Abstract
Background & Aims
Gastric injections of botulinum toxin A (BTA) have been reported to delay gastric emptying, increase satiation, and reduce body weight, but there are few data from randomized, placebo-controlled studies.
Methods
We enrolled 60 obese participants in a 24-week, double-blind, randomized, placebo-controlled, concealed allocation trial to compare the effects of gastric antral injections of BTA (100 U, 300 U, or 500 U) and saline placebo. The study was conducted at an outpatient clinical research unit. Participants were given one set of injections of BTA or placebo into the gastric antral muscularis propria, using endoscopic ultrasound guidance. Gastric emptying of solids (GES) was measured by scintigraphy; we also measured body weight, satiation (maximum tolerated volume in a caloric liquid drink test), calorie intake (by food frequency questionnaire), gastrointestinal symptoms, and psychologic aspects of eating behavior (by rating scale).
Results
Compared with baseline values, 2 weeks after injections, the mean t1/2 for GES increased by 0.8, 14, 24, and 14 minutes among subjects given placebo, 100 U, 300 U, or 500 U of BTA, respectively (P=.24 overall, P=.04 for the group given 300 U vs placebo); 16 weeks after the injections, mean body weights were reduced by 2.2, 0.2, 2.3, and 3.0 kg in these groups, respectively. There were no statistically significant differences in mean body weight change, satiation volume, caloric intake, gastrointestinal symptoms, or psychological aspects of eating behavior among groups.
Conclusions
Gastric antral injections of BTA may delay gastric emptying at a dose of 300 U, but do not cause early satiety, altered eating behaviors, or loss of body weight. Clinicaltrials. gov identifier: NCT00976443
Keywords: obesity, therapy, endoscopy, motility
Introduction
Obesity is an important public health problem. Potential pharmacologic and endoscopic approaches to obesity include treatments that affect gastric emptying, compliance, and satiation. Prior animal and human studies1–11 suggest that gastric botulinum toxin A (BTA) injections slow gastric emptying, increase satiation, and induce weight loss. We investigated the dose-related effects of antral BTA injections on gastric function, body weight loss, and psychological aspects of eating behavior in a placebo-controlled trial in persons with mild to moderate obesity.
Methods and Procedures
Participants
Obese human volunteers were recruited by public advertisement between 9/1/2009 and 1/31/2011 for this study, which was approved by the Mayo Clinic IRB. All participants provided written informed consent. Inclusion criteria included age between 18 and 60 years, BMI ≥ 30 kg/m2 and body weight ≥ 85 kg, and no history of diabetes, gastroparesis, myopathy, or neuromuscular disorder. Potential participants were screened using questions 2, 3, 4, 38, and 39 from the Bowel Disease Questionnaire (BDQ),12 which address symptoms of chronic abdominal pain, nausea, or vomiting; those with positive responses were excluded. Participants received no counseling about diet or exercise, or other interventions for obesity.
Study design
The study utilized a modified group sequential adaptive design. The first 18 participants were randomly assigned to receive BTA 100 U, BTA 300 U, or normal saline placebo (6 participants in each group) according to a computer generated randomization list composed of 2 blocks of 9 participants each. An interim analysis was then conducted by 2 investigators (MC, FE) who had no participant contact. O’Brien Fleming interim analysis methodology was used, with pairwise comparisons with placebo, an endpoint of body weight loss at 8 weeks, and a type I error rate of 0.0415. This showed no significant difference between the placebo and BTA 300 U groups (p=0.33), and a 500 U dose was chosen for the fourth study arm. The other investigators, study staff, and participants remained unaware of the interim findings or the BTA dose chosen for the fourth arm. An additional 42 participants were then randomized to one of the 4 treatment arms (9 additional participants to each of the 3 initial arms, and 15 participants to the fourth arm), utilizing a computer generated randomization list composed of 3 blocks of 9 participants each. In total 15 participants were assigned to each of the 4 study arms. Only the study statistician had knowledge of the randomization lists. All authors had access to study data and reviewed and approved the final manuscript.
Allocation and blinding
Allocation was performed by the Mayo Research Pharmacy staff on the day of the participant’s EUS procedure by matching the participant’s study number with the randomization list. Allocation was concealed from all participants, investigators, study coordinators, and clinicians caring for participants until data ascertainment for the entire trial was complete. Pre-mixed study medication was delivered to the endoscopy suite labeled with participant identifiers and protocol number only. Because reconstituted BTA is a clear solution identical in appearance and viscosity to normal saline, and each participant received the same volume of gastric injectate, study personnel performing EUS procedures remained blinded to participant allocation.
