Abstract
Bisphosphonates (BPs) are chemically stable analogs of pyrophosphate exhibiting strong affinity to bone and have been used for the treatment of diseases characterized by excessive bone resorption. Contrary to the widely accepted BP accumulation model in bone after repeated applications, we report here that an equilibrium-dependent BP-crystalline bone mineral interaction may better explain BP bio-distribution and anti-catabolic bone remodeling and may be relevant to the appearance of osteonecrosis of the jaw (ONJ) in rats. Fluorescent-labeled BP analogs were synthesized and used to evaluate the mode of bone adsorption. After fluorescent-labeled BP adsorbed on crystalline calcium phosphates in vitro, subsequent BP application replaced the previously absorbed BP depending on the dose and the relative binding affinity to hydroxyapatite. The in vivo intravenous zoledronate (ZOL) injection of repeated fractional doses resulted in lower serum CTX and TRAP5b measurements than a single bolus injection in spite of the equivalent cumulative dose. Repeated injections resulted in the distribution of fluorescent-labeled BP on the large area of bone surfaces; whereas the single bolus injection gave rise to the intense BP bio-distribution at selected bone sites such as the alveolar process of jawbones. Necrotic maxillary alveolar bone was predominantly observed in vitamin D deficiency rats treated with bolus ZOL injection. The palatal necrotic bone was characteristically sequestrated by the fistulation of hyperplastic oral epithelium, suggesting the initial development of ONJ-like lesions in rats. Our results suggest that equilibrium-dependent BP-bone interaction may, in part, determine the effectiveness and influence side effects of long-term and repeated applications of BPs.
Keywords: Bisphosphonate, Adsorption, Fluorescence, Equilibrium, Osteonecrosis of the Jaw
1. Introduction
Methylenebisphosphonic acids ((HO)2P(O)CR1R2P(O)(OH)2), commonly referred to as “bisphosphonates” (BPs) are analogs of pyrophosphoric acids in which a carbon atom replaces the bridging oxygen atom between the two phosphate groups. The current generation of BP drugs have in common a hydroxy-R1 side chain and a nitrogen-containing-R2 substituent, and exhibit strong affinity to inorganic mineral in bone [1, 2]. As highly stable chemical compounds, BPs are rapidly adsorbed to bone crystalline mineral after entering the biological system and may remain in skeletal tissues without biological metabolization [3–6]. Once internalized in osteoclasts during bone resorption, nitrogen-containing BPs act as a de-coy substrate of the mevalonate metabolism pathway [7–9], leading to insufficient post-translational prenylation of cell membrane small GTPases [10]. As a result, the osteoclasts undergo premature detachment from bone surface and apoptosis. With this unique pharmacological effect targeting osteoclasts, BPs are among the most widely prescribed drugs for prevention and treatment of hypercalcemia caused by bone resorbing tumors such as multiple myeloma and bone metastases of breast or prostate cancers, as well as for the treatment of osteoporosis.
Since 2003, a phenomenon collectively referred to as osteonecrosis of the jaw (ONJ) has been reported as a rare but potentially severe adverse event in patients receiving BP [11–13]. ONJ is clinically characterized as unresolved exposure of partially necrotic jawbone to the oral cavity and is frequently associated with dentoalveolar procedures in cancer patients receiving high doses of intravenously administered BPs [12, 14–17]. Repeated BP administrations are believed to result in the accumulation of bioavailable doses in bone and thus have been postulated to reach the accumulated “toxic” BP level causing ONJ [18]. This hypothesis currently provides the underlining rationale for assessing the risk of developing ONJ particularly for osteoporosis patients with long-term BP use [19], significantly affecting the dental treatment decisions.
The objective of this study is to establish the bio-distribution of BPs with different administration protocols in vitro and in vivo using fluorescently labeled BP analogs. We report here that unlike currently accepted accumulation kinetics, BP and bone crystalline mineral appear to establish an equilibrium-based molecular interaction and the initially adsorbed BP can be removed and further replaced by the later applied BP in the dose and affinity dependent manner. This “competitive” equilibrium appeared to better explain BP bio-distribution and pharmacological outcomes, and influence the development of ONJ-like lesions in rats.
2. Materials and Methods
2.1. Synthesis and purification of fluorescent BP analogs: 5-FAM-ZOL, 5(6)-FAM-RIS, 6-ROX-RIS
Zoledronate (ZOL) was purchased from Molekula Limited, UK. Risedronate (RIS) was a kind gift from Procter & Gamble Pharmaceuticals. Fluorescent BP analogs: ZOL conjugated with 5-carboxyfluorescein (FAM-ZOL), RIS conjugated with 5(6)-carboxyfluorescein (FAM-RIS) and RIS conjugated with 6-Carboxyl-X-Rhodamine (ROX-RIS); were synthesized via a linker strategy (Fig. 1A) [20]. The conjugates were purified by TLC and HPLC (purity > 95 %), and were characterized by 1H, 31P NMR, HRMS, UV-VIS and fluorescent spectroscopy as described elsewhere [20].
Figure 1.

A: Chemical structures of fluorescent-labeled BP analogs: ZOL conjugated with 5-carboxyfluorescein (FAM-ZOL), RIS conjugated with 5(6)-carboxyfluorescein (FAM-RIS) and RIS conjugated with 6-Carboxyl-X-Rhodamine (ROX-RIS) via a linker strategy. B: The pharmacological function of FAM-ZOL was compared with ZOL in vitamin D deficient (VitD(−)) rats. ZOL, FAM-ZOL or vehicle solution (0.9% NaCl) was injected via tail vein into VitD(−) rats (n=3 per group). Six weeks after the injection, the trabecular bone morphology of femurs was evaluated by micro-CT. FAM-ZOL significantly blocked catabolic bone remodeling resulting in increased trabecular bone structure; however, the effectiveness of FAM-ZOL was reduced approximately 50% of ZOL *: p<0.05 by one-way ANOVA.
2.2. In vivo effect of FAM-ZOL on femur of vitamin D-deficient rats
All animal experiments were approved by the UCLA Animal Research Committee. Eight week-old Sprague-Dawley rats (Charles River Laboratories, Inc., Wilmington, MA) were fed a diet lacking vitamin D (TD.89123: 0.47% calcium, 0.3% phosphorus; Harlan Teklad, Madison, WI) for two weeks. All rats had free access to water and were kept in regular housing with a 12-hr light/dark cycle in a UV-restricted environment; UVB emission (290–305 nm) overlapping the action spectrum. Rats (n=3 in each group) received intravenous injection of the equal molar dose of ZOL or FAM-ZOL. Rats in the control group (n=3) received an intravenous injection of vehicle solution (0.9% NaCl). Six weeks later, rats were euthanized in a carbon dioxide chamber and femur was harvested. The femur specimens were fixed in 10% buffered formalin. Micro-CT scanning (μCT 40, Scanco Medical, Bassersdorf, Switzerland) was performed distally from the growth plate at an x-ray energy level of 55 kVp with a current of 72 μA. Out of this measurement volume, a region located 0.5 mm below the lower end of the growth plate and extending 1.6 mm distally was consistently selected for evaluation. Nominal isotropic resolution was 16 μm, and the analysis region was represented by 100 slices. Three-dimensional image of femur was constructed using a customized computational program by digitally extracting the bone image using the predetermined threshold (220). The trabecular and cortical regions were separated by semi-automatically drawn contours [21], and the trabecular bone structure of the proximal femur was evaluated to assess bone volume ratio (BV/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and trabecular number (Tb.N) [22]. The data were analyzed by one-way analysis of variance (ANOVA) and statistical significance was accepted for p<0.05.
