Abstract
Background
The colon undergoes distension-induced changes in motor activity as luminal contents or feces increases wall pressure. Input from enteric motor neurons regulates motility. Here we examined stretch-dependent responses in circular muscle strips of murine colon.
Methods
Length-ramps (6–31μm s−1) were applied in the axis of the circular muscle layer in a controlled manner until 5 mN isometric force was reached.
Key Results
Length-ramps produced transient membrane potential hyperpolarizations and attenuation of action potential (AP) complexes. Responses were reproducible when ramps were applied every 30s. Stretch-dependent hyperpolarization was blocked by TTX, suggesting AP-dependent release of inhibitory neurotransmitter(s). Atropine did not potentiate stretch-induced hyperpolarizations, but increased compliance of the circular layer. L-NNA inhibited stretch-dependent hyperpolarization and decreased muscle compliance, suggesting release of NO mediates stretch-dependent inhibition. Control membrane potential was restored by the NO donor SNP. Stretch-dependent hyperpolarizations were blocked by L-methionine, an inhibitor of stretch-dependent K+ (SDK) channels in colonic muscles. Loss of ICC, elicited by Kit neutralizing antibody, also inhibited responses to stretch. In presence of L-NNA and apamin, stretch responses became excitatory and were characterized by membrane depolarization and increased AP firing. A neurokinin-1 receptor antagonist inhibited this stretch-dependent increase in excitability.
Conclusions & Inferences
Our data show that stretch-dependent responses in colonic muscles require tonic firing of enteric inhibitory neurons, but reflex activation of neurons does not appear to be necessary. NO causes activation of SDK channels, and stretch of muscles further activates these channels, explaining the inhibitory response to stretch in colonic muscle strips.
Keywords: Stretch, colonic compliance, enteric nervous system, interstitial cells of Cajal, stretch-dependent potassium channels
INTRODUCTION
Several cell types in the gastrointestinal (GI) tract display mechanosensitivity that affect electrical and mechanical responses of GI organs. Smooth muscles of the guinea-pig taenia depolarize in response to stretch,1 and this is similar to the myogenic response in many smooth muscles.2,3 Enteric neurons also display mechanosensitivity to changes in length or tone of smooth muscle tissues. Stretching the small intestine activates mechanosensitive, intrinsic type II primary afferent neurons that respond with sustained AP discharge.4 Maintained stretch generates repetitive and coordinated discharge of ascending excitatory and descending inhibitory neuronal reflex pathways in the distal colon.5,6 Mechanosensitive S-type 1 neurons, with uniaxonal or pseudounipolar morphological features consistent with interneurons, were identified as stretch-sensitive, rather than tone-sensitive in the colon.7
Interstitial cells of Cajal (ICC) also expresses mechanosensitive mechanisms.8,9 Stretch of gastric antrum containing intramuscular ICC (ICC-IM) results in depolarization and increases slow wave frequency. This response may be similar to the myogenic response, depolarization in response to stretch, reported by Bulbring in 1954.1 In regions of the GI tract providing a storage function (e.g. fundus and proximal colon), hyperpolarization in response to stretch would appear as advantageous. Colonic smooth muscle cells respond to stretch via activation of stretch-dependent K+ (SDK) channels,10 and these channels are activated by a cGMP-dependent mechanism.11,12 A stretch-dependent K+ conductance is an appropriate effector to help stabilize resting potentials and reduce the excitability of muscles in organs that provide a reservoir function.
We investigated stretch-dependent responses in murine proximal colon by using precise length ramps to stretch muscles. Our results describe a novel level of mechanosensitive regulation in the gut via ICC. We hypothesized that stretch would elicit hyperpolarization and stabilization of membrane potential in this region and this response might be linked to SDK channels and sensitization of tonic inputs from inhibitory motor neurons.
METHODS
Animals
Forty two Balb/C mice (30–40 days) were obtained from Jackson Laboratory (Bar Harbor, Maine USA). Animals were anesthetized with isoflurane (Baxter, Deerfield, IL, USA) and exsanguinated after cervical dislocation before removal of the entire gastrointestinal tracts. The use and treatment of animals was approved by the Institutional Animal Use and Care Committee at the University of Nevada.
A neutralizing, monoclonal antibody (ACK2) against c-Kit was used to disrupt ICC in the colon, as previously reported.13 Briefly, P0–P0.5 animals were injected intraperitoneally five times on alternate days from P0 to P8 with 100 μg of ACK2. Sibling control animals were injected with 100μg of non-immune serum (rat). After treatment, animals were sacrificed on P10 and colons were removed for physiological studies.
