Abstract
The Saccharomyces cerevisiaeEXO1 gene encodes a 5′ exonuclease that participates in mismatch repair (MMR) of DNA replication errors. Deleting EXO1 was previously shown to increase mutation rates to a greater extent when combined with a mutator variant (pol3-L612M) of the lagging strand replicase, DNA polymerase δ (Pol δ), than when combined with a mutator variant (pol2-M644G) of the leading strand replicase, DNA polymerase ε (Pol ε). Here we confirm that result, and extend the approach to examine the effect of deleting EXO1 in a mutator variant (pol1-L868M) of Pol α, the proofreading-deficient and least accurate of the three nuclear replicases that is responsible for initiating Okazaki fragment synthesis. We find that deleting EXO1 increases the mutation rate in the Pol α mutator strain to a significantly greater extent than in the Pol δ or Pol ε mutator strains, thereby preferentially reducing the efficiency of MMR of replication errors generated by Pol α. Because these mismatches are closer to the 5′ ends of Okazaki fragments than are mismatches made by Pol δ or Pol ε, the results not only support the previous suggestion that Exo1 preferentially excises lagging strand replication errors during mismatch repair, they further imply that the 5′ ends serve as entry points for 5′ excision of replication errors made by Pol α, and possibly as strand discrimination signals for MMR. Nonetheless, mutation rates in the Pol α mutator strain are 5- to 25-fold lower in an exo1Δ strain as compared to an msh2Δ strain completely lacking MMR, indicating that in the absence of Exo1, most replication errors made by Pol α can still be removed in an Msh2-dependent manner by other nucleases and/or by strand displacement.
Keywords: Exo1, mismatch repair, genome stability, replication fidelity
1. Introduction
Because the two strands of duplex DNA are anti-parallel and DNA polymerases only copy DNA in the 5′ to 3′ direction, coordinated replication of the eukaryotic nuclear genome is intrinsically asymmetric, with a leading strand replicated first and a lagging strand replicated slightly thereafter as a series of ~200 base Okazaki fragments (reviewed in [1]). We are interested in understanding relationships between this asymmetry and the fidelity of DNA replication. To investigate this, we are using Saccharomyces cerevisiae strains containing mutant alleles of the POL1 (Pol α), POL2 (Pol ε) and POL3 (Pol δ) genes. These alleles, pol1-L868M [2, 3], pol2-M644G [4] and pol3-L612M ([5] and references therein), encode replicases with single amino acid replacements at the polymerase active site that retain high replicative capacity but have reduced fidelity. Strains harboring these alleles have elevated spontaneous mutation rates and a specific error bias signature, thereby identifying the polymerases responsible for generating the majority of replication errors in vivo. The patterns of mutagenesis in these strains [4–7] and the strand-specific incorporation of ribonucleotides during replication by Pol ε [6, 7], suggest that under normal circumstances, Pol ε is the primary leading strand replicase, while Pol δ primarily participates in lagging strand replication after Pol α-primase initiates the synthesis of Okazaki fragments.
DNA replication fidelity is determined by the nucleotide selectivity of pol α, δ and ε, by the 3′ exonucleolytic proofreading activities intrinsic to Pols δ and ε (but not Pol α), and by mismatch repair (MMR) of replication errors that escape proofreading. MMR begins when a mismatch generated during nuclear DNA replication is recognized either by Msh2-Msh6 (MutSα), which recognizes single base-base and small insertion-deletion (indel) mismatches, or by Msh2-Msh3 (MutSβ), which recognizes indel mismatches with a specificity that is partially redundant with Msh2-Msh6 ([8–10] and reviewed in [11]). Mismatch recognition initiates a series of downstream steps that ultimately remove the replication error from the nascent strand and allow new DNA to be correctly synthesized. In addition to the mismatch, the MMR machinery requires a signal to direct excision to the newly synthesized strand containing the replication error. Studies in vitro show that the signal can be a nick or gap located either 3′ or 5′ to the mismatch, with the protein requirements for MMR differing somewhat depending on the location of the DNA ends relative to the mismatch [12–14]. The origin and exact nature of the strand discrimination signal used for MMR in vivo remains uncertain. Attractive possibilities for this signal include the 3′ ends of growing chains at the replication fork and/or the 5′ ends of Okazaki fragments that are transiently present during lagging strand replication.
