Abstract
Background/Purpose
The objective of this study was identify independent associations between body composition and bone outcomes, including cortical structure and cortical and trabecular volumetric bone mineral density (vBMD) across the adult age spectrum.
Methods
This cross-sectional study evaluated over 400 healthy adults (48% male, 44% black race), ages 21–78 years. Multivariable linear regression models evaluated associations between whole-body DXA measures of lean body mass index (LBMI) and fat mass index (FMI) and tibia peripheral quantitative CT (pQCT) measures of cortical section modulus, cortical and trabecular vBMD and muscle density (as a measure of intramuscular fat), adjusted for age, sex, and race. All associations reported below were statistically significant (p < 0.05).
Results
Older age and female sex were associated with lower LBMI and muscle strength. Black race was associated with greater LBMI but lower muscle density. Greater FMI was associated with lower muscle density. Cortical section modulus was positively associated with LBMI and muscle strength and negatively associated with FMI. Adjustment for body composition eliminated the greater section modulus observed in black participants and attenuated the lower section modulus in females. Greater LBMI was associated with lower cortical BMD and greater trabecular BMD. FMI was not associated with either BMD outcome. Greater muscle density was associated with greater trabecular and cortical BMD. Associations between body composition and bone outcomes did not vary by sex (no significant tests for interaction).
Conclusions
These data highlight age, sex- and race-specific differences in body composition, muscle strength and muscle density, and demonstrate discrete associations with bone density and structure. These data also show that age, sex- and race- related patterns of bone density and strength are independent of differences in body composition. Longitudinal studies are needed to examine the temporal relations between changes in bone and body composition.
Keywords: DXA, lean mass, pQCT, section modulus, bone mineral density, muscle density
Introduction
Body composition varies according to age, sex, and racial background, and contributes to the normal variability in bone structure and bone mineral density (BMD) among adults. Aging is associated with loss of muscle mass and quality, and age-related muscle loss is accompanied by fat gain in older adults [1]. It is well established that low body mass index (BMI) is a risk factor for osteoporosis and fracture [2]; however, studies of the independent contributions of lean mass and fat mass to bone health have yielded conflicting results. For example, an early dual energy x-ray absorptiometry (DXA) report from the Health, Aging, and Body Composition Study (Health-ABC) of men and women, ages 70 to 79 years, concluded that lean mass and fat mass were both positively associated with BMD, however, the associations varied according to sex, measurement site, and the index used to adjust for bone size [3]. A subsequent Women’s Health Initiative study reported that femur BMD and cross-sectional area (CSA) were greater in women with higher BMI and values scaled in proportion to lean mass but not fat or total body mass [4]. More recent studies suggested differential effects of subcutaneous versus visceral fat mass. The majority of studies employing computed tomography (CT) measures of fat distribution demonstrated that visceral adipose tissue was negatively associated with BMD and bone structure [5–8].
The “functional muscle-bone unit” approach posits that bone adapts to the mechanical forces to which it is subjected in order to keep the bone strength at a constant set point [9]. A recent peripheral quantitative computed tomography (pQCT) study in men ages > 65 years, enrolled in the Osteoporotic Fractures in Men Study (MrOS) demonstrated that leg power and physical activity were positively associated with bone size and estimates of bone compressive strength [10]. Muscle metabolism may also affect bone health; fatty infiltration of muscle was associated with a higher risk of fracture in Health-ABC participants, independent of BMD, muscle CSA, and muscle strength [11, 12]. These data support the concept that differences in lean mass and muscle quality between subjects of varying age, sex, race, and total fat mass play a critical role in determining epidemiologic associations between these variables and bone outcomes, including fractures.
Prior studies of the functional muscle-bone unit and the impact of adiposity on BMD and bone structure were largely limited to elderly or adolescent participants, were frequently restricted to males or females, and usually did not examine race differences. A recent study demonstrated positive associations between skeletal muscle mass and bone density and structure at multiple skeletal sites; however, the cohort was > 96% white, and the study did not include measures of adiposity [13]. To our knowledge, no prior studies examined measures of volumetric BMD, cortical structure, body composition, muscle strength, and muscle density across the age range from young adults to the elderly in a multiethnic sample.
This cross-sectional study in 500 adults, ages 21 to 78 years included DXA measures of whole body and regional lean and fat mass, tibia pQCT measures of muscle area, muscle density, trabecular and cortical volumetric BMD and cortical structure, and dynamometric measures of isometric muscle strength. The objectives were to (1) determine the effects of age, sex, and race on lean body mass, muscle strength, and muscle density, and (2) determine the effects of age, sex and race on trabecular and cortical BMD, cortical section modulus (a summary measure of cortical dimensions), and examine associations between body composition and bone outcomes.
Material and Methods
Study Setting and Participants
Adults, ages 21 to 78 years, were enrolled as healthy reference participants for bone studies at the Children’s Hospital of Philadelphia (CHOP) and University of Pennsylvania (UPENN) between March of 2004 and June of 2008. Participants were recruited from UPENN internal medicine clinics and the surrounding community using flyers and newspaper advertisements. Exclusion criteria included a history of chronic diseases or medications known to affect nutrition or bone health, such as a reported history of diabetes, malabsorption syndromes, chronic kidney disease, liver disease, thyroid disease or malignancy. The protocols were approved by the Institutional Review Boards at CHOP and UPENN. Informed consent was obtained from all participants. A total of 90 participants less than 35 years of age were included in a prior study of bone and body composition in children and young adults [14].
Of the 500 total subjects, data were missing for section modulus (15 subjects), cortical density (15 subjects), trabecular density (20 subjects), muscle density (36 subjects), muscle strength (11 subjects), and body composition (43 subjects).
Assessment of Anthropometrics and Race
Weight and height were measured using a digital scale (Scaltronix, White Plains, NY) and stadiometer (Holtain Ltd., Crymych, UK), respectively. Participants self-identified race according to National Institute of Health categories. Among 500 participants, 255 and 221 self-identified as black or white, respectively. The remaining 25 included 20 Asians, one Native American, and three Native Hawaiian or other Pacific Islander.