The success of blinding was evaluated by asking the investigator performing EUS, the study coordinator, and the participant which treatment group the participant had been assigned to (placebo, active drug, or unsure). This was asked during the recovery period on the day of BTA injection as well as 2 and 24 weeks later.
Endoscopic procedures
Four investigators performed endoscopic procedures, and were assigned subjects based on schedule availability. After a 2 week baseline period and an overnight fast, participants underwent esophagogastroduodenoscopy (EGD) under fentanyl and propofol sedation. Those with ulceration or retained food in the stomach were excluded. Endoscopic ultrasound (EUS) was performed, with injection of 30 mL of study medication solution containing either 0, 3.33, 10, or 16.66 units of botulinum toxin A (Botox®, Allergan, Irvine, CA) per mL, corresponding to a total dose of 0, 100, 300, or 500 U BTA per participant. Injections were performed via a 25 gauge EUS needle under real-time EUS guidance, targeting the gastric muscularis propria as previously described.10 Three rings of injections were performed around the antral circumference, with 5 equally spaced 2 mL injections in each ring. The rings were 1 to 2 cm apart, and the most distal ring was 2 to 3 cm proximal to the pylorus. Prophylactic antibiotics (amoxicillin/clavulante or levoquin) were administered for 3 days because of the possibility of intra-peritoneal injection. Participants were followed for 24 weeks after study injections.
Measures
Body weight was measured in the Mayo Center for Translational Scientific Activities (CTSA) Clinical Research Unit at baseline and 1, 2, 3, 4, 6, 8, 10, 12, 14, 16, and 24 weeks after BTA injection. The same calibrated scale was used for all weight measurements. Participants were weighed in street clothes with coats, sweaters, and shoes removed.
Gastric emptying of solids (GES) in a mixed meal was measured over 4 hours using a previously validated scintigraphic method.13 Gastric emptying (GE) tests were performed during the baseline period and 2 weeks after gastric injections, when the maximum effect of BTA was likely to be evident.14 Participants with delayed GES half time (T½) at baseline were excluded from further participation.
Satiation was assessed with the Nutrient Drink Test at baseline and 2 and 16 weeks, following the method of Tack, et al15 as previously reported.16 Participants ingested 120 ml of a nutrient drink (Ensure®) per 4 minutes, and scored their satiety at 5-minute intervals on a scale graded 0–5 (0=no symptoms, 5=maximum or unbearable fullness). Participants stopped meal intake when a score of 5 was reached, and the maximum tolerated volume (MTV) of nutrient drink was recorded. Normal values for MTV in adults in our laboratory are ≥ 850 mL.16
Gastrointestinal symptoms were assessed with the Gastrointestinal Symptom Rating Scale (GSRS).17, 18 Participants completed the GSRS at baseline, weekly for 4 weeks after BTA injection, and at 6, 8, 10, 12, 14, 16, and 24 weeks.
Caloric intake was measured at baseline and at 4, 8, 12, 16 and 24 weeks using VioFFQ, an electronic food frequency questionnaire that queries dietary intake over one month.19
Eating behaviors were assessed at baseline and 4, 16, and 24 weeks. Binge eating episodes were assessed with The Questionnaire on Eating and Weight Patterns-Revised (EWP),20 self-confidence for managing eating was assessed with The Weight Efficacy Life-Style Questionnaire (WEL),21 and Hunger, Cognitive restraint, and Disinhibition were assessed with The Eating Inventory (TEI).22
Statistical methods
Primary study outcome was pre-specified as body weight loss at 16 weeks. Pre-specified secondary outcomes included change in body weight at other time points, as well as changes in GES T½, MTV, caloric intake, and symptoms. Sample size was calculated anticipating a body weight change of −4.9 kg in the 300 U BTA group and −2.5 kg in the placebo group at 16 weeks based on our preliminary data,10 with a standard deviation of 2 kg, a 2-sided type I error rate of 0.0415, and 80% power to demonstrate a treatment effect between placebo and 300 U BTA.
Analysis of variance (ANOVA) models were used to compare the treatment groups for the primary continuous outcomes. Nonparametric Kruskal-Wallis tests were also used to either verify the parametric models or when distributional assumptions were not met. Repeated measures linear models were also explored to provide comparisons at each time point; terms for treatment group, time, and a treatment by time interaction were used. Equal correlations between measurements from the same participant were assumed, and the robust “sandwich” estimate of the covariance matrix for the fixed effects was used.23 Other associations were measured using Pearson correlation coefficients, Fisher’s Exact test, or t-tests, as appropriate.