Physiological studies
Proximal colons (3×7 mm; 1–3 cm below the ileocecal sphincter) were cut parallel to circular muscle and placed into a recording chamber with circular muscle facing upward. A 3×4 mm section of colon from P10 animals was used. At one end of the tissue, pins were used to isolate 3×2.5 mm of tissue (1×1.5 mm for P10 animals) and the other end was attached to a Gould UC3 force transducer. The force transducer was mounted on an electronically controlled platform (Newport 860 SC) so controlled stretch could be applied to the circular layer. Tissues were allowed to equilibrate for 60 min before experiments were initiated. Length ramps were applied at rates of 6–31 μm s−1 until an isometric force of 5mN was reached (approximately 140% of resting length). Reproducible responses were obtained when ramps were applied as rapidly as 31 μm s−1 every 30 seconds. Typically ramps were applied at a rate of 6 μm s−1 every 10 minutes, which generated reproducible responses in murine tissues.9 Three ramps were performed and responses were averaged for each treatment.
Circular muscle cells were impaled with glass microelectrodes, and transmembrane potentials measured with a high impedance electrometer (Intra 767, World Precision Instruments, Sarasota, FL, USA). Electrical signals were recorded by a PC running AxoScope 9.0 acquisition software (Molecular Devices, Downingtown, PA, USA). Data were digitized at 4KHz with a low pass filter @2KHz. Hard copies were printed using Clampfit software (Molecular Devices) and Corel Draw 12 (Corel; Ontario, Canada).
Solutions and drugs
Muscles were maintained in KRB (37.5±0.5°C; pH 7.3–7.4) containing (in mM): Na+ 137.4; K+ 5.9; Ca2+ 2.5; Mg 1.2; Cl− 134; HCO3− 15.5; H2PO4− 1.2; dextrose 11.5 and bubbled with 97% O2-3% CO2. Atropine sulphate, tetrodotoxin and Nω-nitro-L-arginine (L-NNA), L-methionine and apamin and the NK1 antagonist GR82334 (pGlu-Ala-Asp-Pro-Asn-Lys-Phe-Tyr-Pro(spiro-γ-lactam-Leu-Trp-NH2 (Sigma, St Louis, MO, USA) were dissolved in dH2O. Pinacidil and nifedipine (Sigma) were dissolved in ethanol at 1–100mM. The NK2 antagonist SR48968 (Saredutant; Sanofi-Aventis) was dissolved in DMSO. Drugs were diluted in KRB to the stated concentrations and perfused for 15–20 min before length ramps were applied.
Statistical analysis
Data are expressed as means ± standard errors of the mean. Differences in the data were evaluated by Student’s t test. P values <0.05 were taken as statistically significant. The “n values” reported in the text refer to the number of muscles used for each protocol. Each muscle was taken from a separate animal.
RESULTS
Stretch-dependent changes in electrical activity of the proximal colon
Circular muscle cells of proximal colons from Balb/C mice had resting membrane potentials (RMP) averaging −55±1 mV. Spontaneous action potential (AP) complexes, consisting of bursts of APs 23±1.0 mV in amplitude and 3.6±0.3 APs per complex, occurred at 2.3±0.2 min−1. Each AP complex caused contraction of the circular muscle layer; peak force averaged 0.14±0.01 mN (n=26).
Stretching muscles with length ramps (6.0μm sec−1) until 5mN stress was achieved (approx. 140% of resting length) required 330±12.0s. At 5 mN, muscles were stretched to 139.7±1.3% of resting length (n=6). Since the circumference of the proximal colon averaged 10.0±1.0 mm, this would result in a diameter change of 1.3 mm (i.e. from 3.2–4.5 mm). Stretching muscles caused hyperpolarization, averaging 5.0±1.0 mV (from −55±1 mV to −60±1.0 mV; P<0.05) and AP complexes and contractions were inhibited (Fig. 1A–C). Hyperpolarization and inhibition of AP complexes and contractions occurred during lengthening of muscles. When muscle length after stretching was maintained, muscles slowly adapted and stress relaxed by 1.8±0.01 mN (36%) within 5 min. Membrane potential recovered and AP complexes were restored during the period of adaptation. Phasic contractions were also restored (2.0±0.1 mN) when AP complexes recovered (n=25). Restoration of resting muscle length caused membrane depolarization from −55±1.5 mV to −50±1 mV and AP discharge returned to pre-stretch levels (Fig. 1A–C).
