exon 7 region of EMB (Swank et al. 2002; Miller et al. 2005). The chimeric myosins were also expressed in IFM fibers in the Mhc10 background.
IFM fibers were isolated from all four Drosophila lines as previously described (Swank et al. 2002; Swank 2012). The fibers were exchanged into rigor buffer (120 mM KOAc, 5 mM MgCl2, 1 mM EGTA, 40 mM MOPS, pH 7.0) by three centrifugation (12,000×g) and re-suspension steps. The final re-suspension was in rigor buffer with the [KOAc] reduced to 25 mM. Fibers were then spin labeled by addition of 10 μM 2′-SLATP and concentrated by centrifugation (10,000×g). The pellet was isolated and then placed on a quartz flat cell, surrounded by vacuum grease, and covered with a flat cell to prevent dehydration. For experiments involving BeFx, 10 mM NaF and 2 mM BeF3 were added to the buffers resulting in a 2 mM concentration of the fluoride complex.
Myosin subfragment-1 (S-1) was prepared by alpha-chymotrypsin digestion of myosin isolated from IFM fibers as previously described (Miller et al. 2003). S-1 (40 μM) was exchanged into the rigor buffer using a Centricon concentrator followed by three successive washes. 2′-SLATP (25 μM) was added to the resulting myosin S-1 solution. The solution was inserted into a 25-ml capillary for EPR spectra recording. The triphosphate species is hydrolyzed to the diphosphate species by myosin or actomyosin. Thus all spectra shown are of the 2′-SLADP complex. Spin-labeled nucleotides were synthesized as previously described (Crowder and Cooke 1987). The structure of 2′-SLADP is given in (Naber et al. 2007).
EPR spectroscopy
The capillary or flat cell containing the labeled protein sample was placed in the spectrometer cavity. First-derivative, X-band EPR spectra were accumulated in a Bruker EMX spectrometer (Bellerica, MA) using a high-sensitivity microwave cavity and 50-sec, 10-mT-wide sweeps. The instrument settings were: microwave power, 25 mW; time constant, 164 ms; frequency, 9.83 GHz; modulation, 0.1 mT at a frequency of 100 kHz. Each spectrum used in data analysis was an average of 5 to 50 sweeps from an individual experimental preparation. Temperature was controlled by blowing dry air (warm or cool) into the cavity and monitored using a thermistor placed close to the experimental sample. Effective cone angles of mobility can be approximated using the order parameter S=(T||′ − T0)/(T|| − T0) as a measure of probe mobility. Here 2T||′ is the observed splitting, 2T|| is the splitting for an immobilized probe (7.20 mT) and 2T0 is the isotropic hyperfine splitting for freely tumbling 2′-SLADP in solution (3.22 mT). The cone angle is then given by cos θ= −0.5+0.5*(1+8S)1/2, where 2θ is the vertex angle of the cone of mobility (Griffith and Jost 1976; Alessi et al. 1992).
RESULTS
Myosin S-1
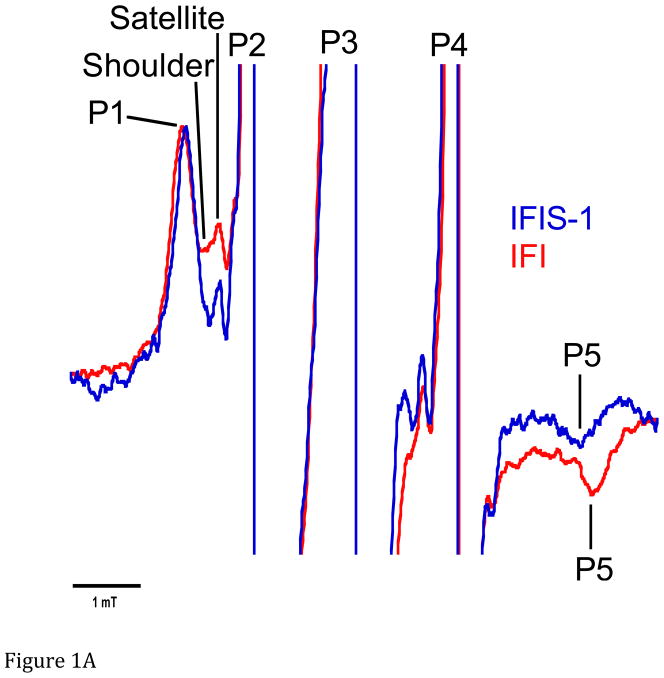
We found that Drosophila indirect flight muscle myosin S-1 bound to 2′-SLADP (IFIS-1•2′-SLADP) produces a characteristic EPR spectrum (Figure 1A) similar to other myosins previously measured. The components include three central large peaks, P2-P4, (truncated to enhance resolution of other spectral components) that are primarily due to unbound 2′-SLADP tumbling rapidly in solution. The sharp, small peak to the immediate left of peak P2 also comes from unbound probe. It is termed a satellite peak. This peak arises from the interaction of the unpaired electron in the spin moiety of unbound probe tumbling in solution with the 13C (methyl groups) isotopes (Nordio 1976). The decreased mobility of the EPR probe bound at the nucleotide site produces a broader EPR spectrum as seen by the addition of the P1 and P5 peaks to the spectrum. The P1-P5 splitting for IFIS-1•2′-SLADP is 6.16 mT (Figure 1A). This corresponds to an effective cone of mobility with a vertex angle of 71°. The shoulder to the right of P1 is also due to bound probe, but these probes have greater mobility than the probes giving rise to P1 and P5. This suggests the pocket also exists in a second, more mobile conformation. While this second population is larger and measurable for myosin bound to actin (see below), in our S-1 spectrum its population, probably <5%, is too small to measure reliably.
