Abstract
Recent behavioral studies suggest that non-selective agonists of cannabinoid receptors may regulate serotonin 2A (5-HT2A) receptor neurotransmission. Two cannabinoids receptors are found in brain, CB1 and CB2 receptors, but the molecular mechanism by which cannabinoid receptors would regulate 5-HT2A receptor neurotransmission remains unknown. Interestingly, we have recently found that certain cannabinoid receptor agonists can specifically upregulate 5-HT2A receptors. Here, we present experimental evidence that rats treated with a non-selective cannabinoid receptor agonist (CP 55,940, 50μg/kg, 7 days) showed increases in 5-HT2A receptor protein levels, 5-HT2A receptor mRNA levels, and 5-HT2A receptor-mediated phospholipase C Beta (PLCβ) activity in prefrontal cortex (PFCx). Similar effects were found in neuronal cultured cells treated with CP 55,940 but these effects were prevented by selective CB2, but not selective CB1, receptor antagonists. CB2 receptors couple to the extracellular kinase (ERK) signaling pathway by Gαi/o class of G-proteins. Noteworthy, GP 1a (selective CB2 receptor agonist) produced a strong upregulation of 5-HT2A receptor mRNA and protein, an effect that was prevented by selective CB2 receptor antagonists and by an ERK1/2 inhibitor, PD 198306. In summary, our results identified a strong cannabinoid-induced upregulation of 5-HT2A receptor signaling in rat PFCx. Our cultured cell studies suggest that selective CB2 receptor agonists upregulate 5-HT2A receptor signaling by activation of the ERK1/2 signaling pathway. Activity of cortical 5-HT2A receptors has been associated with several physiological functions and neuropsychiatric disorders such as stress response, anxiety & depression and schizophrenia. Therefore, these results might provide a molecular mechanism by which activation of cannabinoid receptors might be relevant to the pathophysiology of some cognitive and mood disorders in humans.
Keywords: prefrontal cortex, 5-HT receptor, G protein coupled receptor, ERK MAPK, cannabinoid receptors
Introduction
5-HT2A receptors play an important role in the regulation of stress, mood and impulse control (Kroeze et al., 2002; Magalhaes et al., 2010) and the behavioral effects of several drugs of abuse (Bubar and Cunningham, 2006; Filip et al., 2006; Ross et al., 2006). 5-HT2A receptors are the most abundant serotonin receptor in prefrontal cortex (PFCx) and are predominantly expressed in pyramidal neurons (Jones et al., 2009). Impaired function of cortical 5-HT2A receptors has been identified in several neurological and psychiatric disorders such as schizophrenia, Alzheimer’s disease, depression, and anxiety (Magalhaes et al., 2010; Meltzer et al., 1999; Nacmias et al., 2001).
A recent behavioral report has suggested that repeat exposure to cannabinoid agonists is associated with enhanced activity of 5-HT2A receptors in adult rats (Hill et al., 2006). Specifically, Hill et al. (2006) reported that chronic treatment with HU-210, a CB1/CB2 receptor agonist, led to a significant enhancement of 5-HT2A receptor mediated head-shake responses (Hill et al., 2006). This behavioral test has been widely used as a marker of 5-HT2A receptor function in vivo as this behavior is prevented by pretreatment with selective 5-HT2A receptor antagonists and is absent in 5-HT2A receptor knock out animals (Cheer et al., 1999; Darmani, 2001; Gorzalka et al., 2005). The detailed molecular mechanism by which cannabinoid receptor agonists regulate 5-HT2A receptor signaling in brain remains unknown; however, we have recently reported that selective cannabinoid agonists can upregulate 5-HT2A receptors (Franklin et al., 2012; Franklin and Carrasco, 2012). Nevertheless in those manuscripts we did not assess the effect of cannabinoid agonists on the activity of 5-HT2A receptors in vivo or in vitro.
Two cannabinoid receptors have been identified in the brain, CB1 and CB2 receptors (Brusco et al., 2008; den Boon et al., 2012; Garcia-Gutierrez et al., 2010; Gong et al., 2006; Xi et al., 2011). Endogenous cannabinoids (endocannabinoids), synthetic cannabinoids, and cannabinoids found in nature (such as Δ9-THC) bind to these receptors with high affinity (Childers, 2006; Felder et al., 2006). CB1 and CB2 receptors couple to Gαi/o class of G-proteins and to the extracellular kinase (ERK) signaling (Childers, 2006; Felder et al., 2006). These CB2 receptors have been identified at postsynaptic terminals while CB1 receptors are located at presynaptic terminals (Brusco et al., 2008; Kawamura et al., 2006; Onaivi et al., 2008). While the activation of presynaptic cannabinoid receptors inhibits the release of several neurotransmitters such as serotonin (5-HT) (Nakazi et al., 2000), activation of postsynaptic cannabinoid receptors might modulate the activity of several postsynaptic receptors, including serotonin and dopamine receptors (Demuth and Molleman, 2006; Hermann et al., 2002).
The objectives of the present study were to identify whether repeat exposure to a non-selective cannabinoid agonist can modify the activity of 5-HT2A receptors in rat PFCx. Also two neuronal cell lines were used to better study the mechanisms of cannabinoid-induced upregulation of 5-HT2A receptors after a single and repeated exposure to cannabinoid agonists. These two independent cell lines were utilized to address whether the cannabinoid-induced regulation of serotonin receptors is a generalized phenomenon and not exclusive to a single cell line. Additionally, we investigated the effect of single or repeated exposure to cannabinoids on the ERK1/2 signaling pathway and the role of this signaling protein in the cannabinoid-mediated increases in 5-HT2A receptor protein levels. As 5-HT2A receptors in PFCx have been associated with several physiological functions and neuropsychiatric disorders such as stress response, anxiety & depression and schizophrenia (Carrasco and Van de Kar, 2003; de et al., 2008; Egerton et al., 2006; Swaab et al., 2000), increases in 5-HT2A receptor function in this limbic region may be clinically relevant to the pathophysiology of mood and cognitive disorders.
Materials and Methods
Drugs
(−)-cis-3-[2-Hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclo-hexanol (CP 55,940), a CB1 and CB2 agonist; N-(Piperidin-1-yl)-1-(2,4-dichlorophenyl)-1,4-dihydro-6-methylindeno[1,2-c]pyrazole-3-carboxamide (GP 1a) a highly selective CB2 receptor agonist; N-(2-Chloroethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide (ACEA) a highly selective CB1 receptor agonist; 2-(2-Chlorophenyl)-3-(4-chlorophenyl)-7-(2,2-difluoropropyl)-6,7-dihydro-2H-pyrazolo[3,4-f][1,4]oxazepin-8(5H)-one (PF-514273) a selective and potent CB1 receptor antagonist; N-(1,3-Benzodioxol-5-ylmethyl)-1,2-dihydro-7-methoxy-2-oxo-8-(pentyloxy)-3-quinolinecarboxamide (JTE 907) a selective CB2 receptor antagonist; [6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl](4-methoxyphenyl)-methanone (AM 630) a selective CB2 receptor antagonist; 6-Chloro-2,3-dihydro-5-methyl-N-[6-[(2-methyl-3-pyridinyl)oxy]-3-pyridinyl]-1H-indole-1-carboxyamide dihydrochloride (SB 242084) a selective 5-HT2C receptor antagonist; and N-(Cyclopropylmethoxy)-3,4,5-trifluoro-2-[(4-iodo-2-methylphenyl)amino]-benzamide (PD 198306) a potent and selective ERK1/2 inhibitor were purchased from Tocris (Ellisville, MO, USA). (−) DOI [(−)-1-(2, 5-dimethoxy-4-iodophenyl)-2-aminopropane HCl] was purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA).
Animal Experimental Protocols
Male Sprague-Dawley rats (225–275 g) were purchased from Harlan (Indianapolis, IN, USA). The rats were housed two per cage in a temperature-, humidity-, and light-controlled room (12 hr light/dark cycle, lights on 7:00 AM-19:00 PM). Food and water were available ad libitum. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals as approved by the University of Kansas Institutional Animal Care and Use Committee (IACUC).
After arrival, the rats were allowed to acclimate to their environment for at least 4 days prior to the start of the treatment period. Eight rats were randomly assigned to each group. Cage-mates were assigned to the same treatment group. Rats were injected with either vehicle (Tween-80/ethanol/saline (1:1:18); 1ml/kg, i.p.) or CP 55,940 (0.05 mg/kg, i.p.) once a day for 7 days. Rats were sacrificed by decapitation 48 h after the last CP 55,940 injection. The brains were immediately removed and the PFCx was dissected and frozen in dry ice.
Phospholipase C (PLCβ) Activity assay in rat PFCx
PFCx tissue from treatment groups that received the saline challenge were utilized for measurement of PLCβ activity. PLCβ activity was measured by the amount of inositol 1,4,5 trisphosphate produced by PLCβ in the membrane fraction of the isolated tissue as previously described (Carrasco and Battaglia, 2007; Wolf and Schutz, 1997). 5-HT-stimulated PLCβ activity in PFCx is a selective measure of 5-HT2A receptor function as previously demonstrated using selective antagonists (Wolf and Schutz, 1997). Briefly, membrane protein from PFCx (30 μg) was diluted in 100 μl of buffer (25 mN Hepes-Tris pH 7.4, 3 mM EGTA, 10 mM LiCl, 12 mM MgCl2, 1.44 mM sodium deoxycholate) with 1 μM GTPγS, 300 nM free Ca2+, 0.3 μM 5-HT, and 1 mM unlabeled phosphatidyl inositol. We used 0.3 μM 5-HT to stimulate PLCβ activity because this is the EC50 previously described in the literature (Carrasco and Battaglia, 2007; Wolf and Schutz, 1997). Samples were kept on ice until the reaction was started with 100 μM [3H] phophatidyl inositol at 37°C for 20 minutes. This reaction was then stopped through addition of 0.9 ml CHCL2/ MeOH (1:2) and 0.3 ml of chloroform. Samples were shaken for 90 s and centrifuged at 21,000 g for 90 s at room temperature. Finally, 0.3 ml of the upper aqueous phase was mixed with 6 ml of scintillation cocktail and counted by a scintillation counter for 5 minutes.
Western blots
Proteins isolated from cultured cells and PFCx were used in these experiments. Membrane-associated proteins were isolated using the ProteoExtract™ Native Membrane Protein Extraction kit (Calbiochem, La Jolla, CA, USA). Expression of membrane-associated 5-HT2A receptors were determined by Western blot as previously described (Carrasco et al., 2004). The anti-5-HT2A antibody was a generous gift from Dr. Nancy A. Muma and has been previously validated in the literature (Singh et al., 2007). ERK1/2 and pERK antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Negative controls included either omission of primary antibody or addition of pre-immune rabbit immunoglobulins. β-actin was used as a control for protein loading (approx. 46 kDa molecular weight).