2.3. Dose-dependent adsorption of FAM-ZOL to CaP discs in vitro
Submicron synthetic mineralized calcium phosphate (CaP) thin films coated discs (BioCoat Osteologic Discs, BD Bioscience, San Jose, CA) were used to investigate the adsorption pattern of FAM-ZOL. The CaP disc has been used as an alternative material for direct assessment of osteoclast and osteoblast activity in vitro [23]. Incrementally increased doses (1, 5, 10, 50, 100, 200 and 500 μM) of FAM-ZOL were applied on the discs placed in 24-well plate (n=3 in each group) and incubated for 1 hr at room temperature. After vigorous and repeated washes with phosphate buffered saline (PBS), the standardized fluorescent biophotonics image of the CaP disc was obtained using an excitation wavelength of 460 nm and the 515 nm filter (LAS3000, FUJIFILM Corp, Tokyo, Japan). An image evaluation program (Image J, NIH, Bethesda, MD) was used to determine the fluorescent intensity of each CaP disc.
2.4. FAM-ZOL adsorption to CaP discs after repeated applications
A group of CaP discs (n=3) were incubated with FAM-ZOL (12.5 μM) for 1 hr followed by vigorous washes with PBS. These CaP discs were repeatedly incubated with FAM-ZOL (12.5 μM) for a total of 4 applications (the cumulative dose of 50 μM). In a separate group (n=3), 50 μM FAM-ZOL was applied on the CaP disc followed by vigorous washes with PBS. For a negative control (n=3), the CaP disc was treated with PBS followed by vigorous washes with PBS. The standardized fluorescent images of the CaP disc were assessed as described above. The data were analyzed by Fisher's protected least significance difference (PLSD) test, and statistical significance was accepted for p<0.05.
2.5. Alternate application of BP analogs to CaP discs
A group of CaP discs (n=3) was first incubated with 50μM ZOL. After vigorous washes with PBS, 50 μM FAM-ZOL was then applied followed by vigorous washes. In another group, the CaP discs were first treated with 50 μM FAM-ZOL and then 50 μM ZOL. CaP discs treated only with PBS or 50 μM FAM-ZOL were used as references. The standardized fluorescent images of the CaP disc were assessed. The data were analyzed by Tukey-Kramer test, and statistical significance was accepted for p<0.05. In this study, the wash solutions were collected and fluorescent emission spectra of each wash solution were recorded on Jobin Yvon Nanolog spectrometer, using an excitation wavelength of 490 nm; the excitation and emission slits were set at 5 nm; scan ranges from 495 nm to 700 nm. Spectra were calibrated and the emission peak was found at 522 nm. Fluorescent intensity of each sample at 522 nm was collected and plotted.
In a separate experiment, synthetic CaP-coated wells (Bone Resorption Assay Plate 24, Cosmo Bio Co., LTD, Tokyo, Japan) wells were first incubated with 50 μM ROX-RIS (n=18) or 50 μM FAM-RIS (n=18) for 1 hr at 37°C followed by vigorous and repeated washes in PBS. Then 0, 0.1, 1, 5, 10 or 50 μM FAM-RIS or ROX-RIS, respectively, was applied to CaP-coated wells (n=3 in each condition) and incubated for 1 hr at 37°C. After vigorous and repeated washes in PBS, the fluorescent intensity of CaP plate was examined using an excitation wavelength of 460 nm and the 510 nm filter for FAM fluorescence and an excitation wavelength of 520 nm and the 670 nm filter for ROX fluorescence (LAS3000, FUJIFILM Corp, Tokyo, Japan). An image evaluation program (Image J, NIH, Bethesda, MD) was used to determine the fluorescent intensity of both FAM and ROX in each CaP-coated well.
2.6. FAM-ZOL bio-distribution in rat femur and mandible
Rats fed with vitamin D deficient diet for 2 weeks received either a single bolus FAM-ZOL (180 μg/Kg) intravenous injection or 4 weekly-injections of fractional dose of FAM-ZOL (45 μg/kg per injection; a cumulative dose of 180 μg/Kg). As controls, rats received an intravenous injection of vehicle solution (0.9% NaCl). Six weeks after the single FAM-ZOL injection or 2 weeks after the last weekly injection, rats were euthanized. The femur and mandible specimens were immediately harvested and fixed in 10% buffered formalin. In the standardized fluorescent biophotonics images, fluorescent intensity at proximal and distal areas of femur primary spongiosa; anterior and posterior areas of mandibular ramus; and alveolar bone at the 3rd mandibular molar were quantitatively evaluated. The data were analyzed by Fisher's PLSD test, and statistical significance was accepted for p<0.05.
2.7. Effect of different ZOL administration protocols on femur bone morphometry and serum chemistry as well as on the development of osteonecrosis at the maxillary tooth extraction site
Rats fed with vitamin D deficient diet for 2 weeks received 2 monthly intravenous injections of 70 μg/Kg ZOL (the cumulative dose of 140 μg/Kg) or 8 weekly injections of 17.5 μg/kg ZOL (the cumulative dose of 140 μg/Kg). After the cumulative ZOL dose reached 70 μg/Kg, maxillary left molar teeth were extracted using the previously established procedure [24] under general anesthesia by inhalation of 2% isoflurane. Subcutaneous injections of 0.05 mg/kg buprenorphine were given every 12 hours for 48 hours after tooth extraction, but no antibiotics were administered. Four weeks after tooth extraction, rats were euthanized and approximately 8 ml of periphery blood was extracted from each animal after 6 hours of fasting. Femur and maxillary bones were harvested and fixed in 10% buffered formalin. Serum alkaline phosphatase was measured by a conventional clinical chemistry system (VetACE™, Alfa Wassermann, West Caldwell, USA). C-terminal cross-linking telopeptide of type I collagen (CTX) and serum band 5 of tartrate-resistant acid phosphatase (TRACP-5b) were measured by enzyme-linked immunosorbent assays (RatLaps ELISA, Nordic Bioscience Diagnostics, Herlev, Denmark; RatTRAP Assay, IDS, Inc., Fountain Hills, AZ, USA, respectively). The data were analyzed by Dunnett test, and statistical significance was accepted for p<0.05. The femur specimens were subjected to micro CT evaluation as described previously. The micro CT bone morphometric data of femurs were analyzed by Fisher's PLSD test, and statistical significance was accepted for p<0.05.