Figure 1.
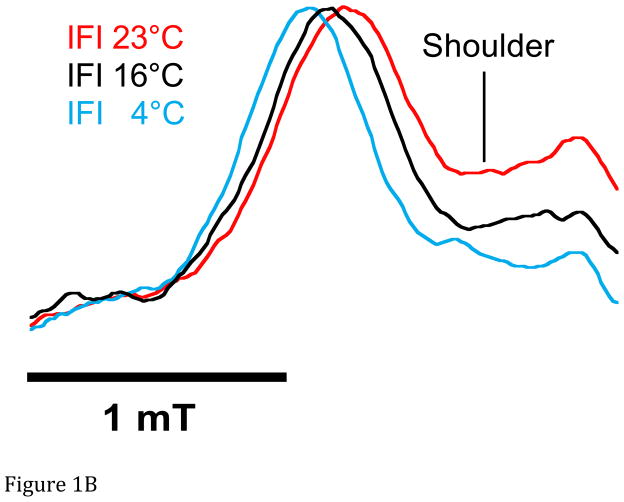
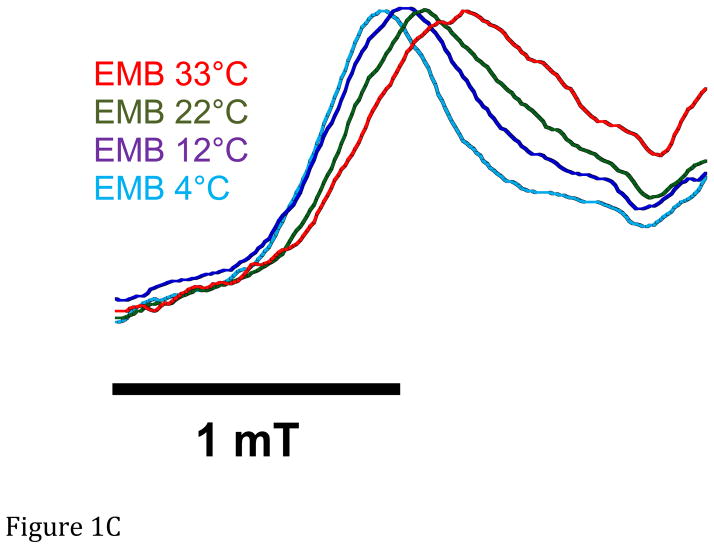
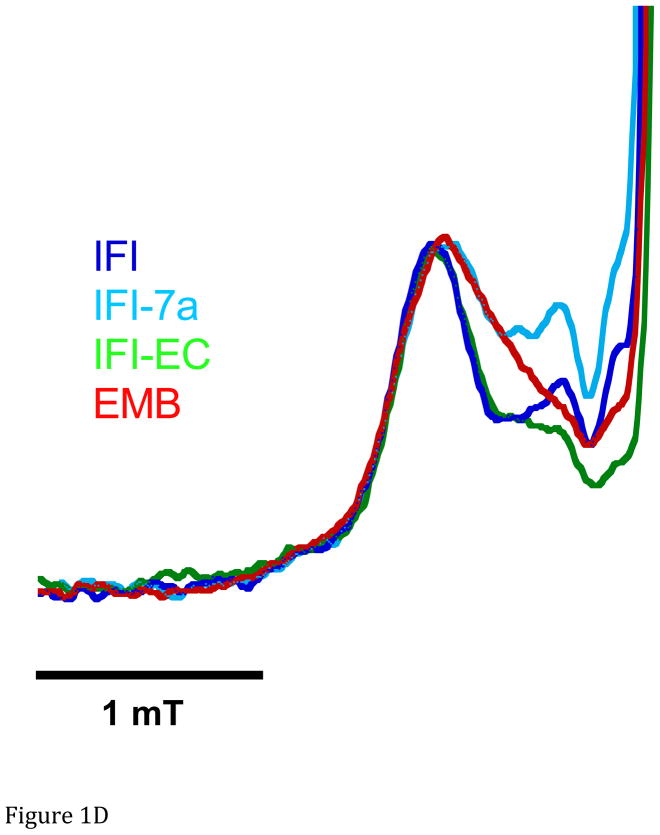
A. Representative spectra of Drosophila indirect flight muscle S1 (IFIS-1) bound to 2′-SLADP (blue) and A•M•2′-SLADP bound to IFI in IFM fibers (red). Both spectra are at 23°C. The vertical axis is the derivative of absorption. The horizontal axis is magnetic field. Center field is 350.6 mT. B. Low field, P1, component of A•M•2′-SLADP bound to IFI in IFM fibers at 4°C (cyan), 16°C (black) and 23°C (red). There is a clear, high-field shoulder to the P1 component of the spectrum that changes with temperature. C. Low field, P1, component of A•M•2′-SLADP bound to embryonic myosin (EMB) in IFM fibers at 4°C (red), 12°C (blue), 22°C (green) and 33°C (cyan). D. Low field component of the A•M•2′-SLADP EPR spectrum comparing four different acto-myosin•SL-ADP complexes, IFI (blue), IFI-7a (cyan) EMB (red) and IFI-EC (green) at 23°C. All spectra are normalized to have the same magnitude for the primary peak in the P1 component.