Results
Flow of participants through the study is shown in supplemental Figure 1. Of the 60 participants 52 were women, mean age was 49 (range 24–59) years, mean baseline BMI was 37.9 (range 30.4–49.7) kg/m2, and mean baseline weight was 106.3 (range 86–159) kg. There was no significant difference in baseline weight or BMI between groups. For each study arm 15 participants were randomly assigned, received the intended treatment, and were analyzed for the primary outcome. Compliance with study visits was 98%.
Gastric Emptying
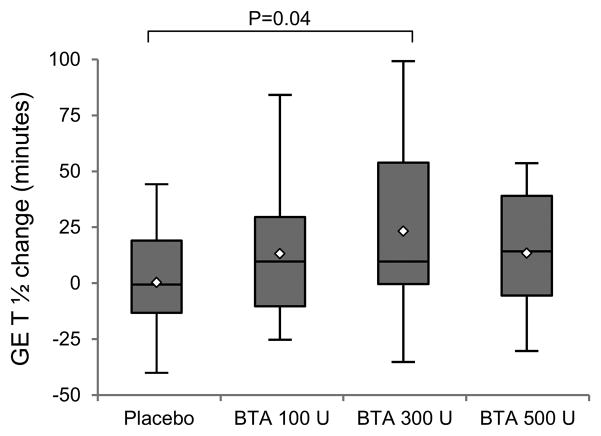
Baseline GES T½ was similar between treatment groups. As shown in Figure 1, the mean change (±SD) in GES T½ two weeks after injections was +0.8 ± 24 minutes for the placebo group and +14 ± 29 minutes, +24 ± 39 minutes, and +14 ± 27 minutes for the BTA 100 U, 300 U, and 500 U groups, respectively. When compared to placebo, changes in GES T½ were statistically significant for the BTA 300 U group only (p=0.04; overall p=0.24). After injections, GES T½ slowed to greater than 160 minutes (the limit of normal with the same test meal in our lab) in 1 placebo participant, 5 BTA 100 U participants, 3 BTA 300 U participants, and no BTA 500 U participants (p=.058 for comparison between groups).
Figure 1.
Change in gastric emptying of solids (GES) T½ two weeks after gastric injections.
Shaded boxes indicate the interquartile range (IQR); the lines and diamonds inside the boxes indicate median and mean values, respectively. p=.24, .04, and .24 for comparison of the 100 U, 300 U, and 500 U groups with placebo, respectively; overall p=0.23.
When comparing this entire obese cohort to sex-matched non-obese normal volunteers previously studied in our unit,24 mean baseline GES for men was 36% vs 20% at 60 minutes (p<.001), 67% vs 58% at 120 minutes (p=0.15), 95% vs 95% at 240 minutes (p=0.79), and T ½ of 89 vs. 110 minutes (p=.003), respectively. Similar values for women were 32% vs 17% (p<.0001), 61% vs 49% (p<.0001), 95% vs 93% (p=.03), and 100 vs 125 minutes (p<.0001), respectively.
Satiation
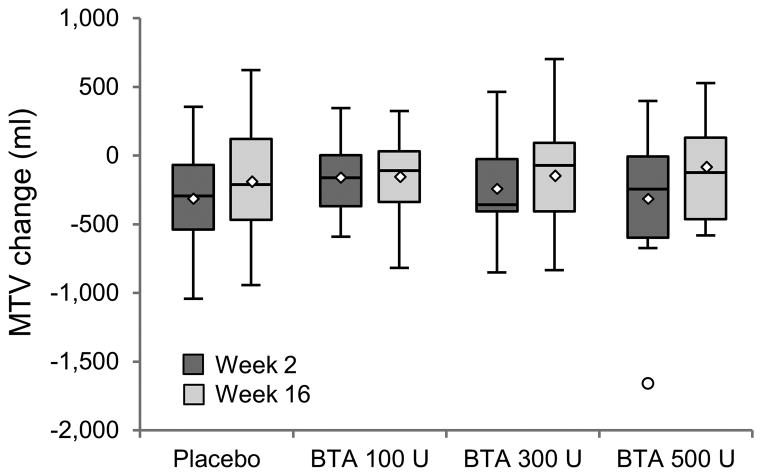
As shown in Figure 2, mean change (±SD) in MTV 2 and 16 weeks after injections was −313 ± 360 mL and −177 ± 408 mL in the placebo group, −154 ± 268 mL and −162 ± 309 mL in the BTA 100 U group, −235 ± 312 mL and −141 ± 359 mL in the BTA 300 U group, and −305 ± 515 mL and −94 ± 361 mL in the BTA 500 U group, respectively. When compared to placebo, changes in MTV over time were not significant in any of the BTA groups.
