Abstract
Radiation oncology modalities such as intensity-modulated and image-guided radiation therapy can reduce the high dose to normal tissue and deliver a heterogeneous dose to tumors focusing on areas deemed at highest risk for tumor persistence. Clinical radiation oncology produces daily doses ranging from 1 to 20 Gy, with tissues being exposed to 30 or more daily fractions. Hypothesizing that cells that survive fractionated radiation therapy have a substantially different phenotype than the untreated cells, which might be exploitable for targeting with molecular therapeutics or immunotherapy, three prostate cancer cell lines (PC3, DU145 and LNCaP) and normal endothelial cells were studied to understand the biology of differential effects of multi-fraction (MF) radiation of 0.5, 1 and/or 2 Gy fraction to 10 Gy total dose, and a single dose (SD) of 5 and 10 Gy. The resulting changes in mRNA, miRNA and phosphoproteome were analyzed. Significant differences were observed in the MF radiation exposures including those from the 0.5 Gy MF that produces little cell killing. As expected, p53 function played a major role in response. Pathways modified by MF include immune response, DNA damage, cell cycle arrest, TGF-β, survival and apoptotic signal transduction. The radiation-induced stress response will set-forth a unique platform for exploiting the effects of radiation therapy as “focused biology” for cancer treatment in conjunction with molecular targeted or immunologically directed therapy. Given that more normal tissue is treated, albeit to lower doses with these newer techniques, the response of the normal tissue may also influence long-term treatment outcome.
Introduction
The biological consequences of radiation exposure are of interest for cancer etiology and treatment. The potential negative consequences of ionizing radiation have been recently highlighted through reports of treatment errors in radiation therapy (RT) (1), risk from CT scans (2) and radionuclide release from the Fukushima Nuclear Power Plant disaster (3–4). On the positive side of the ledger, RT is a mainstay of both curative and palliative cancer treatment used either alone or as a component in adjuvant therapy. Benefits include improved local tumor control (such as in head & neck, breast and lung cancers) survival (such as in breast and brain tumors) and organ preservation (such as in breast, head & neck, gastrointestinal and bladder cancers).
Radiation is administered in multiple fractions usually five days per week for a total of one to seven weeks. As discussed in this review, the tumor and normal tissue cells that survive multi-fraction radiation have a different genetic and proteomic profiles in comparison to the non-irradiated cells at the start of treatment, and the resulting pattern is dependent on the size of the individual radiation dose per fraction. While more studies are needed, it may be possible to take advantage of the new patterns of gene expression and proteomic profiles to select a dose and radiation schedule to increase the efficacy of molecular targeted drugs and immunotherapy, a concept of “focused biology”(5). The impact of the multi-fraction lower dose on the increased volume of irradiated normal tissue remains to be elucidated.
Based on our group's interest in understanding the cellular response to radiation-induced stress, we put forward and tested a hypothesis that the molecular response of cells to single-dose (SD) radiation would be different from the response to multi-fractionated (MF) radiation; and that the cells that survived MF would become more alike by virtue of their adaptation to radiation (6). This review comprises of analyses of the findings from our laboratory (7–9) and published reports by others groups (6, 10–14) on effects of SD and MF on cellular response. There are several translational goals that need to be considered which include:
Understanding the molecular characteristics of tumor cells that survive fractionated radiation and the impact of genetic background impact on MF-induced changes.
What the impact of the size of the dose per fraction, (including low doses that might occur at the tumor periphery and in the surrounding normal tissue) is.
Since molecular-targeted drugs require the presence and persistence of a target, can MF be used to induce such targets rather than depend on its presence and, therefore, make molecular-targeted therapy more effective and broadly applicable?
Is there a preferred “omics” approach or combination of methods (mRNA, miRNA or phosphoproteomics) with which to assess these changes?
Dose-heterogeneity in clinical radiation therapy
In the clinic, MF radiation is used due to its normal tissue sparing effects (15) with the maximum radiation dose delivered based on normal tissue tolerance from clinical experience (16). The less complicated RT modalities including opposed pairs, 4-field configuration and, to some extent, 3-D conformal RT use a limited number of fields with a relatively uniform energy deposition across the beam and a homogeneous dose within the tumor. In contrast, new technological approaches such as Intensity-Modulated Radiation Therapy (IMRT) (17), Image-Guided Radiation Therapy (IGRT) (17) and heavy ion therapy (protons and carbon) (18) are designed to reduce the high doses delivered to normal tissues. IMRT and IGRT utilize dose painting and sculpting to deliver radiation within the target so that dose is often not homogenous within a tumor. A specified target may receive a higher dose based on local tumor burden, the presence of a physiological area at risk for local failure (such as hypoxia) or persistence of an imaging abnormality (such as PET) (19). IMRT and IGRT use multiple and often non-coplanar intensity-modulated beams such that there is more normal tissue treated to some moderate dose. Thus, there is more tissue treated with increased dose heterogeneity. Recently, the advances in diagnostic imaging, the ability to stay on target during radiation treatment by accounting for patient and tumor intra-fraction motion, and an interest in shortening treatment time for biological (prostate cancer) and/or patient convenience (breast cancer) have also led to the use of larger doses-per-fraction (i.e. hypo-fractionation). Thus, in current clinical radiation therapy, tissues are exposed to MF radiation in a range of doses for one to seven weeks.