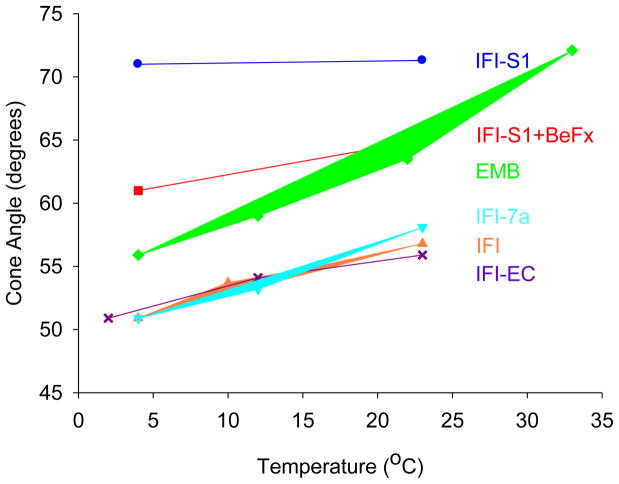
The spectrum of IFIS-1•2′-SLADP•BeFx showed a closed nucleotide pocket with little evidence of the open conformation, although the cone angle associated with the closed state is slightly smaller (Figure 2). Since ADP•BeFx is an analog of ATP, this suggests there is little difference in the conformation of the nucleotide site between the diphosphate and triphosphate states. A similar lack of significant difference between the diphosphate and triphosphate state has been seen with other myosin isoforms (Naber et al. 2007; Naber et al. 2010; Purcell et al. 2011a,b). The spectra of both IFIS-1•2′-SLADP and IFIS-1•2′-SLADP•BeFx showed little to no change with temperature (Figure 2).
Figure 2.
Cone angles of mobility for 2′-SLADP bound to Drosophila S-1, and myosin in skinned IFM fibers as a function of temperature. Effective cone angles of mobility were approximated using the order parameter S=(T||′ − T0)/(T|| − T0) as a measure of probe mobility. 2T||′ is the observed splitting, 2T|| is the splitting for an immobilized probe (7.20 mT) and 2T0 is the isotropic hyperfine splitting for freely tumbling 2′-SLADP in solution (3.22 mT). The cone angle is then given by cos θ = −0.5+0.5*(1+8S)1/2, where 2θ is the vertex angle of the cone of mobility (Griffith and Jost 1976; Alessi et al. 1992). Each value is the mean of 1–4 distinct observations. The standard errors of the EPR cone angles are ± 0.18 degrees.
IFI myosin bound to actin in IFM fibers
We observed two significant changes in the EPR spectrum of IFI A•M•2′-SLADP (SLADP bound to full-length, wild type myosin in IFM fibers) compared to the S-1 spectrum (Figure 1A). An increase in P1-P5 splitting indicated that the nucleotide binding site closes between 16° and 20°, depending on temperature, when IFI binds to actin (Figure 1A and Figure 2). Second, the additional, distinct high-field shoulder of the P1 component becomes more prominent compared to the shoulder in the S-1 spectrum (Figure 1B). This additional shoulder component has a smaller low-field to high-field splitting which indicates greater probe mobility suggesting a more open conformation of the nucleotide pocket exists in equilibrium with a more closed conformation.. This shoulder component has been seen in a variety of myosin types (Purcell et al. 2011a). The intensity of the shoulder is at a different magnetic-field location than that of IFI-S1, indicating it does not come from myosin heads that are not bound to actin.