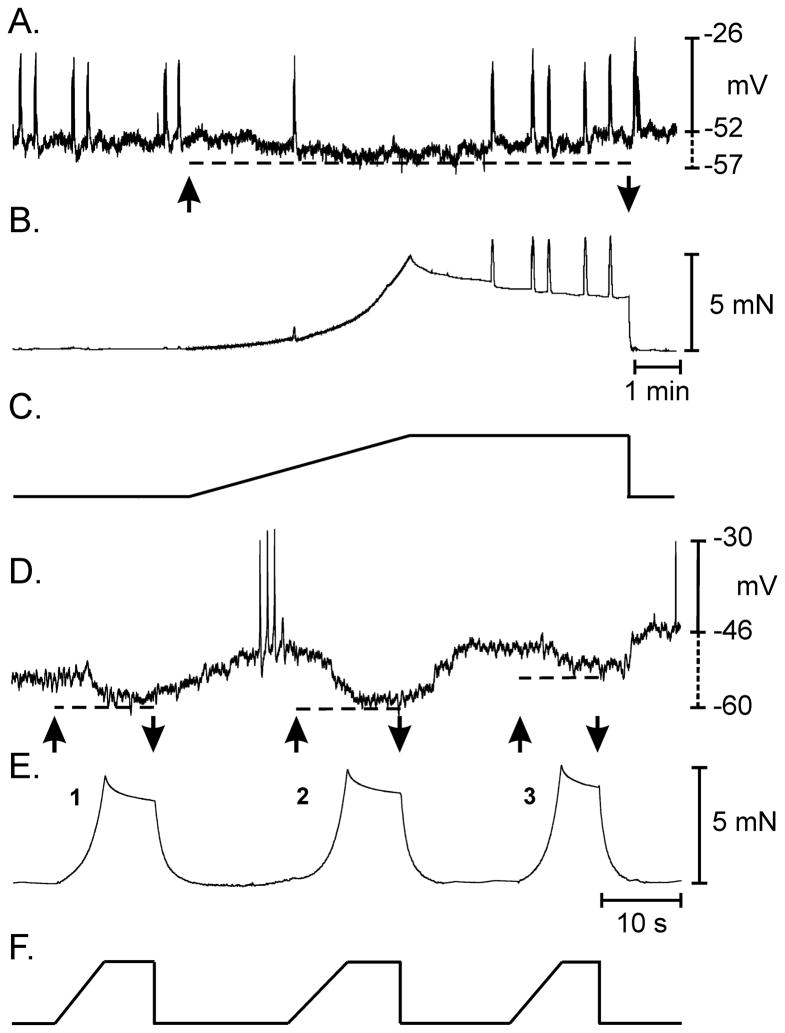
Figure 1.
Stretch-dependent hyperpolarization and inhibition of AP complexes in the murine colon. (A) Membrane potential and superimposed AP complexes of the circular muscle layer, before, during and after application of a muscle stretch ramp that was delivered at 6 μm s−1 until a 5 mN isometric force was reached. (B) Isometric force measurement during applied ramp indicated by the arrows. (C) Stretch ramp applied at a constant rate of 6.0 μm s−1 until 5 mN was reached. The rate of force application was 0.156 mN s−1 and the duration of the active portion of the ramp 320 seconds. Membrane hyperpolarization, indicated by the dashed line in A, and loss of AP complexes occurred during the active change in muscle length. Recovery of membrane potential and return of AP complexes occurred following termination of the active length ramp while muscle length was maintained. Recovery of the change in muscle length often produced a rebound depolarization and an increase in AP discharge for 1–3 cycles before electrical and mechanical activity returned to pre-stretch control activity. (D–F) Effect of repetitive colonic stretch on membrane potential and AP complex discharge. (D & E) Membrane potential and isometric force in response to three rapid stretch applications delivered at 31μm s−1 until an isometric force of 5 mN was reached (arrows in D). Repetitive stretching of the colon (F) caused a reproducible hyperpolarization in membrane potential (dashed lines in D) and inhibition of AP complexes.