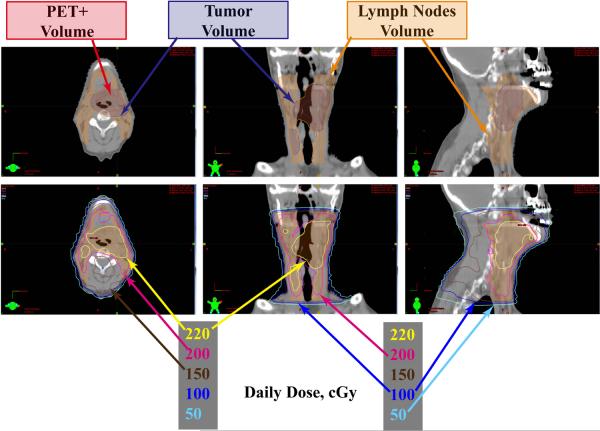
Figure 1 provides an example of dose heterogeneity in clinical radiation oncology. The IMRT plan is for a pharyngeal tumor, which by design covers the tumor well, but also has significant dose distribution to the nearby normal tissue with the majority of the tissue behind the vertebral region receiving >100 cGy/day as a result of multiple field configurations. The daily treatment dose is heterogeneous including a maximum dose of almost 230 cGy/day with much of the tissue receiving as much as 150 cGy/day, and other regions receiving between 50–150 cGy/day.
Figure 1. Dose heterogeneity during IMRT radiation therapy.
Dose distribution from an IMRT treatment plan for a pharyngeal tumor. Top panel: the target includes the PET scan positive region (red outline) that is within the tumor volume (blue outline), and the lymph nodes (orange outline), which receive a lower total dose. Lower panel: the daily tumor dose is 200 cGy (pink outline) with some areas within the tumor receiving higher focal doses (220 cGy, yellow outline). The multiple field approach treats normal tissue to daily doses to 100–150 cGy per day (dark blue and brown lines respectively) and some to even lower doses per fraction of 50 cGy (light blue outline).
Molecular phenotype depends on fractionation, fraction size and underlying genetic profile
In RT, the multiplicity of doses to which patients are exposed may engage different biological processes and molecular pathways. The daily dose ranges include high doses from ablative hypo-fractionation that are >10 Gy (often close to 15–20 Gy) (20–21), moderate dose of 1–10 Gy (22), low doses including treatments designed to take advantage of low-dose hypersensitivity ~0.5 Gy (23). [Note: There are lower doses (~10 cGy) that elicit low dose “adaptive” responses such as the non-targeted/bystander effects in unirradiated cells (24) discussed below]. Differences in the effects of fractionated and single-dose radiation including those from our laboratory have been previously documented, (6–7, 10–12) and include the induction of stress-response (7, 12) and immune-related (6–7, 10–11) genes by fractionated radiation.
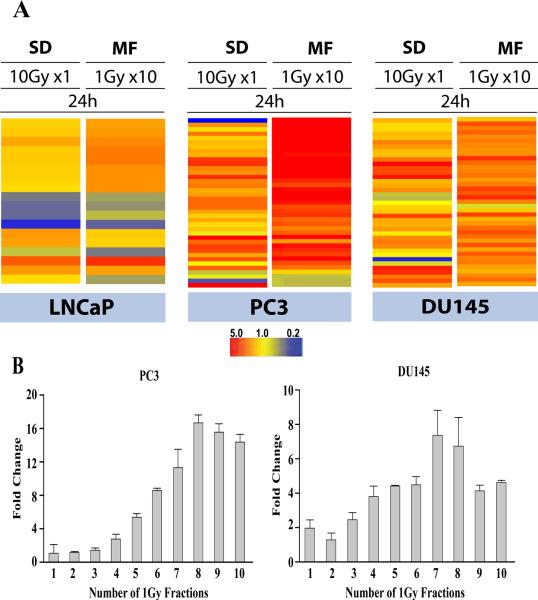
In studies comparing MF and SD from our laboratory, Tsai et al. demonstrated significant differences in the gene expression profiles following MF and SD, with MF uniquely up-regulating interferon-related genes and TGF-β associated genes in a prostate cancer xenograft model and in vitro in prostate, breast and gliosarcoma cells lines (6). Our analyses of the global gene profiles following radiation (MF and SD) show a more robust MF-induced gene expression in HCAEC (in preparation), LNCaP (9) and PC3 (7) in comparison to SD. The observed gene expression patterns have shown MF radiation to be more immune-modulatory in comparison to SD in LNCaP (9) PC3 (7) and normal HCAEC (Human Coronary Artery Endothelial Cells) (in preparation) but not in DU145 cells (7). Figure 2 serves as a representative illustration, highlighting the differences between single and fractionated radiation by depicting differences in expression of immune response gene using SD 10 Gy and MF 1Gy × 10. It also illustrates that there is an inflection point after 6–7 fractions in MF. In comparison to SD, the majority of gene and miRNA changes were also more robust and stable up to 72h after the end of MF (7), suggesting a sufficiently stable phenotype that might affect tissue/tumor response and also potentially be targetable with drug therapy (7).