Peripheral Quantitative Computed Tomography (pQCT)
Bone, muscle and fat measures in the left tibia were obtained by pQCT (Stratec XCT2000 12-detector unit, Orthometrix, Inc.) with a voxel size of 0.4 mm, slice thickness of 2.3 mm, and scan speed of 25 mm/sec. All scans were analyzed with Stratec software version 6.00. A scout view was obtained to place the reference line at the proximal border of the distal endplate. The bone measurements were obtained at 3% and 38% of tibia length proximal to the reference line. At the 3% metaphyseal site, scans were analyzed for trabecular volumetric BMD (mg/cm3) with contour mode 3 threshold 169 mg/cm3, and peel mode 4 threshold 650 mg/cm3 with an additional 10% concentric peel. At the 38% diaphyseal site, scans were analyzed for cortical volumetric BMD (mg/cm3) using a threshold of 710 mg/cm3 to separate bone from soft tissue. Polar section modulus (mm3) was defined using a threshold of 480 mg/cm3. Section modulus provides a composite measure of the effects of cortical periosteal and endosteal dimensions on fracture load [15]. Calf muscle and subcutaneous fat CSA (mm2) were assessed 66% proximal to the distal physis using threshold 40 mg/cm3 for fat/lean separation and 711 mg/cm3 for lean/bone separation. Quality control was monitored daily using a phantom. The in vivo coefficient of variation (CV) ranged from 0.5–1.6% for pQCT outcomes.
The pQCT measure of muscle density (mg/cm3) was used as a composite index of intra and extra-myocellular fat content, as previously described [11, 16]. Prior studies have documented that skeletal muscle attenuation determined by CT was associated with skeletal muscle lipid content on tissue biopsy,[17] and pQCT studies of muscle density demonstrated significant associations with insulin resistance, independent of total body and subcutaneous fat[18]. Edge-detection and threshold techniques were used to separate tissues (fat, muscle, and bone) based on attenuation characteristics that are directly related to tissue composition and density [17, 19]. Images were filtered prior to being analyzed using contour mode 3 (−101 mg/cm3) to find skin, and peel mode 2 (40 mg/cm3) to separate adipose and muscle/bone, respectively. Images were filtered subsequently with a combination 3×3, and double 5×5 kernal image filter that clearly defined the edge of the muscle using contour mode 31 (40 mg/cm3). All bone was identified using a threshold of 150 mg/cm3 and mathematically removed to generate results for muscle density. The CV for muscle density using this method was 0.9% [16].
Dynamometric Measurement of Muscle Strength
Muscle strength was assessed using Biodex Multi-Joint System 3 Pro Dynamometer (Biodex Medical Systems, Inc, Shirley, NY). High intra-rater (0.97 to 0.99) and inter-rater (0.93 to 0.96) intra-class correlation coefficients have been reported [20]. Peak isometric torque [Newton-meters (N-m)] was measured in triplicate at −10, 0, 10, and 20 degrees and the highest value recorded for both dorsiflexion and plantarflexion. We report strength as peak isometric torque (ft-lbs) in dorsiflexion (with the foot placed in 20 degrees of plantarflexion), since this measurement had the best reproducibility in our lab (coefficient of variation, 4.3%) and had the best fit (R2) in prior regression models [21]. Further, the tibialis anterior attaches directly to the tibia (the bone of interest in this study) and causes dorsiflexion of the ankle.
Whole-Body Dual Energy X-Ray Absorptiometry (DXA)
Whole-body fat mass and lean mass were assessed by DXA using a Hologic densitometer (Delphi Systems, Hologic, Inc., Bedford, MA). The measurements were performed in the array mode using standard positioning techniques. Quality control scans were performed daily using a simulated L1–4 lumbar spine made of hydroxyapatite encased in epoxy resin. In our institution, the in vitro coefficient of variation was less than 0.6% and the in vivo coefficient of variation in adults was less than 1% [22]. Fat mass and lean body mass were converted to fat mass index (FMI, kg/m2) and lean body mass index (LBMI, kg/m2) using height. The decision to use body composition indices was based on the observation women had greater tibia length relative to height (compared with men) and black participants had greater tibia length relative to height (compared with non-black participants). We concluded that adjusting for tibia length or height alone would not adequately characterize these effects, potentially resulting in residual confounding in bone models that included measures of body composition.
Physical Activity Questionnaire
Physical activity was assessed using a detailed questionnaire developed for the Multi-Ethnic Study of Atherosclerosis (MESA) [23]. The MESA physical activity questionnaire uses 28 questions to assess physical activity during a typical week in the past month in a number of domains. Participants report the number of days per week and the number of hours and/or minutes per day the activity was performed. Each activity included in the questionnaire has an assigned metabolic equivalent level from the Compendium of Physical Activities. The intra-class correlations compare favorably with other accepted and commonly used physical activity surveys [24]. We used a definition of intentional exercise (the sum of walking for exercise, sports/dancing, and conditioning MET-hours/week) that has been previously defined [25]. The variable was highly skewed, therefore, results were considered in three categories: data were categorized as either no intentional exercise, or as above or below the median number of MET-hours/week among those reporting intentional exercise.
Statistical Analysis
Statistical analysis was performed using Stata 11 software (StataCorp, College Station, TX). Differences in means were assessed using Student’s t test or the Wilcoxon rank sum test as appropriate. Group differences in categorical variables were assessed using the χ2 test.
Natural log transformations resulted in linear relations between tibia length and cortical section modulus, muscle CSA, and muscle strength; therefore, these measures were log transformed in all analyses. The correlations (R) between log transformed tibia length and musculoskeletal outcomes were 0.67 for section modulus, 0.33 for muscle area, and 0.44 for muscle strength (all p< 0.001); therefore, all multivariable models incorporating these outcomes were adjusted for tibia length.
Linear regression models of muscle outcomes (DXA LBMI, pQCT muscle density, and isometric muscle strength) evaluated the independent effects of age, sex, race, DXA FMI, and physical activity. Linear regression models of bone outcomes (cortical section modulus, cortical BMD and trabecular BMD) first examined associations with age, sex and race, and then subsequent models evaluated the independent associations with LBMI, FMI, muscle density, muscle strength, and physical activity. Beta coefficients were converted to standardized coefficients (difference in outcome per every 1 standard deviation difference in the independent variable). Scatter plots were created to illustrate observed associations between body composition and bone outcomes. Multiplicative interaction terms were used to assess interactions between age and sex, age and race, and sex and race for all muscle and bone outcomes. Multiplicative interaction terms were also used to determine if the muscle-bone relations varied according to age, sex and race. Repeated analyses stratified by gender confirmed the study findings.
Multiple comparisons were performed across numerous correlated bone and body composition outcomes. A Bonferroni correction is not appropriate since it assumes the outcomes are independent. Therefore, we interpreted isolated findings with caution and examined the consistency of the overall results.
Results
Subject Characteristics
The participant characteristics are summarized in Table 1 according to sex and race. Black women had significantly greater BMI and FMI, compared to all other groups. Within males and females, tibia length was significantly greater in blacks compared with non-blacks (p<0.001), relative to height.
Table 1.