To probe the enzymology of MMR in vivo and gain insight into the nature of the strand discrimination signal, we are examining the extent to which MMR specificity and efficiency varies as a function of several variables, most especially the polymerase that generates the error. This focus is motivated by a specific prediction that emerges from several related observations. Early studies in E. coli demonstrate that MMR most efficiently corrects those mismatches generated at the highest rates during replication, i.e., transition and indel mismatches [15–17]. If reciprocity between production of mismatches and their repair is evolutionarily conserved in eukaryotes, then MMR should not only more efficiently correct transition and indel mismatches, it may also more efficiently correct mismatches made by Pol α as compared to those made by Pol δ, because Pol α is naturally exonuclease deficient and generates mismatches at substantially higher rates than does proofreading-proficient Pol δ [18]. Early studies also showed that the efficiency of MMR in E. coli is inversely proportional to the distance between the mismatch and the strand discrimination signal [19]. In the initial study showing that PCNA was required for eukaryotic MMR at a step prior to mismatch excision, we proposed [20] that PCNA could physically link MMR and replication in a manner that would allow DNA ends associated with replication to serve as strand discrimination signals. That study was followed by another [21] which led to the more specific hypothesis that the 5′ ends of Okazaki fragments could signal for MMR of replication errors in the nascent lagging strand. If the relationship between distance and bacterial MMR efficiency is conserved in eukaryotes, and if the 5′ ends of Okazaki fragments can serve as strand discrimination signals, this too predicts that MMR might more efficiently correct mismatches made by Pol α as compared to mismatches made by Pol δ. This is because mismatches made as Pol α initiates Okazaki framents will be closer to DNA 5′ termini than would more internal mismatches made by Pol δ. We initially tested this prediction by comparing mutation rates in L612M Pol δ and L868M Pol α strains that were either MMR proficient or deleted for MSH2 (msh2Δ) and therefore lacked both Msh2-Msh6-dependent and Msh2-Msh3-dependent MMR activity. Pairwise comparisons of specific single base mutation rates in these strains provided an apparent MMR efficiency for errors made by each polymerase. We found that mismatches made by Pol δ are repaired efficiently, but the equivalent single base-base mismatches made by Pol α appear to be corrected even more efficiently [22]. Possible explantions for this higher efficiency include use of the 5′ ends of Okazaki fragments to initiate mismatch removal, either by strand displacement [23] or by a 5′ exonuclease.
Based on genetic evidence [22], we previously proposed that one candidate for an exonuclease to perform mismatch excision initiated at the 5′ end of an Okazaki fragment is exonuclease 1 (Exo1), which is well known to participate in eukaryotic MMR [24, 25]. In support of this idea, Kolodner and colleagues [26] recently reported that deletion of EXO1 increases the mutation rate of the pol3-L612M mutator strain to a greater extent than for the pol2-M644G mutator strain. Here we extend this effort by examining the mutagenic consequences of deleting EXO1 (exo1Δ) in all three strains, pol1-L868M, pol2-M644G and pol3-L612M. The hypothesis that the 5′ DNA ends of Okazaki fragments are used for MMR predicts that deleting Exo1 should reduce the efficiency of MMR of errors made by Pol α even more than for errors made by Pol δ. This is exactly what we observe. We also compare the results to those seen upon complete loss of all Msh2-dependent MMR in the same polymerase mutator strains [6, 22]. The results lead to three interpretations. (I) Exo1 has a much greater role in repairing lagging strand replication errors as compared to leading strand replication errors, supporting a similar conclusion by Kolodner and colleagues [26] and extending it to the second essential lagging strand replicase. (II) The 5′ ends of Okazaki fragments are entry points for 5′ to 3′ excision of mismatches by Exo1 during MMR in vivo, and they may also serve as strand discrimination signals. (III) Like DNA replication, MMR enzymology in vivo can differ on the lagging and leading strands.
2. Materials and Methods
Strains, mutation rates, and sequencing ura3 mutants
All strains used in this study are isogenic derivatives of strain Δ|(2)|-7B-YUNI300 (MATa CAN1 his7-2 leu2-Δ::kanMX ura3-Δ trp1-289 ade2-1 lys2-ΔGG2899-2900) [27]. Mutator alleles; pol1-L868M, pol2-M644G, and pol3-L612M have been described previously [2–5]. Heterozygous EXO1/exo1Δ diploids were generated by PCR based targeted gene-disruption. Deletion of EXO1 was verified by phenotype and by PCR across the disrupted region, and haploids were obtained from tetrad dissection. Measurements of spontaneous mutation rates by fluctuation analysis were as described previously [4, 5]. For each ura3 mutant sequenced, an independent colony was patched to YPDA and then replica plated to media containing 5-FOA. Genomic DNA from a single 5-FOA-resistant colony from each patch was isolated, and the URA3 gene was amplified by PCR and sequenced.