Figure 2. Adaptation takes time.
A Heat maps depicting the expression patterns of immune response genes 24h following exposure to 10 Gy as single dose (SD) or 10 fractions of 1 Gy (MF) in LNCaP, PC3 and DU145.B. Inflection point. Samples were collected 24h after the indicated number of 1Gy radiation fractions in PC3 and DU145. For MF, there was at least a 6h window between each fraction; samples were collected 24h after final fraction. Data represents fold change in IFI44 expression determined by RT-PCR. Data were adapted in part from (7).
Dewan et al. demonstrated an MF-induced immune-mediated abscopal response with anti-CTLA-4 antibody, in breast and colon cancer xenograft experimental models. The same abscopal response was not observed with SD; in comparison to SD, MF dramatically improved the local and secondary tumor control (10). More recently, following MF, Postow et al. observed immune activation and elevated antibody levels against a NY-ESO-1(a cancer antigen expressed in 30–40% of melanomas) in a melanoma patient treated with anti-CTLA-4 antibody, ipilimumab (14). Clinical trials combining fractionated radiation and ipilimumab are currently underway in stage IV melanomas (ClinicalTrials.gov number, NCT01449279) (25) and advanced prostate cancer (ClinicalTrials.gov number, NCT00861614).
As much as radiation dose, quality, dose rate and fractionation (10) all play an important role in cellular response to radiation, observed differences in response to SD and MF radiation regimens are also dependent on the underlying genetic profiles. Following radiation (MF and SD), our studies have shown 2255 differential expressed genes in HCAEC (in preparation), 978 in LNCaP (9), 343 in PC3 and 116 in DU145 (7). The genes most altered by both radiation protocols was also different between the three prostate cancer cell lines (7, 9). A list of top 10 upregulated and 10 downregulated genes in the three prostate cancer cell lines are provided as supplementary information. As previously stated, MF altered more genes than SD in HCAEC (normal endothelial cell), LNCaP (p53-wildtype) and PC3 (p53-null). In contrast, SD altered more genes in DU145 (p53-mutant). p53, a vital tumor suppressor, is associated with activation of DNA repair, apoptosis and radiosensitivity. The diverse p53 status of these cells likely plays a central role in their radiation response and could provide a partial explanation as to why different pathways were top-ranked in the functional gene assessments. A more detailed study of the effect of the underlying genotype on the phenotypic change following MF and SD is planned.
In addition to cell cycle, DNA repair, and cell death pathways stress response, signal transduction (6–7, 11), metabolic, respiratory (26–27), immune response(7–8, 10–11) pathways, have been reported to be altered by radiation (SD and MF) in various in vitro and in vivo systems. A list of representative examples of pathways altered by radiation (SD and MF) are listed in table 1; many of which have also been implicated in low dose radiation-induced bystander effects (6–8, 26–27). Klammer et al recently reported a low-dose radiation-induced adaptive phenotype as a bystander effect in mouse fibroblasts (28). An adaptive response is a phenomenon observed after a low dose radiation (<10 cGy) primes cells for a response to a higher radiation dose, such that the cells are more resistant following a priming dose than without it. The genomic alterations resulting from an adaptive response, such as mutations, formation of micronuclei and chromosome aberrations, are often transmitted to the viable progeny or “Radiation Survivors” (24, 28–29). Contributing factors to the maintenance of an adaptive phenotype across generations from both irradiated and non-irradiated bystander cells), include the genetic background of the cells (28) and epigenetic drivers such as histone modifications, DNA methylation and miRNA signaling (29). In the case of fractionated radiation, it is possible that exposure to repeated doses of sublethal radiation could induce an adaptive phenotype different from the classic adaptive response of the “low priming dose +higher dose”. Our data (6) using 1 Gy fractions demonstrate that for some genes there is an inflection point after 6–7 fractions. The MF-induced signal persistence in irradiated cells (7, 30) could be as a result of an “MF-adaptive” response. It is speculative that epigenetic changes from MF could be transmitted across generations; which could also induce a bystander response in the neighboring non-irradiated cells, causing them to send out stress signals and in turn, generate an extended abscopal effect in other tissue and organs.