Temperature influenced both the degree of opening of the active site and the ratio of open to closed sites. As temperature increased, the P1-P5 splitting of spectra from IFI A•M•2′-SLADP in fibers decreased slightly indicating a slight additional opening of the closed conformation of the nucleotide site, a cone angle increase of 7° (Figure 1B and Figure 2). The relative intensity of the high-field shoulder of the P1 peak increased with temperature indicating a shift toward the open conformation (Figure 1B). To quantify the ratio of open to closed states, we deconvolved the spectra into a linear sum of the two respective components as described previously (Sindelar et al. 2002; Naber et al. 2003; Purcell et al. 2011a). Letting Keq = [fraction of myosin showing the more mobile component of the spectrum]/[fraction showing the more immobilize component], the deconvolution of the spectra of IFI A•M•2′-SLADP in fibers shows Keq varies from 0.16 at 4°C to 0.31 at 23°C (Table 1). Thus 86% of the myosin in the strongly bound IFI•2′-SLADP state have nucleotide pockets that are in the more closed conformation at the lower temperature, and 76% are closed at the higher temperature. Correspondingly, with R = Boltzmann’s constant and T= absolute temperature, then ΔG0 = −RTln(Keq) for the closed-to-open transition varies from 4.2 kJ/mol at 4°C to 2.9 kJ/mol at 23°C (Table 1).
EMB myosin bound to actin in IFM fibers
Expressing EMB myosin in Drosophila IFM fibers caused a couple changes in the 2′-SLADP spectra (Figure 1C). We observed a decrease in splitting, indicating a 5° degree increase in probe cone angle compared to IFI (Figure 1D and Figure 2). As was the case for IFI fibers, the P1-P5 splitting decreases with increasing temperature, corresponding to an opening of the immobilized component’s cone angle by 8° for the same temperature range (4° to 22–23° C) as IFI (Figure 2). This was not significantly different compared to the 6° opening observed for IFI. The shoulder on the P1 component was also present in the EMB spectra, again implying an equilibrium between a more closed and more open conformation of the nucleotide site in the A•M•2′-SLADP state (Figure 1C). The equilibrium was the same as observed for IFI, as deconvoluting the spectra showed Keq and ΔG0, at low and high temperatures, were not significantly different compared to IFI values (Table 1).
Chimeric myosins bound to actin in IFM fibers
The two chimeric myosins expressed in IFM fibers showed A•M•2′-SLADP spectra, for the more immobilized (closed) component, that were more similar to spectra from IFI than EMB (Figure 1D and Figure 2). The chimeras’ trend of increased cone angle with temperature did not significantly differ from IFI’s cone angle trend with temperature (Figure 2). As was the case for IFI and EMB, there is a partitioning of the nucleotide sites into more open and more closed conformations. The ratio of the open conformation to closed conformation (Keq) favored the more immobile component, similar to what we observed for IFI and EMB. (Table 1) However, there appears to be a tendency for the more open conformation in IFI-EC when compared to IFI. This tendency is even more pronounced for IFI-7a.
DISCUSSION
We have investigated the conformation dynamics of the nucleotide site of four very fast Drosophila myosin isoforms. Examining the fastest known muscle myosins provides insight into how actin sliding velocity is influenced by conformational changes associated with ADP and actin binding. We found that the nucleotide sites of all four ADP-bound myosin isoforms are in one of two states, one with a highly restricted probe mobility, which we call the closed state, and one with a greater probe mobility, which we call the open state. The closed nucleotide state is strongly favored when myosin in not bound to actin. This is similar to what has been observed for S-1 and myosin isoforms from other species (Purcell et al. 2011a).
Previously, investigators thought that myosin’s nucleotide site should switch to a more open state when myosin binds actin to favor ADP release. This notion was based on EPR studies of slow myosins such as myosin V that showed a switch from a closed to open state upon actin binding (Sun et al. 2006; Purcell et al. 2011a). Our findings showed almost the opposite trend. For Drosophila myosins bound to actin in IFM fibers, we observed a small increase in the percentage of myosins with an open nucleotide site compared to IFI S-1. The increase was from < 5% for IFIS-1 to 15% or 28% for myosin in IFM fibers, depending on temperature and isoform. Thus, the actin-bound equilibrium still strongly favors the closed conformation of the nucleotide site for the Drosophila myosins. Additionally, the closed conformation of the Drosophila myosins is even more tightly closed with actin bound than it is in the absence of actin, a difference in the effective cone angle of mobility of approximately 18°. In all other myosins that have been examined, there are only trivial changes in the cone angle of the closed state following actin binding.
Drosophila myosins are the first observed where a majority (>75%) of the cross-bridges in fibers have a closed nucleotide-binding site when ADP is bound at temperatures at which the myosins typically function, ~37°C for mammals and about 15°C to 25°C for flies. For myosins from all other species that have been studied, the open conformation is more prevalent (Naber et al. 2007; Naber et al. 2010; Purcell et al. 2011a; Purcell et al. 2011b) at higher temperatures. Drosophila myosin’s equilibrium favoring the closed conformation of the nucleotide pocket appears counterintuitive, since ADP release at the end of the working cross-bridge power-stroke is widely assumed to be a significant rate-limiting contributor to active sliding velocity (Siemankowski et al. 1985; Weiss et al. 2001). Additionally, ADP has a weaker affinity for the actomyosin complexes of the Drosophila isoforms compared to most vertebrate myosins. For example, the affinities are 2 to 4 -fold lower than rabbit S-1 (Miller et al. 2003; Silva et al. 2003). An open, not a closed nucleotide-binding site would be expected to weaken nucleotide-protein interactions and thus facilitate ADP release. However, the relationship between nucleotide affinity and the conformation of the nucleotide pocket is clearly complex. The X-ray structures of kinesin•ADP and ncd•ADP show extremely wide-open nucleotide pockets, despite the fact that ADP binding to the proteins is very tight (Kull et al. 1996; Sablin et al. 1996).