Figure 2.
Change (compared to baseline) in maximum tolerated volume (MTV) 2 and 16 weeks after gastric injections.
Shaded boxes indicate the interquartile range (IQR); the lines and diamonds inside the boxes indicate median and mean values, respectively, with outliers represented by open circles. There are no statistically significant differences between groups at either week 2 or week 16.
Body Weight
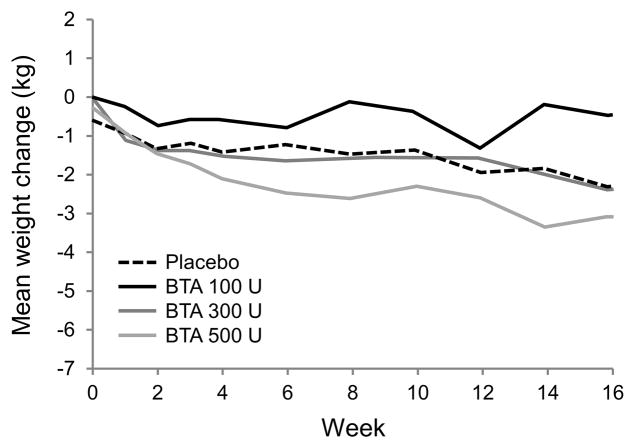
Body weight change over time is shown in Figure 3. At 8 and 16 weeks after injections the mean change in body weight compared to baseline was −1.4 ± 3.5 kg and −2.2 ± 3.5 kg in the placebo group, −0.01 ± 2.7 kg and −0.4 ± 3.1 kg in the BTA 100 U group, −1.5 ± 2.7 kg and −2.3 ± 3.4 kg in the BTA 300 U group, and −2.5 ± 4.3 kg and −3.0 ± 5.1 kg in the BTA 500 U group. There were no statistically significant differences between groups at any time point (ANOVA overall p=0.28 and 0.29 for weeks 8 and 16; p-values ranged from 0.10 to 0.65 for overall treatment effect by week).
Figure 3.
Body weight change after gastric injections.
Lines represent mean body weight change over time for each treatment group. There are no statistically significant differences between groups at any time point.
Among participants not receiving placebo, 7 lost at least 5 kg body weight by week 16. These participants did not differ from other non-placebo participants with regard to age, sex, BMI, baseline body weight, baseline GES T½, or mean change in GES T½ after gastric injections.
Despite blinding there was an association between endoscopist and treatment group assignment (p=0.02), however ANOVA showed no significant difference by endoscopist on GES T ½ change at 2 weeks (p=0.20) or body weight change at 16 weeks (p=0.34).
Caloric Intake
There was no difference in mean daily caloric intake between study groups at baseline or at any subsequent time point.
Symptoms
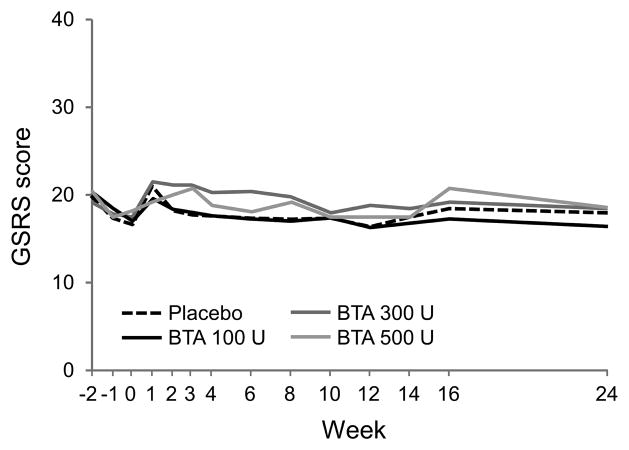
There were no statistically significant differences in GSRS scores between the placebo and treatment groups at any time point (Figure 4). Mean GSRS scores at each time point for each study arm ranged from 19.1 to 20.9 at baseline, and 16.9 to 21.6 between weeks 0 and 24.
Figure 4.
GSRS (Gastrointestinal Symptom Rating Scale) scores over time.