Table 1.
Differences in gene profiles between single dose and multi-fraction radiation. Table summarizes pathway analysis of previously reported gene, following exposure to SD and MF radiation.
| Cell type | Radiation Protocol | Functional Categories | Type of Analysis | Ref | |
|---|---|---|---|---|---|
| MCF-7, MCF-IR20 | MF | 2Gy×20 | Growth regulator, Apoptosis, DNA repair, DNA replication, Cell adhesion, Angiogenesis, regulator, Cell division, Growth factor, Cytokine | in vitro, mRNA | Li, et al. (2001) |
| Cell division, Apoptosis ,GTPase regulator, Growth factor | |||||
|
| |||||
| MCF-7 | MF | 2Gy ×5 | Apoptosis, Cell senescence, Cell Cycle control, DNA damage repair Angiogenesis, Signal transduction and transcription factors | in vitro, mRNA | Madhusoo dhanan, et al. (2009) |
| Angiogenesis, | |||||
| SD | 10Gy×1 | Apoptosis, Cell senescence, Cell Cycle control, DNA damage repair Angiogenesis, Signal transduction and transcription factors | |||
|
| |||||
| MCF-7 | MF | 2Gy ×5 | Cell-To-Cell Signaling and Interaction, DNA Replication, Recombination, and Repair, Nucleic Acid Metabolism | in vitro, mRNA | Tsai, et al. (2007) |
| SF539 | MF | 2Gy ×5 | Cancer, Infectious Disease, Respiratory Disease, Cellular Development, Cellular Growth and Proliferation, Connective Tissue Development and Function | ||
| DU145 | MF | 2Gy ×5 | Cancer, Infectious Disease, Respiratory Disease | ||
| MCF-7, SF539, DU145 | SD | 10Gy×1 | Cancer, Infectious Disease, Respiratory Disease, Molecular Transport, Hematological Disease, Hereditary Disorder | ||
|
| |||||
| DU145 | MF | 1Gy ×5 | Cell-To-Cell Signaling and Interaction, Drug Metabolism, Lipid Metabolism | in vivo, mRNA | |
| Organismal Survival, Cancer, Cell Death, Molecular Transport, Drug Metabolism, Small Molecule Biochemistry, Inflammatory Response, Cellular Development, Cellular Growth and Proliferation, Cancer, Reproductive System Disease, Endocrine System Disorders | |||||
| SD | 10Gy×1 | Organismal Survival, Cancer, Cell Death, Molecular Transport, Drug Metabolism, Small Molecule Biochemistry, Inflammatory Response, Cellular Development, Cellular Growth and Proliferation, Cancer, Reproductive System Disease, Endocrine System Disorders | |||
| Cell Cycle, Gastrointestinal Disease, Hepatic System Disease, Hematological System Development and Function, Hematopoiesis, Tissue Development | |||||
|
| |||||
| LNCaP | MF | 1Gy ×10 | Cell Cycle, DNA Binding, Proliferation, Response to Stress, Transcription Factor, Signal Transduction, DNA Replication, Histone, Cyclin, Immune Resonse | in vitro, mRNA | Simone, et al. (in review) |
| SD | 10Gy ×1 | Cell Cycle, DNA Binding, DNA Replication , Response to Stress, DNA Repair, Proliferation, Transcription Factor, Signal Transduction, Cyclin, Histone | |||
|
| |||||
| PC3 | MF | 1Gy ×10 | Immune Response, DNA Binding, Response to Stress, Interferon, Signal Transduction, Proliferation, Apoptosis, Transcription Factor, Ubiquitin, Inflammatory Response | in vitro, mRNA | John-Aryankala yil, et al. (2010) |
| SD | 10Gy ×1 | DNA Binding, Signal Transduction, Immune Response, Cell Cycle, Response to Stress, Proliferation, Histone, Ubiquitin, Transcription Factor, Interferon | |||
|
| |||||
| DU145 | MF | 1Gy ×10 | DNA Binding, Response to Stress, Apoptosis, Proliferation, Signal Transduction, Immune Response, Interferon, DNA Damage | in vitro, mRNA | |
| SD | 10Gy ×1 | DNA Binding, Response to Stress, Immune Response, Cell Cycle, Transcription Factor, Proliferation, Protease, Histone, Interferon, Cyclin | |||
The role of miRNAs in (radiation and cytotoxic) stress response is becoming increasingly clear (31–32). They are key regulators of gene expression by post-transcriptional interference of mRNAs and function as oncogenes (miR-17–92 cluster, miR21, miR106b-93–25 cluster, miR-155, miR221, miR-222), tumor suppressor (let-7, miR-15–16 cluster, miR-34, miR203) and epigenetic modulators. Mounting evidence associating miRNAs and epigenetics show some miRNAs to be epigenetically regulated (let-7, miR-34 family, miR-124, miR -137, miR -148, miR-203 and miR-200 family) (33–34), while some have been reported to repress epigenetic drivers by directly targeting key genes such as DNA methyl transferases, HDACs and histones(miR-26, miR-29, miR-101, miR-148, mir-203, miR-205, miR-214and miR-449) (30, 33–34).