Film analysis
Films were analyzed densitometrically using Scion Image software (Scion Corporation, Frederick, MD, USA), as previously described (Carrasco et al., 2006). Each sample was measured on three independent gels. All samples were standardized to controls and normalized to their respective actin levels.
qRT-PCR Experiments
Total RNA was isolated from cultured cells and PFCx tissue by using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) protocol as described by the manufacturer. Total RNA was reverse transcribed to generate cDNA. Quantitative real time PCR reactions were prepared using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA), a 4% (v/v) concentration of cDNA product, and forward and reverse primers at a final concentration of 0.35 mM run as we previously described (Singh et al., 2009). All reactions were performed in triplicate using the ABI 7500 fast real time PCR system (Applied Biosystems, Foster City, CA, USA). A negative control lacking cDNA or any known DNA template was included for each primer pair. The primers used in this manuscript were: 5-HT2A (F:5′-AACGGTCCATCCACAGAG-3′ and R:5′-AACAGGAAGAACACGATGC-3′), CB1 (F:5′-CATCATCATCCACACGTCAGAAG-3′ and R:5′-ATCAACACCACCAGGATCAGAAC-3′), CB2 (F:5′-CCAACATGTAGCCAGCTTGACT-3′ and R:5′-TGCAGGAACCAGCATATGA-3′), Gαq (F:5′-AGTTCGAGTCCCCACCACAG-3′ and R : 5′-CCTCCTACATCGACCATTCTGAA-3′), 5-HT1A (F:5′-CCGCACGCTTCCGAATCC-3′ and R:5′-TGTCCGTTCAGGCTCTTCTTC-3′), and GAPDH ( F : 5′-TGGAGTCTACTGGCGTCTTCAC-3′ and R:5′-GGCATGGACTGTGGTCATGA-3′). These primers have been previously validated in the literature (Atkinson et al., 2006; Kindlundh-Hogberg et al., 2006; Mato et al., 2009; Singh et al., 2009).
In all real-time PCR experiments, measurements were made from the number of cycles required to reach the threshold fluorescence intensity [cycle threshold (Ct)]. Ct values for each reaction were subtracted from Ct values for GADPH and then subtracted from Ct values for vehicle-treated controls that served as a baseline, and the result was referred to as ΔΔCt. Fold changes in gene expression were calculated as 2-ΔΔCt to reflect the fact that, under optimal conditions, the amount of PCR product doubles with each amplification cycle.
Cell Culture Protocols
CLU213 and A1A1v cells, neuronal cell lines that endogenously, express CB1, CB2 and 5-HT2A receptors, were either purchased from Cedarlane Laboratories (Burlington, NC, USA) or kindly provided by Dr. William Clarke and Kelly Berg (University of Texas Health Science Center, San Antonio, TX, USA), respectively (Berg et al., 1994; Franklin et al., 2012; Franklin and Carrasco, 2012). We utilized two cells line in our work in order to examine whether our findings could be replicated in two independent neuronal cells lines. These two cell lines were grown on 100-mm2 plates treated with polystyrene (Corning Incorporated, Corning, NY, USA) and maintained in 5% CO2 at 37°C, in Dulbecco’s modified eagle medium (Mediatech Inc, Manassas, VA, USA) containing 10% fetal bovine serum (Thermo Scientific, Logan, UT, USA).
Determination of the effect of CP 55,940 on 5-HT2A receptor mRNA
CLU213 cells were incubated with either vehicle (ethanol 0.01% final concentration) or CP 55,940 (1nM) for 24 hours. Total RNA was isolated and qRT-PCR for 5-HT2A, CB1, and CB2 was performed as described above. Samples were run in triplicate.
Regulation of 5-HT2A receptor protein levels by non-selective cannabinoid receptor agonist
CLU213 cells were incubated with either vehicle (ethanol 0.01% final concentration) or CP 55,940 (1nM) for 72 hours. Cells were washed with PBS (pH 7.4) every 24 hours and fresh vehicle or CP 55,940 (1nM) were added. Expression of membrane-associated 5-HT2A receptors was determined by Western blot as described above.
Phosphoinositol hydrolysis in cultured cells
CLU213 cells were seeded at a density of 10,000 cells/well in 24-well plates in complete medium (day 1). On day 2, cells were placed in serum-free medium and incubated with CP 55,940 (1nM) for 3 days. Cells were washed with PBS every 24 hours and vehicle or CP 55,940 was added. On day 4, Myo-[3H]Inositol (Du Pont NEN, USA) was added to the incubation media (Shi et al., 2007). 5-HT2 receptor-mediated phosphoinositol (PI) hydrolysis assays were performed on day 5 as previously described using (−)DOI (10−6 M), a 5-HT2A/2C receptor agonist (Shi et al., 2007). In all these experiments, cells were pretreated with SB242084 10nM, a 5-HT2C receptor antagonist (Kennett et al., 1997), prior to (−)DOI treatment.
Effect of select cannabinoid receptor antagonists on the CP 55,940-induced upregulation of 5-HT2A receptors
CLU213 cells were pretreated with either vehicle (ethanol 0.01% final concentration), PF-514273 (20nM, CB1 antagonist) (Dow et al., 2009), or JTE 907 (10nM, a CB2 receptor antagonist) (Iwamura et al., 2001; Ueda et al., 2005). Twenty min later cells were incubated with either vehicle (ethanol 0.01% final concentration) or CP 55,940 (1nM) for 24 hours. Total RNA was isolated and qRT-PCR for 5-HT2A was performed as described above.
Regulation of 5-HT2A receptor mRNA transcription by specific CB1 and CB2 receptor agonists
Either CLU213 or A1A1v cells were incubated with either vehicle (ethanol 0.01% final concentration), CP 55,940 1nM (non-selective CB1/CB2 agonist Ki: 0.58nM and 0.68nM for CB1 and CB2 receptors, respectively) (Showalter et al., 1996), ACEA 15nM (selective CB1 agonist, Ki: 1.4nM and 3.1μM for CB1 and CB2 receptors, respectively)(Hillard et al., 1999; Rutkowska and Jachimczuk, 2004) or GP 1a 1nM (selective CB2 agonist, Ki: 0.037nM and 35nM for CB2 and CB1 receptors, respectively) (Gorantla et al., 2010) for 24 hours. Total RNA was isolated and qRT-PCR for 5-HT2A mRNA was performed as described above.
Effect of select cannabinoid receptor antagonists on the CB2 receptor agonist-induced upregulation of 5-HT2A receptors
In a different experiment, CLU213 cells were incubated with either vehicle (ethanol 0.01% final concentration) or one of the following CB2 receptor antagonists, JTE 907 (10nM) or AM 630 (1μM) (Iwamura et al., 2001; Onaivi et al., 2008; Ueda et al., 2005). Twenty minutes later cells were treated with either vehicle or 1nM GP 1a, selective CB2 agonist (Gorantla et al., 2010). Twenty four hours later total RNA was isolated and qRT-PCR for 5-HT2A mRNA was performed as previously described.
Regulation of 5-HT2A receptor protein levels by a CB2 receptor agonist
CLU213 cells were incubated with either vehicle (ethanol 0.01% final concentration) or GP 1a (1nM) for 72 hours. Cells were washed with PBS every 24 hours and fresh vehicle or GP 1a were added. Expression of membrane-associated 5-HT2A receptors was determined by Western blot as described above.
Effect of ERK1/2 inhibition on the GP 1a-induced upregulation of 5-HT2A receptor mRNA
CLU213 or A1A1v cells were incubated with either vehicle (ethanol 0.01% final concentration) or PD 198306 (200nM) for 20 min. PD 198306 is a potent inhibitor of ERK1/2 activation (Crane et al., 2007; Sullivan et al., 2005). Cells were then treated with either vehicle (ethanol 0.01% final concentration) or GP 1a (1nM) and incubated for 24 hours. Total RNA was isolated and 5-HT2A receptor mRNA levels were determined as described above.
Effect of ERK1/2 inhibition on the GP 1a-mediated nuclear-associated levels of pERK
CLU213 cells were incubated with either vehicle (ethanol 0.01% final concentration) or PD 198306 (200nM) for 20 min. Cells were then treated with either vehicle (ethanol 0.01% final concentration) or GP 1a (1nM) and incubated for 24 hours. Cells were then rinsed with PBS, collected and nuclear-associated proteins were isolated using NE-PER R Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific, IL, USA). Expression of nuclear-associated pERK levels were determined by Western blot as previously described.
Effect of ERK1/2 inhibition on the GP 1a-induced increase in 5-HT2A Receptor Protein Levels
CLU213 cells were rinsed with PBS (3x) and incubated with either vehicle (ethanol 0.01% final concentration) or PD 198306 (200nM) for 20 min. Cells were then treated with either vehicle (ethanol 0.01% final concentration) or GP 1a (1nM) and incubated for 24 hours. This procedure was repeated over three days. Cells were collected 72 h after the initial treatment. 5-HT2A receptor protein levels were measured in whole cell lysate by Western blot as described above.
Effect of a single or repeated GP 1a exposure on ERK1/2 or pERK protein levels in CLU213 cells
Cells were treated with either vehicle (ethanol 0.01% final concentration) or GP 1a (1 nM) for 24 hours. The next day, cells were rinsed with PBS (3x) and incubated with either vehicle (ethanol 0.01% final concentration) or GP 1a (1nM) for 15 minutes. Cells were rinsed with PBS, collected and cytosolic and nuclear-associated proteins were isolated using NE-PERR Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific, IL, USA). Expression of either cytosolic ERK1/2 protein or nuclear-associated pERK protein levels were determined by Western blot as previously described.
In a different experiment, CLU213 cells were treated with either vehicle (ethanol 0.01% final concentration) or GP 1a (1nM) for 24 hours. The next day, cells were rinsed with PBS (3x) and incubated with either vehicle (ethanol 0.01% final concentration) or GP 1a (1nM) for 15 minutes. Total RNA was isolated and 5-HT2A receptor mRNA levels were determined as described above.
Statistics
All data are expressed as the mean ± S.E.M., where n indicates the number of rats or cell culture plates per group. Data was analyzed by an unpaired Student’s t-test or ANOVA (Newman-Keuls post-hoc test). GB-STAT software (Dynamic Microsystems, Inc., Silver Spring, MD, USA) was used for all statistical analyses.