There are no statistically significant differences between groups at any time point.
Eating Behavior
Of the 60 study participants, 3 met criteria for binge eating disorder at baseline, too few to detect differences between treatment groups.. TEI and WEL scale scores at baseline were similar between treatment groups, and there was no association between these scores and baseline GES T½. Baseline MTV was inversely correlated with TEI Disinhibition score (r= −0.28, p=.03), total WEL score (r= −0.28, p=.03), and the negative emotions and availability WEL subscale scores (r= −0.27 and −0.30, p=.04 and .02, respectively). There was no correlation between baseline TEI or WEL scale or subscale scores and body weight change at weeks 8, 16, or 24.
Adverse Effects
Study interventions were well tolerated. There were 10 adverse events, 7 of which were possibly or probably related to study interventions. Of these, 6 were judged mild (gastroesophageal reflux symptoms or loose stools that resolved spontaneously) and one moderate (temporary increase in proton pump inhibitor dose for reflux symptoms). Of the 7 adverse events, 2 occurred in the placebo group, 2 in the BTA 100 U group, 2 in the BTA 300 U group, and 1 in the BTA 500 U group.
Success of allocation concealment
The principal investigator and study coordinator were unsure of participant allocation for all participants throughout the study. Immediately following gastric injections 98% (58/59) of participants were also unsure of their allocation, but at 2 and 24 weeks following gastric injections 16% (9/56) and 13% (7/55) of participants thought they had received BTA injections, respectively. There was no association between participant’s guesses about allocation and their actual allocation (p>0.63) or change in GES T½ after injections. Participants who guessed at 2 weeks that they had received BTA did lose more body weight at 8 weeks than other participants (mean −4.0 ± 4.1 vs. −0.9 ± 3.1 kg, p=.01), but not at 16 or 24 weeks.
Discussion
Gastric injections of botulinum toxin A were first reported to induce weight loss in a human in 2003,9 and a subsequent randomized, double-blind trial found that BTA injections were associated with delayed gastric emptying, increased satiation, and body weight loss.4 Our data also suggest the development of delayed gastric emptying after injection of 300 U BTA into the gastric antrum, but this was not accompanied by increased satiation or loss of body weight.
Gastric antral contractility is an important component of normal gastric emptying of solids.25 Reduced antral motility appears to prolong the lag time and gastric emptying T½.26 BTA inhibits cholinergic transmission and impairs contractility of gastric muscle.27 Subserosal injections of BTA in rat gastric antrum induced delayed gastric emptying T½, decreased food intake, and body weight loss.3, 6
Prior human studies of gastric BTA injections have yielded conflicting results (Table 1). Of 5 non-placebo controlled studies, two showed promising changes in gastric emptying and body weight, and this was associated with BTA doses of 200 and 300 U.10 Three previous placebo-controlled trials each tested a dose of 200 U; one demonstrated a decrease in gastric emptying, and two showed a mean decrease in body weight of between 6 and 11 kg in the active treatment arm.
Table 1.
Prior human studies of gastric botulinum toxin injections.
| Author | Trial Type | N | BTA dose | Injection site | # of injections | EUS guided? | Weeks f/u | Δ weight or BMI ± SD, if provided | Δ gastric emptying compared to baseline | Δ satiety | Adverse events |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Garcia Compean 20055 | Open label | 12 | 100 U | Pre-pyloric (1 ring) | 8 | No | 12 | −1 kg (NS c/w baseline) | 4% delay at 4 weeks (NS) | Not measured | None |
| Albani 20051 | Open label | 8 | 500 U Dysport© (= 125 U Botox©) | Pre-pyloric region | 10 | No | 16 | − 1 kg ± 15.5 (NS) | Not measured | Not measured | None |
| Cardoso Junior 20062 | Open label | 6 | 200 U | Antrum (1–2 rings) | 8 – 16 | No | 12 | + 0.1 kg/m2 NS | + 3 min T ½ (NS) | Not measured | None |
| 6 | 300 U | Antrum (2–3 rings) | 16 – 24 | No | 12 | − 0.5 kg/m2 NS | + 2 min T ½ (NS) | Not measured | None | ||