Their dysregulation has been implicated in multiple cancers; the radiation-induced differential expression of miRNA is also well documented (31, 35–36). Mueller and colleagues report that a family of miRNAs (miR-99 family) is upregulated in response to radiation (2Gy x2) and reduces the efficiency of DNA repair in two p53+ breast (MCF7) and prostate (LNCaP) cancer cell lines. They further showed decreased DNA repair efficiency with mir-99 expression following MF (13). miR-99 expression has also been reported to be decreased in advanced and more radioresistant cancers (13, 37). This finding supports earlier reports from our group; we demonstrate increased expression of select miRNA after radiation exposure, especially MF in LNCaP and PC3(8). Further, we showed increased miR-99 expression following MF in PC3 but not in LNCaP. In contrast, let-7, which is also typically underexpressed in cancers and associated with poor prognosis in patients (38), was upregulated after MF in LNCaP and PC3 (8). let-7, a tumor suppressor miRNA, regulates cellular proliferation and expression of oncogenes such as RAS/c-MYC and HMGA-2; its over-expression has been reported to inhibit tumor development and growth (38–40).
miRNAs are crucial to the maintenance of radiation-induced response and although, miRNA profiling is a promising diagnostic tool, as expression levels of specific miR have been associated with major cancer outcomes, it should be noted that the baseline miRNA expression levels in different cancers are not always predictive of response (8, 13) so that post-exposure analysis will be informative in addition to baseline assessment. Further studies are necessary to assess the role of epigenetics and p53 function in radiation-induced miRNA response and to understand the how miRNAs contribute to the maintenance of MF-induced adaptation, as cells pass on their “survivor” phenotype to subsequent generations.
Tumor Heterogeneity
Inter- and intra-tumoral heterogeneity is a feature of carcinogenesis and tumor progression including malignant transformation, metastasis, immune evasion, and treatment resistance. Gerliger et al. recently mapped the heterogeneity in four renal cancer patient samples and the associated metastases using exome-sequencing. They report multiple genetic and functional mutations within a single tumor for mTOR (41) and its downstream targets. Recent reports have also highlighted the heterogeneity within different tumors (42–44). This has implications for biomarkers, diagnostics, therapeutic efficacy and relatively specific molecular-targeted therapies. (41–44). We are interested in exploring the effect of radiation on tumor heterogeneity, particularly how the adaptation to MF could be exploited to therapeutic advantage.
The new perspective for radiation oncology
The novel observation from these studies is that changes in the molecular characteristics of the surviving tumor cells and normal tissues after MF may affect treatment outcome and could potentially be exploited. IMRT and IGRT techniques create a heterogeneous dose distribution within the tumor; hypofractionation uses large doses in either a single or a limited number of fractions. While physical techniques allow for two beneficial outcomes- increasing the dose to regions of interest within the tumor volume and decreasing the maximal normal tissue dose near the tumor, more tissue is exposed to some radiation, much of it receiving approximately half the daily tumor dose and some much lower. The normal tissue and stromal cells that receive repeated lower doses do respond and the impact of this on the tumor microenvironment remains to be elucidated. Our data show that there is a different molecular profile between tumor cell type and differences are dependent on radiation dose per fraction.
Mathematical models based on clinical experience are useful for treatment selection in the clinic (15, 38, 45) but these do not describe biological processes that are changed. Our studies have shown that cells that survive and remain clonogenic after MF are phenotypically different from the starting cells. Furthermore, cells may be lethally irradiated in terms of clonogenic potential yet survive and could influence outcome, such as late effects (46–47). Proteomics data being analyzed indicate that a specific molecular target (such as NF-κB and PARP) may go in opposite directions following SD and MF. Ongoing and future plans include in vivo studies, a broader range of tissue types and genetic background, effect of hypoxia and utilization of MF as part of combined modality treatment and immunotherapy (10).
How does this fit with the newer models for cancer treatment?
New models of molecular-targeted therapy targets both oncogene addiction (OA) and non-oncogene addition (NOA) (48); NOA could be potentially be produced using a specified course of MF radiation (7). Synthetic lethality takes advantage of the cell's susceptibility due to a defective pathway (49–50) and it might be possible to induce cell susceptibility using MF to replace the therapeutic dependence on mutated pathway, thereby broadening the potential for synthetically lethal drugs. The extensive heterogeneity within tumors (41–44) presents a challenge as to what targets can be effectively exploited, so that the cellular adaptation to radiation could generate a target and/or reduce heterogeneity. As noted above, baseline miRNA expression between various cell types is not always predictive of magnitude of response (8, 13) so that sampling a tumor after being challenged by radiation may provide a new approach to molecular targeting. We recognize that there is much to be done; however, there may be very innovative ways of utilizing the ability to target radiation to generate changes that can improve treatment efficacy. It is a new paradigm of using radiation “pharmacokinetics and pharmacodynamics”.