Participant Characteristics According to Sex and Race
|
|
||||
|---|---|---|---|---|
| Males | Females | |||
|
| ||||
| Non-Black | Black | Non-Black | Black | |
| N | 130 | 109 | 150 | 111 |
| Age (yrs) | 46.8 (17.8) | 49.7 (15.2) | 46.3 (15.1) | 50.0 (15.4) |
| Post-menopausal | N/A | N/A | 63 (42%) | 61 (55%) |
| Height (m) | 1.77 (0.07) | 1.75 (0.07) | 1.64 (0.07) | 1.64 (0.07) |
| Weight (kg) | 82.6 (14.5) | 82.6 (15.1) | 65.2 (13.8) | 80.5 (18.2) |
| BMI (kg/m2) | 25.5 (23.0, 27.8) | 26.7 (23.4, 29.5) | 23.0 (20.4, 26.4) | 27.5 (24.7, 32.6) |
| Tibia length (mm) | 410 (394, 423) | 421 (401, 436) | 372 (356, 389) | 394 (376, 410) |
| Calf muscle CSA (mm2) | 80.7 (74.0, 87.0) | 77.5 (68.6, 87.7) | 63.4 (57.2, 70.7) | 64.0 (58.1, 73.0) |
| Muscle strength (N-m) | 40.5 (33.9, 43.7) | 40.3 (33.5, 45.4) | 25.5 (20.7, 29.3) | 25.6 (22.1, 32.1) |
| Calf muscle density (mg/cm3) | 75.5 (74.0, 76.4) | 75.3 (72.9, 76.5) | 75.2 (73.9, 76.4) | 73.7 (71.7, 75.0) |
| Whole body LBMI (kg/m2) | 18.4 (2.3) | 18.9 (2.5) | 14.9 (2.0) | 16.4 (2.3) |
| Whole body FMI (kg/m2) | 5.8 (2.9) | 5.4 (2.4) | 7.4 (3.2) | 10.6 (4.0) |
| Trabecular BMD (mg/cm3) | 264 (236, 293) | 244 (215, 265) | 222 (203, 245) | 231 (209, 257) |
| Cortical BMD (mg/cm3) | 1176 (1157, 1196) | 1200 (1182, 1211) | 1203 (1182, 1219) | 1201 (1172, 1220) |
| Cortical section modulus (mm3) | 2230 (1110, 3230) | 2420 (1240, 3500) | 1480 (919, 2310) | 1760 (1190, 3540) |
| Regular exercise (n, %) | 113 (87%) | 87 (80%) | 135 (90%) | 90 (81%) |
| Exercise (MET-hr/week) | 31 (10.5, 72) | 32.7 (5.5, 99.8) | 24.5 (10.5, 52.8) | 24.5 (5.3, 65.5) |
Results are presented as mean (SD) for normally distributed data and median (inter-quartile range) for skewed data.
CSA: cross-sectional area
Muscle Outcomes
Table 2 summarizes the multivariable models for DXA whole body LBMI, pQCT muscle density, and muscle strength. LBMI was lower in older participants and women, greater in blacks, and positively associated with FMI (Table 2). The greater LBMI among black participants was significantly less pronounced among women (test for interaction, p<0.05), compared with men. Tests for age-by-sex and age-by-race interactions were not significant.
Table 2.
Multivariable Linear Regression Models of Muscle Outcomes
| DXA LBMI (R2 = 0.63) N = 457 |
pQCT Muscle Density (R2 = 0.43) N = 417 |
Muscle Strength (R2 = 0.41) N = 417 |
|
|---|---|---|---|
|
| |||
| β (95% CI) | β (95% CI) | β (95% CI) | |
| Age | −0.51 (−0.68, −0.35) c | −0.014 (−0.017, −0.012) c | −0.069 (−0.10, −0.035) c |
| Female sex | −1.70 (−1.85, −1.55) c | −0.00079 (−0.0091, 0.0076) | −0.14 (−0.25, −0.035) b |
| Black race | 0.84 (0.38, 1.30) c | −0.0073 (−0.012, −0.0022) b | −0.030 (−0.094, 0.034) |
| Black race * Female | −0.67 (−1.33, −0.015) a | -- | -- |
| FMI | 1.80 (1.61, 1.99) c | −0.012 (−0.015, −0.0080) c | −0.072 (−0.12, −0.025) b |
| LBMI | -- | 0.00052 (−0.0034, 0.0044) | 0.12 (0.071, 0.17) c |
| Muscle Density | -- | -- | 0.65 (−0.50, 1.79) |
| Tibia Length | -- | -- | 0.080 (0.044, 0.12) c |
Results are presented as standardized beta-coefficients for continuous variables (effect per 1 SD). Tibia length, muscle strength, and muscle density were log-transformed in all models.
p<0.05,
p<0.01,
p<0.001
Muscle density was significantly lower in older adults, women and blacks. However, in models adjusted for FMI (Table 2) muscle density did not differ according to sex. Greater FMI was significantly associated with lower muscle density.
Muscle strength was significantly lower in older participants and females in models adjusted for LBMI, but did not vary significantly according to race (Table 2). Greater LBMI was positively associated with muscle strength and greater FMI was negatively associated with muscle strength. When pQCT muscle CSA was substituted for DXA LBMI in the muscle strength models, similar results were observed.
Reported exercise was not associated with LBMI, muscle strength, or muscle density in any of the models described above.
