Abstract
BACKGROUND
Alcohol-induced blackouts are associated with the development of alcohol abuse and dependence, so it is important to consider potential neurobiological risk factors for experiencing this problem prior to the onset of substance use. This study examines whether neural activity during inhibitory processing might be atypical in substance-naïve youth who later experience alcohol-induced blackouts.
METHODS
We examined inhibitory processing during fMRI with a go/no-go task that requires withholding a prepotent response in substance-naïve youth who would later transition into heavy drinking (n=40) and youth who remain abstinent (n=20). After approximately 5 years of annual follow-up assessments, youth were classified as nondrinkers (n=20), and heavy drinking youth were classified as having experienced an alcohol-induced blackout (blackout+; n=20) or not (blackout−; n=20). Groups were matched on demographic variables, and youth who experienced blackouts were matched on follow-up substance use.
RESULTS
Prior to initiating substance use, blackout+ youth showed greater activation during inhibitory processing than nondrinkers and blackout− youth in frontal and cerebellar brain regions. Mean activation during correct inhibitory responses relative to go responses in the left and right middle frontal gyri at baseline predicted future blackout experience, after controlling for follow-up externalizing behaviors and lifetime alcohol consumption.
CONCLUSIONS
Substance-naïve adolescents who later experience alcohol-induced blackouts show increased neural effort during inhibitory processing, as compared to adolescents who go on to drink at similar levels but do not experience blackouts and healthy, nondrinking controls, suggesting a neurobiological vulnerability to alcohol-induced memory impairments.
Keywords: Adolescence, Substance use, Neuroimaging, Blackouts, Alcohol, fMRI
1. INTRODUCTION
Alcohol use during adolescence is a widespread problem in most Western societies with a range of adverse consequences. Alcohol initiation typically occurs between ages 15 to 17 and escalates throughout adolescence and early adulthood (Sartor et al., 2009, 2007; York et al., 2004). By 12th grade, nearly 25% of high school seniors report engaging in heavy episodic drinking (i.e., consuming five or more drinks on one occasion) (Johnston et al., 2011), which has been linked to an increased likelihood of alcohol-related problems, such as alcohol-induced blackouts (Rose and Grant, 2010; White et al., 2004).
Alcohol-induced blackouts, or episodes of memory loss for a drinking occasion, have been associated with negative consequences and behavioral risks, including criminal activity, unprotected sex, and driving after drinking (White et al., 2002). Interestingly, some individuals may be more likely to experience an alcohol-induced blackout than others, even when matched on blood alcohol concentrations (BAC) reached (Hartzler and Fromme, 2003; Wetherill and Fromme, 2011; Wetherill et al., 2012). Two alcohol challenge studies found that, while sober, individuals with alcohol-induced blackout histories showed no memory task performance differences from those without blackout history, but after a moderate dose of alcohol, previous blackout experience was linked to poorer recollection performance (Hartzler and Fromme, 2003; Wetherill and Fromme, 2011).
Recent functional magnetic resonance imaging (fMRI) studies extend the above findings and provide insight into potential neural mechanisms underlying alcohol-induced blackouts. Wetherill and colleagues (2012) examined the acute effects of alcohol on neural correlates of contextual memory among 24 individuals with (n = 12) and without (n = 12) a history of alcohol-induced blackouts, matched on demographic and drinking variables. Participants underwent two fMRI scanning sessions: one while sober and one after consuming a moderate dose of alcohol (.08% BAC). During each scan session, participants completed a contextual memory task. Groups did not differ on memory-related activation while sober, but after alcohol consumption, those with histories of blackouts showed less activation during encoding and recollection in prefrontal and posterior parietal regions. Although individuals were well-matched and drank similarly, these findings suggest that alcohol had differential effects on frontoparietal activity, and consequently, may place some individuals at greater risk for experiencing alcohol-induced blackouts.
Similar frontoparietal brain abnormalities have been observed among youth at risk for alcohol use disorders while completing inhibition tasks (Norman et al., 2011; Schweinsburg et al., 2004). Disinhibition, the impaired ability to inhibit an inappropriate response, has been associated with substance misuse (Bogg et al., 2011; Iacono et al., 2008; Tarter et al., 2004) and a wide range of maladaptive behaviors (Kirisci et al., 2009; Young et al., 2009). Disinhibition may contribute to blackout occurrence through disinhibited drinking (e.g., drinking rapidly, gulping drinks), as opposed to drinking more steadily (Goodwin, 1995; Perry et al., 2006; Ryback, 1970). Further, deficits in the ability to inhibit distractors affects memory performance (Conway and Engle, 1994; Unsworth and Engle, 2007), and consequently, some individuals may be more likely to experience alcohol-induced memory impairments as a result, particularly after alcohol intoxication (Wetherill and Fromme, 2011; Wetherill et al., 2012). Collectively, atypical frontoparietal activity during inhibitory processing and inhibitory control deficits may place some individuals at greater risk for experiencing an alcohol-induced blackout and associated negative consequences.