Statistical analysis
To determine if differences in mutation rates between strains are significant we used a one-sided Mann-Whitney t-test.
3. Results and Discussion
3.1. The exo1Δ mutator effect is greater for Pol α than for Pol δ or Pol ε
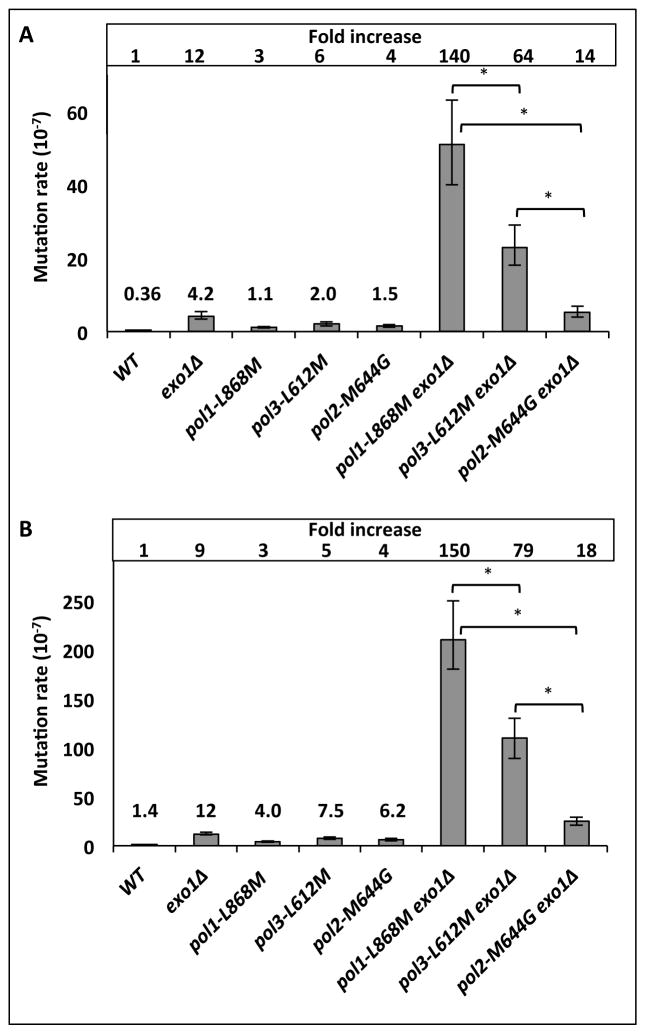
Mutation rates for resistance to 5-fluoro-orotic acid (5-FOA) were determined in EXO1 versus exo1Δ strains that encode either wild type DNA polymerases or mutator variants of Pol α (pol1-L868M), Pol δ (pol3-L612M) or Pol ε (pol2-M644G). Compared to the wild type (POLEXO1) strain, the mutation rate of the exo1Δ single mutant strain was elevated by 12-fold, and as previously observed, the mutation rates in the pol1-L868M, pol2-M644G and pol3-L612M [4, 5] single mutant strains were each elevated by a few-fold (Fig. 1A). The mutation rate in the double mutant pol1-L868M exo1Δ strain was increased by 140-fold, to 51 ×10−7. This is greater than the 64-fold increase observed in the pol3-L612M exo1Δ strain (rate = 23 × 10−7). The rates in these double mutant strains are much more than the sum of rates in the individual single mutant strains, consistent with the idea that two processes are acting in series, i.e., Exo1-dependent MMR is correcting replication errors that escape the fork. In contrast, the mutation rate in the pol2-M644G exo1Δ strain was 5.2 × 10−7, which is remarkably close to the sum of the increases observed for the pol2-M644G and exo1Δ single mutants (5.7 × 10−7). When mutation rates were measured at the CAN1 locus in these same strains, similar relative differences among the double mutant strains were obtained (Fig 1B). At both URA3 and CAN1, the rates in the pol1-L868M exo1Δ strain were significantly higher than the rates in the pol3-L612M exo1Δ and pol2-M644G exo1Δ strains (p values < 0.05, Fig. 1A).
Figure 1. Polymerase-specific mutator effects in exo1Δ strains.