Cortical Section Modulus
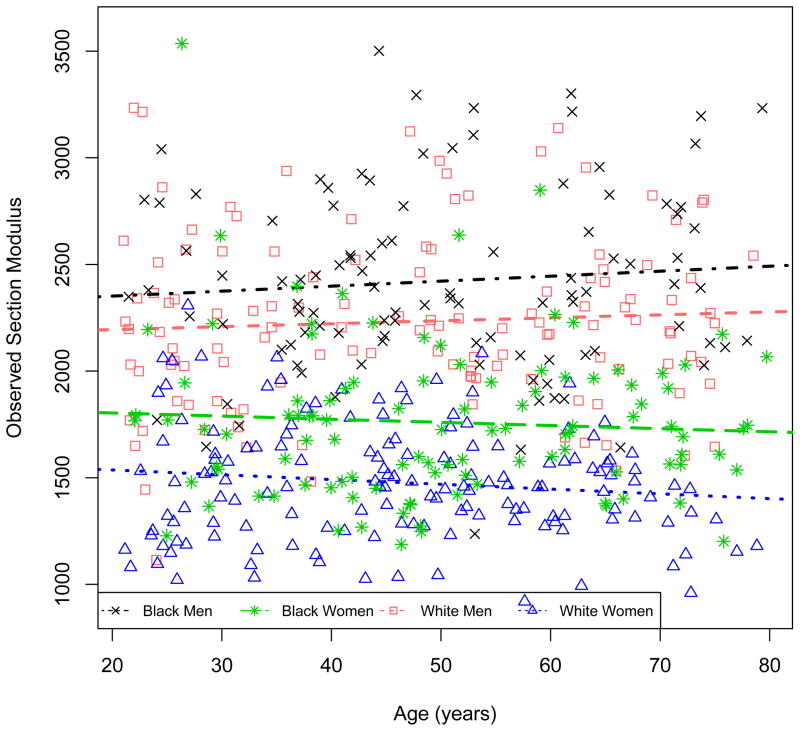
Table 3 summarizes the model for cortical section modulus, adjusted for age, sex, race and tibia length (Model 1) with sequential adjustment for muscle and fat covariates (Models 2–4). In Model 1, female sex was associated with a lower section modulus with a significant interaction with age, indicating that the difference between men and women was more pronounced with greater age. The positive β-coefficient for age indicates that section modulus is greater in older males. Section modulus was significantly greater in black, compared with non-black participants. The adjustment for tibia length confirmed that the lower section modulus in females was independent of their significantly shorter tibia length, and the greater section modulus in black participants was independent of their greater tibia length. Figure 1 demonstrates the association between observed section modulus and age, according to sex and race.
Table 3.
Multivariable Models Assessing the Impact of Muscle and Fat Covariates on Natural Log-Transformed Cortical Section Modulus.
| Section Modulus Model 1 R2 = 0.64 N = 405 |
Section Modulus Model 2 R2 = 0.71 N = 405 |
Section Modulus Model 3 R2 = 0.72 N= 405 |
Section Modulus Model 4 R2 = 0.73 N = 405 |
|
|---|---|---|---|---|
|
| ||||
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| Age | 0.020 (−0.0029, 0.042) | 0.030 (0.010, 0.051) b | 0.036 (0.013, 0.058) b | 0.040 (0.017, 0.062) b |
| Female sex | −0.16 (−0.27, −0.058) b | −0.018 (−0.11, 0.079) | 0.00062 (−0.098, 0.099) | 0.066 (−0.034, 0.17) |
| Female * Age | −0.038 (−0.070, −0.0048) a | −0.050 (−0.080, −0.021) b | −0.051 (−0.080, −0.023) c | −0.049 (−0.078, −0.021) b |
| Black race | 0.065 (0.030, 0.10) c | 0.022 (0.010, 0.054) a | 0.023 (−0.0089, 0.056) | 0.021 (−0.010, 0.053) |
| Tibia length | 0.10 (0.084, 0.12) c | 0.11 (0.088, 0.12) c | 0.10 (0.083, 0.12) c | 0.11 (0.088, 0.12) c |
| LBMI | -- | 0.095 (0.077, 0.11) c | 0.090 (0.071, 0.11) c | 0.12 (0.098, 0.14) c |
| Muscle strength | -- | -- | 0.026 (0.0074, 0.045) b | 0.021 (0.0022, 0.040) a |
| Muscle density | -- | -- | −0.0035 (−0.022, 0.015) | −0.015 (−0.034, 0.0040) |
| FMI | -- | -- | -- | −0.049 (−0.072, −0.026) c |
Results are presented as standardized beta-coefficients for continuous variables (effect per 1 SD). Tibia length, muscle strength, and muscle density, were log-transformed in all models.
p <0.05,
p<0.01,
p<0.001
Figure 1.
Age-related differences in section modulus by sex and race group.
Model 2 demonstrates that greater LBMI was strongly associated with greater section modulus, and adjustment for LBMI markedly attenuated the race differences observed in Model 1. In Model 3, LBMI and muscle strength, but not muscle density, were independently and positively associated with section modulus. Similar results were obtained when pQCT muscle CSA was substituted for DXA LBMI (R2 = 0.71, model not shown). The association between DXA LBMI and section modulus was similar in men and women; however, the association was slightly attenuated among black participants [Interaction β: −0.038 (−0.068, −0.0090) p=0.01], compared with non-black participants.
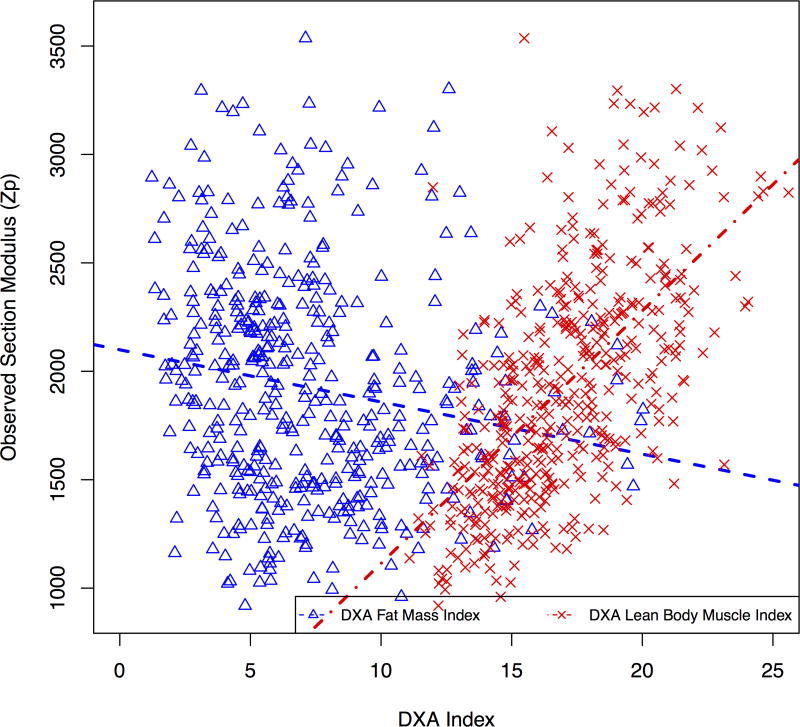
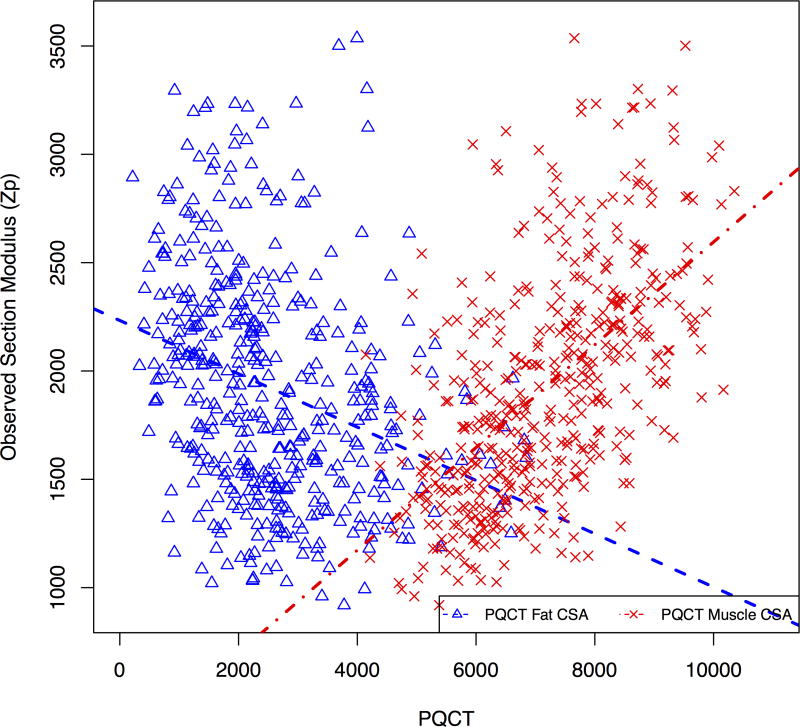
Model 4 incorporated measures of fat mass, demonstrating that greater FMI was associated with significantly lower section modulus, while greater LBMI and muscle strength were independently associated with greater section modulus. Similar results were obtained when pQCT muscle and subcutaneous fat CSA, or BMI were substituted for DXA LBMI and FMI (R2 = 0.71). Figure 2a illustrates the unadjusted opposing associations of fat mass and lean mass with cortical section modulus using DXA FMI and LBMI. Figure 2b illustrates unadjusted associations of pQCT muscle and subcutaneous fat CSA with section modulus.