To this end, a critical unanswered question is whether response inhibition and frontoparietal abnormalities exist among substance-naive youth who not only later transition into heavy drinking, but also experience alcohol-induced blackouts. We performed blood oxygen level dependent (BOLD) fMRI during a go/no-go response inhibition task, a task shown to engage frontal and posterior parietal cortical brain regions (Norman et al., 2011; Tapert et al., 2007), to characterize response inhibition among substance-naïve youth who were then followed annually. After approximately 5 years, youth had either remained substance-naïve (CON) or had transitioned into heavy drinking and were classified as either alcohol-induced blackout positive (B+) or alcohol-induced blackout negative (B−). Based on previous findings (Norman et al., 2011; Schweinsburg et al., 2004; Wetherill et al., 2012), we hypothesized that those who would experience alcohol-induced blackouts would show different, and most likely less, frontal and parietal activation during response inhibition prior to initiating substance use than youth who would remain substance-naïve and youth who would not go on to experience alcohol-induced blackouts. By identifying potential neurobiological antecedents of alcohol-induced blackouts, this study will point to risk factors for alcohol-induced impairments and related problems.
2. Methods
2.1. Participants
Youth in the current study (N=60) were identified from a sample of 296 adolescents participating in longitudinal study on neural effects of adolescent drinking (Squeglia et al., 2011, 2012; Wetherill et al., 2012). Youth were recruited through flyer distribution at local public schools when youth were ages 12–14 and had minimal, if any, substance use (Bava et al., 2011; Pulido et al., 2009; Squeglia et al., 2009). Parents and the youth were screened by phone then each privately completed semi-structured diagnostic interviews to confirm eligibility. Participants provided assent, and parents provided consent, as approved by the University of California, San Diego Human Research Protections Program.
Baseline exclusion criteria that helped rule out potential confounds were parental history of bipolar, psychotic, or antisocial personality disorder; any indication of in utero alcohol, tobacco, or illicit drug exposure; complicated or premature birth (<34 weeks gestation); left-handedness; history of chronic medical illness, any neurological or Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV, (Association, 1994) Axis I disorder; traumatic brain injury with loss of consciousness > 2 min., or learning disabilities; use of psychoactive medications; more than 6 lifetime alcohol, cigarette, marijuana, or other illicit drug uses, or more than 1 drink per drinking occasion; MRI contraindications (e.g., braces, positive pregnancy test); sensory impairments; and non-fluency in English. At baseline, participants (N=40) were ages 12–14 years with minimal substance use (≤1 total lifetime drinks; ≤1 lifetime uses of marijuana; ≤1 lifetime cigarette uses; and no history of other illicit substance use).
2.2. Measures
2.2.1. Substance use
The Customary Drinking and Drug Use Record (CDDR; Brown et al., 1998) assessed lifetime and past year information on alcohol, nicotine, and other drug use, DSM-IV abuse and dependence criteria, withdrawal/hangover symptomatology, blackout, and other substance-related negative consequences. Adolescent self-report data were verified by Breathalyzer screens, urine toxicology analyses, and parent reports on youth substance use.
2.2.2. Blackouts
Questions from the CDDR ascertained alcohol-induced blackout experience with the following questions: “I’m going to ask about some problems you may have had while drinking alcohol. I would like to know if you have had this problem in the past year. Have you experienced periods of time that later you could not remember (blackouts)? If yes, how long was your longest blackout ever in the past 3 months?” Youth were classified as blackout+ if he/she provided a positive response.
2.2.3. Family background
The Hollingshead scale provided a measure of socioeconomic status that incorporates level of education and occupation of each parent (Hollingshead, 1965). The Family History Assessment Module (FHAM; Rice et al., 1995) assessed DSM-IV criteria of alcohol and other drug abuse and dependence in first and second-degree relatives. An index of family history density was created by compositing youth and parent reports: each parent with a history of substance use disorder was assigned a 0.5 and each such grandparent added 0.25 (range: 0–2; Zucker et al., 1994).
2.2.4. Development
The Pubertal Development Scale (Petersen et al., 1988) assessed current levels of pubertal development with five sex-specific items (e.g., “Would you say that your body hair growth: has not begun yet to grow; has barely started to grow; is definitely underway; seems complete?”; “Have you noticed that your breasts are starting to grow: have not yet started growing; have barely started growing; breast growth is definitely underway; breast growth seems complete?”). Body mass index (BMI) was calculated using the standard formula incorporating height and weight.
2.2.5. Neurocognition
At baseline, verbal IQ was estimated with the Vocabulary subtest of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) and reading ability was determined using the Wide Range Achievement Test, 3rd Edition (Wilkinson, 1993).
2.2.6. Psychopathology
At baseline and follow-ups until age 18, the Child Behavior Checklist (CBCL; Achenbach and Rescorla, 2001) provided a level of adolescent psychopathological syndromes (e.g., internalizing and externalizing behaviors). Similar self-report data were obtained from the Youth Self-Report (YSR) for those age 18 and under, and with the Adult Self-Report (ASR) after turning 18 (Achenbach and Rescorla, 2003) to provide a quantitative measure of psychopathological syndromes including externalizing and internalizing behaviors.
2.2.7. Self-rating of effects of alcohol
The Self-Rating of the Effects (SRE) of alcohol form assessed the number of drinks required to feel specific effects of alcohol on first five times the participant ever consumed more than a taste or a sip (Schuckit et al., 1997).
2.2.8. Experimental paradigm
A go/no-go event-related paradigm (Norman et al., 2011; Tapert et al., 2007) measured response inhibition during fMRI scanning. During the task, participants viewed a serial presentation of blue shapes on a screen, comprised of large circles (n=64), small circles (n=16), large squares (n=43), and small squares (n=57) with each stimulus appearing for 200 msec. Participants were instructed to press a button each time a large circle, small circle, or large square (go stimuli) appeared and to not press when shown a small square (the no-go stimulus, 32% of trials). The intertrial interval was 1500 msec. Total task duration was 6 minutes and 24 seconds.