Responses to stretch were reproducible with repetitive length ramps
Application of multiple length ramps (applied every 30s until 5mN of stress was obtained during each ramp) showed that responses were reproducible over a wide range in ramp rates (i.e. 6–31μm s−1). Each time muscles were stretched, cells hyperpolarized to the same level, and membrane potential recovered to pre-stretch levels between each length ramp. During each stretch, AP complexes and contractions were inhibited and recovered between length ramps (Fig. 1D–F). Membrane depolarization following return of the length ramp to control levels was observed occasionally. Thus, responses to stretch recovered rapidly between ramps and responses were reproducible, suggesting a robust mechanism that can regulate colonic excitability on a moment-to-moment basis through cycles of contraction and distension.
Muscle tone and stretch-dependent responses in the proximal colon
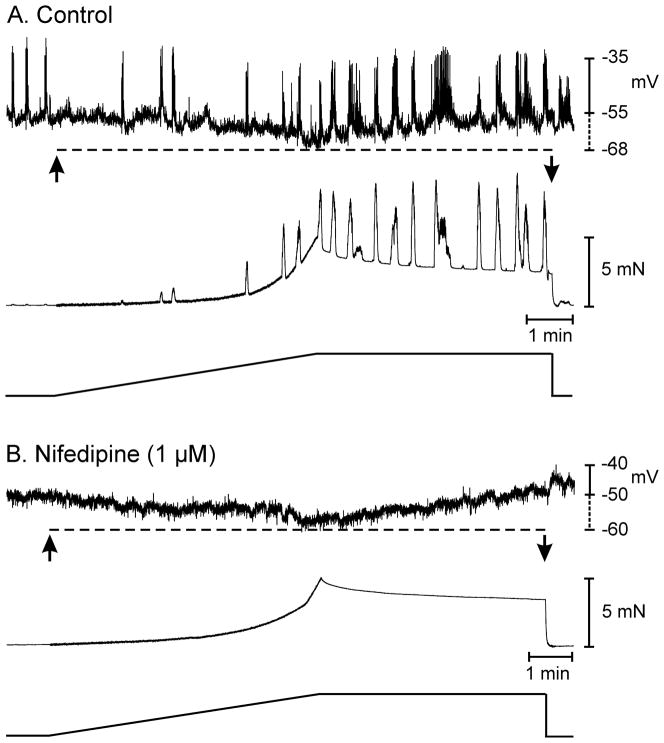
Experiments comparing stretch responses before and in the presence of nifedipine were performed to determine whether muscle tone affected responses to stretch. Nifedipine (1μM) inhibited AP complexes and contractions and increased muscle compliance. In these experiments 5mN of stress developed in 314±8s when muscles were stretched at 6μm s−1. After nifedipine, 5mN of stress was reached in 349±9s during stretch at 6μm s−1 (P<0.05). Before nifedipine, stretch caused hyperpolarization from −53±0.4 mV to −60±1.4 mV (n=4; P<0.05), and the same magnitude of hyperpolarization occurred in nifedipine (7.0±1 mV; n=4, P>0.05). Restoration of resting length resulted in repolarization before and after nifedipine. Thus, resting tone did not influence stretch-dependent hyperpolarization responses (Fig. 2).
Figure 2.
Muscle tone does not alter colonic responses to stretch. (A) Shows membrane potential and isometric force in response to a stretch ramp under control conditions. Application of the stretch ramp (arrows) produced membrane hyperpolarization (dashed line) and inhibition of AP complexes. (B) In the presence of nifedipine, AP complexes were abolished but application of the stretch ramp still produced membrane hyperpolarization (dashed line) in response to stretch dependent changes in muscle length. Nifedipine also increased the compliance of the colonic tissue. In this example, under control conditions 5 mN of force was reached in 335 seconds (A) and after nifedipine 5mN of force was reached in 372 seconds (B).