The maxillary bones harvested 4 weeks after tooth extraction were scanned by micro CT and then decalcified with 10% ethylenediaminetetraacetic acid (EDTA) followed by paraffin embedding. A series of 4-mm-thick sections in the frontal plane were prepared through the second to third molars of the non-extraction side as the reference. The palatal and buccal alveolar processes of the tooth extraction side were subjected for further investigations. Osteonecrosis was histologically defined by the presence of empty or pyknotic osteocyte lacunae. The bone sequestration was histologically determined by a piece of separated necrotic bone surrounded by the abnormal elongation of hyperplastic oral epithelium [25]. The effect of different ZOL administration protocols was compared by the percentage of animals positive for histologic necrotic bone sequestration lesion(s) in the group. Fisher's exact test was used for statistical evaluation and statistical significance was accepted for p<0.05.
3. Results
3.1. FAM-ZOL retained the pharmacological effect of ZOL in vivo
In this study, ZOL was conjugated with 5-caroxyfluorescein (FAM-ZOL) through `magic linker' chemistry as previously described [20]. The approach is based on the use of a functionalized epoxide to attach a universal linker group to the heterocyclic nitrogen of BP under mild reaction conditions (aqueous, near neutral pH, 21–40 °C). The drug-linker is facilely conjugated to succinimidyl ester of FAM, yielding FAM-ZOL (Fig. 1A). Previously, FAM-RIS has been shown to prevent prenylation of Rap1A of J774.2 macrophages in vitro [20, 26, 27]. In the present study, to evaluate the effect of FAM conjugation on the overall pharmacological efficacy of ZOL, we carried out a direct comparison of FAM-ZOL vs. ZOL for relative anti-resorptive activity in vivo. Nutritionally induced vitamin D deficiency increased osteoporosis-like catabolic bone remodeling in rats [25, 28, 29]. Compared to the control VitD(−) group, the FAM-ZOL group shows a significant (p < 0.05) increase in the trabecular bone volume and improvements of trabecular structural indices, while the anti-resorptive effect of FAM-ZOL in vivo was approximately 50% of that of ZOL (Fig. 1B). This study provided direct evidence that the dye-drug conjugate can maintain anti-resorptive drug activity in vivo albeit at reduced rates.
3.2. BP absorption kinetics to CaP disc in vitro
When increasing concentrations of FAM-ZOL in PBS were incubated, commercially available synthetic calcium phosphate (CaP)-coated quartz substrate discs adsorbed FAM-ZOL in a dose-dependent linear increase manner (Fig. 2A) and did not show any sign of adsorption saturation up to 500 μM of applied drug. Our data suggested that the binding characteristics of BP to hydroxyapatite crystals follow the classical Langmuir adsorption isotherm [30] and that CaP discs are an appropriate model for BP adsorption studies.
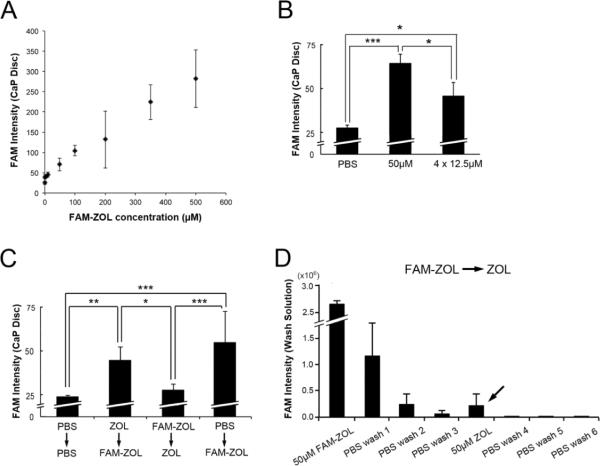
Figure 2.
A: FAM-ZOL showed a dose-dependent linear absorption to CaP discs and did not saturate below 500 μM in PBS. B: Although the 4 repeated applications of 12.5μM FAM-ZOL should deliver the equivalent cumulative dose of the single application of 50μM FAM-ZOL, the CaP discs showed less fluorescent intensity level in the former protocol than those of single high-dose application. *: p<0.05 by Fisher's PLSD test. C: Unlabeled ZOL (50μM) and FAM-ZOL (50μM) were alternately applied to CaP discs. The fluorescence intensity of CaP discs was largely influenced by the lastly applied compound. *: p<0.05 by Tukey-Kramer test. D: The PBS wash solutions collected from the group of FAM-ZOL followed by ZOL application in Fig. 2C revealed a distinct peak of FAM fluorescence (arrow) during the second ZOL application, suggesting the displacement of FAM-ZOL from CaP discs.
One-time application of 50 μM FAM-ZOL consistently gave rise to approximately 70 pixel-intensity fluorescence in CaP discs. Four repeated applications of 12.5 μM FAM-ZOL resulted in significantly less FAM fluorescence intensity in the CaP discs than the single bolus application of 50 μM FAM-ZOL (Fig. 2B). Cumulative doses of FAM-ZOL were equivalent in these groups; however, repeated applications of a fractional FAM-ZOL dose did not reach the adsorption level of a high dose-single time application.
Because the Langmuir isotherm is influenced by the adsorption affinity, we postulated that the first application of BP to the CaP disc might condition the adsorption affinity and thus interfere with the subsequent BP adsorption. To test this hypothesis, CaP discs were alternately treated with ZOL, then FAM-ZOL or FAM-ZOL, then ZOL. In the group of CaP discs pretreated with 50 μM ZOL followed by a second application of 50 μM FAM-ZOL, the CaP disc fluorescent intensity reached nearly at the level of FAM-ZOL application alone (Fig. 2C). In the reverse addition order group, the FAM-derived fluorescence intensity of the CaP discs from pre-adsorbed FAM-ZOL decreased to an undetectable level after the second application of unlabeled ZOL (Fig. 2C).
The applied solutions were saved from the above experiment and the FAM fluorescence intensity of these solutions was determined. Non-adsorbed FAM-ZOL appeared to be effectively removed by sequential PBS washes to a negligible level (Fig. 2D). After unlabeled ZOL solution was applied, a small but distinct peak of FAM fluorescence intensity was detected in the ZOL solution (arrow in Fig. 2D), suggesting that the loss of FAM fluorescence intensity in the CaP discs was likely to be due to displacement of previously adsorbed FAM-ZOL from the discs.