Figure 2.
Associations between predicted cortical section modulus and (a) DXA measures of LBMI and FMI, and (b) pQCT measures of muscle and subcutaneous fat CSA.
Cortical and Trabecular BMD
Table 4 summarizes the models for cortical and trabecular BMD, adjusted for age, sex, race, with subsequent adjustment for muscle and fat covariates. In the first model, cortical BMD was greater in females, and declined with age in females only (age-sex interaction p<0.001). This interaction demonstrated that post-menopausal females had lower cortical density than men. Within females, the inverse association of cortical BMD with age was greater among post-menopausal women compared with pre-menopausal women (interaction term p<0.01). Cortical BMD was greater in black men compared to non-black men, but this race effect was not present among females (interaction p < 0.001).
Table 4.
Multivariable Models of Log-Transformed Cortical and Trabecular BMD
| Cortical BMD Model 1 R2 = 0.23 N = 415 |
Cortical BMD Model 2 R2 = 0.27 N = 415 |
Trabecular BMD Model 1 R2 = 0.21 N = 411 |
Trabecular BMD Model 2 R2 = 0.27 N = 411 |
|
|---|---|---|---|---|
|
|
||||
| β (95% CI) | β (95% CI) | |||
| Age | −0.0036 (−0.0070, −0.00020) a | −0.0017 (−0.0054, 0.0021) | −0.044 (−0.059, −0.029) c | −0.028 (−0.045, −0.011) c |
| Female sex | 0.053 (0.038, 0.069) c | 0.048 (0.032, 0.065) c | −0.16 (−0.20, −0.12) c | −0.11 (−0.16, −0.056) c |
| Female sex * Age | −0.012 (−0.017, −0.0073) c | −0.012 (−0.017, −0.0072) c | -- | -- |
| Black race | 0.019 (0.012, 0.026) c | 0.014 (0.0089, 0.019) c | −0.088 (−0.13, −0.045) c | −0.088 (−0.13, −0.046) c |
| Female sex * Black race | −0.017 (−0.026, −0.0067) c | −0.013 (−0.023, −0.0026) a | 0.12 (0.060, 0.18) c | 0.10 (0.045, 0.16) c |
| LBMI | −0.0044 (−0.0077, −0.00046) a | 0.044 (0.021, 0.067) c | ||
| Muscle strength | -- | -- | ||
| Muscle density | 0.0044 (0.0012, 0.0076) b | 0.033 (0.014, 0.052) c | ||
| FMI | −0.00047 (−0.0044, 0.0036) | 0.015 (−0.0086, 0.039) | ||
| Exercise (v. 0 MET-hr/wk) | ||||
| <27 MET-hr/wk | 0.0018 (−0.0059, 0.0094) | 0.048 (0.0036, 0.093) a | ||
| ≥27 MET-hr/wk | 0.0014 (−0.0060, 0.0088) | 0.054 (0.011, 0.098) a | ||
Results are presented as standardized beta-coefficients for continuous variables (effect per 1 SD). Tibia length, muscle strength, and muscle density, were log-transformed in all models.
p≤0.05,
p<0.01,
p<0.001
The second cortical BMD model examined associations with body composition. Greater LBMI was associated with significantly lower cortical BMD. Similar results were obtained when pQCT muscle CSA was substituted for DXA LBMI (model R2=0.30). Muscle strength and physical activity were not associated with cortical BMD with or without adjustment for measures of muscle mass (DXA FMI or pQCT muscle CSA). Greater muscle density was associated with greater cortical BMD. LBMI did not modify the association of cortical BMD with sex or race.
Trabecular BMD was lower in older participants and was lower in females (Table 4). The impact of age did not vary according to sex. Black race was associated with lower trabecular BMD in men, but not women (interaction p < 0.001). The second trabecular BMD model examined the effect of body composition. Greater LBMI was significantly associated with greater trabecular BMD; however, FMI was not. Greater reported exercise and greater muscle density were also associated with greater trabecular BMD. In contrast, muscle strength was not associated with trabecular BMD, independent of age, sex, and race effects. LBMI did not modify the association of cortical BMD with sex or race.
Discussion
These data demonstrated distinct associations between body composition and cortical dimensions, trabecular BMD and cortical BMD (summarized in Table 5). Greater LBMI and muscle strength were independently associated with greater section modulus, while greater FMI was associated with lower section modulus after adjustment for LBMI. Travison, et al reported a positive effect of LBMI and negative effect of FMI on DXA-based estimates of cortical structure in the proximal femur in men [26]. Similar to our results, the study demonstrated that adjustment for lean mass significantly attenuated the greater cortical dimensions observed in black participants. Our study extends these findings to women, and is strengthened by the use of three-dimensional QCT methods to measure cortical dimensions. Furthermore, our data demonstrated that age was inversely associated with section modulus in women only, and this sex effect was not explained by sex- or age- related differences in LBMI.