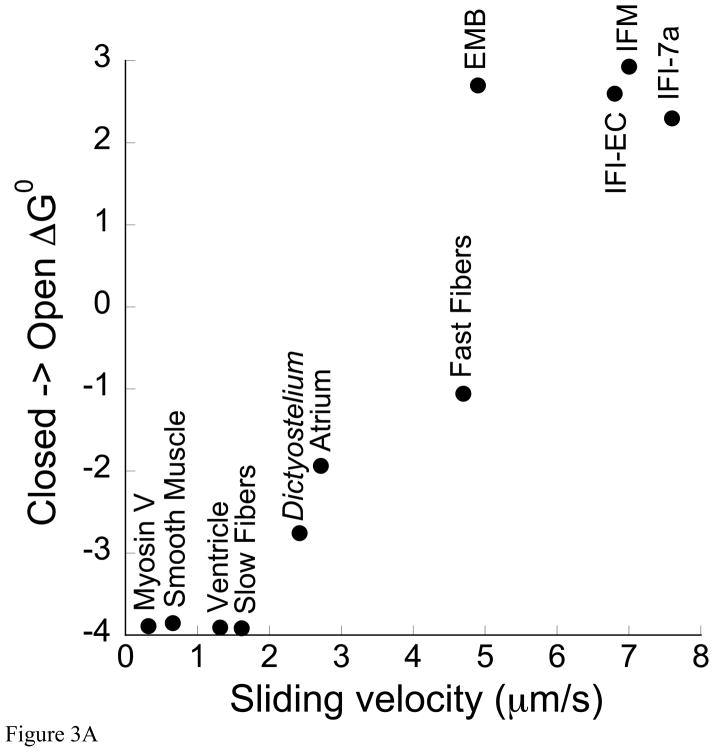
Data from different myosin isoforms with a 20-fold difference in sliding velocities indicated that the relationship between sliding velocity and the ΔG0 associated with the transition between the closed and open conformations of the nucleotide site could be reconciled by invoking a partitioning of free energy between work generating actomyosin•ADP states prior to ADP release (Purcell et al. 2011a). This analysis showed that the degree to which the nucleotide pocket stays open (measured by the ΔG0 for the closed-to-open transition) inversely correlates with sliding velocity (Purcell et al. 2011a). IFI was included in this analysis and appeared to fit the correlation (Purcell et al. 2011a).
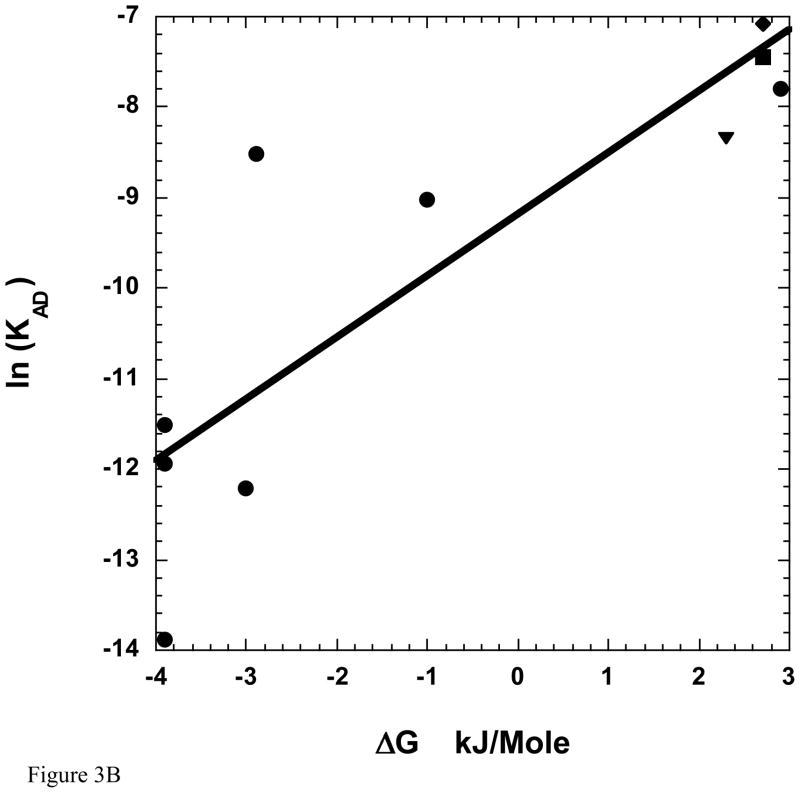
Our more extensive analysis here of IFI and three additional Drosophila myosins suggests that at least at the high end of the correlation, velocity is not influenced by the conformation of the ADP bound nucleotide site. Compared to vertebrate myosin isoforms, all four Drosophila isoforms tested should be considered very fast isoforms, and all four favor the closed conformation (Table 2). Thus, these myosins fit the overall trend observed previously by Purcell and colleagues (Purcell et al. 2011a) that as myosin speed increases, the closed conformation becomes dominant, and the ΔG0 difference between the closed and open states becomes more positive (Figure 3A). However, the inverse correlation between velocity and ΔG0 does not hold within this group of fast myosins (Table 1, Figure 3A). This result suggests the nucleotide site conformation in the ADP bound state is not important for setting Drosophila myosin velocity. Similarly, the Drosophila myosin ΔG0 differences do not appear critical for influencing ADP release rate (Figure 3B). The ADP dissociation constants (KAD) (higher KAD = lower affinity) for these myosins range from 838 μM to 239 μM (Miller et al. 2003; Miller et al. 2007) and the ΔG0 values range from 2.3 to 2.9 (Table 1), but the two sets of values do not positively or negatively correlate (Figure 3B). Although there is no systematic correlation of KAD with ΔG0 within the four Drosophila isoforms, the values of KAD are clustered at the weak end of the range of myosins from a variety of species (Figure 3B), and the ΔG0 values are clustered at the high end of the range. Thus, the correlation of KAD with ΔG0 for myosins from a variety of species is better than the correlation with velocity.
Figure 3.