While the so-called tridentate structure of BP is thought to play the primary role in the binding to bone mineral, it is well established that the chemical moiety of R2 chain can also influence the binding affinity. The addition of chemical structures such as FAM and ROX to the R2 chain is anticipated to affect the binding affinity. Roelofs et al. (2012) demonstrated that ROX-RIS has significantly higher affinity than FAM-RIS in mouse tibiae [31]. Taking advantage of the relative difference in affinities of ROX-RIS (higher affinity) and FAM-RIS (lower affinity), we further investigated whether the BP affinity influenced the adsorption to CaP discs. CaP-coated culture wells were pre-treated by 50 μM ROX-RIS or FAM-RIS, giving rise to uniform adsorption as detected by the RIS or FAM fluorescence intensity. When the pre-adsorbed 50 μM ROX-RIS was challenged by FAM-RIS, ROX-RIS was not completely replaced even at the same molar concentration (Fig. 3A). On the contrary, when the pre-adsorbed 50 μM FAM-RIS was challenged by ROX-RIS, FAM-RIS was completely removed (Fig. 3A). ROX-RIS showed initial increase in CaP adsorption and reached the plateau between 10 μM and 50μM (Fig. 3B). It was noted that the adsorption concentration of ROX-RIS did not seem to increase beyond the previously established adsorption concentration between FAM-RIS and CaP.
Figure 3.
CaP-coated wells were pre-treated with either ROX-RIS or FAM-RIS (50μM). After challenged by serially diluted doses of FAM-RIS or ROX-RIS (from 0 to 50μM), respectively, ROX and FAM fluorescence intensities of the CaP-coated wells were measured. A: ROX-RIS in CaP (red bars) remained near the pre-adsorbed 50 μM level until 5 μM or greater concentrations of FAM-RIS challenged. On the contrary, FAM-RIS (green bars) was rapidly displaced by as low as 1 μM ROX-RIS. B: The pre-adsorbed FAM-RIS was completely replaced by the challenged ROX-RIS between 10 μM to 50 μM range.
3.3. Bio-distribution pattern of FAM-ZOL with different administration protocols in vivo
The in vivo bio-distribution of FAM-ZOL was examined in rats with nutritionally induced vitamin D deficiency. A single injection of 180 μg/Kg FAM-ZOL resulted in localized intense fluorescence in the femur trabecular bone proximal to the poorly labeled primary sponsiosa located at the distal end of epiphysis (Fig. 4A). The fluorescence intensity varied significantly in the femur of this group. Contrarily, 4-weekly injections of 45 μg/Kg FAM-ZOL showed more diffused and uniform fluorescent labeling in femurs (Fig. 4A). In the mandibular ramus, repeated injections of 45 μg/Kg FAM-ZOL resulted in uniform but less intense fluorescent labeling, whereas a single 180 μg/Kg FAM-ZOL injection generated a line of intense fluorescent labeling (Fig. 4B). In the mandibular alveolar bone, the fluorescence intensity was found to be much stronger in the single injection group than in the repeated injection group, despite the fact that both groups received the equivalent cumulative FAM-ZOL dose (Fig. 4C).
Figure 4.
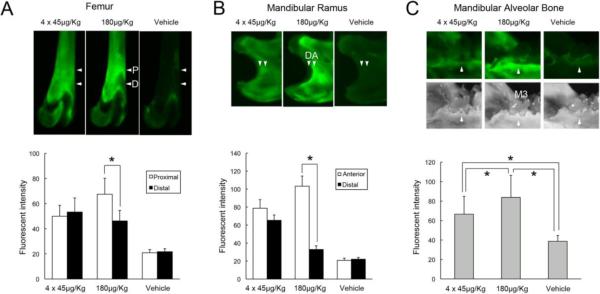
In vivo absorption pattern of FAM-ZOL in rat bone tissue. Through the tail vein, a group of vitamin D-deficient rats (n=3) received 4 repeated-weekly injections of 45μg/Kg FAM-ZOL, another group (n=3) received a single-injection of 180μg/Kg FAM-ZOL and the control rats (n=3) received a single-injection of vehicle solution (0.9% NaCl). Rat femurs and mandibles were harvested 8 weeks after the initial injection and subjected to the fluorescent CCD detector imaging analysis. A: Fluorescence distribution in femurs showed more diffused and less intense adsorption of FAM-ZOL in the repeated injection group. In the single application group, an intense fluorescence signal was found localized at the proximal (P) area of femur primary spongiosa (arrowheads) contrasted to lesser labeling in the distal (D) primary sponsiosa of the more recent growth area. B: Mandibular ramus specimens similarly indicated bone growth-dependent discrepancy in FAM-ZOL bio-distributions at the anterior (A) and distal (D) areas (arrowheads). C: Mandibular molar alveolar bone (arrowheads; M3: the 3rd molar) completed the growth by 6 to 8 weeks of age and showed the intense FAM-ZOL labeling in the single-injection group. *: p<0.05 by Fisher's PLSD test.
3.4. Effect of different ZOL administration protocols on anti-catabolic bone remodeling
The biological effect of different administration protocols with the equivalent cumulative dose was examined in vitamin D deficient rats. All rats were treated by the extraction of left maxillary molars. One group received 2 monthly-injections (70 μg/Kg ZOL per injection) and the other group received 8 weekly-injections (17.5 μg/Kg ZOL per injection). At the end of the experiment, whole blood, femur and maxillary bone samples were harvested (Fig. 5A).
Figure 5.

Different biological consequences to the modulated administration of ZOL. A: Experimental protocols involving vitamin D deficient treatment and ZOL injections in rats. The equal cumulative ZOL dose of 140μg/Kg was given in 2 rat groups; however, one group received 8 weekly-injections of 17.5μg/Kg ZOL (n=8), whereas the other group received 2 monthly-injections of 70μg/Kg ZOL (n=6). Control rats (n=4) received injections of vehicle solution (0.9% NaCl). B: Micro-CT bone morphometry of femur distal primary spongiosa indicated significantly greater anti-catabolic effect by 70μg/Kg monthly-injections than by 17.5μg/Kg weekly-injections. *: p<0.05 by Fisher's PLSD test.
The serum samples were examined for bone remodeling markers: alkaline phosphatase [32], carboxy-terminal collagen crosslinks (CTX) [32, 33], and secreted tartrate-resistant acid phosphatase 5b (TRACP-5b) [34]. The weekly injection protocol significantly decreased all serum bone remodeling markers compared to the monthly injection protocol (Table 1). On the contrary, the micro CT bone morphometry data of the femur trabecular bone clearly indicated the more robust increase in bone mass in the monthly injection group (Fig. 5B).
Table 1.