2.3. Procedures
Eligible youth completed baseline (i.e., prior to onset of substance use) interviews, urine toxicology, Breathalyzer screens, neuropsychological testing, and neuroimaging, and parents provided collateral reports of youth substance use, general life functioning, and behavior. Quarterly thereafter, participants were contacted for follow-up assessments on substance use and related problems. For the larger study, follow-up rates exceeded 95% at each annual time point. At the time of the current study, 185 of the 296 adolescents (62.5%) remained substance-naïve, 28 adolescents (9.5%) transitioned into moderate drinking, and 83 adolescents (28%) transitioned into heavy drinking (see Figure 1 for classifications). After approximately 5 years, we identified 20 substance-naïve youth, 20 heavy drinkers who reported experiencing an alcohol-induced blackout, and 20 additional heavy drinkers with matching demographics and drinking patterns who had not experienced an alcohol-induced blackout. Groups were similar on baseline age, gender, ethnicity, pubertal maturation status, body mass index, family history of substance use disorder, socioeconomic status, and heavy-drinking youth reported similar maximum number of drinks consumed in one time period during the year that they transitioned into heavy drinking and/or when they experienced their first alcohol-induced blackout (see Table 1).
Figure 1.
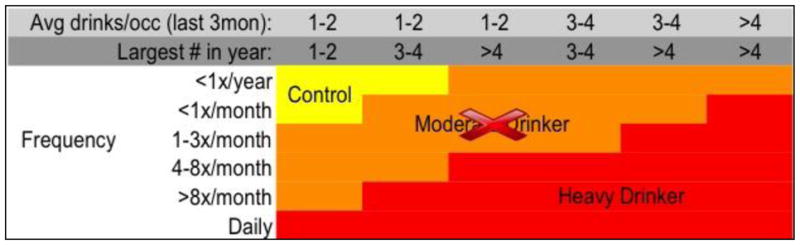
Drinking classification for heavy drinking adolescents, based on Cahalan et al., 1969 and Squeglia et al., 2009. Note that Moderate Drinkers were not included in this study; B− and B+ participants were all in the Heavy Drinker category.
Table 1.
Baseline and follow-up demographic characteristics for controls and heavy drinkers who experienced a blackout and those who did not.
| Controls (n = 20) | Blackout − (n = 20) | Blackout + (n = 20) | |
|---|---|---|---|
| M (SD) | M (SD) | M (SD) | |
| Baseline | |||
| Age | 13.3 (0.8) | 13.6 (0.7) | 13.2 (0.7) |
| % female | 45% | 45% | 45% |
| Hollingshead SES | 26.9 (15.3) | 21.3 (13.6) | 23.6 (15.7) |
| Familial substance use disorder density | 0.3 (0.5) | 0.2 (0.2) | 0.4 (0.5) |
| Achenbach externalizing T-score | 42.7 (7.4) | 44.8 (6.8) | 45.0 (7.3) |
| Achenbach internalizing T-score | 46.2 (10.3) | 44.7 (7.6) | 45.4 (9.1) |
| Reading standard score | 108.3 (9.0) | 110.7 (7.0) | 111.2 (8.0) |
| Vocabulary T-score | 55 (10.4) | 56.0 (6.6) | 58.4 (5.5) |
| Females’ pubertal stage | 2.4 (0.6) | 3.0 (0.6) | 2.5 (0.7) |
| Males’ pubertal stage | 2.1 (0.6) | 2.4 (0.5) | 2.4 (0.5) |
| Body mass index | 20.0 (3.3) | 19.3 (3.0) | 18.1 (2.5) |
| % correct inhibitions (no-go) | 74.2 (4.0) | 73.8 (2.9) | 73.9 (3.1) |
| % correct hits (go) | 98.0 (2.3) | 99.2 (2.4) | 99.1 (1.1) |
| D-prime (D′) | 2.9 (0.6) | 3.3 (0.5) | 3.2 (0.5) |
| Response bias (β) | 0.2 (0.1) | 0.2 (0.1) | 0.2 (0.1) |
| GO trial RTs (ms) | 761.7 (78.0) | 730.0 (70.4) | 726.3 (98.5) |
| NO-GO trial RTs (ms) | 665.2 (65.1) | 641.5 (83.0) | 633.7 (99.6) |
| Follow-up | |||
| Age | 18.3 (1.4) | 18.4 (2.1) | 18.7 (1.5) |
| Age of alcohol use onset | N/A | 17.2 (3.0) | 17.5 (1.8) |
| Age of transition to heavy drinking | N/A | 18.3 (1.8) | 18.2 (1.4) |
| Age of first blackout experience | N/A | N/A | 18.8 (1.4) |
| Years between baseline and follow-up | 4.6 (1.2) | 4.6 (2.0) | 5.3 (1.2) |
| Achenbach externalizing T-score** | 38.4 (5.5) | 43.7 (9.1) | 52.1 (8.0) |
| Achenbach internalizing T-score | 40.3 (7.1) | 39.5 (8.0) | 42.6 (10.1) |
| Past year drinking days per month | N/A | 4.6 (3.9) | 4.9 (3.6) |
| Past year average drinks/episode | N/A | 5.1 (2.7) | 5.7 (3.2) |
| Past year peak drinks/episode | N/A | 9.7 (6.2) | 10.3 (4.4) |
| Past year hangover symptoms** | N/A | 0.2 (0.5) | 1.7 (2.0) |
| Past year alcohol use disorder symptoms | N/A | 0.7 (1.1) | 1.5 (1.8) |
| Lifetime number of drinks* | N/A | 243.3 (162.3) | 433.8 (348.7) |
| Lifetime cannabis use episodes | N/A | 7.8 (12.9) | 7.5 (10.0) |
| Lifetime other drug use episodes | N/A | 0.3 (1.1) | 2.0 (4.8) |
| Drinks needed to feel alcohol effects (1st 5 times ever drank) | N/A | 2.8 (1.6) | 2.6 (1.2) |
p < .05 refers to the corresponding ANOVA.