Table 5.
Summary of Associations between Body Composition and Bone Outcomes
| Section Modulus Model 4 R2 = 0.73 N = 405 |
Cortical BMD Model 2 R2 = 0.27 N = 415 |
Trabecular BMD Model 2 R2 = 0.27 N = 411 |
|
|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | |
| Age | Positive in men | Negative | Negative |
| Female sex and age effects | Lower in females and the sex differences are more pronounced with greater age | Greater in younger females but the decline with age is more pronounced in females. Lower in post-menopausal women compared with men | Lower in females |
| Black race and sex effects | Greater in blacks compared with non-blacks; however these differences are eliminated with adjustment for LBMI | Greater in black men compared with non-black men | Lower in black men |
| Tibia Length | Positive | NS | NS |
| LBMI | Positive | Negative | Positive |
| Muscle Strength | Positive | NS | NS |
| Muscle Density | NS | Positive | Positive |
| FMI | Negative | NS | NS |
| Exercise (v. 0 MET-hr/wk) | |||
| <27 MET-hr/wk | NS | NS | Positive |
| ≥27 MET-hr/wk | NS | NS | Positive |
The mechanisms underlying the association between greater LBMI and superior cortical dimensions and trabecular BMD have not been established; potential mediators include mechanical and biologic signals [27–31]. Sex steroids (estrogen and testosterone) and other hormonal signaling pathways, including growth hormone and insulin-like growth factors, have been implicated [13, 32, 33]. Physical forces applied to bone may also regulate bone modeling through mechanical signals including mechanical receptors and hemichannels on osteocytes [34, 35]. Finally, other, more novel biologic mechanisms are being studied including sclerostin [36], myokines [29, 37], and muscle-derived progenitor cells [38].
The mechanism for the negative association between FMI and cortical dimensions is not known. Gilsanz, et al recently used QCT to measure cortical dimensions in the femur, and visceral and subcutaneous adipose tissue in the abdomen in young women, ages 15 to 25 years. In multivariate models, thigh muscle and abdominal subcutaneous fat CSA were positively associated with cortical dimensions while visceral fat CSA was negatively associated with cortical area. Visceral adiposity is associated with increased levels of inflammatory cytokines that may have adverse effects on bone metabolism [39]. A recent longitudinal study in elderly adults demonstrated that, although greater fat mass was associated with greater lean mass at baseline, greater fat mass was associated with greater declines in leg lean mass over an 8-year period [1]. Therefore, the negative effect of FMI on section modulus observed here may reflect declines in muscle mass. Longitudinal studies are needed to assess the independent effects of changes in lean mass, visceral fat, and subcutaneous fat on changes in cortical dimensions in adults.
Our study showed that cortical BMD was greater in females, compared with males, and declined with age to a greater extent in females. These data extend our observation that female sex was associated with greater cortical BMD in children, adolescents and young women (age < 30 years) [14]. This is also consistent with estrogen effects on bone turnover and cortical density. Greater LBMI was associated with lower cortical BMD. Since calcium apatite is primarily important in resisting compression, and the primary role of cortical bone is to resist torque, less mineralized cortical bone may not always represent an adverse outcome [40]. In individuals with greater LBMI, less dense cortical bone may be evidence of greater ductility which is compensated to absorb energy through greater compliance [41].
Trabecular BMD was lower in females than males and declined with age. After multivariable adjustment, trabecular BMD was positively associated with LBMI, but not FMI. These data confirm similar observations made recently by LeBrasseur et al. This group also utilized high-resolution techniques to show subjects with greater appendicular muscle mass index had greater trabecular number and thickness. PQCT measures of trabecular compartment BMD do not distinguish between trabecular bone volume fraction and material BMD. This may explain the opposing associations of LBMI with cortical (lower material density) and trabecular (lower material density but greater trabecular microarchitecture) BMD.
While our data do not support an additional independent association of total FMI with trabecular or cortical BMD, we found that muscle density (an inverse correlate of intramuscular fat) was independently associated with BMD. This supports previous data pointing to the importance of fat distribution. These findings are similar to results in a recent study of adolescent girls [16]. Previously reported associations between muscle density at the thigh and subsequent fracture risk [11] could thus be related to greater mineralization of bone in those with greater muscle density. Low muscle density may be associated with greater visceral fat and clinical comorbidities such as insulin resistance and the metabolic syndrome [18]. Fracture risk could be increased in these participants through associations with these comorbidities. Lang et al. noted that inclusion of clinical comorbidities attenuated the association between muscle density and fracture risk [11]. Further study is needed to further clarify the mechanism by which muscle density is associated with cortical and trabecular density and with fracture risk. In contrast to the study in young girls [16], we found that lower muscle density (greater intramuscular fat) was not associated with cortical dimensions (section modulus), adjusted for LBMI and muscle strength among adults.
Prior DXA based studies suggested that the relations between lean mass and bone outcomes (bone mineral content or areal BMD) differed according to sex [42, 43]. Ferretti, et al hypothesized that estrogen modified the mechanical set-point for bone remodeling in response to biomechanical loads [42]. LeBrasseur also found evidence of an interaction between sex and muscle on pQCT bone outcomes [13]. Our analyses did not provide evidence of a sex-muscle interaction in the models for cortical section modulus, cortical BMD or trabecular BMD. For example, the highly significant positive association between LBMI and section modulus did not differ significantly between males and females in the multivariate models adjusted for age, race, muscle strength, and tibia length. Possible explanations for our different results include different age of subjects and the use of different measurement sites and techniques. In addition, differences in the statistical methods may have contributed to the observed differences. The use of ratios to assess bone mineral content – lean mass relations does not incorporate adjustments for sex differences in tibia lengths, and potentially introduces statistical errors since muscle area and bone measures do not scale isometrically [44]. In our dataset, no interaction was identified after adjusting for sex differences in tibia length. Our observations among adult participants across the age spectrum confirm our previous findings in children, adolescents and young adults [14].
The greatest limitation of this study is the cross-sectional design. Longitudinal studies are needed to establish the temporal relations between body composition and bone density and structure. The fracture implications of these findings are not known and measures in the tibia may not reflect bone strength at the hip or other sites. PQCT does not have sufficient resolution to assess trabecular microarchitecture or cortical porosity. Additional limitations include lack of dietary intake and measures of vitamin D, parathyroid hormone, sex hormones, and growth factors (such as IGFBP-2) [13] to potentially explain the age, sex and race differences in BMD and dimensions. Last, visceral fat may have distinct effects on bone and these data were not available. However, to our knowledge, this is the first study to examine relations of fat and lean mass with bone density and structure in a diverse sample of men and women from young adulthood through the elderly. These data highlight age, sex- and race-specific differences in body composition, muscle strength and muscle density, and demonstrated discrete associations with bone density and structure.