Serum bone remodeling markers of vitamin D deficient rats with different ZOL injection protocols.
| Parameters |
|||
|---|---|---|---|
| ZOL Treatment | ALP (U/L) | CTX (ng/mL) | TRACP-5b (U/L) |
| 8 × 17.5μg/Kg | 89.50±10.75 | 16.61±4.82 | 2.95±0.69 |
| ]* | ]* | ||
| 2 × 70.0μg/Kg | 145.17±70.17 | 39.44±14.35 | 4.63±1.22 |
|
| |||
| Reference (VD- rats) | 270.20±46.82 | 63.85±13.26 | 14.37±3.81 |
p<0.05 Student t-test
3.5. Effect of different ZOL administration protocols on the development of ONJ-like lesions in the rat maxilla
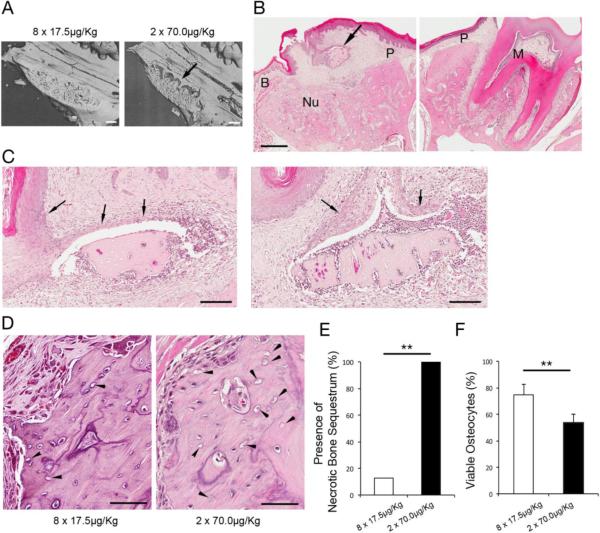
At the time of maxillary molar extraction and the end of the experimental period, the cumulative ZOL doses in both groups were equivalent (Fig 5A). Four weeks after maxillary molar extraction, the maxillary tissues were harvested. The micro CT scanning indicated that all of 6 rats received 2 injections of high dose ZOL (70 μg/Kg per injection) developed the abnormal bone sequestration, which appeared to be separated from the palatal alveolar process at the maxillary molar extraction wound (Fig. 6A). The histological cross section of the maxilla revealed the bone sequestrum was indeed necrotic and associated with oral epithelial fistulation and inflammatory cell infiltration (Fig. 6B and 6C). There was 1 out of 8 rats (or 12.5%) received the 8 repeated weekly injections of low dose ZOL (17.5 μg/Kg per injection) developed the bone sequestration. Fisher's exact test showed the significant difference on the presence of necrotic bone sequestration between 2 different ZOL administration groups (p<0.01) (Fig. 6E). The buccal alveolar process of the tooth extraction side was covered by the buccinators muscle and separated from the oral epithelium (Fig. 6B). There were clusters of empty osteocyte lacunae in the buccal alveolar process (Fig. 6D). The percent of viable osteocytes were significantly lower in rats received the bolus injection of high dose ZOL (Fig. 6F).
Figure 6.
A. C: Micro-CT evaluations of maxillary alveolar bone after molar extraction revealed the partially necrotic bone sequestra (arrow) in rats received 70.0 μg/Kg monthly injections. (Bar = 500 μm) B. Histological cross section of maxilla of rats received 70.0 μg/Kg monthly injections showed the necrotic bone sequestrum (arrow) that appeared to be derived from palatal alveolar bone (P). The bony socket housed molar (M) was filled with new bone (Nu) between palatal (P) and buccal (B) alveolar bone. (H & E staining; Bar = 500 μm) C. Necrotic bone sequestrum was associated with abnormal hyperplasia of oral epithelium (arrows). (H & E staining; Bar = 100 μm) D. Buccal alveolar process of rat maxilla received weekly injections of 17.5 μg/Kg ZOL or monthly injections of 70.0 μg/Kg ZOL showed the different distributions of empty osteocyte lacunae (arrowhead). (H & E staining; Bar = 100 μm) E. The rate of rats developed necrotic bone sequestrum. ** p<0.01 by Fisher's Exact test. F. The rate of viable osteocytes in the buccal alveolar process. ** p<0.01 by Student's t test.
4. Discussion
The results from this study collectively suggest that the establishment of a dynamic equilibrium between BP and the CaP substrate may better explain the BP adsorption pattern to bone tissue and its pharmacological effects than a simple dose accumulation model. Repeated applications of low-dose FAM-ZOL did not produce the adsorption level observed for a single high-dose FAM-ZOL application in vitro (Fig. 2) as well as in vivo (Fig. 4) albeit at equivalent cumulative doses. Repeated vigorous washes in vitro or creatinine clearance in rats [35] should not be the primary cause of the removal of the adsorbed BP. Instead, our in vitro data suggest the possibility that the initially adsorbed BP can be removed by freshly applied BP (Fig. 2C and 2D) and further replaced by the later applied BP in the dose and affinity dependent manner (Fig. 3).
Russell et al (2008) reported that near saturation occurred at bisphosphonate concentrations greater than 10 μM [36]; however, in the present study, FAM-ZOL did not demonstrate adsorption saturation within our study conditions (Fig. 2A). The addition of a bulky chemical moiety such as fluorescent molecules by conjugation to ZOL will influence the skeletal distribution [31, 37]. As previously reported [20], the FAM conjugate of risedronate (FAM-RIS) showed a moderate decrease (30 %) in retention time (Rt = 7 min) on a hydroxyapatite column compared to risedronate (Rt = 10 min). Duan estimated bone mineral binding of several clinically used bisphosphonates and related fluorescent conjugates using a HAP powder adsorption assay and subsequently reported a smaller decrease (10 %) [38]. The derived Kd (equilibrium dissociation constant) value for 5(6)-FAM-RIS (i.e., as a mixture, thus the Kd is apparent) was Kd = 14.56±3.65 μM, while risedronate had a Kd = 6.63±0.43 μM. Despite differences in methodologies, the results are consistent with the FAM conjugates having comparable but slightly reduced HAP binding affinity, relative to the parent BP drug. Roelofs et al. (2012) tested the bone mineral affinity of a wider range of BP conjugates with several fluorescent dyes including FAM, ROX, and Alexa Fluor 647 (AF647) using a dentine disc adsorption assay [31]. The addition of FAM to various BP derivatives gave rise to lowered affinity, although the exact mechanism is unknown [31]. In the present study, the FAM conjugation apparently attenuated the anti-resorptive activity of ZOL (Fig. 1B). This may also explain the discrepancy of the FAM-ZOL binding saturation pattern with the published data of unmodified FAM. We further found that ROX-RIS with higher affinity was not completely replaced by FAM-RIS with lower affinity (Fig. 3). These observations are consistent with the establishment of a dynamic equilibrium during the initial adsorption of BP to bone mineral.