In summary, understanding and utilizing the radiation stress response and the adaptation to MF radiation for both tumors and normal tissues coupled with the advances in imaging and technological delivery of radiation provides potential for a unique targeting situation for combined modality therapy and immunotherapy, an approach we call “focused biology” (5).
Supplementary Material
Table 2.
Differences in miRNAprofiles between single dose and multi-fraction radiation. Table summarizes pathway analysis of previously reported microRNAs, following exposure to SD and MF radiation.
| Cell type | Radiation Protocol | Functional Categories | Type of Analysis | Ref | |
|---|---|---|---|---|---|
| LNCaP | MF | 0.5Gy ×10 | Genetic disorder, skeletal and muscular disorders, inflammatory disease | in vitro, miRNA | John-Aryankala yil, et al. (2012) |
| 1Gy ×10 | Reproductive system disease, genetic disorder, skeletal and muscular disorder | ||||
| SD | 5Gy ×1 | Cell cvcle cellular orowtli and proliferation cellular development | in vitro, miRNA | ||
| 10Gy ×1 | Cellular development, cellular growth and proliferation, cell cycle | ||||
|
| |||||
| PC3 | MF | 0.5Gy ×10 | Cell death, liver necrosis/cell death, cell cycle | in vitro, miRNA | |
| 1Gy ×10 | Reproductive system disease, genetic disorder, skeletal and muscular disorders | ||||
| SD | 5Gy ×1 | Cellular development, embryonic development, nervous system development and function | in vitro, miRNA | ||
| 10Gy ×1 | Cellular assembly and organization, cell death, skeletal and muscular system development and function | ||||
|
| |||||
| DU145 | MF | 0.5Gy ×10 | Cell cycle, cell death, cell morphology | in vitro, miRNA | |
| 1Gy ×10 | DNA replication, recombination and repair, cell death, liver necrosis/cell death, inflammatory response, antigen presentation, cell-to-cell signaling and interaction | ||||
| SD | 5Gy ×1 | None | in vitro, miRNA | ||
| 10Gy ×1 | Cell cycle, cell death, cell morphology | ||||
Acknowledgement
Barbara Arora for preparing the radiation therapy treatment plan images and James B. Mitchell and Mansoor Ahmed for editorial advice.
Footnotes
Disclosure of Potential Conflicts of Interest: None
References
- 1.Bogdanich W. Radiation Offers New Cures, and Ways to Do Harm. 2010 Jan 23; 2010. [cited October 16, 2011]; Available from: http://www.nytimes.com/2010/01/24/health/24radiation.html?pagewanted=all.
- 2.Brenner DJ. Should we be concerned about the rapid increase in CT usage? Rev Environ Health. 2010;25:63–8. doi: 10.1515/reveh.2010.25.1.63. [DOI] [PubMed] [Google Scholar]
- 3.Kinoshita N, Sueki K, Sasa K, Kitagawa J, Ikarashi S, Nishimura T, et al. Assessment of individual radionuclide distributions from the Fukushima nuclear accident covering central-east Japan. Proc Natl Acad Sci U S A. 2011;108:19526–9. doi: 10.1073/pnas.1111724108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yasunari TJ, Stohl A, Hayano RS, Burkhart JF, Eckhardt S, Yasunari T. Cesium-137 deposition and contamination of Japanese soils due to the Fukushima nuclear accident. Proc Natl Acad Sci U S A. 2011;108:19530–4. doi: 10.1073/pnas.1112058108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman CN. Linking radiation oncology and imaging through molecular biology (or now that therapy and diagnosis have separated, it's time to get together again!) Radiology. 2003;228:29–35. doi: 10.1148/radiol.2281021567. [DOI] [PubMed] [Google Scholar]
- 6.Tsai MH, Cook JA, Chandramouli GV, DeGraff W, Yan H, Zhao S, et al. Gene expression profiling of breast, prostate, and glioma cells following single versus fractionated doses of radiation. Cancer Res. 2007;67:3845–52. doi: 10.1158/0008-5472.CAN-06-4250. [DOI] [PubMed] [Google Scholar]
- 7.John-Aryankalayil M, Palayoor ST, Cerna D, Simone CB, 2nd, Falduto MT, Magnuson SR, et al. Fractionated radiation therapy can induce a molecular profile for therapeutic targeting. Radiat Res. 2010;174:446–58. doi: 10.1667/RR2105.1. [DOI] [PubMed] [Google Scholar]