RESULTS
CP 55,940 exposure enhances 5-HT2A receptor signaling and expression in rat PFCx
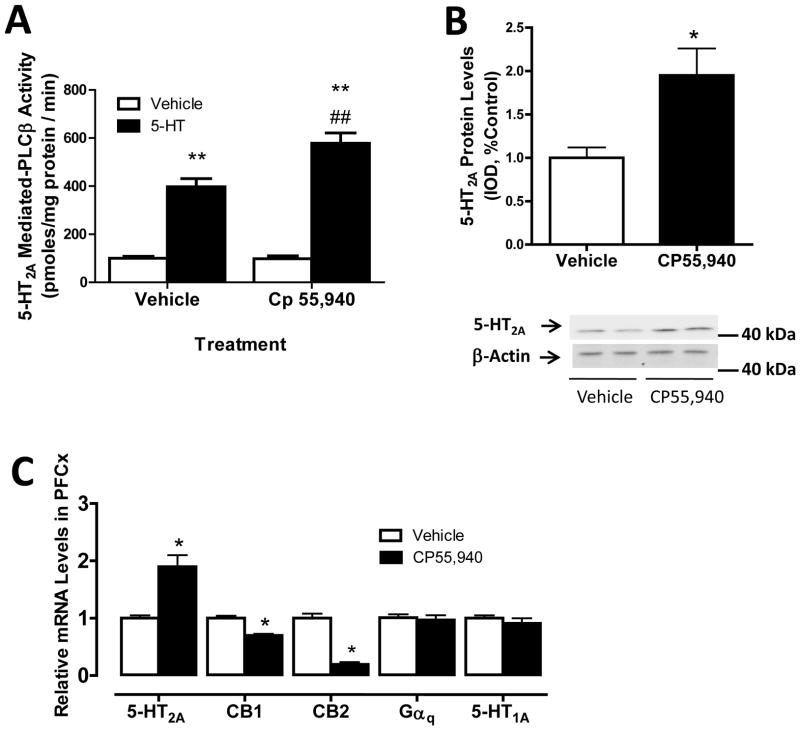
We first examined the effect of chronic administration of CP 55,940 (50μg/kg for 7 days), a CB1/CB2 receptor agonist (Bouaboula et al., 1996), on the activity and expression of 5-HT2A receptors in rat PFCx. Previously we have found that chronic CP 55,940 treatment increases 5-HT2A receptor expression but the effect on 5-HT2A receptor activity is unknown (Franklin and Carrasco, 2012). Initially, we measured activity of 5-HT2A receptors in PFCx because 5-HT-stimulated phosphoinositol hydrolysis in this brain area has been reported to be mediated primarily by activation of 5-HT2A receptors (Carrasco and Battaglia, 2007; Wolf and Schutz, 1997). 5-HT2A receptor stimulated PLCβ activity was significantly (p<0.01) greater in CP 55,940-treated rats compared with controls (578 ± 44 and 397 ± 34 pmoles/mg protein/min for CP 55,940 and vehicle-treated rats, respectively) (Fig. 1A). The two-way ANOVA detected a main effect of treatment with CP 55,940 (F1,58.18, p<0.0001) and 5-HT (F1,1000.48, p<0.0001) on the PLCβ activity and a main interaction between these two factors (F1,58,45, p<0.0001). Noteworthy, this CP 55,940-induced enhanced PLCβ activity was associated with a significant (p<0.05) two-fold increase in the membrane-associated levels of 5-HT2A receptors in PFCx compared to controls (Fig. 1B). Here, 5-HT2A receptors were identified as a single and prominent band with a molecular mass of approximately 42–43 kDa as previously described (Singh et al., 2007).
Figure 1.
CP 55,940-induced enhanced activity and upregulation of 5-HT2A receptors in PFCx. (A) 5-HT2A receptor stimulated PLCβ activity in PFCx of rats treated with either vehicle or CP 55,940 (50μg/kg, i.p.) for 7 days and withdrawn for 48 hours. We detected an increased 5-HT-stimulated PLCβ activity in rats treated with CP 55,940 compared to control rats (**p <0.01, significant effect compared to vehicle-treated rats; ## p <0.01, significant effect of 5-HT-stimulated PLCβ activity compared to vehicle-treated rats). (B) Increased membrane-associated 5-HT2A receptor protein levels in PFCx of CP 55,940-treated rats. β-actin was used as a loading control. Representative Western blots are shown in this figure and IOD was calculated as described in Experimental Procedures (*p<0.05, significant effect of CP 55,940 treatment compared to vehicle-treated animals). (C) Increased 5-HT2A receptor mRNA levels and reduced CB1 and CB2 mRNA levels in PFCx of CP 55,940 treated rats compared to controls (*p <0.05, significant effect compared to vehicle-treated rats). The data represent mean ± SEM (n=4–6).
5-HT2A receptor mRNA was significantly (p<0.05) increased in PFCx of CP 55,940-treated rats compared to vehicle-treated controls (approx. 90% increase, Fig. 1C). No significant changes in the levels of 5-HT1A receptor or 5-HT2A receptor coupled-Gαq G-protein mRNAs were detected in PFCx of CP 55,940-treated animals. This highlights the specificity of the effect of CP 55,940 on 5-HT2A receptor signaling. Also, we found a significant (p<0.05) downregulation of CB1 and CB2 receptor mRNA in PFCx of CP 55,940-treated rats compared to vehicle controls (Fig. 1C). CB1 and CB2 mRNA levels were reduced by 35% and 60% in PFCx of CP 55,940-treated animals, respectively.
CP 55,940 exposure enhances 5-HT2A receptor signaling and expression in a neuronal cell model, CLU213 cells
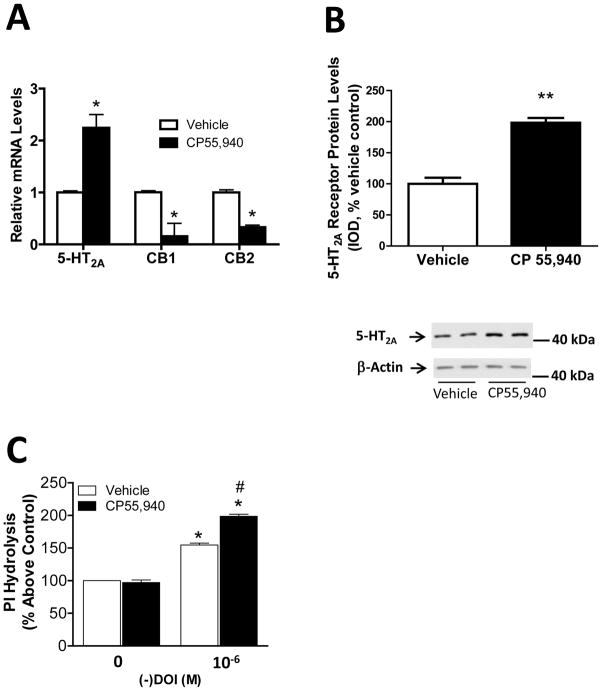
CLU213 cells were used to better study the mechanisms involved in the upregulation of 5-HT2A receptors. Initially, we examined the effect of chronic incubation with CP 55,940 on the mRNA levels of 5-HT2A and cannabinoid receptors. CP 55,940 produced a significant (p<0.05) upregulation of 5-HT2A mRNA levels in CLU213 cells (Fig. 2A). Cells treated with CP 55,940 showed an approximate two-fold increase in 5-HT2A receptor mRNA levels compared to controls. CB1 and CB2 mRNA levels were significantly (p<0.05) downregulated in CP 55,940-treated cells (Fig. 2A). CB1 and CB2 mRNA levels were 80% and 65% lower in CP 55,940-treated cells compared to vehicle-treated cells. CP 55,940-treated cells also showed a significant (p<0.01) increase in the membrane-associated levels of 5-HT2A receptors compared to vehicle treated cells (approx. 80% increase, Fig. 2B). Indeed, CLU213 cells exposed to CP 55,940 for 72 hours showed a two-fold increase in the membrane-associated levels of 5-HT2A receptors compared to controls (Fig. 2B). No significant effect on the protein levels of 5-HT2A receptors were detected after 24 hours of exposure to CP 55,940 (data not shown). We also studied the effect of CP 55,940 on the activity of 5-HT2 receptors in CLU213 cells by measuring the (−)DOI-induced phosphoinositol (PI) hydrolysis (Shi et al., 2007) (Fig. 2C). In this assay, we measured the (minus;)DOI-stimulation of PI hydrolysis in cells incubated with either vehicle or CP 55,940 (1nM) for 72 hours. We found that (−)DOI stimulated PI hydrolysis in vehicle- and CP 55,940-treated cells (Fig. 2C). Indeed, 10−6 M (−)DOI produced a 60% and 98% increase in PI hydrolysis compared to controls (vehicle- and CP 55,940-treated cells, respectively). Importantly, (−)DOI-induced PI hydrolysis was significantly (p<0.05) higher in CP 55,940-treated cells compared to vehicle-treated controls.
Figure 2.
CP 55,940-induced enhanced activity and upregulation of 5-HT2A receptors in CLU213 cells. (A) CP 55,940-induced increased 5HT2A receptor mRNA levels and reduced CB1 and CB2 receptor mRNA levels in CLU213 cells. *p<0.05 significant effect of CP 55,940 treatment compared to controls. (B) Increased membrane-associated 5-HT2A receptor protein levels in CP 55,940 treated cells. **p<0.01, significant effect of CP 55,940 compared to vehicle-treated controls (C) CP 55,940-induced increases in 5-HT2A receptor-mediated phosphoinositol (PI) hydrolysis in CLU213 cells. *p<0.05, significant effect of (−)DOI compared to vehicle-treated controls. #p<0.05, significant effect of (−)DOI on CP 55,940-treated cells compared with (−)DOI-treated cells. The data represent mean ± SEM (n=3).