Cremers et al. (2005) estimated the uptake of BP to skeletal tissue from 24-hr urine excretion data in humans [39]. Repeated intravenous administration of pamidronate appeared to be accumulated in skeletal tissue, which mirrored progressive decrease in bone resorption assessed by urine N-terminal telopeptide of type I collagen and serum alkaline phosphatase. In the present study, rat femurs in the group that received repeated injections of low dose ZOL exhibited more reduction in catabolic bone remodeling assessed by serum CTX and TRAP5b assays (Table 1) than those treated with less frequent injections of high dose ZOL. Fluorescent imaging of the rat bones revealed that the repeated injections resulted in wide distribution of FAM-ZOL on bone surfaces, suggesting a net increase in the amount of BP adsorbed in the skeletal tissues (Fig. 4), which might have contributed to the significant reduction of serum bone remodeling markers as reported by Cremers et al. (2005) [39]. Also, in the clinical context, some of the adsorbed bisphosphonate may be buried by new bone formation, and virgin surfaces are continually being laid down on which subsequent bisphosphonate doses can be adsorbed. This would, of course, decrease the total fluorescence signal intensity. On the contrary, a high local BP concentration established by the bolus injection appears to contribute to the retention of bone mass in femurs as measured by micro CT (Fig. 5B).
A single bolus injection resulted in highly localized FAM-ZOL adsorption in the alveolar process of jawbone, where bone remodeling was thought to be active [40, 41] (Fig. 4). Therefore, we hypothesized that the probability of developing ONJ, if associated with a BP, might be influenced by different administration protocols. The extended exposure of necrotic bone in patients treated with BP but not with radiation therapy has been proposed as the clinical definition of ONJ [12]. ONJ biopsy specimens frequently demonstrated the presence of hyperplastic epithelium surrounding the bone sequestrum [25, 42]. In our rat model study, the bolus injections of high dose ZOL resulted in this abnormal extension of oral epithelium that sequestrated the necrotic alveolar bone (Fig. 6B and 6C). The sign of osteonecrosis was also observed in the buccal alveolar process in this group (Fig. 6D). Furthermore, our studies may suggest that bolus injections of high dose ZOL can establish a BP-bone equilibrium during the initial exposure and the resulting high concentration of ZOL bio-distribution at the alveolar bone may contribute to the development of ONJ-like lesions (Fig. 6).
Palaska et al. (2009) summarized the published literatures on the clinical incidences of ONJ and reported that the mean duration of ZOL treatment was 1.8 years but the range varied significantly [43]. Van der Wyngaert et al. (2006) reported that the duration of bisphosphonate therapy at the time of diagnosis of ONJ ranged from 1 to 94 months with the average 2.5 years [44]. Hoff et al. (2008) reported that the duration of bisphosphonate treatment in ONJ patients was 1 to 50 months (median 1.67 years) for pamidronate; and 2 to 71 months (median 2.02 years) for ZOL [15]. These studies suggest that ONJ can occur without a clear time-dependency.
Saad et al (2011) reported a three-blinded active-controlled phase III trial of humanized anti-RANKL antibody (denosumab) compared to ZOL in cancer patients [45]. The development of ONJ-like lesions was reported in both denosumab- and ZOL-treated patients and the cumulative prevalence at the end of trial or 36 months was 1.8% and 1.3%, respectively. Among ZOL-treated patients, ONJ cases were steadily reported during 0–12 months, 12–24 months and 24–36 months of treatment. The time-independent pattern of ONJ occurrences appeared to be consistent with previous reports. The development of ONJ may associate with other drugs such as denosumab and its etiology is not clearly established. It must be noted that among denosumab-treated patients, all of the ONJ cases were reported during the first 24-month period and none was found in the 24–36-month period [45]. Although preliminary, the possible difference in the timing of ONJ development in denosumab- and ZOL-treated patients may provide a clue to elucidate the similarity and differences in the pathological mechanism contributed by these anti-resorptive agents.
Currently, there are no definitive causal relationship between low dose BP treatment and the development of ONJ in osteoporosis patients. Within the limited experimental period of our study, repeated injections of low dose BPs did not result in the accumulation of BP at the alveolar process of rat jawbone and the infrequent development of ONJ-like lesions.
In conclusion, this study suggests evidence for an equilibrium process of BP adsorption to bone tissue. The postulated equilibrium model may better explain BP bio-distribution, anti-catabolic bone remodeling and the prevalence of ONJ. Furthermore, the possibility to remove adsorbed BP molecules may suggest a novel opportunity for widening the therapeutic options.
Highlights
Repeated applications of fluorescent-labeled BPs to CaP substrate demonstrated the removal of pre-adsorbed BP in an equilibrium-dependent manner.
Single bolus and repeated fractional doses of ZOL demonstrated different pharmacological effects in vivo despite the equivalent cumulative dose.
The prevalence of ONJ-like lesion in a vitamin D deficient rat model was dependent on the initial ZOL dose.
The equilibrium-dependent BP-bone interaction model suggests the possibility of removing pre-adsorbed BP from skeletal tissues by displacement.
Acknowledgements
We thank very capable laboratory assistance by Briella McDaniel and Michaela Scott of the Howard Hughes Medical Institute Pre-College Program, UCLA School of Dentistry. This study was supported, in part, by the UCLA Clinical and Translational Science Institute (NIH 1UL1RR033176), the UCLA Academic Senate Faculty Research Program, a Grant-in-Aid from the Japan Society for the Promotion of Science and R01DC009837. This investigation was conducted in part at the UCLA facility constructed with support from Research Facilities Improvement Program (NIH C06RR014529).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions A.H. performed in vitro experiments and animal experiments. S.S. synthesized and characterized fluorescent-labeled bisphosphonates. S.S. also performed the fluorescence measurement of liquid samples. S.P. and I.N. performed animal experiments. C.E.M. and I.N. jointly conceived the study, analyzed the data and wrote the paper.
Competing financial interests C.E.M. previously served as consultant for Warner Chilcott and received funding from Alliance for Better Bone Health and Warner Chilcott. I.N. has received funding from Merck & Co. and Biomet3I.
