Abstract
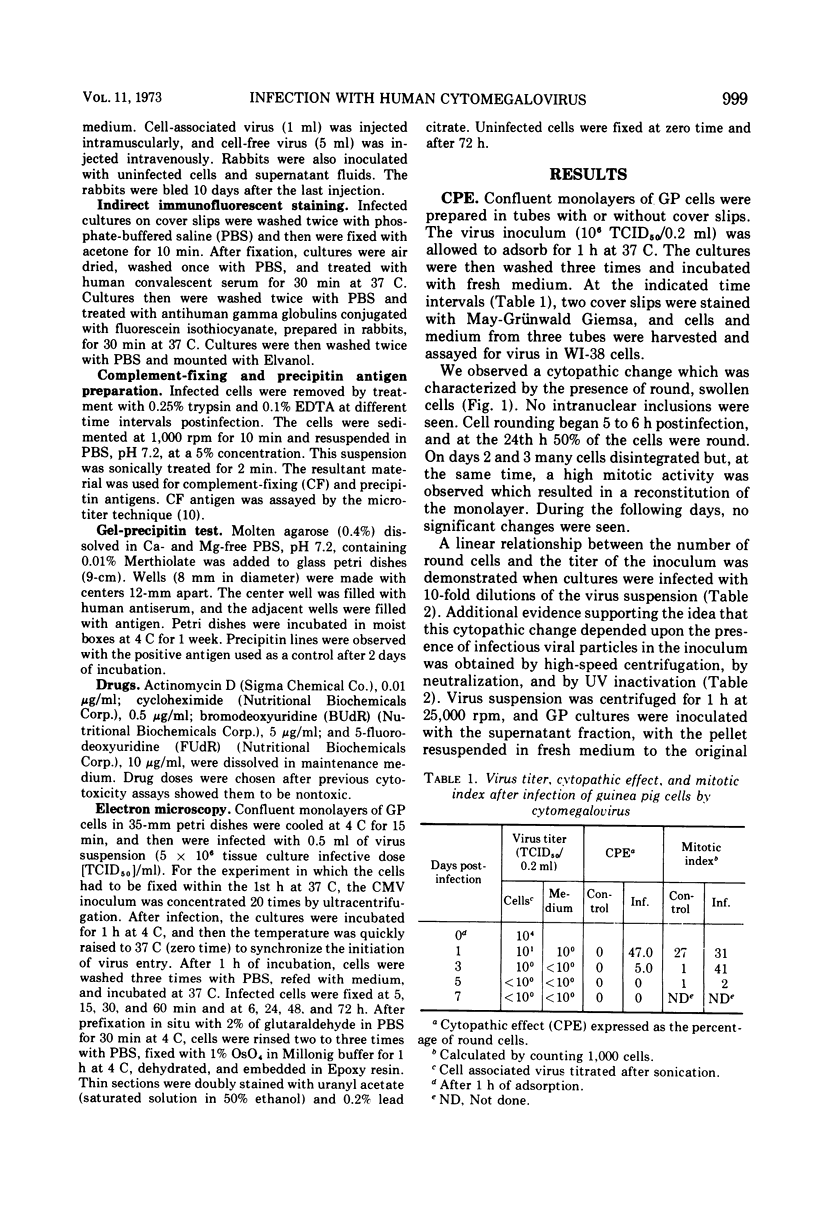
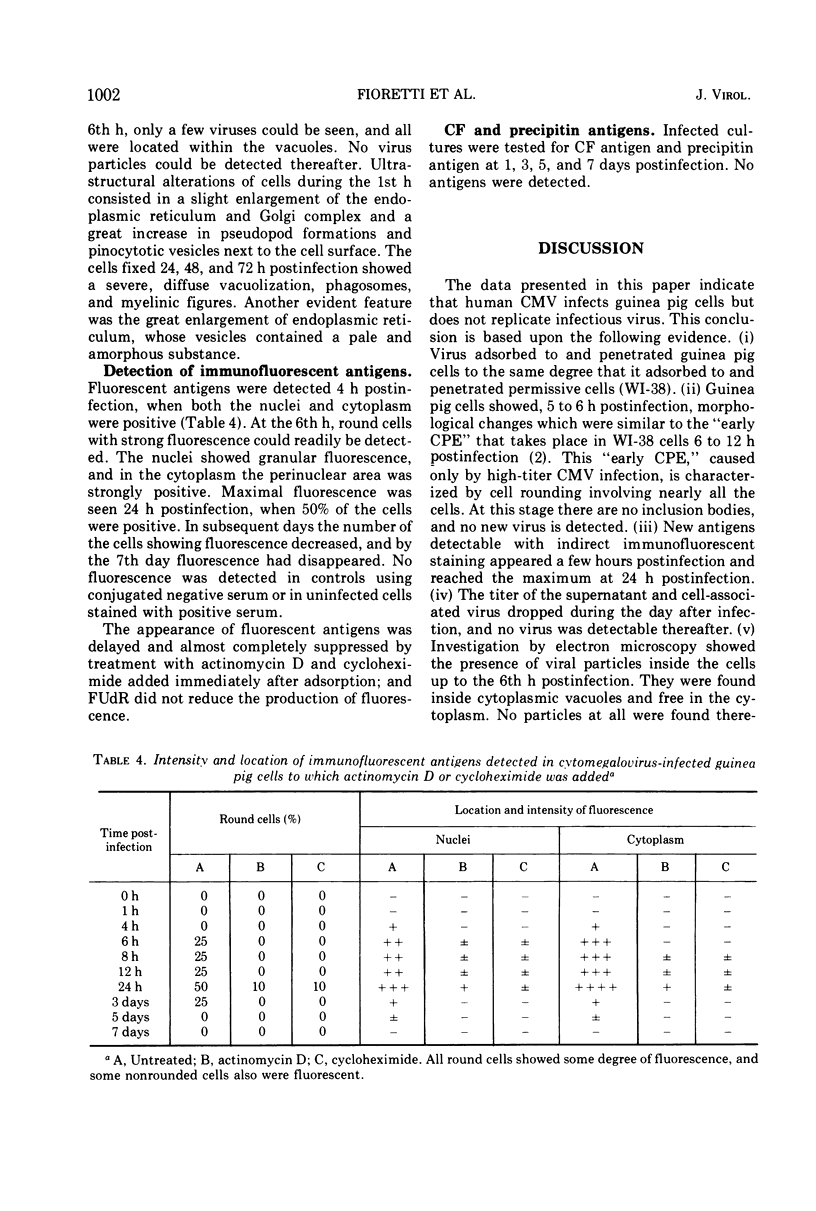
Human cytomegalovirus was capable of adsorbing to and penetrating guinea pig cells, but was unable to replicate new virus. Cultures infected with virus inoculum of high titer showed a cytopathic effect (CPE) characterized by cell rounding. This CPE depended upon the presence of infectious virus, and its extent was directly related to the multiplicity of infection. Staining by indirect immunofluorescence by using human convalescent sera was positive as early as 4 h postinfection. Maximal fluorescence was observed 24 h postinfection when 50% of the cells contained fluorescent antigens both in nuclei and cytoplasm. No evidence for viral replication was found, and no defective particles were detected by electron microscopy. Treatment with actinomycin D or with cycloheximide strongly inhibited both the fluorescent antigens and the CPE, whereas 5-fluorodeoxyuridine and bromodeoxyuridine were ineffective.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AURELIAN L., ROIZMAN B. ABORTIVE INFECTION OF CANINE CELLS BY HERPES SIMPLEX VIRUS. II. ALTERNATIVE SUPPRESSION OF SYNTHESIS OF INTERFERON AND VIRAL CONSTITUENTS. J Mol Biol. 1965 Mar;11:539–548. doi: 10.1016/s0022-2836(65)80009-2. [DOI] [PubMed] [Google Scholar]