| Gui 20067 | RCT | 4 | Placebo | Incisura (1 ring) | 8 | No | 5 | −0.4 kg ± 5 (NS) | − 8 min T ½ ± 7 (NS) | Not measured | None |
| 6 | 133 U | − 7.8 kg ± 8.5 (NS) | + 1 min T ½ ± 24 (NS) | ||||||||
| 4 | 200 U | − 6 kg ± 5 (NS) | − 8 min T ½ ± 13 (NS) | ||||||||
| Foschi 20074 | RCT | 12 | Placebo | Antrum (3 rings), Cardia (1 ring), Fundus (1 ring) | 20 | No | 8 | − 5.7 kg ± 1 | − 2 min T ½ ± 7 | ? | None |
| 12 | 200 U | − 11 kg ± 1 (p <.01 c/w placebo) | + 19 min T ½ ± 8 (p < .05 c/w placebo) | Early satiety on VAS (p < .001) | |||||||
| Mittermair 20078 | RCT | 5 | Placebo | 4 rings 4,6,8, and 12 cm from pylorus | 16 | No | 24 | 0 kg ± 2 | Not measured | Not measured | None |
| 5 | 200 U | + 0.3 kg ± 0.3 | |||||||||
| Topazian 200810 | Open label | 10 | 100 U | Antrum (1 ring) | 5 | Yes | 16 | − 4.9 kg ± 6.3 (NS) | − 5 min T ½ | Decreased maximum tolerated volume | None |
| 300 U | + 10 min T ½ | ||||||||||
| Li 201211 | RCT | 9 | 200 U | Antrum, body, and fundus (5 rings) | 20 | No | 12 | −3.5 kg (p<.05) | + 35 min T ½ at 4 weeks (p<.05) | Not measured | None |
| 10 | 300 U | 20 | No | 12 | −5.95 kg (p<.05) | +46.7 min T ½ at 4 weeks (p<.05) | Not measured | None |
RCT = randomized controlled trial, NS=not statistically significant, SD=standard deviation, T ½ = half time, U=units, VAS=visual analog scale
We found a dose-response relationship in GES T½ changes following BTA injections, with slowest gastric emptying in the group receiving 300 U. It is unclear why 500 U had less effect on gastric emptying. One possible explanation is diffusion of BTA to the pylorus in the higher dose group, relaxing the pylorus and promoting gastric emptying. BTA-induced changes in gastric emptying were well tolerated.
Unlike the largest previous placebo-controlled study,4 which randomly assigned 24 participants to BTA 200 U vs. placebo, we did not observe significant changes in body weight after BTA injections. This discrepancy is not due to differing effects on gastric motility: the mean delay in GES T½ in our participants was similar to the previous study. Furthermore, participants who lost the most weight in our study did not have correspondingly longer delays in gastric emptying. Large and consistent changes in body weight were obtained in the previous study, with narrow standard deviations that we were unable to reproduce. There are several methodological differences between studies. In the previous work small aliquots were injected in the gastric cardia and body in addition to several rings of antral injections; we did not perform gastric body BTA injections. EUS guidance was not utilized in the previous work, so it is unclear which layer of the gastric wall (or peritoneum) was injected. The previous investigators found decreased gastric capacity for liquids after BTA injection; in contrast, we observed no change in gastric capacity for liquids. Finally there is no mention of concealed allocation in the previous report. Failure to conceal allocation could result in a placebo effect.
Previous research has found an association between gastrointestinal symptoms and eating behaviors.28 We found that baseline MTV, a physiologic measure of satiation, correlated with eating behavior: higher MTV was associated with less self-efficacy for control of eating. These findings suggest that educating obese patients about physiologic differences in stomach volume might help them improve their self-efficacy for eating.
In summary, we found that injections of 300 U BTA into gastric antral muscularis propria under EUS guidance may induce delays in GES T½, but do not cause body weight loss. It is unlikely that higher doses of BTA than those tested here would induce additional delays in gastric emptying.
Acknowledgments
Grant support: This study was supported by NIH DK 1R21DK079903-01A2 and 3R21DK079903-02S1 grants from the National Institutes of Health to Dr Topazian, and by NIH CTSA grant RR0024150. Dr. M. Camilleri’s participation was supported in part by NIH DK-067071.
The authors thank Duane Burton and the Endoscopy and Imaging core of the Mayo Clinic CTSA for assistance in the conduct of gastric physiology testing, Amy Boldingh for study coordination, and Brigitte Syhakhoun for secretarial support.