Spontaneous mutation rates (with 95% confidence intervals) to (A) 5-FOA resistance and (B) canavanine resistance. Differences in mutation rates between the pol1-L868M exo1Δ and other two double mutant strains are statistically significant (p <0.05), as indicated by asterisks. Deleting EXO1 results in a 46-fold and a 12-fold increase in mutation rates to 5-FOA resistance compared to the corresponding single pol mutants; pol1-L868M and pol3-L612M, respectively. For canavanine resistance, deletion of EXO1 in a pol mutator background causes a mutation rate increase of 52-fold and 14-fold, compared to pol1-L868M and pol3-L612M, respectively.
3.2. Determining the specificity of exo1Δ mutator effects
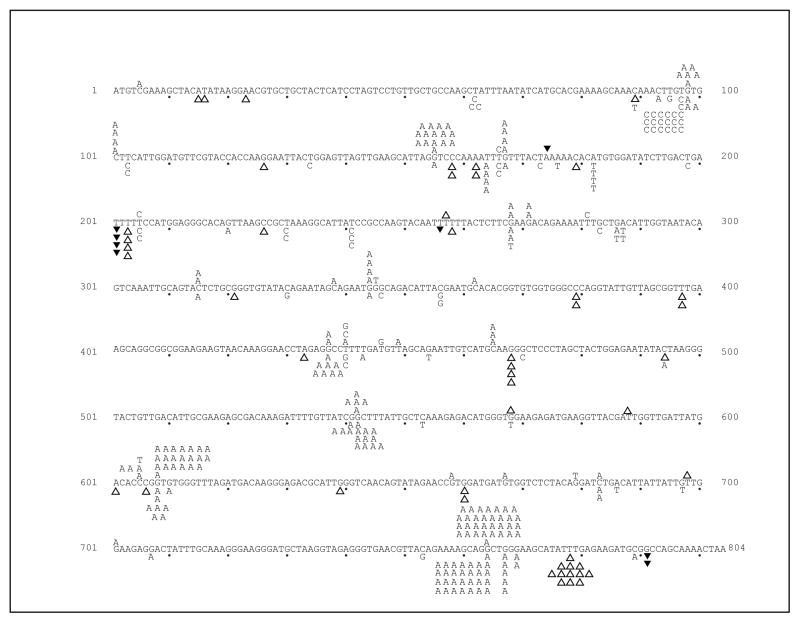
The above interpretation depends on a demonstration that the mutations arising in the pol1-L868M exo1Δ and pol3-L612M exo1Δ strains are characteristic of partial loss of MMR of DNA replication errors rather than a defect in other DNA transactions in which Exo1 participates [28]. One advantage of the polymerase variants used here is that, in the absence of Msh2-dependent MMR, they have mutational signatures characteristic of common replication errors [22]. To determine if these signatures are observed in the exo1Δ strains, we examined the DNA sequence changes in the URA3 gene responsible for 5-FOA-resistance, in independent mutants collected from the pol1-L868M exo1Δ and pol3-L612M exo1Δ strains. The majority of mutants did indeed contain single base substitutions and deletions (Table 1), and these were non-randomly distributed throughout the URA3 open reading frame (Figure 2).
Table 1.
Specific mutation rates for strains used in this study
| pol1-L868M | po13-L612M | |||||
|---|---|---|---|---|---|---|
| MMR* | msh2Δ* | exolΔ | MMR* | msh2Δ* | exolΔ | |
| Total rate 95 % CI | 1.7 (1.2–4.9) | 1000 (960–2100) | 51 (40–63) | 2.7 (1.9–7.3) | 560 (500–750) | 23 (18–29) |
| ura3 mutants sequenced | 175 | 180 | 162 | 217 | 174 | 241 |
|
| ||||||
| All point mutations | 100(1.0) | 172 (960) | 129(41) | 144 (1.8) | 172 (550) | 201 (19) |
| Total G to A | 34 (0.33) | 112 (620) | 114 (36) | 50 (0.6) | 49 (160) | 77 (7.4) |
| G to A 98 | 0 (≤0.01) | 9 (50) | 6 (1.9) | n.a. | n.a. | n.a. |
| G to A 155 | 1 (0.01) | 13 (72) | 15 (4.7) | n.a. | n.a. | n.a. |
| G to A 167 | 3 (0.03) | 6 (33) | 4 (1.3) | n.a. | n.a. | n.a. |
| G to A 437 | n.a. | n.a. | n.a. | 4 (0.05) | 8 (26) | 8 (0.8) |
| G to A 542 | n.a. | n.a. | n.a. | 6 (0.07) | 3 (9.7) | 11 (1.0) |
| G to A 608 | 6 (0.06) | 6 (33) | 21 (6.6) | 6 (0.07) | 4 (13) | 7 (0.7) |
| G to A 764 | 11 (0.1) | 51 (280) | 32 (10) | 16 (0.2) | 16 (52) | 29 (2.8) |
The number of events are shown and the specific rates are displayed in parenthesis
From Nick McElhinny et al., 2010. Mutation rates and detailed error specificity for the wild type strain has been published in Nick McElhinny et al., 2010.
n.a.: no events or not a significant number of events.