Selective CB2 receptor agonists upregulate 5-HT2A receptor mRNA in two neuronal cell models, CLU213 and A1A1v cells
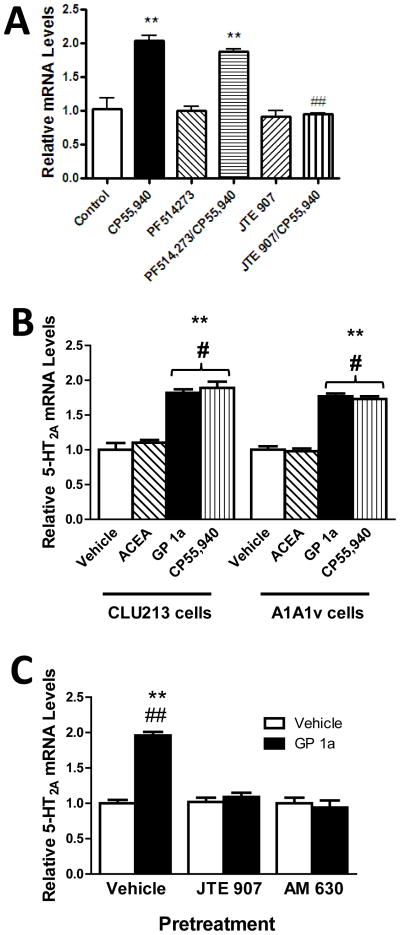
We then aimed to identify the effect of selective cannabinoid receptor antagonists, PF-514 (CB1 antagonist) and JTE907 (CB2 antagonist), on the CP 55,940-induced upregulation of 5-HT2A receptor signaling in CLU213 cells as described in methods (Fig. 3A). CP 55,940 produced an approximate two-fold increase in 5-HT2A mRNA levels (Fig. 3A). CP 55,940-induced 5-HT2A receptor upregulation was not significantly (p>0.05) modified in cells pretreated with PF-514273. Moreover, CP 55,940-induced a 93% increase in 5-HT2A mRNA compared to vehicle controls, suggesting that the CP 55,940-induced 5-HT2A receptor upregulation would be independent of CB1 receptors. Noteworthy, the CP 55,940-induced 5-HT2A receptor upregulation was prevented in CLU213 cells pretreated with JTE 907. Furthermore, we did not detect significant (p>0.05) differences in 5-HT2A mRNA levels between vehicle controls and cells pretreated with JTE 907 and treated with CP 55,940. This suggests that CP 55,940-induced upregulation of 5-HT2A receptors might be mediated by CB2 receptors in CLU213 cells (Fig. 3A). Neither JTE 907 nor PF-514273 modified 5-HT2A receptor mRNA basal levels (Fig. 3A). The two-way ANOVA did not show a significant main effect of CP 55,940 treatment (F1,2.36, p>0.15) but found a main effect of pretreatment with cannabinoid receptor antagonists (F2,20.65, p<0.0001). There was a significant interaction between pretreatment and treatment on 5-HT2A receptor mRNA levels (F2,63.25, p<0.0001)
Figure 3.
Selective CB2 receptor antagonists prevent selective cannabinoid agonist-induced upregulation of 5-HT2A receptors in neuronal cells (A) Effect of antagonists of CB1 and CB2 receptors on the CP 55,940-induced upregulation of 5-HT2A receptor mRNA. JTE 907 pretreatment, a CB2 receptor antagonist, prevented the 5-HT2A receptor upregulation in CP 55,940-treated cells. PF514273 pretreatment, a CB1 receptor antagonist, did not modify the effect of CP 55,940-treatment on 5-HT2A mRNA levels. **p<0.01, significant effect of CP 55,940 on 5-HT2A receptor mRNA levels compared with vehicle-treated cells. ##p<0.01, significant effect of JTE 907 pretreatment on the CP 55,940-induced upregulation of 5-HT2A mRNA. (B) Effect of CP 55,940 (nonselective cannabinoid agonist), ACEA and GP 1a, selective CB1 and CB2 receptor agonists, respectively, on 5-HT2A receptor mRNA levels in CLU213 and A1A1v cells. **p<0.01, significant effect of GP 1a or CP 55,940 treatment on 5-HT2A receptor mRNA levels compared with vehicle-treated CLU213 or A1A1v cells. #p<0.05, significant effect of GP 1a or CP 55,940 treatment compared to ACEA treatment on 5-HT2A receptor mRNA levels in CLU213 or A1A1v cells. (C) JTE 907 and AM 630, selective CB2 receptor antagonists, prevented the GP 1a-induced upregulation of 5-HT2A receptor mRNA. **p<0.01, significant effect of GP 1a treatment on 5-HT2A receptor mRNA levels compared to vehicle treated cells. ##p<0.01, significant effect of JTE 907 or AM 630 pretreatment on the GP 1a-induced upregulation of 5-HT2A receptor mRNA in CLU213 cells. The data represent mean ± SEM (n=3).
We also used the non-selective or selective CB1 or CB2 receptor agonists, CP 55,940, ACEA or GP 1a respectively, to study their effect on 5-HT2A receptor upregulation on either CLU213 or A1A1v cells (Abood and Martin, 1992; Di et al., 2007; Gong et al., 2006; Moore et al., 2007). This experiment was designed to study whether these cannabinoid agonists can mediate similar effects in two different and independent neuronal cell lines that endogenously express 5-HT2A receptors. CP 55,940 and GP 1a significantly (p<0.01) upregulated 5-HT2A mRNA in both CLU213 and A1A1v cells (Fig. 3B). Specifically, CP 55,940 increased 5-HT2A receptor mRNA levels by 89 ± 9% or 73 ± 4% in CLU213 or A1A1v cells compared to vehicle controls, respectively. GP 1a increased 5-HT2A receptor mRNA levels by 82 ± 5% or 77 ± 3% in CLU213 or A1A1v cells compared to controls, respectively (Fig. 3B). ACEA was unable to upregulate 5-HT2A receptor mRNA levels in either cell type compared to controls. No significant (p>0.05) differences in either the CP 55,940- or the GP 1a-induced upregulation of 5-HT2A receptor mRNA was detected between CLU213 and A1A1v cells. The two-way ANOVA analysis did not show a main effect on cell type used (F1,.12, p>0.1645) but found a main effect on the cannabinoid agonists used (F3,167.98, p<0.0001) and a main interaction between these two factors (F3,167.98, p<0.0001).
Our previous data suggest that selective CB2 receptor agonists induce a strong regulation of 5-HT2A receptors. JTE 907 and AM 630 are two specific CB2 receptor antagonists used in the literature to study the specific effects of CB2 receptors in different animal or cell culture models (Iwamura et al., 2001; Onaivi et al., 2008; Ueda et al., 2005). In these experiments we studied the effect of these two antagonists on the GP 1a-induced upregulation of 5-HT2A receptors (Fig. 3C). GP 1a induced a significant (p<0.01) approximately two-fold increase in 5-HT2A mRNA levels in CLU213 cells compared to vehicle controls (Fig. 3C). This effect was significantly (p<0.01) prevented in cells pretreated with either JTE 907 or AM 630 prior to the GP 1a treatment. The two-way ANOVA found a main effect of antagonist pretreatment (F2,35.76, p<0.0001) and agonist treatment (F1,43.63, p>0.0001) and a significant interaction between these two factors (F2,36.91, p<0.0001).
GP 1a-induces upregulation of 5-HT2A receptor mRNA via ERK1/2 signaling pathway in two neuronal cell models
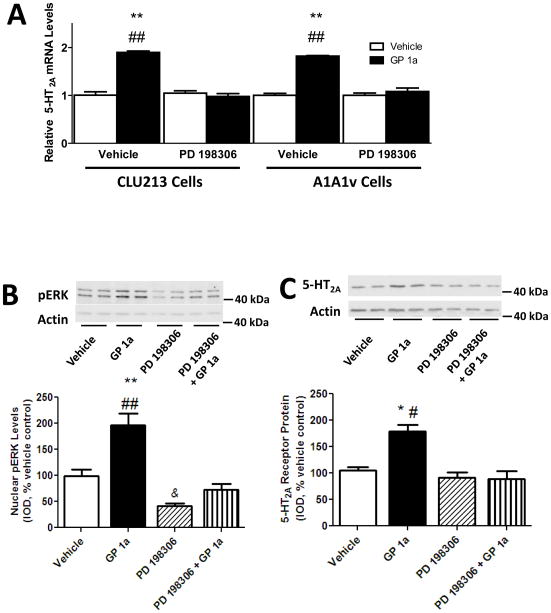
CB2 receptors couple through the Gi/o class of G-proteins to the ERK signaling pathway (Childers, 2006). We aimed to identify the effect of a selective ERK1/2 inhibitor on the GP1a-induced upregulation of 5-HT2A receptor signaling in CLU213 cells as described in the methods (Fig. 4A). GP 1a mediated a significant (p<0.01) upregulation of 5-HT2A receptor mRNA in both cell types. Indeed, cells incubated with GP 1a (1nM) exhibited a 90 ± 4% and 84 ± 4% increase in 5-HT2A mRNA in CLU213 and A1A1v cells (Fig. 4A). This strong GP 1a-induced 5-HT2A receptor upregulation was significantly (p<0.01) prevented in both cell types by PD 198306 pretreatment, suggesting that the GP 1a-induced activation of ERK1/2 signaling mediates the 5-HT2A receptor upregulation in both CLU213 and A1A1v cell lines. No significant differences in the basal levels of 5-HT2A receptor mRNA was detected between cells pretreated with either vehicle or PD 198306. The three-way ANOVA found a main effect of pretreatment with PD 198306 (F1,0.4524, p<0.0001) and treatment with GP 1a (F1,116.332, p<0.0001) but not of the cell type used (F1,0.4524, p>0.8343). No main interaction was detected between either pretreatment (F1,0.7159, p<0.4099) or treatment (F1,21316, p<0.6505) with the cell type used. A main interaction was detected between pretreatment and treatment (F1,115.2387, p<0.0001). No main interaction was detected between these three factors (F1,2.0105, p<0.1754).
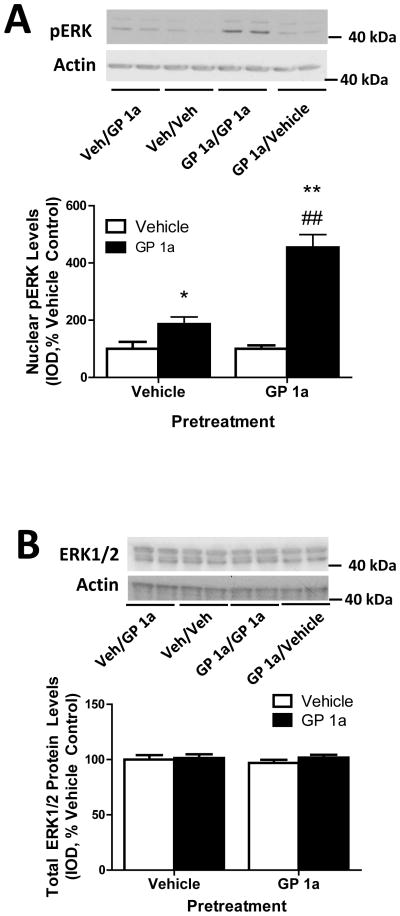
Figure 4.