p < .01 refers to the corresponding ANOVA.
2.4. MR Acquisition
Youth were imaged on a 3.0-Tesla General Electric Excite MR system with an 8-channel phase-array head coil (General Electric Medical System, Milwaukee, WI, USA). Scan sessions involved a 10-second scout scan to assure good head placement and slice selection covering the whole brain, and a sagittally-acquired high-resolution 3d T1-weighted anatomical image (FOV 24 cm, 256 × 256 × 192 matrix, 0.94 × 0.94 × 1 mm voxels, 176 slices; TR=8 ms, TE=3 ms, TI=450 ms, flip angle 12°, 7:19 minutes). Whole-brain echo planar images were collected axially (FOV 24 cm, 64 × 64 matrix, 3.75 × 3.75 × 3.8 mm voxels, TR = 2000 ms, TE = 30 ms, 90° flip angle, 32 slices no gap, slice thickness = 3.8 mm).
2.5. Data Processing and Analysis
fMRI data were processed using Analysis of Functional NeuroImages (AFNI) software (Cox, 1996). Two trained raters visually inspected time series data for gross movement artifacts and removed repetitions containing visible head motions. All data sets included in analyses retained >85% of repetitions. Small movements were corrected by registering image repetitions to a selected base volume using the AFNI 3D volume registration program (Cox and Jesmanowicz, 1999). Separate hemodynamic task response functions for successful inhibitions and response selections were calculated using deconvolution based on each participant’s behavioral data, while accounting for hemodynamic delays (Bandettini et al., 1993) and covarying for motion and linear trends. This yielded fit coefficients representing BOLD response specific to task stimulus type for each voxel within the brain. Voxel-wise fit-coefficient data were transformed to standard coordinates (Talairach and Tournoux, 1988), resampled into 3 mm3 isotropic voxels, and smoothed spatially with a 5 mm full-width half-maximum Gaussian filter.
Analyses examined baseline (i.e., prior to substance use) BOLD response contrast differences between successful no-go trials (small square; STOPS) and go trials (all other shapes) and were performed on whole-brain fMRI data using one-sample t-tests to examine group activation and a one-way analysis of variance (ANOVA) (AFNI 3dANOVA) comparing adolescents who would remain substance-naïve, heavy drinking adolescents who would later go on to experience an alcohol-induced blackout, and heavy drinking adolescents who would not experience an alcohol-induced blackout. Monte Carlo simulations using the AFNI 3dClustSim program were conducted to guard against false positive activations. Based on these analyses, a voxel-wise α of .01 would result in a corrected cluster-wise activation probability of .01 with at least 33 contiguous activated voxels (891 μl). BOLD response data were exported to PASW 18.0 (Chicago, IL) to conduct analyses.
Groups did not differ on baseline characteristics (described below); thus, analyses of variance (ANOVAs) were conducted for each cluster found to differ to between groups, and pairwise post hoc differences between groups were assessed using Tukey’s Honest Significant Difference test (HSD). For each analysis, the mean activation from each functional cluster was entered as the independent variable, and the follow-up group membership (i.e., CON, B+, or B−) as the dependent variable. Among the heavy-drinking blackout groups, follow-up logistic regression analyses examined baseline BOLD response contrast (no-go versus go) in significant clusters from the ANOVA analysis as predictors of experiencing an alcohol-induced blackout at follow-up, with blackout status as the dependent variable, mean activation from significant functional clusters of the BOLD response contrast as the independent variable, and variables in which groups differed (i.e., externalizing behaviors T-score, lifetime alcohol consumption) as covariates.
Demographic variables, substance use, and task performance variables were compared between groups with ANOVAs (e.g., age, pubertal maturation) and chi-square (e.g., gender) tests. Go/no-go task performance measures were go and no-go trial reaction times, percent correct, D′ (a measure of accuracy in discriminating between go and no-go stimuli, and β (a measure of response bias; Green and Swets, 1966).