Stretch responses depend upon input from intrinsic nerves
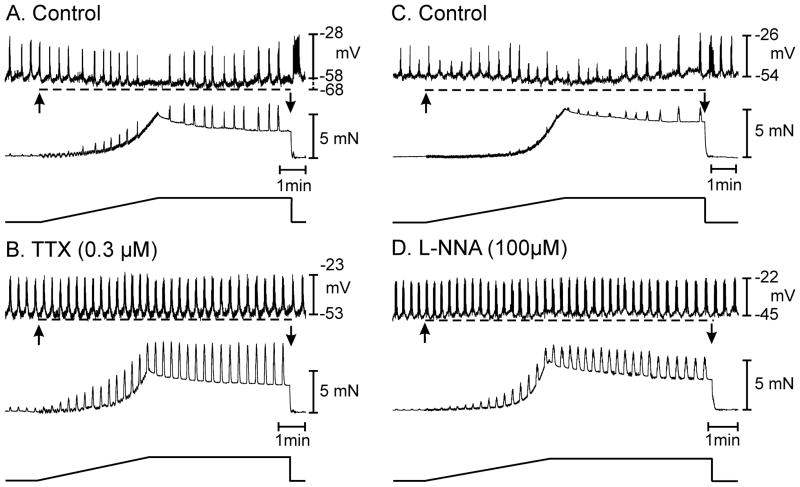
Stretch-dependent regulation of colonic muscle excitability might result from neural reflexes or direct effects of stretch on other cells within the tunica muscularis, such as smooth muscle cells or ICC.4,6,8–10,14,15 We attempted to dissect the nature of the response by applying length ramps before and during exposure to tetrodotoxin (TTX; 0.3μM). TTX caused membrane depolarization, from −58±1.0 to −55±1.0 mV (P<0.05), had a slight but insignificant increase in frequency of AP complexes, from 2.2±0.2 to 2.8±0.4 min−1 (P=0.21), and increased the amplitude and frequency of phasic contractions (Fig. 3A, B; n=6). These results demonstrate the dominant inhibitory drive on colonic muscles from intrinsic motor neurons reported previously.14,16–18 Stretching muscles in TTX had either no effect on membrane potential (n=3 of 5 muscles) or caused depolarization of about 1.5 mV (n=2 of 5 muscles). The effects of TTX on the duration of stretch to reach 5mN were mixed. Length ramps of shorter duration than in control conditions resulted in development of 5mN stress in the 3 muscles that responded to stretch with no change in membrane potential. There was no change in ramp duration to reach 5mN in 1 muscle, and a slightly longer duration ramp to reach this level of stress in the final muscle. Thus, regulation of colonic excitability in response to stretch depends upon input from motor neurons.
Figure 3.
Neuronal mechanisms underlie stretch dependent responses in the proximal colon. (A) Shows typical membrane hyperpolarization (dashed line) and reduction in AP complexes associated with an increase in isometric tension in response to a 5 mN stretch of the circular muscle layer (arrows in A). (B) In the presence of tetrodotoxin (TTX; 0.3 μM) there was a depolarization in membrane potential and an increase in muscle excitability. Application of the stretch ramp in the presence of TTX did not cause membrane hyperpolarization or a decrease in the discharge of AP complexes (dashed line) or a reduction in phasic mechanical activity (B). (C & D) show the role of nitric oxide in stretch dependent membrane hyperpolarization and inhibition of AP complexes. (C) Membrane hyperpolarization, reduction in AP complex discharge and reduction in spontaneous mechanical activity to the stretch ramp under control conditions (C) was abolished by the addition of the nitric oxide synthase inhibitor L-NNA (D). L-NNA (100 μM) caused membrane depolarization from −54 to −45 mV and increased the spontaneous electrical and mechanical activities. During active change in muscle length when the tension ramp was applied there was no change in membrane potential or AP complex discharge (dashed line in D). L-NNA also decreased the compliance of the circular muscle. In the presence of atropine the tension ramp applied at 6 μm s−1 produced force at a rate of 0.015 mN s−1 and required 330 seconds to reach the maximal 5 mN force took 330 seconds to reach 5 mN of force however, after L-NNA this was reached in 246 seconds.
If responses to stretch depended upon afferent and interneuron components of a reflex, then synaptic signaling would be necessary to initiate APs in motor neurons. Afferent input in the enteric nervous system are linked to motor output, in part, via nicotinic, cholinergic receptors on dendrites or somas of motor neurons.19,20 Thus, we tested the effects of hexamethonium on the stretch-dependent responses in the proximal colon. Hexamethonium (300μM) caused depolarization of smooth muscle cells from −53±1 mV to −44±3 mV and increased the frequency of AP complexes (2.1±0.13 to 2.7±0.1 cycles min−1; p<0.05). Stretch in the presence of hexamethonium resulted in membrane hyperpolarization of 5 ± 1 mV compared to control responses of 8±3 mV (n=4; p=0.3). AP complexes and associated contractions persisted in the presence of hexamethonium (i.e. 0.35±0.2 during stretch under control conditions compared to 2.6±0.3 cycles min−1 in hexamethonium; P<0.05). Hexamethonium also slightly decreased the compliance of the circular layer from 330±7s to 305±5s to reach 5mN stress (P<0.05; n=4). These data show that blocking nicotinic receptors did not mimic the effect of TTX, and a portion of the stretch response in the colon is down-stream of an intrinsic reflex (e.g. mediated by responses of motor neurons and/or by responses in post-junctional, non-neural cells).