GP 1a, a selective CB2 receptor agonist, upregulates 5-HT2A receptors via ERK1/2 signaling in CLU213 and A1A1v cells. (A) PD198306, a selective ERK1/2 inhibitor, prevented the effect of GP 1a on 5-HT2A receptor mRNA levels in CLU213 and A1A1v cells. **p<0.01, significant effect of GP 1a treatment on 5-HT2A receptor mRNA levels compared to vehicle treated CLU213 or A1A1v cells. ##p<0.01, significant effect of PD 198306 pretreatment on the GP 1a-induced upregulation of 5-HT2A receptor mRNA in CLU213 cells. (B) PD198306 prevented GP 1a-induced ERK1/2 activation. **p<0.01, significant effect of GP 1a treatment on nuclear pERK levels compared to vehicle control. ##p<0.01, significant effect of GP 1a treatment on nuclear pERK levels compared to the effect of GP 1a treatment in PD198306 pretreated cells. &p<0.05, significant effect of PD198306 pretreatment compared to vehicle controls (C) PD198306 prevented the GP 1a-induced increases in 5-HT2A receptor protein levels in CLU213 cells. *p<0.05, significant effect of GP 1a treatment on 5-HT2A receptor protein levels compared to vehicle treated cells. #p<0.05, significant effect of GP 1a treatment on 5-HT2A receptor protein levels compared to the effect of GP 1a treatment in PD198306 pretreated cells. The data represent mean ± SEM (n=3).
We measured nuclear levels of pERK to verify the effect of PD 198306 on preventing the activation of ERK signaling. Fig. 4B illustrates the effect of PD 198306 on the GP 1a-induced increase of nuclear-associated pERK protein levels. GP 1a produced a significant (p<0.01) increase in the nuclear levels of pERK compared to vehicle-treated cells (97% increase over control, Fig. 4B). While CLU213 cells pretreated with PD 198306 and treated with vehicle showed a 55% reduction (p<0.05) in nuclear pERK protein levels compared to controls, cells pretreated with PD 198306 and treated with GP 1a showed a 23% reduction (p>0.05) compared to vehicle controls. The two-way ANOVA showed a main effect of pretreatment (F1,616.80, p<0.0001), treatment (F1,563.85, p<0.0001), and a main interaction (F1,244.71, p<0.0001) between pretreatment and treatment on nuclear pERK levels. We then studied whether ERK1/2 inhibition with PD 198306 was able to prevent the GP 1a-induced increase on 5-HT2A receptor protein levels (Fig. 4C). CLU213 cells were pretreated with either vehicle or PD 198306 prior to a treatment with either vehicle or GP 1a (1nM). This process was repeated for 3 days and cells were collected 24 h after the last incubation. We found that GP 1a produced a significant (p<0.05) increase in membrane-associated 5-HT2A receptor protein levels compared to controls (71% increase over controls, Fig. 4C). PD 198306 pretreatment significantly (p<0.05) inhibited this GP 1a-induced upregulation of 5-HT2A receptors (Fig. 4C). Furthermore, pretreatment with PD 198306 significantly (p<0.05) reduced the membrane-associated protein levels of 5-HT2A receptors compared with vehicle controls. The two-way ANOVA showed a main effect of pretreatment (F1,451.110, p<0.0001), treatment (F1,370.16, p<0.0001) and a main interaction (F1,452.63, p<0.0001) between pretreatment and treatment on membrane-associated 5-HT2A receptor protein levels.
Repeat GP 1a exposure enhances ERK1/2 activation in a neuronal cell model
Finally, we studied whether the GP 1a-induced activation of ERK in CLU213 cells was modified by a previous exposure to GP 1a 24 hours earlier (Fig. 5). Here, cells were treated with either vehicle or GP 1a (1nM) for 24h. After rinsing the cells, they were treated with either vehicle or GP 1a (1nM) for 15 min and nuclear and cytosolic proteins were isolated as previously described. We found that a single treatment with GP 1a induced a significant (p<0.05) activation of ERK1/2 in CLU213 cells and this response was significantly enhanced (p<0.01) in cells that were exposed to GP 1a 24 hours earlier (Fig. 5A). Nuclear levels of pERK were increased by 86% or 350% after either a single or repeated exposure to GP 1a. The two-way ANOVA detected a main effect of pretreatment (F1,5.231, p>0.0332), treatment (F1,16.82, p>0.0006) and an interaction between pretreatment and treatment (F1,5.188, p>0.0339) on nuclear levels of pERK. We measured cytosolic levels of ERK1/2 protein as an index of the overall levels of this protein. We found that cytosolic ERK1/2 protein levels were not significantly (p>0.05) affected by repeated exposure to the CB2 agonist GP 1a (Fig. 5B). The two-way ANOVA did not find a main effect of pretreatment (F1,0.166, p>0.687) or treatment (F1,0.87635, p>0.3594) nor an interaction between pretreatment and treatment (F1,0.27146, p>0.6076) on cytosolic levels of ERK1/2 protein.
Figure 5.
Multiple GP 1a treatments enhance nuclear-associated pERK levels in CLU213 cells. (A) Repeat GP 1a treatment enhances nuclear pERK protein levels over a single GP 1a treatment. *p<0.05, significant effect of one GP 1a treatment on nuclear associated pERK levels compared to vehicle treated controls. **p<0.01, significant effect of two GP 1a treatments on nuclear associated pERK levels compared to vehicle treated controls. ##p<0.01, significant effect of two GP 1a treatments on nuclear associate pERK levels compared to one GP 1a treatment in CLU213 cells. (B) GP 1a treatment does not significantly (p>0.05) alter total cytosolic ERK1/2 protein levels. The data represent mean ± SEM (n=3).
Discussion
Several behavioral reports suggest that cannabinoids can regulate 5-HT neurotransmission, and more specifically 5-HT2A receptors (Cheer et al., 1999; Darmani, 2001; Gorzalka et al., 2005; Hill et al., 2006). However, these studies did not identify the molecular mechanisms by which cannabinoid receptors would modify 5-HT2A receptor signaling and/or activity. Here we used CP 55,940, a synthetic CB1/CB2 receptor agonist, that displays high and roughly similar affinity for both cannabinoid receptors to study the cannabinoid regulation of 5-HT2A receptors in rats and culture cells (Gatley et al., 1997; Thomas et al., 1998). Indeed, we found that CP 55,940 induced a significant increase in the 5-HT2A receptor-mediated PLCβ activity in rat PFCx that was associated with increased 5-HT2A receptor protein and mRNA levels in this brain area (Fig. 1). 5-HT2A receptor-mediated PLCβ activity measures the phosphoinositide breakdown to diacylglycerol (DAG) and inositol trisphosphate (IP3) via stimulation of phosphoinositide-specific PLCβ (Carrasco and Battaglia, 2007; Wolf and Schutz, 1997). This assay involves measuring receptor-stimulated PLCβ activity in brains via the conversion of radiolabeled phosphatidylinositol (3H-PI) to inositol monophosphate (3H-IP) (Carrasco and Battaglia, 2007; Wolf and Schutz, 1997) and makes it feasible to investigate treatment-induced changes in various components of the 5-HT2A receptor effector systems in a well-controlled in vitro situation that is entirely mediated by activation of 5-HT2A receptors in this brain region (Carrasco and Battaglia, 2007; Wolf and Schutz, 1997).
The 5-HT2A receptor-mediated increases in PLCβ activity seem to be mediated by increased levels of membrane-associated 5-HT2A receptors in PFCx. Enhanced transcription of the 5-HT2A receptor gene and translation of the 5-HT2A receptor mRNA could explain this increase in 5-HT2A receptor protein levels as we detected an approximate two-fold increase in 5-HT2A receptor mRNA in PFCx (Fig. 1). Noteworthy, this increase in mRNA levels seems to be specific for 5-HT2A receptors as no upregulation of 5-HT1A receptors and Gαq was found. 5-HT1A and 5-HT2A receptors exhibit overlapping distributions in various brain regions, including frontal and prefrontal cortex (Amargos-Bosch et al., 2004; Araneda and Andrade, 1991) and Gαq is a G-protein that couples 5-HT2A receptors to the PLCβ signaling pathway. The lack of changes in Gαq seems to indicate that the cannabinoid-induced increases in membrane-associated 5-HT2A receptors are sufficient to enhance PLCβ activity in this brain area. We also noted decreased CB1 and CB2 receptor mRNA levels if PFCx of CP 55,940-treated rats. This could explain how chronic exposure to CP 55,940 is associated with downregulation and a significant loss of CB1 and CB2 receptors in cortex as measured by receptor binding experiments (Rubino et al., 2000; Shoemaker et al., 2005).
We used CLU213 cells as cellular model to investigate the molecular mechanism by which cannabinoid receptors might mediate the upregulation and enhanced activity of 5-HT2A receptor signaling in a neuronal cell line. This neuronal cell line endogenously expresses 5-HT2A receptors coupled to the stimulation of PI hydrolysis. Interestingly, we detected CP 55,940-induced increases in 5-HT2A receptor mRNA and protein levels and enhanced 5-HT2 receptor-mediated PI hydrolysis in these neuronal cells (Fig. 2). Although, SB242084, a selective 5-HT2C receptor antagonist (Kennett et al., 1997), was added to prevent the activation of 5-HT2C receptors, the lack of selective 5-HT2A receptor agonist hindered our ability to identify a CP 55,940-mediated specific increase in 5-HT2A receptor activity in this cell line. The two-fold increase in both 5-HT2A receptor mRNA and protein levels suggest that an increase in the activity of 5-HT2A receptors might be associated with the exposure to the non-selective cannabinoid agonist, CP 55,940.
We used PF-514273 (CB1 antagonist) (Dow et al., 2009), and JTE 907 (CB2 receptor antagonist) (Iwamura et al., 2001; Ueda et al., 2005) to study the involvement of cannabinoid receptors in the CP 55,940-induced increases in 5-HT2A receptor mRNA (Fig. 3A). We found that JTE 907, but not PF-514273, produced a complete inhibition of the CP 55,940-induced increases in 5-HT2A receptormRNA. These results suggest that blockade of the CB2 receptors, but not CB1 receptors, with selective antagonists can prevent the CP 55,940-induced upregulation of 5-HT2A receptors in CLU213 cells. We also studied the effect of CP 55,940, ACEA and GP 1a (non-selective and selective CB1 and CB2 receptor agonists, respectively) on 5-HT2A receptor upregulation in both CLU213 and A1A1v cells (Fig. 3B). Noteworthy, we detected similar upregulation of 5-HT2A receptors in both CLU213 and A1A1v cells. This suggests that selective CB2 receptor agonists can upregulate 5-HT2A receptors in two independent neuronal cells that endogenously express 5-HT2A receptors. We also found confirmatory evidence of the role of CB2 receptor agonists on the upregulation of 5-HT2A receptors in CLU213 cells through studying the effect of two selective CB2 receptor antagonists (JTE 907 and AM 630) on cells exposed to the selective CB2 receptor agonist, GP 1a (Fig. 3C). Both CB2 receptor antagonists completely prevented the GP 1a-induced upregulation of 5-HT2A receptors in this neuronal cell line, supporting the role of the CB2 receptor agonist in this phenomenon. In summary, the previous results suggest that cannabinoid agonists would upregulate 5-HT2A receptor mRNA via activation of CB2 receptors in neuronal cells that endogenously express 5-HT2A and CB2 receptors.