A number of 5-FOA resistant mutants had no sequence change in the 804 base pair URA3 open reading frame. These mutants were not investigated further, but they may result from epigenetic silencing, they may contain sequence change in the promoter or the 3* untranslated region of URA3, or they may contain mutations in other genes that result in 5-FOA resistance.
Figure 2. Spectra of ura3 mutations in exo1Δ strains.
The coding strand of the URA3 ORF is shown, with every tenth base indicated by a closed dot. Single letters represent base substitutions, open triangles represent single-base deletions, and closed triangles represent single base insertions. Spectra for the pol1-L868Mexo1 Δ and pol3-L612Mexo1 Δ strains are shown above and below the URA3 ORF, respectively.
3.3. Exo1 preferentially participates in MMR of Pol α errors
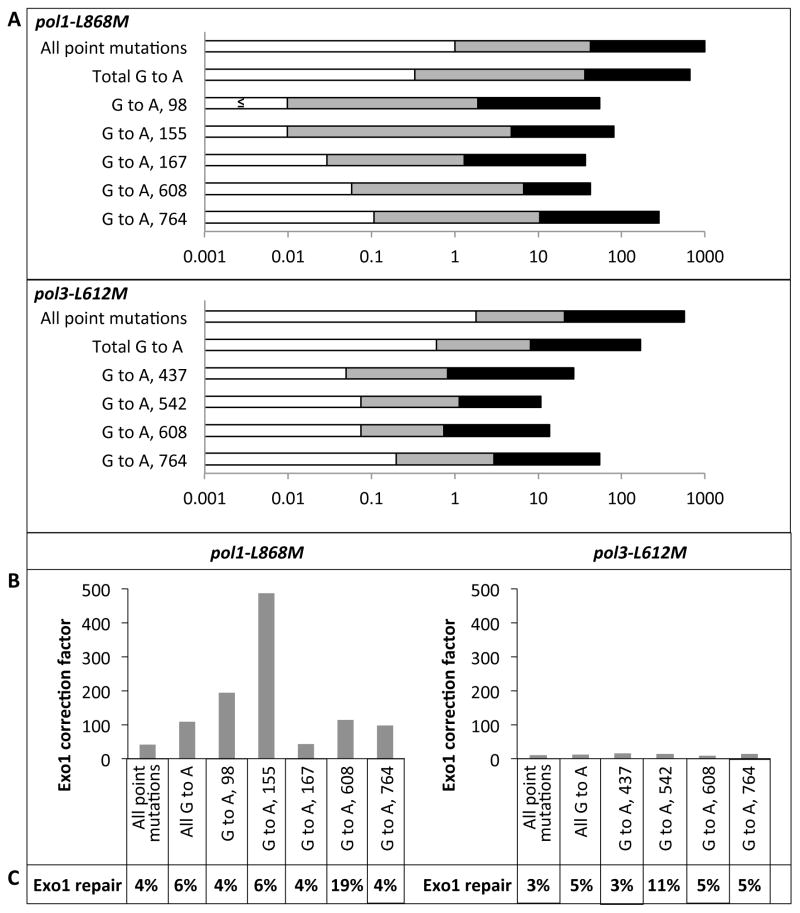
Focused on the hypothesis that deleting Exo1 would reduce the efficiency of MMR of lagging strand replication errors made by Pol α more than for errors made by Pol δ, the data in Figures 1 and 2 were used to calculate specific mutation rates (Table 1 and Fig. 3A) and MMR correction factors (Fig. 3B) for three classes of mutations that are signatures of a complete loss of MMR in pol1-L868Mmsh2Δ strains [22]. These classes are (i) total single base errors (base substitutions and indels), (ii) total G to A transitions, which comprise 74% of all single base errors in pol1-L868Mmsh2Δ and pol3-L612M msh2Δ strains and are generated by misincorporation of dTTP opposite template G during lagging strand replication by Pol α (pol1-L868M) or Pol δ (pol3-L612M) and (iii) G to A transitions at characteristic hotspots in the URA3 reporter gene (Figure 2 and 3B) [22]. Mutation rates for these events are shown on a log scale in Figure 3A, where rates for MMR-proficient strains are depicted as open bars, rates for exo1Δ strains are represented by open + grey bars, and rates for msh2Δ strains are represented by open + grey + black bars. Dividing the rates in the exo1Δ strains by the rates in the MMR-proficient strains provides the correction factor (CF values in Fig. 3B) for Exo1-dependent MMR of each type of error. Correction efficiencies for these mismatches in the pol1-L868M strain range from 43 to 490. These values are consistently higher than the 9- to 15-fold correction efficiencies for the same classes of mismatches generated in the pol3-L612M strain (Fig. 3B), thus supporting the hypothesis.