Abbreviations
- BDQ
Bowel Disease Questionnaire
- BMI
body mass index
- BTA
botulinum toxin A
- EUS
endoscopic ultrasound
- EWP
Questionnaire on Eating and Weight Patterns-Revised
- GES
gastric emptying of solids
- GS
gastrointestinal symptoms
- GSRS
Gastrointestinal Symptoms Rating Scale
- MTV
maximum tolerated volume
- T ½
half time
- TEI
The Eating Inventory
- U
units
- VioFFQ
Vio Food Frequency Questionnaire
- WEL
Weight Efficacy Life-Style Questionnaire
Footnotes
Disclosures: The authors have no relevant disclosures or conflicts of interest.
Writing Assistance: None
Author Contributions:
Mark Topazian: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtained funding; study supervision Michael Camilleri and Matthew Clark: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content Felicity Enders and Ross Dierkhising: study concept and design; statistical analysis; critical revision of the manuscript for important intellectual content All other authors: acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albani G, Petroni M, Mauro A, Liuzzi A, Lezzi G, Verti B, Marzullo P, Cattani L. Safety and efficacy of therapy with botulinum toxin in obesity: a pilot study. J Gastroenterol. 2005;40:833–5. doi: 10.1007/s00535-005-1669-x. [DOI] [PubMed] [Google Scholar]
- 2.Cardoso A, Junior, Savassi-Rocha P, Vaz Coelho L, de Mello Sposito M, Albuquerque W, Costa Diniz M, de Mattos Paixao A, Duarte Garcia F, Faria Lasmar L. Botulinum A toxin injected into the gastric wall for the treatment of class III obesity: a pilot study. Obes Surg. 2006;16:335–43. doi: 10.1381/096089206776116408. [DOI] [PubMed] [Google Scholar]
- 3.Coskun H, Duran Y, Dilege E, Mihmanli M, Seymen H, Demirkol M. Effect on gastric emptying and weight reduction of botulinum toxin-A injection into the gastric antral layer: an experimental study in the obese rat model. Obes Surg. 2005;15:1137–43. doi: 10.1381/0960892055002275. [DOI] [PubMed] [Google Scholar]
- 4.Foschi D, Corsi F, Lazzaroni M, Sangaletti O, Riva P, La Tartara G, Bevilacqua M, Osio M, Alciati A, Bianchi Porro G, Trabucchi E. Treatment of morbid obesity by intraparietogastric administration of botulinum toxin: a randomized, double-blind, controlled study. Int J Obes. 2007;31:707–12. doi: 10.1038/sj.ijo.0803451. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Compean D, Mendoza-Fuerte E, Martinez J, Villarreal I, Maldonado H. Endoscopic injection of botulinum toxin in the gastric antrum for the treatment of obesity. Results of a pilot study. Gastroenterol Clin Biol. 2005;29:789–91. doi: 10.1016/s0399-8320(05)86349-3. [DOI] [PubMed] [Google Scholar]
- 6.Gui D, Da Gaetano A, Spara P, Viggiano A, Cassetta E, Albanese A. Botulinum toxin injected in the gastric wall reduces body weight and food intake in rats. Aliment Pharmacol Ther. 2000;14:829–834. doi: 10.1046/j.1365-2036.2000.00765.x. [DOI] [PubMed] [Google Scholar]
- 7.Gui D, Mingrone G, Valenza V, Spada P, Mutignani M, Runfola M, Scarfone A, Di Mugno M, Panunzi S. Effect of botulinum toxin antral injection on gastric emptying and weight reduction in obese patients: a pilot study. Aliment Pharmacol Ther. 2006;23:675–80. doi: 10.1111/j.1365-2036.2006.02773.x. [DOI] [PubMed] [Google Scholar]
- 8.Mittermair R, Keller C, Geibel J. Intragastric injection of botulinum toxin A for the treatment of obesity. Obesity Surgery. 2007;17:732–6. doi: 10.1007/s11695-007-9135-x. [DOI] [PubMed] [Google Scholar]
- 9.Rollnik JDMP, Manns MP, Goke M. Antral injections of botulinum A toxin for the treatment of obesity. Annals of Internal Medicine. 2003;138:359–360. doi: 10.7326/0003-4819-138-4-200302180-00026. [DOI] [PubMed] [Google Scholar]
- 10.Topazian M, Camilleri M, De La Mora-Levy J, Enders FB, Foxx-Orenstein AE, Levy MJ, Nehra V, Talley NJ. Endoscopic ultrasound-guided gastric botulinum toxin injections in obese subjects: a pilot study. Obesity Surgery. 2008;18:401–7. doi: 10.1007/s11695-008-9442-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Liu QS, Liu WH, Yang YS, Yan D, Peng LH, Li LY, Meng JY, Wang XD, Ke M. Treatment of obesity by endoscopic gastric intramural injection of botulinum toxin a: a randomized clinical trial. Hepato-gastroenterology. 2012;59:2003–7. doi: 10.5754/hge11755. [DOI] [PubMed] [Google Scholar]