The CB2 receptor is a seven transmembrane receptors that was initially identified in the periphery but not in the brain (Abood and Martin, 1996; Demuth and Molleman, 2006). Indeed, brain expression of CB2 receptors was much less well established and characterized in comparison to the expression of brain CB1 receptors. However, more recent reports have established the expression of CB2 receptors in normal neurons in PFCx, amygdala, hypothalamus, hippocampus, etc. (den Boon et al., 2012; Gong et al., 2006; Onaivi et al., 2008; Xi et al., 2011). Furthermore, recent studies indicate that CB2 receptors are mainly localized in post synaptic neurons (Brusco et al., 2008; den Boon et al., 2012) where they might mediate their effects by activation of Gi/o G-proteins, ERK signaling and Jun N-terminal kinase (JNK) which are signaling pathways that regulate nuclear transcription factors (Bouaboula et al., 1996; Childers, 2006; Howlett, 2005). Interestingly, the ERK1/2 signaling cascade can regulate transcription factors which have consensus sequences within the rat 5-HT2A receptor promoter region (Du et al., 1995; Ferry and Molinoff, 1996).
In our experiments we tested the effect of PD 198306, a potent ERK1/2 inhibitor, on the GP 1a-induced increases in 5-HT2A receptor mRNA levels in CLU213 and A1A1v cells (Crane et al., 2007; Sullivan et al., 2005). We found that ERK1/2 inhibitor pretreatment prevented the GP 1a-induced increases in 5-HT2A receptor mRNA levels in both CLU213 and A1A1v cells (Fig 4A). This would indicate that the GP 1a-induced upregulation of 5-HT2A receptors might require ERK1/2 activation and suggests similarities in the mechanism involved in the upregulation of 5-HT2A receptors in both cell types. Since ERK1/2 is activated (phosphorylated) in the cytoplasm and then translocates to the nucleus, we measured nuclear-associated levels of pERK as an index of CB2 receptor-induced ERK activation (Aksamitiene et al., 2010; Howlett, 2005). We found that PD 198306 pretreatment also prevented GP 1a-induced increases in nuclear associated pERK protein levels and membrane-associated 5-HT2A receptor protein levels in CLU213 cells (Fig 4B and 4C). This evidence might indicate that CB2 receptor agonists would induce an increase in the nuclear levels of activated ERK (pERK) that would mediate the upregulation of 5-HT2A receptor mRNA and increase synthesis of the 5-HT2A receptor protein. Agonists of CB2 receptors might upregulate 5-HT2A receptor mRNA, transcription factors that are located downstream of ERK and that target the promoter region of the 5-HT2A receptor gene, such as cyclic AMP response element binding protein (CREB), specificity protein 1 (SP-1), and activator protein 1 (AP-1), could be involved (Chang et al., 2003; Zhu et al., 1995). Indeed, we have recently found that AP-1, but not CREB, plays a role in the cannabinoid-induced upregulation of 5-HT2A receptors (Franklin and Carrasco, 2012).
Interestingly, we found that repeated stimulation of CB2 receptors produced an enhanced activation of ERK1/2 in CLU213 cells (Fig. 5). While a single administration of GP 1a triggered an increase in the nuclear-associated levels of pERK, we observed a greater response in cells that were exposed to GP 1a 24 hours earlier (Fig. 5A). Furthermore, GP 1a treatment did not have an effect on the total ERK1/2 protein levels (Fig 5B). Previous reports that predominantly examined the ability of cannabinoid agonists to stimulate [(35)S]guanylyl-5′-O-(gamma-thio)-triphosphate binding to the Gα subunits of the G-proteins indicated that short exposure to cannabinoids produces desensitization of cannabinoid receptors (Childers, 2006). However, our results would suggest that repeat exposure to cannabinoid agonists might mediate enhanced responses of the CB2 receptor-mediated regulation of ERK activation. Interestingly, studies by Lefkowitz et al. (2005) suggest that seven transmembrane receptors can regulate activation of ERK1/2 through either G-proteins or β-arrestins (Lefkowitz and Shenoy, 2005). Indeed, G-protein coupled receptors, such as CB2 receptors, could regulate long-term activation of ERK1/2, independent of G-proteins through β-arrestins which can form scaffolding complexes with several kinases such as ERK1/2 (Lefkowitz and Shenoy, 2005; Shenoy and Lefkowitz, 2011). Therefore, it is possible that while repeated exposure induces the desensitization of CB2 receptor-mediated activation of G-proteins, the CB2 receptor-mediated activation of ERK1/2 signaling could be enhanced by a mechanism that is mediated by β-arrestins and is independent of G-protein activation. Future experiments in our lab will try to identify the mechanisms involved in ERK1/2 activation after repeated exposure to CB2 receptor agonists.
The results presented here suggest that exposure to selective CB2 receptor agonists can induce the upregulation of 5-HT2A receptors by a mechanism that involves ERK1/2 activation. Recent reports have provided new evidence linking chronic use of cannabinoids to an earlier onset of psychiatric disorders, including psychosis, schizophrenia and anxiety (Garcia-Gutierrez et al., 2012; Henquet et al., 2005; Kuepper et al., 2011; Large et al., 2011). Indeed, these studies proposed that repeated exposure to cannabinoids might precipitate the onset of cognitive disorders in individual predispose to developing them (Henquet et al., 2005; Kuepper et al., 2011; Large et al., 2011). Interestingly, preclinical evidence links CB2 receptors to several neuropsychiatric disorders such as schizophrenia, anxiety, and depression (Garcia-Gutierrez et al., 2010; Garcia-Gutierrez et al., 2012; Onaivi et al., 2008; Ortega-Alvaro et al., 2011). This evidence highlights that need to identify the mechanism by which CB2 receptors may be inducing and/or contributing to neuropsychiatric disorders. The CB2 receptor agonist-induced upregulation of 5-HT2A receptors in PFCx could provide a molecular mechanism by which exposure to cannabinoid receptor agonists might contribute to the pathophysiology of cognitive disorders, since psychosis and schizophrenia are disorders associated with dysfunction of 5-HT2A receptor signaling in PFCx (Bubar and Cunningham, 2006; Carrasco and Van de Kar, 2003; de et al., 2008; Magalhaes et al., 2010).
5-HT2A receptors, which has been shown to regulate the dopamine mesoaccumbens pathway, play a critical role in the regulation of stress, mood, impulse control and the behavior effects of drugs of abuse (Bubar and Cunningham, 2006; Carrasco and Van de Kar, 2003; Magalhaes et al., 2010). Furthermore, impaired function of cortical 5-HT2A receptors has been identified in several neurological and psychiatric disorders such as schizophrenia, depression, anxiety and eating disorders (Roth, 2011). Moreover, the therapeutic benefits of atypical antipsychotics, which are more potent 5-HT2A receptor antagonists than dopamine D2 receptor antagonists (de et al., 2008; Ichikawa et al., 2001), are proposed to be mediated by antagonism and subsequent desensitization of 5-HT2A receptor signaling in PFCx (Singh et al., 2009). Actually, it has been suggested that blockade of pyramidal neurons in PFCx (particularly enriched in 5-HT2A receptors) may underlie the beneficial effects of atypical antipsychotic drugs (de et al., 2008; Singh et al., 2009). Additionally, a recent report suggests that crosstalk between 5-HT2A and corticotrophin receptors in PFCx regulates anxiety-like behaviors and mood disorders as well (Magalhaes et al., 2010). Indeed, they provide evidence that overexpression of 5-HT2A receptors in the PFCx can contribute to anxiety-like behaviors (Magalhaes et al., 2010). Therefore, the cannabinoid receptor-mediated upregulation of 5-HT2A receptor neurotransmission in PFCx could be responsible for anxiety-like behaviors reported after exposure to cannabinoid agonists (Rubino et al., 2011). Indeed, a recent report indicates that while chronic exposure to selective CB2 receptor agonists induced anxiogenic-like behaviors, chronic antagonism of CB2 receptors induces anxiolytic-like behaviors in rodents (Garcia-Gutierrez et al., 2012).
Emerging evidence indicates that selective CB2 receptor agonists might have wide therapeutic application in the treatment of stroke, neurogenerative diseases, neuropathic pain, etc (Morales and Bonci, 2012; Zarruk et al., 2012). However, the cannabinoid-induced upregulation of 5-HT2A receptors might represent a potential negative side-effect of the long term exposure to selective CB2 receptor agonists. Our studies might provide a molecular mechanism by which chronic use of cannabinoids might precipitate the onset of some psychiatric disorders in people predisposed to developing them and contribute to the mechanisms associated with the therapeutic use of selective cannabinoid agonists.