Acknowledgments
Funding: This work was supported by NIH grants R01DK064966 and K24DK076808, and the University of Pennsylvania Clinical Translational Research Center (UL1-RR024134). Dr. Alexander was supported by the Bertha Dagan Berman-FOCUS Medical Student Fellowship.
Abbreviations
- BMD
bone mineral density
- BMI
body mass index
- DXA
dual energy x-ray absorptiometry
- CSA
cross-sectional area
- CT
computed tomography
- pQCT
peripheral quantitative computed tomography
- MrOS
Osteoporotic Fractures in Men Study
- CHOP
Children’s Hospital of Pennsylvania
- UPENN
University of Pennsylvania
- N-m
Newton-meters
- CV
coefficient of variation
- FMI
fat mass index
- LBMI
lean body mass index
- MESA
Multi-ethnic Study of Atherosclerosis
Footnotes
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koster A, Ding J, Stenholm S, Caserotti P, Houston DK, Nicklas BJ, You T, Lee JS, Visser M, Newman AB, Schwartz AV, Cauley JA, Tylavsky FA, Goodpaster BH, Kritchevsky SB, Harris TB. Does the amount of fat mass predict age-related loss of lean mass, muscle strength, and muscle quality in older adults? J Gerontol A Biol Sci Med Sci. 2011;66:888–95. doi: 10.1093/gerona/glr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanis JA, Johnell O, Oden A, Johannson H, McCloskey E. FRAX™ and the assessment of fracture probability in men and women from the UK. Osteoporosis International. 2008;19:385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taaffe DR, Cauley JA, Danielson M, Nevitt MC, Lang TF, Bauer DC, Harris TB. Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: the Health, Aging, and Body Composition Study. J Bone Miner Res. 2001;16:1343–52. doi: 10.1359/jbmr.2001.16.7.1343. [DOI] [PubMed] [Google Scholar]
- 4.Beck TJ, Petit MA, Wu G, LeBoff MS, Cauley JA, Chen Z. Does obesity really make the femur stronger? BMD, geometry, and fracture incidence in the women’s health initiative-observational study. J Bone Miner Res. 2009 Aug;24(8):1369–79. doi: 10.1359/JBMR.090307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheu Y, Cauley JA. The role of bone marrow and visceral fat on bone metabolism. Curr Osteoporos Rep. 2011;9:67–75. doi: 10.1007/s11914-011-0051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi HS, Kim KJ, Kim KM, Hur NW, Rhee Y, Han DS, Lee EJ, Lim SK. Relationship between visceral adiposity and bone mineral density in Korean adults. Calcif Tissue Int. 2010;87:218–25. doi: 10.1007/s00223-010-9398-4. [DOI] [PubMed] [Google Scholar]
- 7.Russell M, Mendes N, Miller KK, Rosen CJ, Lee H, Klibanski A, Misra M. Visceral fat is a negative predictor of bone density measures in obese adolescent girls. J Clin Endocrinol Metab. 2010;95:1247–55. doi: 10.1210/jc.2009-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilsanz V, Chalfant J, Mo AO, Lee DC, Dorey FJ, Mittelman SD. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab. 2009;94:3387–93. doi: 10.1210/jc.2008-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnham JM, Shults J, Dubner SE, Sembhi H, Zemel BS, Leonard MB. Bone density, structure, and strength in juvenile idiopathic arthritis: importance of disease severity and muscle deficits. Arthritis Rheum. 2008;58:2518–27. doi: 10.1002/art.23683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cousins JM, Petit MA, Paudel ML, Taylor BC, Hughes JM, Cauley JA, Zmuda JM, Cawthon PM, Ensrud KE. Muscle power and physical activity are associated with bone strength in older men: The osteoporotic fractures in men study. Bone. 2010;47:205–11. doi: 10.1016/j.bone.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang T, Cauley JA, Tylavsky F, Bauer D, Cummings S, Harris TB. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res. 2010;25:513–9. doi: 10.1359/jbmr.090807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schafer AL, Vittinghoff E, Lang TF, Sellmeyer DE, Harris TB, Kanaya AM, Strotmeyer ES, Cawthon PM, Cummings SR, Tylavsky FA, Scherzinger AL, Schwartz AV. Fat infiltration of muscle, diabetes, and clinical fracture risk in older adults. J Clin Endocrinol Metab. 2010;95:E368–72. doi: 10.1210/jc.2010-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lebrasseur NK, Achenbach SJ, Melton LJ., 3rd . Skeletal muscle mass is associated with bone geometry and microstructure and serum IGFBP-2 levels in adult women and men. In: Amin S, Khosla S, editors. J Bone Miner Res. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leonard MB, Elmi A, Mostoufi-Moab S, Shults J, Burnham JM, Thayu M, Kibe L, Wetzsteon RJ, Zemel BS. Effects of sex, race, and puberty on cortical bone and the functional muscle bone unit in children, adolescents, and young adults. J Clin Endocrinol Metab. 2010;95:1681–9. doi: 10.1210/jc.2009-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu D, Manske SL, Kontulainen SA, Tang C, Guy P, Oxland TR, McKay HA. Tibial geometry is associated with failure load ex vivo: a MRI, pQCT and DXA study. Osteoporos Int. 2007;18:991–997. doi: 10.1007/s00198-007-0325-0. [DOI] [PubMed] [Google Scholar]
- 16.Farr JN, Funk JL, Chen Z, Lisse JR, Blew RM, Lee VR, Laudermilk M, Lohman TG, Going SB. Skeletal muscle fat content is inversely associated with bone strength in young girls. J Bone Miner Res. 2011;414 doi: 10.1002/jbmr.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89:104–10. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 18.Miljkovic I, Cauley JA, Petit MA, Ensrud KE, Strotmeyer E, Sheu Y, Gordon CL, Goodpaster BH, Bunker CH, Patrick AL, Wheeler VW, Kuller LH, Faulkner KA, Zmuda JM. Greater adipose tissue infiltration in skeletal muscle among older men of African ancestry. J Clin Endocrinol Metab. 2009;94:2735–42. doi: 10.1210/jc.2008-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelley DE, Slasky BS, Janosky J. Skeletal muscle density: effects of obesity and non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1991;54:509–515. doi: 10.1093/ajcn/54.3.509. [DOI] [PubMed] [Google Scholar]