Figure 3. Rates and Exo1-dependent MMR correction factors for single base mutations in pol1-L868M and pol3-L612M strains.
(A) The base substitution and its position in URA3 are indicated on the left. Mutation rates (×10−7) for specific base substitutions in EXO1 MSH2 (open bars), exo1Δ (open + grey bars), and msh2Δ strains (open + grey + black bars) are shown on a log scale (x-axis). In cases where no occurrences were observed (see Table 1), the rate is estimated as (≤).(B) Exo1-dependent MMR correction factors are the rate in exo1Δ strains divided by the rate in EXO1 strains. (C) The ratios of Exo1-dependent repair compared to Msh2-dependent repair, shown as percentages.
3.4. The role of Exo1 in MMR of lagging strand errors is important, but redundant
Dividing the mutation rates in the pol1-L868M exo1Δ strain by the rates in the pol1-L868M msh2Δ strain that is completely defective in MMR reveals yields values of 4% to 19% (Fig. 3C). Thus efficient MMR still occurs in the absence of Exo1.
4. Discussion
About 10% of Okazaki fragment synthesis is catalyzed by Pol α. This presents a strong challenge to nuclear genome stability because Pol α is a naturally proofreading-deficient replicase that is substantially less accurate than is proofreading-proficient Pol δ. An early idea for eliminating errors made by Pol α was that the mismatches it generates are removed by strand displacement synthesis during normal Okazaki fragment maturation, a process that historically has not invoked participation of MMR proteins [29]. However, canonical Okazaki fragment maturation proteins alone are insufficient to remove many Pol α errors, as clearly illustrated by the fact that the mutation rate of the pol1-L868M strain is very strongly elevated by deleting Msh2 (see [2, 5] and Fig. 3). Now, in addition to Msh2, the present study demonstrates the involvement of Exo1 in MMR of Pol α errors. That Exo1 is indeed correcting Pol α replication errors, rather than affecting mutation rates via another DNA transaction, is clearly illustrated by the increase in single base mutations characteristic of loss of MMR in the Pol α mutator strain (Figs. 2 and 3). Because mismatches generated by Pol α are closer to the 5′ ends of Okazaki fragments than are mismatches made by Pol δ, the stronger mutator effect of deleting Exo1 in the pol1-L868M strain strongly support the hypothesis that the 5′ ends of Okazaki fragments are entry points for Exo1-dependent excision of a mismatch, and may possibly serve as strand discrimination signals for MMR. The fact that deleting EXO1 is much less mutagenic than deleting MSH2indicates that Pol α errors can also be corrected by an Exo1-independent MMR process. This repair may involve partially redundant nucleases, and/or a strand displacement mechanism to remove mismatches [13, 14, 23, 30–32]. The possibility that Exo1 is redundant with other exonucleases has been suggested before [11, 32–34], and our data extend this idea to include replication errors made by both lagging strand replicases. Finally, the fact that Exo1 influences MMR of errors made by the two lagging strand replicases to a greater extent than the leading strand replicase illustrates that, like DNA replication, eukaryotic DNA mismatch repair is enzymologically asymmetric.
Highlights.
Deleting EXO1 increases the mutation rate in the Pol α mutator strain to a significantly greater extent than in the Pol δ or Pol ε mutator strains.
Exo1 preferentially excises lagging strand replication errors during mismatch repair
Transient lagging strand 5′ ends serve as entry points for 5′ excision of replication errors made by Pol α, and possibly as strand discrimination signals for MMR.
In the absence of Exo1, most replication errors made by Pol α can still be removed in an Msh2-dependent manner.