3. RESULTS
3.1. Participant characteristics
At baseline, groups did not differ significantly on age (F(2,57) = 2.38, p = 0.10; η2 = 0.08), gender (χ2 = 0.14, p = 0.93), socioeconomic status (F(2,57) = 0.73, p = 0.49; η2 = 0.03), pubertal development (females: F(2,24) = 1.52, p = 0.25; η2 = 0.14; males: F(2,30) = 0.85, p = 0.44; η2= 0.06), body mass index (F(2,57) = 2.23, p = 0.12; η2 = 0.07), externalizing (F(2,57) = 0.97, p = 0.39; η2 = 0.04) or internalizing behaviors (F(2,57) = 0.26, p = 0.78; η2 = 0.01), or familial substance use disorders (F(2,57) = 0.23, p = 0.79; η2 = 0.01) (see Table 1). At follow-up, heavy-drinking blackout groups were similar on alcohol use (maximum number of drinks during one drinking episode during past year (F(1,38) = 0.14, p = 0.72; η2 = 0.004), number of drinking days per month (F(1,38) = 0.04, p = 0.84; η2 = 0.001), and average number of drinks per drinking episode (F(1,38)= 0.45, p = 0.51; η2 = 0.01), substance use (number of times used marijuana in the past year (F(1,38) = 0.01, p = 0.94; η2 = 0.000); number of times used other substances in the past year (F(1,38)= 2.59, p = 0.12; η2 = 0.06), but blackout+ youth showed greater number of lifetimes drinks (F(1,37) = 4.45, p = 0.04; η2 = 0.11). At follow-up, CON, B+, and B− groups were similar on internalizing behaviors, but blackout+ youth were higher on externalizing behaviors (F(2,57) = 13.46, p < 0.001; η2 = 0.35). Tukey’s HSD revealed that the control group differed significantly from the B+ group (p < 0.001), the B− and B+ groups also differed significantly (p < 0.01), and the control and B− groups were not significantly different (p > 0.05). No group differences were found on go/no-go task performance (ps > 0.05)(see Table 1)
3.2. Event-related fMRI results
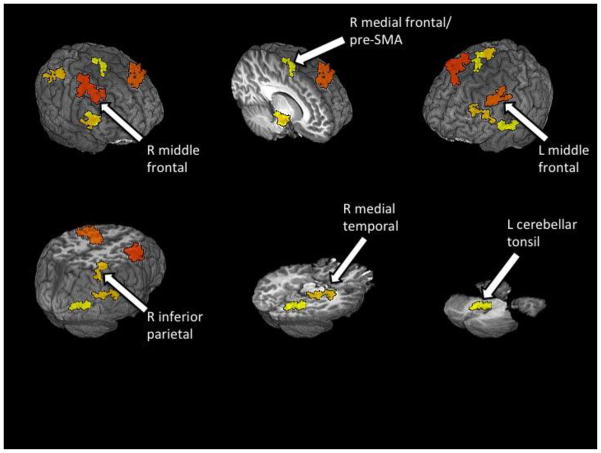
Whole-brain analyses of no-go versus go contrast revealed frontal activation in middle, superior, and medial frontal gyri and non-frontal activation in temporal, parietal, and cerebellar regions (Table 2, Figure 2). Analyses of group differences revealed that B+ youth typically had greater activation or comparable activation with controls followed by B− youth. Pairwise post hoc tests showed B+ youth had greater activity than controls in the left middle frontal gyrus (LMFG), right medial temporal lobe (RMTL), and left cerebellar tonsil. The B− youth showed less activation than controls in the right middle frontal gyrus (RMFG) and rostromedial prefrontal cortex (pre-SMA). Both B+ and B− youth showed less activation than controls in the right inferior parietal lobule (RIPL).
Table 2.
Inhibition results: cluster volumes are in micro-liters and center-of-mass coordinates are in the Talairach & Tournoux atlas.
| Anatomical region | Brodmann area | Volume (μl) | RL | AP | IS | Pairwise differences |
|---|---|---|---|---|---|---|
| R middle frontal gyrus | 6 | 4671 | −37 | −4 | 48 | B+ > B−; C > B− |
| L middle frontal gyrus | 9 | 2592 | 39 | −9 | 39 | B+ > C; B+ > B− |
| R medial temporal lobe | 28 | 1404 | −13 | 14 | −14 | B+ > C; B+ > B− |
| R inferior parietal lobule | 40 | 1350 | −37 | 50 | 45 | C > B− |
| L cerebellar tonsil | 1269 | 12 | 36 | −30 | B+ > C; B+ > B− | |
| R medial frontal gyrus (pre-SMA) | 6 | 1080 | −7 | 5 | 66 | B+ > B−; C > B− |
RL: right-left, AP: anterior-posterior, IS: inferior-superior. For the pairwise contrasts (Tukey’s Honest Significant Difference test) C = controls, B+ = blackout+ and B− = blackout− and p < 0.05.
Figure 2.
Group differences in no-go versus go BOLD response contrast (p < .01, clusters > 891 μl) between youth who go on to experience blackouts (B+; n = 20), youth who do not experience blackouts (B−; n = 20), and controls (CON; n = 20).
With respect to pairwise comparisons between B+ and B− groups, the B+ group displayed greater activity than the B− group in the RMFG, LMFG, RMTL, left cerebellar tonsil, and pre-SMA. There were no regions where B− youth showed greater activation compared with B+ youth.
Follow-up analyses were conducted to determine whether differential brain responses in the clusters shown to differ between B+ and B− youth (i.e., RMFG, LMFG, RMTL, left cerebellar tonsil, and pre-SMA) predicted alcohol-induced blackouts at follow-up after controlling for externalizing behaviors and lifetime alcohol consumption. Logistic regressions (Type I error controlled with α = 0.05/5 = 0.01) revealed that BOLD response contrast at baseline in the RMFG (Wald’s χ2 = 6.86, p = 0.01; OR = 1.52, CI: 1.11–2.09; Cox & Snell R2= 27%) and LMFG (Wald’s χ2 = 9.01, p = 0.003; OR = 1.71, CI: 1.20–2.42; Cox & Snell R2= 41%) predicted blackouts after controlling for covariates (Figure 3).