- 8.John-Aryankalayil M, Palayoor ST, Makinde AY, Cerna D, Simone CB, 2nd, Falduto MT, et al. Fractionated Radiation Alters Oncomir and Tumor Suppressor miRNAs in Human Prostate. Cancer Cells Radiat Res. 2012;178:105–17. doi: 10.1667/rr2703.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simone CB, 2nd, John-Aryankalayil M, Palayoor ST, Makinde AY, Cerna D, Falduto MT, et al. mRNA Expression Profiles for Prostate Cancer Following Fractionated Radiation are Influenced by p53 Status. Radiat Res. doi: 10.1593/tlo.13241. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–88. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khodarev NN, Minn AJ, Efimova EV, Darga TE, Labay E, Beckett M, et al. Signal transducer and activator of transcription 1 regulates both cytotoxic and prosurvival functions in tumor cells. Cancer Res. 2007;67:9214–20. doi: 10.1158/0008-5472.CAN-07-1019. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Xia L, Lee LM, Khaletskiy A, Wang J, Wong JY, et al. Effector genes altered in MCF-7 human breast cancer cells after exposure to fractionated ionizing radiation. Radiat Res. 2001;155:543–53. doi: 10.1667/0033-7587(2001)155[0543:egaimh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Mueller AC, Sun D, Dutta A. The miR-99 family regulates the DNA damage response through its target SNF2H. Oncogene. 2012 doi: 10.1038/onc.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eric J, Hall AJG. Radiobiology for the Radiologist. Sixth Edition ed Lippincott Williams and Wilkins; 2006. [Google Scholar]
- 16.Bentzen SM, Constine LS, Deasy JO, Eisbruch A, Jackson A, Marks LB, et al. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): an introduction to the scientific issues. Int J Radiat Oncol Biol Phys. 2010;76:S3–9. doi: 10.1016/j.ijrobp.2009.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Hu C, Eisbruch A. Organ-sparing radiation therapy for head and neck cancer. Nat Rev Clin Oncol. 2011;8:639–48. doi: 10.1038/nrclinonc.2011.106. [DOI] [PubMed] [Google Scholar]
- 18.Allen C, Borak TB, Tsujii H, Nickoloff JA. Heavy charged particle radiobiology: using enhanced biological effectiveness and improved beam focusing to advance cancer therapy. Mutat Res. 2011;711:150–7. doi: 10.1016/j.mrfmmm.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wahl RL, Herman JM, Ford E. The promise and pitfalls of positron emission tomography and single-photon emission computed tomography molecular imaging-guided radiation therapy. Semin Radiat Oncol. 2011;21:88–100. doi: 10.1016/j.semradonc.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Barros M, Thin TH, Maj J, Cordon-Cardo C, Haimovitz-Friedman A, Fuks Z, et al. Impact of stromal sensitivity on radiation response of tumors implanted in SCID hosts revisited. Cancer Res. 2010;70:8179–86. doi: 10.1158/0008-5472.CAN-10-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zelefsky MJ, Greco C, Motzer R, Magsanoc JM, Pei X, Lovelock M, et al. Tumor control outcomes after hypofractionated and single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases from renal cell carcinoma. Int J Radiat Oncol Biol Phys. 2012;82:1744–8. doi: 10.1016/j.ijrobp.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coleman CN, Blakely WF, Fike JR, MacVittie TJ, Metting NF, Mitchell JB, et al. Molecular and cellular biology of moderate-dose (1–10 Gy) radiation and potential mechanisms of radiation protection: report of a workshop at Bethesda, Maryland, December 17-18, 2001. Radiat Res. 2003;159:812–34. doi: 10.1667/rr3021. [DOI] [PubMed] [Google Scholar]
- 23.Turesson I, Nyman J, Qvarnstrom F, Simonsson M, Book M, Hermansson I, et al. A low-dose hypersensitive keratinocyte loss in response to fractionated radiotherapy is associated with growth arrest and apoptosis. Radiother Oncol. 2010;94:90–101. doi: 10.1016/j.radonc.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Morgan WF. Communicating non-targeted effects of ionizing radiation to achieve adaptive homeostasis in tissues. Curr Mol Pharmacol. 2011;4:135–40. [PubMed] [Google Scholar]
- 25.Hiniker SM, Chen DS, Knox SJ. Abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:2035. doi: 10.1056/NEJMc1203984. author reply -6. [DOI] [PubMed] [Google Scholar]
- 26.Azzam EI, de Toledo SM, Little JB. Oxidative metabolism, gap junctions and the ionizing radiation-induced bystander effect. Oncogene. 2003;22:7050–7. doi: 10.1038/sj.onc.1206961. [DOI] [PubMed] [Google Scholar]
- 27.Mothersill C, Stamato TD, Perez ML, Cummins R, Mooney R, Seymour CB. Involvement of energy metabolism in the production of 'bystander effects' by radiation. Br J Cancer. 2000;82:1740–6. doi: 10.1054/bjoc.2000.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klammer H, Zhang LH, Kadhim M, Iliakis G. Dependence of adaptive response and its bystander transmission on the genetic background of tested cells. Int J Radiat Biol. 2012 doi: 10.3109/09553002.2012.691613. [DOI] [PubMed] [Google Scholar]