Acknowledgments
We thank Kasia Bebenek and Dmitry Gordenin for thoughtful comments on the manuscript, and the NIEHS Molecular Genetics Core Facility for sequencing mutants. This work was supported by Project Z01 ES065089 to TAK from the Division of Intramural Research of the National Institutes of Health, National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burgers PM. Polymerase dynamics at the eukaryotic DNA replication fork. J Biol Chem. 2009;284:4041–4045. doi: 10.1074/jbc.R800062200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niimi A, Limsirichaikul S, Yoshida S, Iwai S, Masutani C, Hanaoka F, Kool ET, Nishiyama Y, Suzuki M. Palm mutants in DNA polymerases alpha and eta alter DNA replication fidelity and translesion activity. Mol Cell Biol. 2004;24:2734–2746. doi: 10.1128/MCB.24.7.2734-2746.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pavlov YI, Frahm C, Nick McElhinny SA, Niimi A, Suzuki M, Kunkel TA. Evidence that errors made by DNA polymerase alpha are corrected by DNA polymerase delta. Curr Biol. 2006;16:202–207. doi: 10.1016/j.cub.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Pursell ZF, Isoz I, Lundstrom EB, Johansson E, Kunkel TA. Yeast DNA polymerase epsilon participates in leading-strand DNA replication. Science. 2007;317:127–130. doi: 10.1126/science.1144067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nick McElhinny SA, Gordenin DA, Stith CM, Burgers PM, Kunkel TA. Division of labor at the eukaryotic replication fork. Mol Cell. 2008;30:137–144. doi: 10.1016/j.molcel.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lujan SA, Williams JS, Pursell ZA, Abdulovic-Cui AA, Clark AB, Nick McElhinny SA, Kunkel TA. Mismatch repair balances leading and lagging strand DNA replication fidelity. PLoS Genet. 2012 doi: 10.1371/journal.pgen.1003016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyabe I, Kunkel TA, Carr AM. The major roles of DNA polymerases epsilon and delta at the eukaryotic replication fork are evolutionarily conserved. PLoS Genet. 2011;7:e1002407. doi: 10.1371/journal.pgen.1002407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strand M, Earley MC, Crouse GF, Petes TD. Mutations in the MSH3 gene preferentially lead to deletions within tracts of simple repetitive DNA in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1995;92:10418–10421. doi: 10.1073/pnas.92.22.10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greene CN, Jinks-Robertson S. Frameshift intermediates in homopolymer runs are removed efficiently by yeast mismatch repair proteins. Mol Cell Biol. 1997;17:2844–2850. doi: 10.1128/mcb.17.5.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marsischky GT, Kolodner RD. Biochemical characterization of the interaction between the Saccharomyces cerevisiae MSH2-MSH6 complex and mispaired bases in DNA. J Biol Chem. 1999;274:26668–26682. doi: 10.1074/jbc.274.38.26668. [DOI] [PubMed] [Google Scholar]
- 11.Kunkel TA, Erie DA. DNA mismatch repair. Annu Rev Biochem. 2005;74:681–710. doi: 10.1146/annurev.biochem.74.082803.133243. [DOI] [PubMed] [Google Scholar]
- 12.Dzantiev L, Constantin N, Genschel J, Iyer RR, Burgers PM, Modrich P. A defined human system that supports bidirectional mismatch-provoked excision. Mol Cell. 2004;15:31–41. doi: 10.1016/j.molcel.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Constantin N, Dzantiev L, Kadyrov FA, Modrich P. Human mismatch repair: reconstitution of a nick-directed bidirectional reaction. J Biol Chem. 2005;280:39752–39761. doi: 10.1074/jbc.M509701200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Yuan F, Presnell SR, Tian K, Gao Y, Tomkinson AE, Gu L, Li GM. Reconstitution of 5′-directed human mismatch repair in a purified system. Cell. 2005;122:693–705. doi: 10.1016/j.cell.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 15.Kramer B, Kramer W, Fritz HJ. Different base/base mismatches are corrected with different efficiencies by the methyl-directed DNA mismatch-repair system of E. coli. Cell. 1984;38:879–887. doi: 10.1016/0092-8674(84)90283-6. [DOI] [PubMed] [Google Scholar]
- 16.Dohet C, Wagner R, Radman M. Repair of defined single base-pair mismatches in Escherichia coli. Proc Natl Acad Sci U S A. 1985;82:503–505. doi: 10.1073/pnas.82.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaaper RM. Base selection, proofreading, and mismatch repair during DNA replication in Escherichia coli. J Biol Chem. 1993;268:23762–23765. [PubMed] [Google Scholar]