Figure 3.
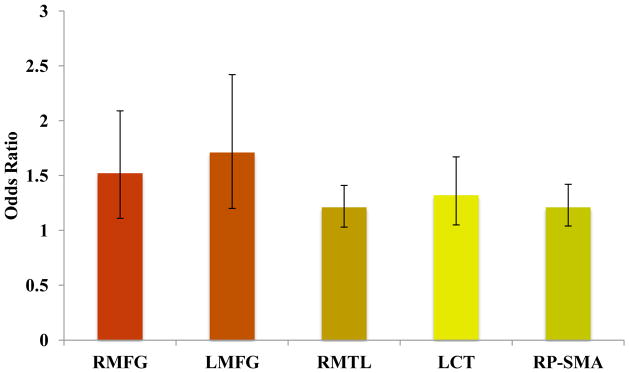
Odds ratios for functional clusters that significantly differ between B+ and B− youth as predictors of alcohol-induced blackouts. RMFG: right middle frontal gyrus; LMFG: left middle frontal gyrus; RMTL: right medial temporal lobe; LCT: left cerebellar tonsil; RP-SMA: right pre-supplementary motor area.
4. DISCUSSION
The present study examined whether future heavy-drinking adolescents who experience alcohol-induced blackouts show altered brain response during inhibitory processing. We hypothesized, based on previous findings (Norman et al., 2011; Schweinsburg et al., 2004; Wetherill et al., 2012), that adolescents who would go on to experience alcohol-induced blackouts would show less frontal and parietal activation during response inhibition prior to initiating substance use than youth who would remain substance-naïve and youth who transition into heavy drinking but would not experience alcohol-induced blackouts. Contrary to our hypothesis, adolescents who would later experience alcohol-induced blackouts showed greater brain response in frontal regions commonly associated with inhibitory processing (Buchsbaum et al., 2005; Garavan et al., 2006, Simmonds et al., 2008). Greater activation in the context of comparable behavioral performance is consistent with the notion of functional compensation (Rao et al., 2011; Suskauer et al., 2008; Tsapkini et al., 2011), which could explain these findings. As such, blackout+ youth may need to recruit more brain areas or increase use of specific regions associated with inhibitory processing to respond and inhibit successfully.
Differential activation was prominent in bilateral frontal brain regions, right parietal, right temporal, and left cerebellar brain regions. Further, greater activation in the right and left middle frontal brain regions in future heavy-drinking youth predicted almost a 2-fold chance of blackouts in the subsequent 5 years, after controlling for follow-up externalizing behaviors and lifetime alcohol consumption. Thus, prior to initiating substance use, blackout+ youth showed atypical neural activity in brain regions associated with inhibitory processing, decision-making, and substance use risk (Adleman et al., 2002; Lundqvist, 2010; Squeglia et al., 2009; Tapert et al., 2004). In fact, our follow-up measures show that blackout+ adolescents had increased externalizing behaviors and greater lifetime alcohol consumption, which supports this idea. Thus, these youth may be more vulnerable to maladaptive behaviors and subsequent consequences due to the fact that inhibition is more difficult to achieve due to underlying differences, which may be exacerbated by cognitive loads, such as alcohol.
Furthermore, our finding that greater baseline activation during inhibitory processing predicting follow-up alcohol-induced memory impairment raises the question of whether this activation pattern is a marker for heavy drinking, or if this pattern is marker of a vulnerable memory system. Given that our blackout groups show similar drinking patterns at follow-up, these findings suggest the latter. Specifically, atypical frontoparietal activation during inhibitory processing may indicate a vulnerability to alcohol’s effects on encoding or inhibiting distractors (a common source of encoding failure), and as such, memory difficulties and impairments may become evident after alcohol consumption or additional cognitive demands.
To our knowledge, this is the first fMRI investigation of response inhibition among youth who experience alcohol-induced blackouts prior to substance use initiation, and as such, there is no extant literature to compare our findings. Our findings are, however, consistent with studies examining inhibitory processing in youth with attention-deficit hyperactivity disorder (ADHD; Durston et al., 2003) where youth with ADHD show greater dorsolateral prefrontal and posterior parietal BOLD response during response inhibition compared to their healthy counterparts. As such, the frontoparietal abnormalities observed in the current study may reflect deficits in attention and vigilance. Although the current sample is comprised of youth who do not meet criteria for ADHD, blackout+ youth did show greater, yet within the clinically normal range, externalizing problems at follow-up, which has been linked to other behavioral problems, including substance use (Norman et al., 2011).
The current findings should be considered in light of possible limitations. Although groups were matched on several baseline and follow-up demographic and substance use variables, blackout+ youth had greater externalizing behaviors and lifetime alcohol consumption at follow-up. While differential activation patterns remained after statistically controlling for these factors, such differences may contribute to the current findings. Thus, the BOLD differences observed may be indicative of overall behavioral problems, not solely alcohol-induced blackouts. Continued longitudinal analyses with larger sample sizes and follow-up imaging data will help clarify BOLD response differences during inhibitory processing among substance-naïve youth and whether these differences are associated with subsequent behavioral risks, including alcohol-related problems.