- Furukawa T., Fioretti A., Plotkin S. Growth characteristics of cytomegalovirus in human fibroblasts with demonstration of protein synthesis early in viral replication. J Virol. 1973 Jun;11(6):991–997. doi: 10.1128/jvi.11.6.991-997.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Carp R. I. Abortive infection of human diploid cells by murine cytomegalovirus. Infect Immun. 1972 Nov;6(5):793–797. doi: 10.1128/iai.6.5.793-797.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Carp R. I. Growth of murine cytomegalovirus in various cell lines. J Virol. 1971 Jun;7(6):720–725. doi: 10.1128/jvi.7.6.720-725.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane D. E., Sommerville R. G. VERO cells (Cercopithecus aethiops kidney)--growth characteristics and viral susceptibility for use in diagnostic virology. (Brief report). Arch Gesamte Virusforsch. 1969;27(2):379–385. doi: 10.1007/BF01249659. [DOI] [PubMed] [Google Scholar]
- Nii S. Abortive infection of LLC-MK2 cells by Herpes simplex virus. Biken J. 1972 Mar;15(1):43–47. [PubMed] [Google Scholar]
- Plummer G., Goodheart C. R., Henson D., Bowling C. P. A comparative study of the DNA density and behavior in tissue cultures of fourteen different herpesviruses. Virology. 1969 Sep;39(1):134–137. doi: 10.1016/0042-6822(69)90355-9. [DOI] [PubMed] [Google Scholar]
- Raynaud J., Atanasiu P., Virat J. Nouvelles observations sur l'adaptation aux cellules humaines, d'une souche de virus cytomégalique provenant du mulot (Apodemussylvaticus. C R Acad Sci Hebd Seances Acad Sci D. 1972 May 24;274(21):2920–2923. [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]