- 12.Talley N, Phillips S, Melton, Wiltgen C, Zinsmeister A. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111:671–4. doi: 10.7326/0003-4819-111-8-671. [DOI] [PubMed] [Google Scholar]
- 13.Cremonini F, Mullan B, Camilleri M, Burton D, Rank M. Performance characteristics of scintigraphic transit measurements for studies of experimental therapies. Aliment Pharmacol Ther. 2002;16:1781–90. doi: 10.1046/j.1365-2036.2002.01344.x. [DOI] [PubMed] [Google Scholar]
- 14.Abbruzzese G, Berardelli A. Neurophysiological effects of botulinum toxin type A. Neurotox Res. 2006;9:109–14. doi: 10.1007/BF03033927. [DOI] [PubMed] [Google Scholar]
- 15.Tack JPH, Coulie B, Caenepeel P, Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346–1352. doi: 10.1016/s0016-5085(98)70012-5. [DOI] [PubMed] [Google Scholar]
- 16.Delgado-Aros S, Cremonini F, Castillo J, Chial H, Burton D, Ferber I, Camilleri M. Independent influences of body mass and gastric volumes on satiation in humans. Gastroenterology. 2004;126:432–440. doi: 10.1053/j.gastro.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Dimenas E, Glise H, Hallerback B, Hernqvist H, Svedlund J, Wiklund I. Quality of life in patients with upper gastrointestinal symptoms. An improved evaluation of treatment regimens? Scand J Gastroenterol. 1993;28:681–687. doi: 10.3109/00365529309098272. [DOI] [PubMed] [Google Scholar]
- 18.Talley N, Fullerton S, Junghard O, Wiklund I. Quality of life in patients with endoscopy-negative heartburn: reliability and sensitivity of disease-specific instruments. Am J Gastroenterol. 2001;96:1998–2004. doi: 10.1111/j.1572-0241.2001.03932.x. [DOI] [PubMed] [Google Scholar]
- 19.Patterson R, Kristal A, Tinker L, Carter R, Bolton M, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–87. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 20.Yanovski S. Binge eating disorder: Current knowledge and future directions. Obesity Research. 1993;1:306–24. doi: 10.1002/j.1550-8528.1993.tb00626.x. [DOI] [PubMed] [Google Scholar]
- 21.Clark M, Abrams D, Niaura R, Eaton C, Rossi J. Self-efficacy in weight management. Journal of Consulting and Clinical Psychology. 1991;59:739–44. doi: 10.1037//0022-006x.59.5.739. [DOI] [PubMed] [Google Scholar]
- 22.Stunkard A, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. Journal of Psychosomatic Research. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 23.Diggle P, Liang K, Zeger S. Analysis of longitudinal data. Clarendon Press; 1994. [Google Scholar]
- 24.Camilleri M, Iturrino J, Bharucha AE, Burton D, Shin A, Jeong ID, Zinsmeister AR. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participants. Neurogastroenterol Motil. 2012 doi: 10.1111/j.1365-2982.2012.01972.x. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camilleri M, Malagelada JR, Brown ML, Becker G, Zinsmeister AR. Relation between antral motility and gastric emptying of solids and liquids in humans. Am J Physiol. 1985;249:G580–5. doi: 10.1152/ajpgi.1985.249.5.G580. [DOI] [PubMed] [Google Scholar]
- 26.Camilleri M, Brown ML, Malagelada JR. Relationship between impaired gastric emptying and abnormal gastrointestinal motility. Gastroenterology. 1986;91:94–9. doi: 10.1016/0016-5085(86)90444-0. [DOI] [PubMed] [Google Scholar]
- 27.James A, Ryan J, Parkman H. Inhibitory effects of botulinum toxin on pyloric and antral smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2003;285:G291–G297. doi: 10.1152/ajpgi.00296.2002. [DOI] [PubMed] [Google Scholar]
- 28.Cremonini F, Camilleri M, Clark MM, Beebe TJ, Locke GR, Zinsmeister AR, Herrick LM, Talley NJ. Associations among binge eating behavior patterns and gastrointestinal symptoms: a population-based study. International Journal of Obesity. 2009;33:342–53. doi: 10.1038/ijo.2008.272. [DOI] [PMC free article] [PubMed] [Google Scholar]