References
- [1].Russell RG, Xia Z, Dunford JE, Oppermann U, Kwaasi A, Hulley PA, Kavanagh KL, Triffitt JT, Lundy MW, Phipps RJ, Barnett BL, Coxon FP, Rogers MJ, Watts NB, Ebetino FH. Bisphosphonates: an update on mechanisms of action and how these relate to clinical efficacy. Ann N Y Acad Sci. 2007;1117:209–57. doi: 10.1196/annals.1402.089. [DOI] [PubMed] [Google Scholar]
- [2].Ebetino FH, Hogan AM, Sun S, Tsoumpra MK, Duan X, Triffitt JT, Kwaasi AA, Dunford JE, Barnett BL, Oppermann U, Lundy MW, Boyde A, Kashemirov BA, McKenna CE, Russell RG. The relationship between the chemistry and biological activity of the bisphosphonates. Bone. 2011;49:20–33. doi: 10.1016/j.bone.2011.03.774. [DOI] [PubMed] [Google Scholar]
- [3].Peris P, Torra M, Olivares V, Reyes R, Monegal A, Martinez-Ferrer A, Guanabens N. Prolonged bisphosphonate release after treatment in women with osteoporosis. Relationship with bone turnover. Bone. 2011;49:706–9. doi: 10.1016/j.bone.2011.06.027. [DOI] [PubMed] [Google Scholar]
- [4].Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC, Cummings SR. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–38. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- [5].Bauer DC, Garnero P, Hochberg MC, Santora A, Delmas P, Ewing SK, Black DM. Pretreatment levels of bone turnover and the antifracture efficacy of alendronate: the fracture intervention trial. J Bone Miner Res. 2006;21:292–9. doi: 10.1359/JBMR.051018. [DOI] [PubMed] [Google Scholar]
- [6].Sebba A. Osteoporosis: how long should we treat? Curr Opin Endocrinol Diabetes Obes. 2008;15:502–7. doi: 10.1097/MED.0b013e328317ca83. [DOI] [PubMed] [Google Scholar]
- [7].Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581–9. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- [8].van Beek E, Pieterman E, Cohen L, Lowik C, Papapoulos S. Farnesyl pyrophosphate synthase is the molecular target of nitrogen-containing bisphosphonates. Biochem Biophys Res Commun. 1999;264:108–11. doi: 10.1006/bbrc.1999.1499. [DOI] [PubMed] [Google Scholar]
- [9].van Beek E, Pieterman E, Cohen L, Lowik C, Papapoulos S. Nitrogen-containing bisphosphonates inhibit isopentenyl pyrophosphate isomerase/farnesyl pyrophosphate synthase activity with relative potencies corresponding to their antiresorptive potencies in vitro and in vivo. Biochem Biophys Res Commun. 1999;255:491–4. doi: 10.1006/bbrc.1999.0224. [DOI] [PubMed] [Google Scholar]
- [10].Rogers MJ, Crockett JC, Coxon FP, Monkkonen J. Biochemical and molecular mechanisms of action of bisphosphonates. Bone. 2011;49:34–41. doi: 10.1016/j.bone.2010.11.008. [DOI] [PubMed] [Google Scholar]
- [11].Marx RE, Cillo JE, Jr., Ulloa JJ. Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J Oral Maxillofac Surg. 2007;65:2397–410. doi: 10.1016/j.joms.2007.08.003. [DOI] [PubMed] [Google Scholar]
- [12].Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, Gagel RF, Gilsanz V, Guise T, Koka S, McCauley LK, McGowan J, McKee MD, Mohla S, Pendrys DG, Raisz LG, Ruggiero SL, Shafer DM, Shum L, Silverman SL, Van Poznak CH, Watts N, Woo SB, Shane E. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–91. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- [13].Ruggiero SL. Bisphosphonate-related osteonecrosis of the jaw (BRONJ): initial discovery and subsequent development. J Oral Maxillofac Surg. 2009;67:13–8. doi: 10.1016/j.joms.2008.10.005. [DOI] [PubMed] [Google Scholar]
- [14].Reid IR, Cornish J. Epidemiology and pathogenesis of osteonecrosis of the jaw. Nat Rev Rheumatol. 2011 doi: 10.1038/nrrheum.2011.181. [DOI] [PubMed] [Google Scholar]
- [15].Hoff AO, Toth BB, Altundag K, Johnson MM, Warneke CL, Hu M, Nooka A, Sayegh G, Guarneri V, Desrouleaux K, Cui J, Adamus A, Gagel RF, Hortobagyi GN. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008;23:826–36. doi: 10.1359/JBMR.080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Urade M, Tanaka N, Furusawa K, Shimada J, Shibata T, Kirita T, Yamamoto T, Ikebe T, Kitagawa Y, Fukuta J. Nationwide Survey for Bisphosphonate-Related Osteonecrosis of the Jaws in Japan. J Oral Maxillofac Surg. 2011 doi: 10.1016/j.joms.2011.03.051. [DOI] [PubMed] [Google Scholar]
- [17].Barasch A, Cunha-Cruz J, Curro FA, Hujoel P, Sung AH, Vena D, Voinea-Griffin AE, Beadnell S, Craig RG, DeRouen T, Desaranayake A, Gilbert A, Gilbert GH, Goldberg K, Hauley R, Hashimoto M, Holmes J, Latzke B, Leroux B, Lindblad A, Richman J, Safford M, Ship J, Thompson VP, Williams OD, Yin W. Risk factors for osteonecrosis of the jaws: a case-control study from the CONDOR dental PBRN. J Dent Res. 2011;90:439–44. doi: 10.1177/0022034510397196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Allen MR, Ruggiero SL. Higher bone matrix density exists in only a subset of patients with bisphosphonate-related osteonecrosis of the jaw. J Oral Maxillofac Surg. 2009;67:1373–7. doi: 10.1016/j.joms.2009.03.048. [DOI] [PubMed] [Google Scholar]
- [19].Hellstein JW, Adler RA, Edwards B, Jacobsen PL, Kalmar JR, Koka S, Migliorati CA, Ristic H. Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: executive summary of recommendations from the American Dental Association Council on Scientific Affairs. J Am Dent Assoc. 2011;142:1243–51. doi: 10.14219/jada.archive.2011.0108. [DOI] [PubMed] [Google Scholar]
- [20].Kashemirov BA, Bala JL, Chen X, Ebetino FH, Xia Z, Russell RG, Coxon FP, Roelofs AJ, Rogers MJ, McKenna CE. Fluorescently labeled risedronate and related analogues: “magic linker” synthesis. Bioconjug Chem. 2008;19:2308–10. doi: 10.1021/bc800369c. [DOI] [PubMed] [Google Scholar]
- [21].Laib A, Kumer JL, Majumdar S, Lane NE. The temporal changes of trabecular architecture in ovariectomized rats assessed by MicroCT. Osteoporos Int. 2001;12:936–41. doi: 10.1007/s001980170022. [DOI] [PubMed] [Google Scholar]
- [22].Hildebrand T, Ruegsegger P. A new method for the model-independent assessment of thickness in three-dimensional images. J Microsc. 1997;185:67–75. [Google Scholar]
- [23].Cao JJ, Wronski TJ, Iwaniec U, Phleger L, Kurimoto P, Boudignon B, Halloran BP. Aging increases stromal/osteoblastic cell-induced osteoclastogenesis and alters the osteoclast precursor pool in the mouse. J Bone Miner Res. 2005;20:1659–68. doi: 10.1359/JBMR.050503. [DOI] [PubMed] [Google Scholar]