A. In vitro motility versus ΔG0. Three additional Drosophila myosin isoforms (EMB, IFI-EC, IFI-7a) have been added to Figure 4 from (Purcell et al. 2011a) showing the relationship between ΔG0 for the open-to-closed transition between the A•M•D states in fibers and sliding velocity over a 25-fold range of velocities. Drosophila isoform velocities are from the references cited in Table 1. ΔG0 = −RTln(Keq) where Keq = [fraction of myosin showing the more mobile component of the spectrum]/[fraction showing the more immobilize component], R = Boltzmann’s constant, and T= absolute temperature. B. The ADP dissociation constants (KAD) for the binding of ADP to the actomyosin complex are plotted as a function of the free energy for closing of the nucleotide site of myosin, ΔG0, measured at 23°C. The values for EMB, IFI-EC, IFI-7a have been added to the plot in Figure 7 from (Purcell et al. 2011a). Symbols for the Drosophila myosins are circle for IFI, square for EMB, diamond for IFI-7a, and triangle for IFI-7a. The other circles are the same myosins as in Figure 3A. While there appears to be a general trend over a wide range of isoforms (black line), there was no correlation within the Drosophila myosin isoforms between ΔG0 and KAD values. KAD values are from references listed in the legend for Table 1.
The lack of correlations support previous work suggesting that ADP release rate is not important for setting Drosophila myosin velocity. S-1 ADP affinities measured in solution (Miller et al. 2003; Miller et al. 2007) did not correlate with actin sliding velocity in the motility assay (Swank et al. 2002; Swank et al. 2003) or with the frequency of maximum work production of isolated muscle fibers as measured by sinusoidal analysis (Swank et al. 2002; Swank et al. 2004). Additional sinusoidal experiments on wild-type IFM fibers also suggested that ADP release is not limiting under oscillatory length change conditions (Swank et al. 2006; Yang et al 2010). Instead, the results suggested that either Pi release is rate limiting or that a low IFI ATP affinity may make ATP binding and subsequent detachment from actin limiting (Swank et al. 2006). That ATP binding can limit velocity has also been suggested by experiments involving some fast skeletal myosins (Nyitrai et al 2005). These findings are counter to the general assumption that ADP release rate sets velocity for all skeletal muscles (Siemankowski et al. 1985; Weiss et al. 2001). Thus, studying fast Drosophila myosins is revealing new insights into the mechanochemistry of fast motors and ATPases.
Acknowledgments
This work was supported by National Instates of Health grants AR042895 (R.C., N.N.), AR053720 (E.P., N.N.), and AR055611 (D.S.)