- 18.Kunkel TA, Burgers PM. Dividing the workload at a eukaryotic replication fork. Trends Cell Biol. 2008;18:521–527. doi: 10.1016/j.tcb.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu AL. Influence of GATC sequences on Escherichia coli DNA mismatch repair in vitro. J Bacteriol. 1987;169:1254–1259. doi: 10.1128/jb.169.3.1254-1259.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umar A, Buermeyer AB, Simon JA, Thomas DC, Clark AB, Liskay RM, Kunkel TA. Requirement for PCNA in DNA mismatch repair at a step preceding DNA resynthesis. Cell. 1996;87:65–73. doi: 10.1016/s0092-8674(00)81323-9. [DOI] [PubMed] [Google Scholar]
- 21.Pavlov YI, Mian IM, Kunkel TA. Evidence for preferential mismatch repair of lagging strand DNA replication errors in yeast. Curr Biol. 2003;13:744–748. doi: 10.1016/s0960-9822(03)00284-7. [DOI] [PubMed] [Google Scholar]
- 22.Nick McElhinny SA, Kissling GE, Kunkel TA. Differential correction of lagging-strand replication errors made by DNA polymerases {alpha} and {delta} Proc Natl Acad Sci U S A. 2010;107:21070–21075. doi: 10.1073/pnas.1013048107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadyrov FA, Genschel J, Fang Y, Penland E, Edelmann W, Modrich P. A possible mechanism for exonuclease 1-independent eukaryotic mismatch repair. Proc Natl Acad Sci U S A. 2009;106:8495–8500. doi: 10.1073/pnas.0903654106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tishkoff DX, Boerger AL, Bertrand P, Filosi N, Gaida GM, Kane MF, Kolodner RD. Identification and characterization of Saccharomyces cerevisiae EXO1, a gene encoding an exonuclease that interacts with MSH2. Proc Natl Acad Sci U S A. 1997;94:7487–7492. doi: 10.1073/pnas.94.14.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tishkoff DX, Amin NS, Viars CS, Arden KC, Kolodner RD. Identification of a human gene encoding a homologue of Saccharomyces cerevisiae EXO1, an exonuclease implicated in mismatch repair and recombination. Cancer Res. 1998;58:5027–5031. [PubMed] [Google Scholar]
- 26.Hombauer H, Campbell CS, Smith CE, Desai A, Kolodner RD. Visualization of eukaryotic DNA mismatch repair reveals distinct recognition and repair intermediates. Cell. 2011;147:1040–1053. doi: 10.1016/j.cell.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pavlov YI, Newlon CS, Kunkel TA. Yeast origins establish a strand bias for replicational mutagenesis. Mol Cell. 2002;10:207–213. doi: 10.1016/s1097-2765(02)00567-1. [DOI] [PubMed] [Google Scholar]
- 28.Tran PT, Erdeniz N, Symington LS, Liskay RM. EXO1-A multi-tasking eukaryotic nuclease. DNA Repair (Amst) 2004;3:1549–1559. doi: 10.1016/j.dnarep.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Balakrishnan L, Bambara RA. Eukaryotic lagging strand DNA replication employs a multi-pathway mechanism that protects genome integrity. J Biol Chem. 2011;286:6865–6870. doi: 10.1074/jbc.R110.209502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Genschel J, Modrich P. Mechanism of 5′-directed excision in human mismatch repair. Mol Cell. 2003;12:1077–1086. doi: 10.1016/s1097-2765(03)00428-3. [DOI] [PubMed] [Google Scholar]
- 31.Kadyrov FA, Dzantiev L, Constantin N, Modrich P. Endonucleolytic function of MutLalpha in human mismatch repair. Cell. 2006;126:297–308. doi: 10.1016/j.cell.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 32.Amin NS, Nguyen MN, Oh S, Kolodner RD. exo1-Dependent mutator mutations: model system for studying functional interactions in mismatch repair. Mol Cell Biol. 2001;21:5142–5155. doi: 10.1128/MCB.21.15.5142-5155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harfe BD, Jinks-Robertson S. DNA mismatch repair and genetic instability. Annu Rev Genet. 2000;34:359–399. doi: 10.1146/annurev.genet.34.1.359. [DOI] [PubMed] [Google Scholar]
- 34.Tran HT, Gordenin DA, Resnick MA. The 3′-->5′ exonucleases of DNA polymerases delta and epsilon and the 5′-->3′ exonuclease Exo1 have major roles in postreplication mutation avoidance in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:2000–2007. doi: 10.1128/mcb.19.3.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]