- [24].Sukotjo C, Lin A, Song K, Ogawa T, Wu B, Nishimura I. Oral fibroblast expression of wound-inducible transcript 3.0 (wit3.0) accelerates the collagen gel contraction in vitro. J Biol Chem. 2003;278:51527–34. doi: 10.1074/jbc.M309616200. [DOI] [PubMed] [Google Scholar]
- [25].Hokugo A, Christensen R, Chung EM, Sung EC, Felsenfeld AL, Sayre JW, Garrett N, Adams JS, Nishimura I. Increased prevalence of bisphosphonate-related osteonecrosis of the jaw with vitamin D deficiency in rats. J Bone Miner Res. 2010;25:1337–49. doi: 10.1002/jbmr.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Roelofs AJ, Coxon FP, Ebetino FH, Lundy MW, Henneman ZJ, Nancollas GH, Sun S, Blazewska KM, Bala JL, Kashemirov BA, Khalid AB, McKenna CE, Rogers MJ. Fluorescent risedronate analogues reveal bisphosphonate uptake by bone marrow monocytes and localization around osteocytes in vivo. J Bone Miner Res. 2010;25:606–16. doi: 10.1359/jbmr.091009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sun S, Blazewska KM, Kashemirov BA, Roelofs AJ, Coxon FP, Rogers MJ, Ebetino FH, McKenna MJ, McKenna CE. Synthesis and characterization of novel fluorescent nitrogen-containing bisphosphonate imaging probes for bone active drugs. Phosphorus Sulfur Silicon Relat Elem. 2011;186:970–971. doi: 10.1080/10426507.2010.526674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vandersteenhoven JJ, DeLustro FA, Bell NH, Turner RT. Osteoinduction by implants of demineralized allogeneic bone matrix is diminished in vitamin D-deficient rats. Calcif Tissue Int. 1988;42:39–45. doi: 10.1007/BF02555837. [DOI] [PubMed] [Google Scholar]
- [29].Turner RT, Farley J, Vandersteenhoven JJ, Epstein S, Bell NH, Baylink DJ. Demonstration of reduced mitogenic and osteoinductive activities in demineralized allogeneic bone matrix from vitamin D-deficient rats. J Clin Invest. 1988;82:212–7. doi: 10.1172/JCI113573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Henneman ZJ, Nancollas GH, Ebetino FH, Russell RG, Phipps RJ. Bisphosphonate binding affinity as assessed by inhibition of carbonated apatite dissolution in vitro. J Biomed Mater Res A. 2008;85:993–1000. doi: 10.1002/jbm.a.31599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Roelofs AJ, Stewart CA, Sun S, Blazewska KM, Kashemirov BA, McKenna CE, Russell RG, Rogers MJ, Lundy MW, Ebetino FH, Coxon FP. Influence of bone affinity on the skeletal distribution of fluorescently labeled bisphosphonates in vivo. J Bone Miner Res. 2012;27:835–47. doi: 10.1002/jbmr.1543. [DOI] [PubMed] [Google Scholar]
- [32].Brown JP, Albert C, Nassar BA, Adachi JD, Cole D, Davison KS, Dooley KC, Don-Wauchope A, Douville P, Hanley DA, Jamal SA, Josse R, Kaiser S, Krahn J, Krause R, Kremer R, Lepage R, Letendre E, Morin S, Ooi DS, Papaioaonnou A, Ste-Marie LG. Bone turnover markers in the management of postmenopausal osteoporosis. Clin Biochem. 2009;42:929–42. doi: 10.1016/j.clinbiochem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- [33].Rosen HN, Moses AC, Garber J, Iloputaife ID, Ross DS, Lee SL, Greenspan SL. Serum CTX: a new marker of bone resorption that shows treatment effect more often than other markers because of low coefficient of variability and large changes with bisphosphonate therapy. Calcif Tissue Int. 2000;66:100–3. doi: 10.1007/pl00005830. [DOI] [PubMed] [Google Scholar]
- [34].Rissanen JP, Suominen MI, Peng Z, Halleen JM. Secreted tartrate-resistant acid phosphatase 5b is a Marker of osteoclast number in human osteoclast cultures and the rat ovariectomy model. Calcif Tissue Int. 2008;82:108–15. doi: 10.1007/s00223-007-9091-4. [DOI] [PubMed] [Google Scholar]
- [35].Geng Z, Monier-Faugere MC, Bauss F, Malluche HH. Short-term administration of the bisphosphonate ibandronate increases bone volume and prevents hyperparathyroid bone changes in mild experimental renal failure. Clin Nephrol. 2000;54:45–53. [PubMed] [Google Scholar]
- [36].Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19:733–59. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- [37].Turek J, Ebetino FH, Lundy MW, Sun S, Kashemirov BA, McKenna CE, Gallant MA, Plotkin LI, Bellido T, Duan X, Triffitt JT, Russell RG, Burr DB, Allen MR. Bisphosphonate binding affinity affects drug distribution in both intracortical and trabecular bone of rabbits. Calcif Tissue Int. 2012;90:202–10. doi: 10.1007/s00223-012-9570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Duan X. Medical Sciences Division. University of Oxford; Oxford: 2010. Physiological and biological mechanisms of bisphosphonate action. [Google Scholar]
- [39].Cremers SC, Papapoulos SE, Gelderblom H, Seynaeve C, den Hartigh J, Vermeij P, van der Rijt CC, van Zuylen L. Skeletal retention of bisphosphonate (pamidronate) and its relation to the rate of bone resorption in patients with breast cancer and bone metastases. J Bone Miner Res. 2005;20:1543–7. doi: 10.1359/JBMR.050522. [DOI] [PubMed] [Google Scholar]
- [40].Kozloff KM, Volakis LI, Marini JC, Caird MS. Near-infrared fluorescent probe traces bisphosphonate delivery and retention in vivo. J Bone Miner Res. 2010;25:1748–58. doi: 10.1002/jbmr.66. [DOI] [PubMed] [Google Scholar]
- [41].Wen D, Qing L, Harrison G, Golub E, Akintoye SO. Anatomic site variability in rat skeletal uptake and desorption of fluorescently labeled bisphosphonate. Oral Dis. 2011;17:427–32. doi: 10.1111/j.1601-0825.2010.01772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hansen T, Kunkel M, Weber A, James Kirkpatrick C. Osteonecrosis of the jaws in patients treated with bisphosphonates - histomorphologic analysis in comparison with infected osteoradionecrosis. J Oral Pathol Med. 2006;35:155–60. doi: 10.1111/j.1600-0714.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- [43].Palaska PK, Cartsos V, Zavras AI. Bisphosphonates and time to osteonecrosis development. Oncologist. 2009;14:1154–66. doi: 10.1634/theoncologist.2009-0115. [DOI] [PubMed] [Google Scholar]
- [44].Van den Wyngaert T, Huizing MT, Vermorken JB. Bisphosphonates and osteonecrosis of the jaw: cause and effect or a post hoc fallacy? Ann Oncol. 2006;17:1197–204. doi: 10.1093/annonc/mdl294. [DOI] [PubMed] [Google Scholar]
- [45].Saad F, Brown JE, Van Poznak C, Ibrahim T, Stemmer SM, Stopeck AT, Diel IJ, Takahashi S, Shore N, Henry DH, Barrios CH, Facon T, Senecal F, Fizazi K, Zhou L, Daniels A, Carriere P, Dansey R. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2012;23:1341–7. doi: 10.1093/annonc/mdr435. [DOI] [PubMed] [Google Scholar]