In summary, the current data shows that atypical brain response during inhibitory processing may be a neural risk factor for the occurrence of alcohol-induced blackouts. These findings indicate that some individuals appear to have inherent vulnerabilities to inhibitory processing difficulties that likely contribute alcohol-induced memory impairments. Together, these findings contribute to the growing literature identifying neural risk factors for alcohol use and alcohol-related problems and provide information on potential targets for intervention programs.
Acknowledgments
Role of funding source
This research was supported by National Institute of Alcohol Abuse and Alcoholism grant R01 AA13419 to Susan Tapert and from grant T32 AA013525 to Edward Riley. The NIAAA had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
The authors thank Dr. Sandra Brown, Dr. M.J. Meloy, Dr. Carmen Pulido, Dr. Omar Mahmood, Sonja Eberson, Veronique Boucquey, and Alejandra Infante for assistance with subject recruitment and data management, and the participating families.
Footnotes
Contributors
Authors Wetherill and Tapert designed the study and wrote the protocol. Authors Wetherill, Castro, and Squeglia contributed to the acquisition and analyses of data. Author Wetherill managed the literature search and drafted the manuscript with assistance from authors Castro, Squeglia, and Tapert. All authors contributed to and have approved the final manuscript.
Conflict of interest
All authors declare they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms and Profiles. University of Vermont, Research Center for Children, Youth, and Families; Burlington, VT: 2001. [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA Adult Forms and Profiles. University of Vermont, Research Center for Children, Youth, and Families; Burlington, VT: 2003. [Google Scholar]
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, Reiss AL. A developmental fMRI study of the Stroop color-word task. Neuroimage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 1994. (DSM-IV) [Google Scholar]
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magn Reson Med. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF. Longitudinal characterization of white matter maturation during adolescence. Brain Res. 2011;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogg T, Fukunaga R, Finn PR, Brown JW. Cognitive control links alcohol use, trait disinhibition, and reduced cognitive capacity: evidence for medial prefrontal cortex dysregulation during reward-seeking behavior. Drug Alcohol Depend. 2011;122:112–118. doi: 10.1016/j.drugalcdep.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum Brain Mapp. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan D, Cisin I, Crossley H. American Drinking Practices. Rutgers Center of Alcohol Studies; New Brunswick, NJ: 1969. [Google Scholar]
- Conway ARA, Engle RW. Working memory and retrieval: a resource-dependent inhibition model. J Exp Psychol Gen. 1994;123:354–373. doi: 10.1037//0096-3445.123.4.354. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn Reson Med. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang Y, Ulug AM, Casey BJ. Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry. 2003;53:871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- Garavan H, Hester R, Murphy K, Fassbender C, Kelly C. Individual differences in the functional neuroanatomy of inhibitory control. Brain Res. 2006;1105:130–142. doi: 10.1016/j.brainres.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Goodwin DW. Alcohol amnesia. Addiction. 1995;90:315–317. doi: 10.1111/j.1360-0443.1995.tb03779.x. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal-detection Theory and Psychophysics. Wiley; New York: 1966. [Google Scholar]
- Hartzler B, Fromme K. Fragmentary blackouts: their etiology and effect on alcohol expectancies. Alcohol Clin Exp Res. 2003;27:628–637. doi: 10.1097/01.ALC.0000062743.37558.C8. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Two-factor Index of Social Position. Yale University; New Haven, CT: 1965. [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annu Rev Clin Psychol. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. National Institute on Drug Abuse; Bethesda, MD: 2011. Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings, 2010. [Google Scholar]
- Kirisci L, Mezzich AC, Reynolds M, Tarter RE, Aytaclar S. Prospective study of the association between neurobehavior disinhibition and peer environment on illegal drug use in boys and girls. Am J Drug Alcohol Abuse. 2009;35:145–150. doi: 10.1080/00952990902825405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist T. Imaging cognitive deficits in drug abuse. Curr Top Behav Neurosci. 2010;3:247–275. doi: 10.1007/7854_2009_26. [DOI] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend. 2011;119:216–223. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry PJ, Argo TR, Barnett MJ, Liesveld JL, Liskow B, Hernan JM, Trnka MG, Brabson MA. The association of alcohol-induced blackouts and grayouts to blood alcohol concentrations. J Forensic Sci. 2006;51:896–899. doi: 10.1111/j.1556-4029.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pulido C, Anderson KG, Armstead AG, Brown SA, Tapert SF. Family history of alcohol-use disorders and spatial working memory: effects on adolescent alcohol expectancies. J Stud Alcohol Drugs. 2009;70:87–91. doi: 10.15288/jsad.2009.70.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao LL, Zhou Y, Xu L, Liang ZY, Jiang T, Li S. Are risky choices actually guided by a compensatory process? New insights from FMRI. PloS One. 2011;6:e14756. doi: 10.1371/journal.pone.0014756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Rose ME, Grant JE. Alcohol-induced blackout. Phenomenology, biological basis, and gender differences. J Addict Med. 2010;4:61–73. doi: 10.1097/ADM.0b013e3181e1299d. [DOI] [PubMed] [Google Scholar]