Acknowledgments
Supported by: NIH/NIDA DA024329 and University of Kansas Startup Funds
Reference List
- Abood ME, Martin BR. Neurobiology of marijuana abuse. Trends Pharmacol Sci. 1992;13:201–206. doi: 10.1016/0165-6147(92)90064-d. [DOI] [PubMed] [Google Scholar]
- Abood ME, Martin BR. Molecular neurobiology of the cannabinoid receptor. Int Rev Neurobiol. 1996;39:197–221. doi: 10.1016/s0074-7742(08)60667-4. [DOI] [PubMed] [Google Scholar]
- Aksamitiene E, Kholodenko BN, Kolch W, Hoek JB, Kiyatkin A. PI3K/Akt-sensitive MEK-independent compensatory circuit of ERK activation in ER-positive PI3K-mutant T47D breast cancer cells. Cell Signal. 2010;22:1369–1378. doi: 10.1016/j.cellsig.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amargos-Bosch M, Bortolozzi A, Puig MV, Serrats J, Adell A, Celada P, Toth M, Mengod G, Artigas F. Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cereb Cortex. 2004;14:281–299. doi: 10.1093/cercor/bhg128. [DOI] [PubMed] [Google Scholar]
- Araneda R, Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- Atkinson PJ, Young KW, Ennion SJ, Kew JN, Nahorski SR, Challiss RA. Altered expression of G(q/11alpha) protein shapes mGlu1 and mGlu5 receptor-mediated single cell inositol 1,4,5-trisphosphate and Ca(2+) signaling. Mol Pharmacol. 2006;69:174–184. doi: 10.1124/mol.105.014258. [DOI] [PubMed] [Google Scholar]
- Berg KA, Clarke WP, Chen Y, Ebersole BJ, McKay RDG, Maayani S. 5-hydroxytryptamine type 2A receptors regulate cyclic AMP accumulation in a neuronal cell line by protein kinase C- dependent and calcium/calmodulin-dependent mechanisms. Mol Pharmacol. 1994;45:826–836. [PubMed] [Google Scholar]
- Bouaboula M, Poinot-Chazel C, Marchand J, Canat X, Bourrie B, Rinaldi-Carmona M, Calandra B, Le FG, Casellas P. Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor. Involvement of both mitogen-activated protein kinase and induction of Krox-24 expression. Eur J Biochem. 1996;237:704–711. doi: 10.1111/j.1432-1033.1996.0704p.x. [DOI] [PubMed] [Google Scholar]
- Brusco A, Tagliaferro PA, Saez T, Onaivi ES. Ultrastructural localization of neuronal brain CB2 cannabinoid receptors. Ann N Y Acad Sci. 2008;1139:450–457. doi: 10.1196/annals.1432.037. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Serotonin 5-HT2A and 5-HT2C receptors as potential targets for modulation of psychostimulant use and dependence. Curr Top Med Chem. 2006;6:1971–1985. doi: 10.2174/156802606778522131. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Battaglia G. Withdrawal from a single exposure to cocaine increases 5-HT2A receptor and G protein function. Neuroreport. 2007;18:51–55. doi: 10.1097/01.wnr.0000246324.43567.55. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Damjanoska KJ, D’Souza DN, Zhang Y, Garcia F, Battaglia G, Muma NA, Van de Kar LD. Short-Term Cocaine Treatment Causes Neuroadaptive Changes in G{alpha}q and G{alpha}11 Proteins in Rats Undergoing Withdrawal. J Pharmacol Exp Ther. 2004;311:349–355. doi: 10.1124/jpet.104.069807. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Van de Kar LD, Sullivan NR, Landry M, Garcia F, Muma NA, Battaglia G. Cocaine-mediated supersensitivity of 5-HT2A receptors in hypothalamic paraventricular nucleus is a withdrawal-induced phenomenon. Neuroscience. 2006;143:7–13. doi: 10.1016/j.neuroscience.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL, Franklin RA, McCubrey JA. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia. 2003;17:1263–1293. doi: 10.1038/sj.leu.2402945. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Cadogan AK, Marsden CA, Fone KC, Kendall DA. Modification of 5-HT2 receptor mediated behaviour in the rat by oleamide and the role of cannabinoid receptors. Neuropharmacology. 1999;38:533–541. doi: 10.1016/s0028-3908(98)00208-1. [DOI] [PubMed] [Google Scholar]
- Childers SR. Activation of G-proteins in brain by endogenous and exogenous cannabinoids. AAPS J. 2006;8:E112–E117. doi: 10.1208/aapsj080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JW, Shimizu K, Carrasco GA, Garcia F, Jia C, Sullivan NR, D’Souza DN, Zhang Y, Van de Kar LD, Muma NA, Battaglia G. 5-HT1A receptors mediate (+)8-OH-DPAT-stimulation of extracellular signal-regulated kinase (MAP kinase) in vivo in rat hypothalamus: time dependence and regional differences. Brain Res. 2007;1183:51–59. doi: 10.1016/j.brainres.2007.07.101. [DOI] [PubMed] [Google Scholar]
- Darmani NA. Cannabinoids of diverse structure inhibit two DOI-induced 5-HT(2A) receptor-mediated behaviors in mice. Pharmacol Biochem Behav. 2001;68:311–317. doi: 10.1016/s0091-3057(00)00477-9. [DOI] [PubMed] [Google Scholar]
- de AJ, Palacios JM, Mengod G. Distribution of 5-HT and DA receptors in primate prefrontal cortex: implications for pathophysiology and treatment. Prog Brain Res. 2008;172:101–115. doi: 10.1016/S0079-6123(08)00905-9. [DOI] [PubMed] [Google Scholar]
- Demuth DG, Molleman A. Cannabinoid signalling. Life Sci. 2006;78:549–563. doi: 10.1016/j.lfs.2005.05.055. [DOI] [PubMed] [Google Scholar]
- den Boon FS, Chameau P, Schaafsma-Zhao Q, van AW, Bari M, Oddi S, Kruse CG, Maccarrone M, Wadman WJ, Werkman TR. Excitability of prefrontal cortical pyramidal neurons is modulated by activation of intracellular type-2 cannabinoid receptors. Proc Natl Acad Sci U S A. 2012;109:3534–3539. doi: 10.1073/pnas.1118167109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di FM, Morrison PD, Butt A, Murray RM. Cannabis use and psychiatric and cogitive disorders: the chicken or the egg? Curr Opin Psychiatry. 2007;20:228–234. doi: 10.1097/YCO.0b013e3280fa838e. [DOI] [PubMed] [Google Scholar]
- Dow RL, Carpino PA, Hadcock JR, Black SC, Iredale PA, Silva-Jardine P, Schneider SR, Paight ES, Griffith DA, Scott DO, O’Connor RE, Nduaka CI. Discovery of 2-(2-chlorophenyl)-3-(4-chlorophenyl)-7-(2,2-difluoropropyl)-6,7-dihydro-2 H-pyrazolo[3,4-f][1,4]oxazepin-8(5H)-one (PF-514273), a novel, bicyclic lactam-based cannabinoid-1 receptor antagonist for the treatment of obesity. J Med Chem. 2009;52:2652–2655. doi: 10.1021/jm900255t. [DOI] [PubMed] [Google Scholar]
- Du Y-L, Wilcox BD, Jeffrey JJ. Regulation of rat 5-hydroxytryptamine type 2 receptor gene activity: Identification of cis elements that mediate basal and 5-hydroxytryptamine-dependent gene activation. Mol Pharmacol. 1995;47:915–922. [PubMed] [Google Scholar]
- Egerton A, Allison C, Brett RR, Pratt JA. Cannabinoids and prefrontal cortical function: insights from preclinical studies. Neurosci Biobehav Rev. 2006;30:680–695. doi: 10.1016/j.neubiorev.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Felder CC, ckason-Chesterfield AK, Moore SA. Cannabinoids biology: the search for new therapeutic targets. Mol Interv. 2006;6:149–161. doi: 10.1124/mi.6.3.6. [DOI] [PubMed] [Google Scholar]
- Ferry RC, Molinoff PB. Regulation of 5-HT2A receptor mRNA in P11 cells. Behav Brain Res. 1996;73:187–191. doi: 10.1016/0166-4328(96)00094-0. [DOI] [PubMed] [Google Scholar]
- Filip M, Bubar MJ, Cunningham KA. Contribution of serotonin (5-HT) 5-HT2 receptor subtypes to the discriminative stimulus effects of cocaine in rats. Psychopharmacology (Berl) 2006;183:482–489. doi: 10.1007/s00213-005-0197-y. [DOI] [PubMed] [Google Scholar]
- Franklin JM, Carrasco GA. Cannabinoid-induced enhanced interaction and protein levels of serotonin 5-HT2A and dopamine D2 receptors in rat prefrontal cortex. Journal of Psychopharmacology. 2012;26:915–929. doi: 10.1177/0269881112450786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin JM, Vasiljevik T, Prisinzano TE, Carrasco GA. Cannabinoid 2 Receptor- and Beta Arrestin 2-Dependent Upregulation of Serotonin 2A Receptors. European Neuropsychopharmacology. 2012 doi: 10.1016/j.euroneuro.2012.06.012. in press: http://dx.doi.org/10.1016/j.euroneuro.2012.06.012. [DOI] [PMC free article] [PubMed]
- Garcia-Gutierrez MS, Garcia-Bueno B, Zoppi S, Leza JC, Manzanares J. Chronic blockade of cannabinoid CB2 receptors induces anxiolytic-like actions associated with alterations in GABA(A) receptors. Br J Pharmacol. 2012;165:951–964. doi: 10.1111/j.1476-5381.2011.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gutierrez MS, Perez-Ortiz JM, Gutierrez-Adan A, Manzanares J. Depression-resistant endophenotype in mice overexpressing cannabinoid CB(2) receptors. Br J Pharmacol. 2010;160:1773–1784. doi: 10.1111/j.1476-5381.2010.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatley SJ, Lan R, Pyatt B, Gifford AN, Volkow ND, Makriyannis A. Binding of the non-classical cannabinoid CP 55,940, and the diarylpyrazole AM251 to rodent brain cannabinoid receptors. Life Sci. 1997;61:PL191–PL197. doi: 10.1016/s0024-3205(97)00690-5. [DOI] [PubMed] [Google Scholar]
- Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Gorantla S, Makarov E, Roy D, Finke-Dwyer J, Murrin LC, Gendelman HE, Poluektova L. Immunoregulation of a CB2 receptor agonist in a murine model of neuroAIDS. J Neuroimmune Pharmacol. 2010;5:456–468. doi: 10.1007/s11481-010-9225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorzalka BB, Hill MN, Sun JC. Functional role of the endocannabinoid system and AMPA/kainate receptors in 5-HT2A receptor-mediated wet dog shakes. Eur J Pharmacol. 2005;516:28–33. doi: 10.1016/j.ejphar.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Henquet C, Murray R, Linszen D, van OJ. The environment and schizophrenia: the role of cannabis use. Schizophr Bull. 2005;31:608–612. doi: 10.1093/schbul/sbi027. [DOI] [PubMed] [Google Scholar]
- Hermann H, Marsicano G, Lutz B. Coexpression of the cannabinoid receptor type 1 with dopamine and serotonin receptors in distinct neuronal subpopulations of the adult mouse forebrain. Neuroscience. 2002;109:451–460. doi: 10.1016/s0306-4522(01)00509-7. [DOI] [PubMed] [Google Scholar]