- 29.Mothersill C, Seymour C. Are epigenetic mechanisms involved in radiation-induced bystander effects? Front Genet. 2012;3:74. doi: 10.3389/fgene.2012.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ilnytskyy Y, Koturbash I, Kovalchuk O. Radiation-induced bystander effects in vivo are epigenetically regulated in a tissue-specific manner. Environ Mol Mutagen. 2009;50:105–13. doi: 10.1002/em.20440. [DOI] [PubMed] [Google Scholar]
- 31.Simone NL, Soule BP, Ly D, Saleh AD, Savage JE, Degraff W, et al. Ionizing radiation-induced oxidative stress alters miRNA expression. PLoS One. 2009;4:e6377. doi: 10.1371/journal.pone.0006377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell. 2012;148:1172–87. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malumbres M. miRNAs and cancer: An epigenetics view. Mol Aspects Med. 2012 doi: 10.1016/j.mam.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato F, Tsuchiya S, Meltzer SJ, Shimizu K. MicroRNAs and epigenetics. FEBS J. 2011;278:1598–609. doi: 10.1111/j.1742-4658.2011.08089.x. [DOI] [PubMed] [Google Scholar]
- 35.Dickey JS, Zemp FJ, Martin OA, Kovalchuk O. The role of miRNA in the direct and indirect effects of ionizing radiation. Radiat Environ Biophys. 2011;50:491–9. doi: 10.1007/s00411-011-0386-5. [DOI] [PubMed] [Google Scholar]
- 36.Cha HJ, Shin S, Yoo H, Lee EM, Bae S, Yang KH, et al. Identification of ionizing radiation-responsive microRNAs in the IM9 human B lymphoblastic cell line. Int J Oncol. 2009;34:1661–8. doi: 10.3892/ijo_00000297. [DOI] [PubMed] [Google Scholar]
- 37.Sun D, Lee YS, Malhotra A, Kim HK, Matecic M, Evans C, et al. miR-99 family of MicroRNAs suppresses the expression of prostate-specific antigen and prostate cancer cell proliferation. Cancer Res. 2011;71:1313–24. doi: 10.1158/0008-5472.CAN-10-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nair VS, Maeda LS, Ioannidis JP. Clinical outcome prediction by microRNAs in human cancer: a systematic review. J Natl Cancer Inst. 2012;104:528–40. doi: 10.1093/jnci/djs027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson CD, Esquela-Kerscher A, Stefani G, Byrom M, Kelnar K, Ovcharenko D, et al. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007;67:7713–22. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- 40.Nana-Sinkam SP, Croce CM. MicroRNAs as therapeutic targets in cancer. Transl Res. 2011;157:216–25. doi: 10.1016/j.trsl.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–92. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hou Y, Song L, Zhu P, Zhang B, Tao Y, Xu X, et al. Single-Cell Exome Sequencing and Monoclonal Evolution of a JAK2-Negative Myeloproliferative Neoplasm. Cell. 2012;148:873–85. doi: 10.1016/j.cell.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 43.Xu X, Hou Y, Yin X, Bao L, Tang A, Song L, et al. Single-cell exome sequencing reveals single-nucleotide mutation characteristics of a kidney tumor. Cell. 2012;148:886–95. doi: 10.1016/j.cell.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson K, Lutz C, van Delft FW, Bateman CM, Guo Y, Colman SM. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011;469:356–61. doi: 10.1038/nature09650. [DOI] [PubMed] [Google Scholar]
- 45.Glatstein E. The omega on alpha and beta. Int J Radiat Oncol Biol Phys. 2011;81:319–20. doi: 10.1016/j.ijrobp.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 46.Papadopoulou A, Kletsas D. Human lung fibroblasts prematurely senescent after exposure to ionizing radiation enhance the growth of malignant lung epithelial cells in vitro and in vivo. Int J Oncol. 2011;39:989–99. doi: 10.3892/ijo.2011.1132. [DOI] [PubMed] [Google Scholar]
- 47.Yarnold J, Brotons MC. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol. 2010;97:149–61. doi: 10.1016/j.radonc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and nononcogene addiction. Cell. 2009;136:823–37. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iglehart JD, Silver DP. Synthetic lethality--a new direction in cancer-drug development. N Engl J Med. 2009;361:189–91. doi: 10.1056/NEJMe0903044. [DOI] [PubMed] [Google Scholar]
- 50.Kaelin WG., Jr. Synthetic lethality: a framework for the development of wiser cancer therapeutics. Genome Med. 2009;1:99. doi: 10.1186/gm99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.