- 20.Leggin BG, Neuman RM, Iannotti JP, Williams GR, Thompson EC. Intrarater and interrater reliability of three isometric dynamometers in assessing shoulder strength. J Shoulder Elbow Surg. 1996;5:18–24. doi: 10.1016/s1058-2746(96)80026-7. [DOI] [PubMed] [Google Scholar]
- 21.Wetzsteon RJ, Kalkwarf HJ, Shults J, Zemel BS, Foster BJ, Griffin L, Strife CF, Foerster DL, Jean-Pierre DK, Leonard MB. Volumetric bone mineral density and bone structure in childhood chronic kidney disease. J Bone Miner Res. 2011 doi: 10.1002/jbmr.427. 10./jbmr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leonard MB, Shults J, Elliott DM, Stallings VA, Zemel BS. Interpretation of whole body dual energy X-ray absorptiometry measures in children: comparison with peripheral quantitative computed tomography. Bone. 2004;34:1044–52. doi: 10.1016/j.bone.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 24.Pereira MA, FitzerGerald SJ, Gregg EW, Joswiak ML, Ryan WJ, Suminski RR, Utter AC, Zmuda JM. A collection of Physical Activity Questionnaires for health-related research. Med Sci Sports Exerc. 1997;29:S1–205. [PubMed] [Google Scholar]
- 25.Bertoni AG, Whitt-Glover MC, Chung H, Le KY, Barr RG, Mahesh M, Jenny NS, Burke GL, Jacobs DR. The association between physical activity and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2009;169:444–54. doi: 10.1093/aje/kwn350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Travison TG, Araujo AB, Esche GR, Beck TJ, McKinlay JB. Lean mass and not fat mass is associated with male proximal femur strength. J Bone Miner Res. 2008;23:189–98. doi: 10.1359/JBMR.071016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ausk BJ, Huber P, Poliachik SL, Bain SD, Srinivasan S, Gross TS. Cortical bone resorption following muscle paralysis is spatially heterogeneous. Bone. 2011 doi: 10.1016/j.bone.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamrick MW, Samaddar T, Pennington C, McCormick J. Increased muscle mass with myostatin deficiency improves gains in bone strength with exercise. J Bone Miner Res. 2006;21:477–83. doi: 10.1359/JBMR.051203. [DOI] [PubMed] [Google Scholar]
- 29.Hamrick MW. A role for myokines in muscle-bone interactions. Exerc Sport Sci Rev. 2011;39:43–7. doi: 10.1097/JES.0b013e318201f601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moustafa A, Sugiyama T, Prasad J, Zaman G, Gross TS, Lanyon LE, Price JS. Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos Int. 2012;23:1225–34. doi: 10.1007/s00198-011-1656-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perrini S, Laviola L, Carreira MC, Cignarelli A, Natalicchio A, Giorgino F. The GH/IGF1 axis and signaling pathways in the muscle and bone: mechanisms underlying age-related skeletal muscle wasting and osteoporosis. J Endocrinol. 2010;205:201–10. doi: 10.1677/JOE-09-0431. [DOI] [PubMed] [Google Scholar]
- 32.Khosla S, Melton LJ, 3rd, Riggs BL. The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: is a revision needed? J Bone Miner Res. 2011;26:441–51. doi: 10.1002/jbmr.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bechtold S, Ripperger P, Bonfig W, Pozza RD, Haefner R, Schwarz HP. Growth hormone changes bone geometry and body composition in patients with juvenile idiopathic arthritis requiring glucocorticoid treatment: a controlled study using peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005;90:3168–73. doi: 10.1210/jc.2004-1603. [DOI] [PubMed] [Google Scholar]
- 34.Jiang JX, Siller-Jackson AJ, Burra S. Roles of gap junctions and hemichannels in bone cell functions and in signal transmission of mechanical stress. Front Biosci. 2007;12:1450–62. doi: 10.2741/2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts MD, Santner TJ, Hart RT. Local bone formation due to combined mechanical loading and intermittent hPTH-(1–34) treatment and its correlation to mechanical signal distributions. J Biomech. 2009;42:2431–8. doi: 10.1016/j.jbiomech.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 36.Agholme F, Isaksson H, Li X, Ke HZ, Aspenberg P. Anti-sclerostin antibody and mechanical loading appear to influence metaphyseal bone independently in rats. Acta Orthop. 2011;82:628–32. doi: 10.3109/17453674.2011.625539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elkasrawy MN, Hamrick MW. Myostatin (GDF-8) as a key factor linking muscle mass and bone structure. J Musculoskelet Neuronal Interact. 2010;10:56–63. [PMC free article] [PubMed] [Google Scholar]
- 38.Henrotin Y. Muscle: a source of progenitor cells for bone fracture healing. BMC Med. 2011;9:136. doi: 10.1186/1741-7015-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halade GV, El Jamali A, Williams PJ, Fajardo RJ, Fernandes G. Obesity-mediated inflammatory microenvironment stimulates osteoclastogenesis and bone loss in mice. Exp Gerontol. 2011;46:43–52. doi: 10.1016/j.exger.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turner CH. Bone strength: current concepts. Ann N Y Acad Sci. 2006;1068:429–46. doi: 10.1196/annals.1346.039. [DOI] [PubMed] [Google Scholar]
- 41.Tommasini SM, Nasser P, Schaffler MB, Jepsen KJ. Relationship between bone morphology and bone quality in male tibias: implications for stress fracture risk. J Bone Miner Res. 2005;20:1372–80. doi: 10.1359/JBMR.050326. [DOI] [PubMed] [Google Scholar]
- 42.Ferretti JL, Capozza RF, Cointry GR, Garcia SL, Plotkin H, Alvarez-Filgueira ML, Zanchetta JR. Gender-related differences in the relationship between densitometric values of whole-body bone mineral content and lean body mass in humans between 2 and 87 years of age. Bone. 1998;22:683–690. doi: 10.1016/s8756-3282(98)00046-5. [DOI] [PubMed] [Google Scholar]
- 43.Taafe DR, Cauley JA, Danielson M, Nevitt MC, Lang T, Bauer DC, Harris TB. Race and Sex Effects on the Association Between Muscle Strength, Soft Tissue, and Bone Mineral Density in Healthy Elders: The Health, Aging, and Body Composition Study. JBMR. 2001;16 doi: 10.1359/jbmr.2001.16.7.1343. [DOI] [PubMed] [Google Scholar]
- 44.Allison DB, Paultre F, Goran MI, Poehlman ET, Heymsfield SB. Statistical considerations regarding the use of ratios to adjust data. Int J Obes Relat Metab Disord. 1995;19:644–52. [PubMed] [Google Scholar]