- Hill MN, Sun JC, Tse MT, Gorzalka BB. Altered responsiveness of serotonin receptor subtypes following long-term cannabinoid treatment. Int J Neuropsychopharmacol. 2006;9:277–286. doi: 10.1017/S1461145705005651. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Manna S, Greenberg MJ, DiCamelli R, Ross RA, Stevenson LA, Murphy V, Pertwee RG, Campbell WB. Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1) J Pharmacol Exp Ther. 1999;289:1427–1433. [PubMed] [Google Scholar]
- Howlett AC. Cannabinoid receptor signaling. Handb Exp Pharmacol. 2005:53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Ishii H, Bonaccorso S, Fowler WL, O’Laughlin IA, Meltzer HY. 5-HT2A and D2 receptor blockade increases cortical DA release via 5-HT1A receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. Journal of Neurochemistry. 2001;76:1521–1531. doi: 10.1046/j.1471-4159.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- Iwamura H, Suzuki H, Ueda Y, Kaya T, Inaba T. In vitro and in vivo pharmacological characterization of JTE-907, a novel selective ligand for cannabinoid CB2 receptor. J Pharmacol Exp Ther. 2001;296:420–425. [PubMed] [Google Scholar]
- Jones KA, Srivastava DP, Allen JA, Strachan RT, Roth BL, Penzes P. Rapid modulation of spine morphology by the 5-HT2A serotonin receptor through kalirin-7 signaling. Proc Natl Acad Sci U S A. 2009;106:19575–19580. doi: 10.1073/pnas.0905884106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett GA, Wood MD, Bright F, Trail B, Riley G, Holland V, Avenell KY, Stean T, Upton N, Bromidge S, Forbes IT, Brown AM, Middlemiss DN, Blackburn TP. SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology. 1997;36:609–620. doi: 10.1016/s0028-3908(97)00038-5. [DOI] [PubMed] [Google Scholar]
- Kindlundh-Hogberg AM, Svenningsson P, Schioth HB. Quantitative mapping shows that serotonin rather than dopamine receptor mRNA expressions are affected after repeated intermittent administration of MDMA in rat brain. Neuropharmacology. 2006;51:838–847. doi: 10.1016/j.neuropharm.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Kroeze WK, Kristiansen K, Roth BL. Molecular biology of serotonin receptors structure and function at the molecular level. Curr Top Med Chem. 2002;2:507–528. doi: 10.2174/1568026023393796. [DOI] [PubMed] [Google Scholar]
- Kuepper R, van OJ, Lieb R, Wittchen HU, Hofler M, Henquet C. Continued cannabis use and risk of incidence and persistence of psychotic symptoms: 10 year follow-up cohort study. BMJ. 2011;342:d738. doi: 10.1136/bmj.d738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis Use and Earlier Onset of Psychosis: A Systematic Meta-analysis. Arch Gen Psychiatry. 2011;68:555–561. doi: 10.1001/archgenpsychiatry.2011.5. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Magalhaes AC, Holmes KD, Dale LB, Comps-Agrar L, Lee D, Yadav PN, Drysdale L, Poulter MO, Roth BL, Pin JP, Anisman H, Ferguson SS. CRF receptor 1 regulates anxiety behavior via sensitization of 5-HT2 receptor signaling. Nat Neurosci. 2010;13:622–629. doi: 10.1038/nn.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato S, Alberdi E, Ledent C, Watanabe M, Matute C. CB1 cannabinoid receptor-dependent and -independent inhibition of depolarization-induced calcium influx in oligodendrocytes. Glia. 2009;57:295–306. doi: 10.1002/glia.20757. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Price JC, Mathis CA, Greer PJ, Cantwell MN, Houck PR, Mulsant BH, Ben Eliezer D, Lopresti B, DeKosky ST, Reynolds CF., III PET imaging of serotonin type 2A receptors in late-life neuropsychiatric disorders. American Journal of Psychiatry. 1999;156:1871–1878. doi: 10.1176/ajp.156.12.1871. [DOI] [PubMed] [Google Scholar]
- Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Morales M, Bonci A. Getting to the core of addiction: Hooking CB2 receptor into drug abuse? Nat Med. 2012;18:504–505. doi: 10.1038/nm.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacmias B, Tedde A, Forleo P, Piacentini S, Guarnieri BM, Bartoli A, Ortenzi L, Petruzzi C, Serio A, Marcon G, Sorbi S. Association between 5-HT2A receptor polymorphism and psychotic symptoms in Alzheimer’s disease. Biol Psychiatry. 2001;50:472–475. doi: 10.1016/s0006-3223(01)01114-3. [DOI] [PubMed] [Google Scholar]
- Nakazi M, Bauer U, Nickel T, Kathmann M, Schlicker E. Inhibition of serotonin release in the mouse brain via presynaptic cannabinoid CB1 receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:19–24. doi: 10.1007/s002109900147. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, Perchuk A, Mora Z, Tagliaferro PA, Gardner E, Brusco A, Akinshola BE, Hope B, Lujilde J, Inada T, Iwasaki S, Macharia D, Teasenfitz L, Arinami T, Uhl GR. Brain Neuronal CB2 Cannabinoid Receptors in Drug Abuse and Depression: From Mice to Human Subjects. PLoS ONE. 2008;3:e1640. doi: 10.1371/journal.pone.0001640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Alvaro A, Aracil-Fernandez A, Garcia-Gutierrez MS, Navarrete F, Manzanares J. Deletion of CB2 cannabinoid receptor induces schizophrenia-related behaviors in mice. Neuropsychopharmacology. 2011;36:1489–1504. doi: 10.1038/npp.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JD, Herin DV, Frankel PS, Thomas ML, Cunningham KA. Chronic treatment with a serotonin(2) receptor (5-HT(2)R) agonist modulates the behavioral and cellular response to (+)-3,4-methylenedioxymethamphetamine [(+)-MDMA] Drug Alcohol Depend. 2006;81:117–127. doi: 10.1016/j.drugalcdep.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Roth BL. Irving Page Lecture: 5-HT(2A) serotonin receptor biology: interacting proteins, kinases and paradoxical regulation. Neuropharmacology. 2011;61:348–354. doi: 10.1016/j.neuropharm.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Vigano D, Massi P, Parolaro D. Changes in the cannabinoid receptor binding, G protein coupling, and cyclic AMP cascade in the CNS of rats tolerant to and dependent on the synthetic cannabinoid compound CP55,940. J Neurochem. 2000;75:2080–2086. doi: 10.1046/j.1471-4159.2000.0752080.x. [DOI] [PubMed] [Google Scholar]
- Rubino T, Zamberletti E, Parolaro D. Adolescent exposure to cannabis as a risk factor for psychiatric disorders. J Psychopharmacol. 2011;26:177–88. doi: 10.1177/0269881111405362. [DOI] [PubMed] [Google Scholar]
- Rutkowska M, Jachimczuk O. Antidepressant--like properties of ACEA (arachidonyl-2-chloroethylamide), the selective agonist of CB1 receptors. Acta Pol Pharm. 2004;61:165–167. [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. beta-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci. 2011;32:521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Zemaitaitis B, Muma NA. Phosphorylation of Galpha11 protein contributes to agonist-induced desensitization of 5-HT2A receptor signaling. Mol Pharmacol. 2007;71:303–313. doi: 10.1124/mol.106.028241. [DOI] [PubMed] [Google Scholar]
- Shoemaker JL, Ruckle MB, Mayeux PR, Prather PL. Agonist-directed trafficking of response by endocannabinoids acting at CB2 receptors. J Pharmacol Exp Ther. 2005;315:828–838. doi: 10.1124/jpet.105.089474. [DOI] [PubMed] [Google Scholar]
- Showalter VM, Compton DR, Martin BR, Abood ME. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther. 1996;278:989–999. [PubMed] [Google Scholar]
- Singh R, Jia C, Garcia F, Carrasco G, Battaglia G, Muma N. Activation of the JAK-STAT pathway by olanzapine is necessary for desensitization of serotonin2A receptor-stimulated phospholipase C signaling in rat frontal cortex but not serotonin2A receptor-stimulated hormone release. J Psychopharmacol. 2009;24:1079–88. doi: 10.1177/0269881109103090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RK, Shi J, Zemaitaitis BW, Muma NA. Olanzapine increases RGS7 protein expression via stimulation of the Janus tyrosine kinase-signal transducer and activator of transcription signaling cascade. J Pharmacol Exp Ther. 2007;322:133–140. doi: 10.1124/jpet.107.120386. [DOI] [PubMed] [Google Scholar]
- Sullivan NR, Crane JW, Damjanoska KJ, Carrasco GA, D’Souza DN, Garcia F, Van de Kar LD. Tandospirone activates neuroendocrine and ERK (MAP kinase) signaling pathways specifically through 5-HT1A receptor mechanisms in vivo. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:18–26. doi: 10.1007/s00210-004-1005-7. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Fliers E, Hoogendijk WJ, Veltman DJ, Zhou JN. Interaction of prefrontal cortical and hypothalamic systems in the pathogenesis of depression. Prog Brain Res. 2000;126:369–396. doi: 10.1016/S0079-6123(00)26025-1. [DOI] [PubMed] [Google Scholar]
- Thomas BF, Gilliam AF, Burch DF, Roche MJ, Seltzman HH. Comparative receptor binding analyses of cannabinoid agonists and antagonists. J Pharmacol Exp Ther. 1998;285:285–292. [PubMed] [Google Scholar]
- Ueda Y, Miyagawa N, Matsui T, Kaya T, Iwamura H. Involvement of cannabinoid CB(2) receptor-mediated response and efficacy of cannabinoid CB(2) receptor inverse agonist, JTE-907, in cutaneous inflammation in mice. Eur J Pharmacol. 2005;520:164–171. doi: 10.1016/j.ejphar.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Wolf WA, Schutz LJ. The 5-HT2C receptor is a prominent 5-HT receptor in basal ganglia: evidence from functional studies on 5-HT-mediated phosphoinositide hydrolysis. J Neurochem. 1997;69:1449–1458. doi: 10.1046/j.1471-4159.1997.69041449.x. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, Yang HJ, Bi GH, Li J, Gardner EL. Brain cannabinoid CB receptors modulate cocaine’s actions in mice. Nat Neurosci. 2011;14:1160–1166. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarruk JG, Fernandez-Lopez D, Garcia-Yebenes I, Garcia-Gutierrez MS, Vivancos J, Nombela F, Torres M, Burguete MC, Manzanares J, Lizasoain I, Moro MA. Cannabinoid type 2 receptor activation downregulates stroke-induced classic and alternative brain macrophage/microglial activation concomitant to neuroprotection. Stroke. 2012;43:211–219. doi: 10.1161/STROKEAHA.111.631044. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Chen K, Shih JC. Characterization of the human 5-HT2A receptor gene promoter. J Neurosci. 1995;15:4885–4895. doi: 10.1523/JNEUROSCI.15-07-04885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]