References
- Alessi DR, Corrie JE, Fajer PG, Ferenczi MA, Thomas DD, Trayer IP, Trentham DR. Synthesis and properties of a conformationally restricted spin-labeled analog of ATP and its interaction with myosin and skeletal muscle. Biochemistry. 1992;31:8043–8054. doi: 10.1021/bi00149a039. [DOI] [PubMed] [Google Scholar]
- Cooke R. Force generation in muscle. Cur Opinion Cell Biol. 1990;2:62–66. doi: 10.1016/s0955-0674(05)80032-8. [DOI] [PubMed] [Google Scholar]
- Cripps R, Bernstein SI. Generation of transgenic Drospohila melanogaster. In: Norton P, Steel L, editors. Introducing DNA into living cells and organisms. Natick, MA: Eaton Publishing; 2000. pp. 93–125. [Google Scholar]
- Crowder MS, Cooke R. Orientation of spin-labeled nucleotides bound to myosin in glycerinated muscle fibers. Biophys J. 1987;51:323–333. doi: 10.1016/S0006-3495(87)83338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith OH, Jost PC. In: Spin Labeling Theory and Applications. Berliner LJ, editor. New York, NY: Academic Press; 1976. pp. 454–523. [Google Scholar]
- Higashi-Fujime S, Ishikawa R, Iwasawa H, Kagami O, Kurimoto E, Kohama K, Hozumi T. The fastest actin-based motor protein from the green algae, Chara, and its distinct mode of interaction with actin. FEBS Let. 1995;375:151–154. doi: 10.1016/0014-5793(95)01208-v. [DOI] [PubMed] [Google Scholar]
- Inoue A, Saito J, Ikebe R, Ikebe M. Myosin-IXb is a single-headed minus-end-directed processive motor. Nat Cell Biol. 2002;4:302–306. doi: 10.1038/ncb774. [DOI] [PubMed] [Google Scholar]
- Kull FJ, Sablin EP, Lau R, Fletterick RJ, Vale RD. Crystal structure of the kinesin motor domain reveals a structural similarity to myosin. Nature. 1996;380:550–555. doi: 10.1038/380550a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BM, Bloemink MJ, Nyitrai M, Bernstein SI, Geeves MA. A variable domain near the ATP-binding site in Drosophila muscle myosin is part of the communication pathway between the nucleotide and actin-binding sites. J Mol Biol. 2007;368:1051–1066. doi: 10.1016/j.jmb.2007.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BM, Nyitrai M, Bernstein SI, Geeves MA. Kinetic analysis of Drosophila muscle myosin isoforms suggests a novel mode of mechanochemical coupling. J Biol Chem. 2003;278:50293–50300. doi: 10.1074/jbc.M308318200. [DOI] [PubMed] [Google Scholar]
- Miller BM, Zhang S, Suggs JA, Swank DM, Littlefield KP, Knowles AF, Bernstein SI. An alternative domain near the nucleotide-binding site of Drosophila muscle myosin affects ATPase kinetics. J Mol Biol. 2005;353:14–25. doi: 10.1016/j.jmb.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Naber N, Cooke R, Pate E. Conformational changes at the nucleotide pocket of motor proteins moitored by electron parmagnetic resonance spectroscopy. Pure Appl Chem. 2011;83:1675–1684. [Google Scholar]
- Naber N, Malnasi-Csizmadia A, Purcell TJ, Cooke R, Pate E. Combining EPR with fluorescence spectroscopy to monitor conformational changes at the myosin nucleotide pocket. Journal of molecular biology. 2010;396:937–948. doi: 10.1016/j.jmb.2009.12.035. [DOI] [PubMed] [Google Scholar]
- Naber N, Purcell TJ, Pate E, Cooke R. Dynamics of the nucleotide pocket of myosin measured by spin-labeled nucleotides. Biophys J. 2007;92:172–184. doi: 10.1529/biophysj.106.090035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naber N, Rice S, Matuska M, Vale RD, Cooke R, Pate E. EPR spectroscopy shows a microtubule-dependent conformational change in the kinesin switch 1 domain. Biophys J. 2003;84:3190–3196. doi: 10.1016/S0006-3495(03)70043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordio PL. General magnetic resonance theory. In: Berliner LJ, editor. Spin Labeling Theory and Applications. New York, NY: Academic Press; 1976. pp. 5–52. [Google Scholar]
- Nyitrai M, Rossi R, Adamek N, Pellegrino MA, Bottinelli R, Geeves MA. What limits the velocity of fast-skeletal muscle contraction in mammals? J Mol Biol. 2006;355:432–42. doi: 10.1016/j.jmb.2005.10.063. [DOI] [PubMed] [Google Scholar]
- Post PL, Tyska MJ, O’Connell CB, Johung K, Hayward A, Mooseker MS. Myosin-IXb is a single-headed and processive motor. J Biol Chem. 2002;277:11679–11683. doi: 10.1074/jbc.M111173200. [DOI] [PubMed] [Google Scholar]