- Ryback RS. Alcohol amnesia: observations in seven drinking inpatient alcoholics. Q J Stud Alcohol. 1970;31:616–632. [PubMed] [Google Scholar]
- Sartor CE, Lynskey MT, Bucholz KK, Madden PA, Martin NG, Heath AC. Timing of first alcohol use and alcohol dependence: evidence of common genetic influences. Addiction. 2009;104:1512–1518. doi: 10.1111/j.1360-0443.2009.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, Lynskey MT, Heath AC, Jacob T, True W. The role of childhood risk factors in initiation of alcohol use and progression to alcohol dependence. Addiction. 2007;102:216–225. doi: 10.1111/j.1360-0443.2006.01661.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Tipp JE. The Self-Rating of the Effects of alcohol (SRE) form as a retrospective measure of the risk for alcoholism. Addiction. 1997;92:979–988. [PubMed] [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, Brown SA, Tapert SF. An FMRI study of response inhibition in youths with a family history of alcoholism. Ann N Y Acad Sci. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrtating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadoni AD, Norman AL, Schweinsburg AD, Tapert SF. Effects of family history of alcohol use disorders on spatial working memory BOLD response in adolescents. Alcohol Clin Exp Res. 2008;32:1135–1145. doi: 10.1111/j.1530-0277.2008.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Tapert SF. The influence of substance use on adolescent brain development. Clin EEG Neurosci. 2009;40:31–38. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Schweinsburg AD, Pulido C, Tapert SF. Adolescent binge drinking linked to abnormal spatial working memory brain activation: differential gender effects. Alcohol Clin Exp Res. 2011;35:1831–1841. doi: 10.1111/j.1530-0277.2011.01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Sorg SF, Schweinsburg AD, Wetherill RR, Pulido C, Tapert SF. Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology. 2012;220:529–539. doi: 10.1007/s00213-011-2500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol Addict Behav. 2009;23:715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suskauer SJ, Simmonds DJ, Caffo BS, Denckla MB, Pekar JJ, Mostofsky SH. fMRI of intrasubject variability in ADHD: anomalous premotor activity with prefrontal compensation. J Am Acad Child Adolesc Psychiatry. 2008;47:1141–1150. doi: 10.1097/CHI.0b013e3181825b1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. An Approach to Cerebral Imaging. Thieme Medical Publishers; New York: 1988. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System. [Google Scholar]
- Tapert SF, Pulido C, Paulus MP, Schuckit MA, Burke C. Level of response to alcohol and brain response during visual working memory. J Stud Alcohol. 2004;65:692–700. doi: 10.15288/jsa.2004.65.692. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology. 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Habeych M, Reynolds M, Vanyukov M. Neurobehavior disinhibition in childhood predisposes boys to substance use disorder by young adulthood: direct and mediated etiologic pathways. Drug Alcohol Depend. 2004;73:121–132. doi: 10.1016/j.drugalcdep.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Tsapkini K, Vindiola M, Rapp B. Patterns of brain reorganization subsequent to left fusiform damage: fMRI evidence from visual processing of words and pseudowords, faces and objects. NeuroImage. 2011;55:1357–1372. doi: 10.1016/j.neuroimage.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth N, Engle RW. The nature of individual differences in working memory capacity: Active maintenance in primary memory and controlled search from secondary memory. Psychol Rev. 2007;114:104–132. doi: 10.1037/0033-295X.114.1.104. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Wetherill RR, Bava S, Thompson WK, Boucquey V, Pulido C, Yang TT, Tapert SF. Frontoparietal connectivity in substance-naive youth with and without a family history of alcoholism. Brain Res. 2012;1432:66–73. doi: 10.1016/j.brainres.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Fromme K. Acute alcohol effects on narrative recall and contextual memory: an examination of fragmentary blackouts. Addict Behav. 2011;36:886–889. doi: 10.1016/j.addbeh.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Schnyer DM, Fromme K. Acute alcohol effects on contextual memory on BOLD response: differences based on fragmentary blackout history. Alcohol Clin Exp Res. 2012;36:607–17. doi: 10.1111/j.1530-0277.2011.01702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Jamieson-Drake DW, Swartzwelder HS. Prevalence and correlates of alcohol-induced blackouts among college students: results of an e-mail survey. J Am Coll Health. 2002;51:117–119. 122–131. doi: 10.1080/07448480209596339. [DOI] [PubMed] [Google Scholar]
- White AM, Signer ML, Kraus CL, Swartzwelder HS. Experiential aspects of alcohol-induced blackouts among college students. Am J Drug Alcohol Abuse. 2004;30:205–224. doi: 10.1081/ada-120029874. [DOI] [PubMed] [Google Scholar]
- Wilkinson G. Jastak Associates; Wilmington, DE: 1993. The Wide Range Achievement Test-3 Administration Manual. [Google Scholar]
- York JL, Welte J, Hirsch J, Hoffman JH, Barnes G. Association of age at first drink with current alcohol drinking variables in a national general population sample. Alcohol Clin Exp Res. 2004;28:1379–1387. doi: 10.1097/01.alc.0000139812.98173.a4. [DOI] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral disinhibition: liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. J Abnorm Psychol. 2009;118:117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RA, Ellis DA, Fitzgerald HE. Developmental evidence for at least two alcoholisms. I. Biopsychosocial variation among pathways into symptomatic difficulty. Ann N Y Acad Sci. 1994;708:134–146. doi: 10.1111/j.1749-6632.1994.tb24706.x. [DOI] [PubMed] [Google Scholar]