- Purcell TJ, Naber N, Franks-Skiba K, Dunn AR, Eldred CC, Berger CL, Malnasi-Csizmadia A, Spudich JA, Swank DM, Pate E, Cooke R. Nucleotide pocket thermodynamics measured by EPR reveal how energy partitioning relates myosin speed to efficiency. J Mol Biol. 2011a;407:79–91. doi: 10.1016/j.jmb.2010.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell TJ, Naber N, Sutton S, Cooke R, Pate E. EPR spectra and molecular dynamics agree that the nucleotide pocket of myosin V is closed and that it opens on binding actin. J Mol Biol. 2011b;411:16–26. doi: 10.1016/j.jmb.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablin EP, Kull FJ, Cooke R, Vale RD, Fletterick RJ. Crystal structure of the motor domain of the kinesin-related motor ncd. Nature. 1996;380:555–559. doi: 10.1038/380555a0. [DOI] [PubMed] [Google Scholar]
- Siemankowski RF, Wiseman MO, White HD. ADP dissociation from actomyosin subfragment 1 is sufficiently slow to limit the unloaded shortening velocity in vertebrate muscle. Proc Natl Acad Sci USA. 1985;82:658–662. doi: 10.1073/pnas.82.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva R, Sparrow JC, Geeves MA. Isolation and kinetic characterisation of myosin and myosin S1 from the Drosophila indirect flight muscles. J Muscle Research Cell Motil. 2003;24:489–498. doi: 10.1023/b:jure.0000009809.69829.74. [DOI] [PubMed] [Google Scholar]
- Sindelar CV, Budny MJ, Rice S, Naber N, Fletterick R, Cooke R. Two conformations in the human kinesin power stroke defined by X-ray crystallography and EPR spectroscopy. Nature Struct Biol. 2002;9:844–848. doi: 10.1038/nsb852. [DOI] [PubMed] [Google Scholar]
- Sun M, Oakes JL, Ananthanarayanan SK, Hawley KH, Tsien RY, Adams SR, Yengo CM. Dynamics of the upper 50-kDa domain of myosin V examined with fluorescence resonance energy transfer. J Biol Chem. 2006;281:5711–5717. doi: 10.1074/jbc.M508103200. [DOI] [PubMed] [Google Scholar]
- Swank DM. Mechanical analysis of Drosophila indirect flight and jump muscles. Methods. 2012;56:69–77. doi: 10.1016/j.ymeth.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank DM, Knowles AF, Kronert WA, Suggs JA, Morrill GE, Nikkhoy M, Manipon GG, Bernstein SI. Variable N-terminal regions of muscle myosin heavy chain modulate ATPase rate and actin sliding velocity. J Biol Chem. 2003;278:17475–17482. doi: 10.1074/jbc.M212727200. [DOI] [PubMed] [Google Scholar]
- Swank DM, Knowles AF, Kronert WA, Suggs JA, Morrill GE, Nikkhoy M, Manipon GG, Bernstein SI. Variable N-terminal regions of muscle myosin heavy chain modulate ATPase rate and actin sliding velocity. J Biol Chem. 2003;278:17475–17482. doi: 10.1074/jbc.M212727200. [DOI] [PubMed] [Google Scholar]
- Swank DM, Knowles AF, Suggs JA, Sarsoza F, Lee A, Maughan DW, Bernstein SI. The myosin converter domain modulates muscle performance. Nature Cell Biol. 2002;4:312–316. doi: 10.1038/ncb776. [DOI] [PubMed] [Google Scholar]
- Swank DM, Kronert WA, Bernstein SI, Maughan DW. Alternative N-terminal regions of Drosophila myosin heavy chain tune muscle kinetics for optimal power output. Biophys J. 2004;87:1805–1814. doi: 10.1529/biophysj.103.032078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank DM, Vishnudas VK, Maughan DW. An exceptionally fast actomyosin reaction powers insect flight muscle. Proc Natl Acad Sci USA. 2006;103:17543–17547. doi: 10.1073/pnas.0604972103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank DM, Wells L, Kronert WA, Morrill GE, Bernstein SI. Determining structure/function relationships for sarcomeric myosin heavy chain by genetic and transgenic manipulation of Drosophila. Microsc Res Techniq. 2000;50:430–442. doi: 10.1002/1097-0029(20000915)50:6<430::AID-JEMT2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Sweeney HL, Houdusse A. Structural and functional insights into the myosin motor mechanism. Ann Rev Biophys. 2010;39:539–557. doi: 10.1146/annurev.biophys.050708.133751. [DOI] [PubMed] [Google Scholar]
- Weiss S, Rossi R, Pellegrino MA, Bottinelli R, Geeves MA. Differing ADP release rates from myosin heavy chain isoforms define the shortening velocity of skeletal muscle fibers. J Biol Chem. 2001;276:45902–45908. doi: 10.1074/jbc.M107434200. [DOI] [PubMed] [Google Scholar]
- Wells L, Edwards KA, Bernstein SI. Myosin heavy chain isoforms regulate muscle function but not myofibril assembly. EMBO J. 1996;15:4454–4459. [PMC free article] [PubMed] [Google Scholar]
- Yang C, Kaplan C, Thatcher M, Swank DM. The influence of myosin converter and relay domains on cross-bridge kinetics of Drosophila indirect flight muscle. Biophys J. 2010;99:1546–1555. doi: 10.1016/j.bpj.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]