Inhibitory neural pathways involved in stretch responses
Experiments with TTX suggested that responses to stretch depend upon inputs from neural pathways. We tested whether inhibitory transmitter(s) are responsible for the stretch-dependent effects. Lω-nitro-L-arginine (L-NNA; 100μM) depolarized cells by 9.0±1.3 mV (i.e. from −51±2.0 to −42±2 mV), increased the frequency of AP complexes from 2.5±0.2 to 3.4±0.3 cycles min−1, and increased the force of phasic contractions. Stretching muscles in the presence of L-NNA did not cause significant hyperpolarization (i.e. −42±2.0 mV before stretch and −43±2.0 mV at the peak of the ramp; n=5; P>0.05), and there was no significant effect on the frequency of AP complexes (i.e. from 3.4±0.3 to 3.9±0.3 cycles min−1; P=0.22; n=5). However, the number of APs per complex, increased significantly in response to stretch in the presence of L-NNA (i.e. from 8.7±0.4 before to 11.0±0.9 during stretch; P<0.05; n=5;). L-NNA also decreased compliance of muscle strips. In these experiments, muscles reached 5mN of stress in 325±16s under control conditions and after 278±11s in the presence of L-NNA (P<0.05; n=9). Thus, input from nitrergic neurons regulates basal compliance of colonic muscles and is largely responsible for hyperpolarization and inhibitory responses to stretch (Fig. 3C, D).
The depolarization in membrane potential, observed in the presence of L-NNA could possibly inhibit the stretch-dependent inhibitory responses observed under control conditions. To rule out this possibility we performed an additional series of experiments where muscles were stretched under control conditions, then stretched in the presence of L-NNA, followed by a third stretch in the continued presence of L-NNA and pinacidil in order to restore membrane potential to control levels. Stretching colonic muscles under control conditions hyperpolarized membrane potential from membrane potential from −54.0±1.7 mV to −60.0±1.6 mV (n=5; P=0.02) and abolished action potential complexes. In L-NNA membrane potential depolarized to −42.5±1.1 mV and stretch had no effect (i.e. −41.3±1.4 mV; P=0.52). In the continued presence of L-NNA, pinacidil (0.1μM) restored RMP to −58.8±2.9 mV, but stretch did not affect RMP (−57.7±2.7 mV; P=0.95). Therefore, membrane depolarization alone did not block stretch-dependent inhibitory responses (Fig. S1).
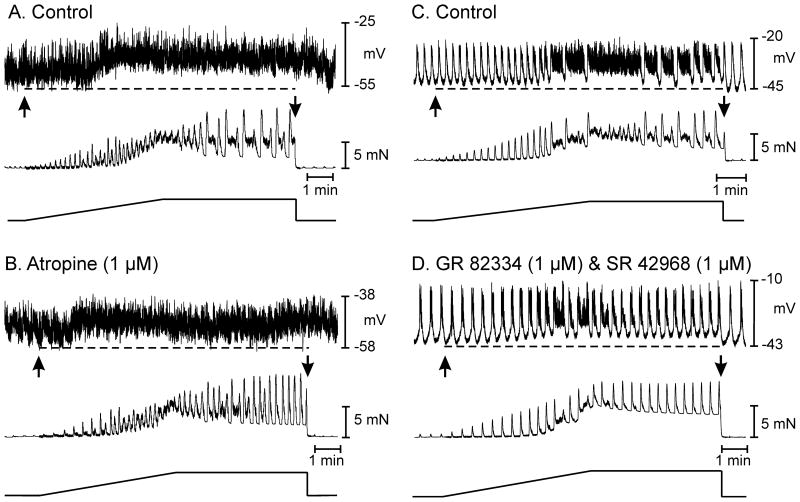
Blockade of muscarinic receptors had little effect on membrane potential or AP complexes. Addition of L-NNA in the presence of atropine (1μM) caused depolarization of 8±1mV (i.e. from −55±1 to −46±1 mV; n=6; P<0.001) and increased AP complexes (from 1.3±0.2 to 3.4±0.2 cycles min−1; P<0.001). Stretch-dependent responses were blocked in the presence of atropine and L-NNA (i.e. RMP was −46±1 mV with atropine present and −44±2 mV during stretch after atropine and L-NNA; n=6; P=0.4; Fig. S2).
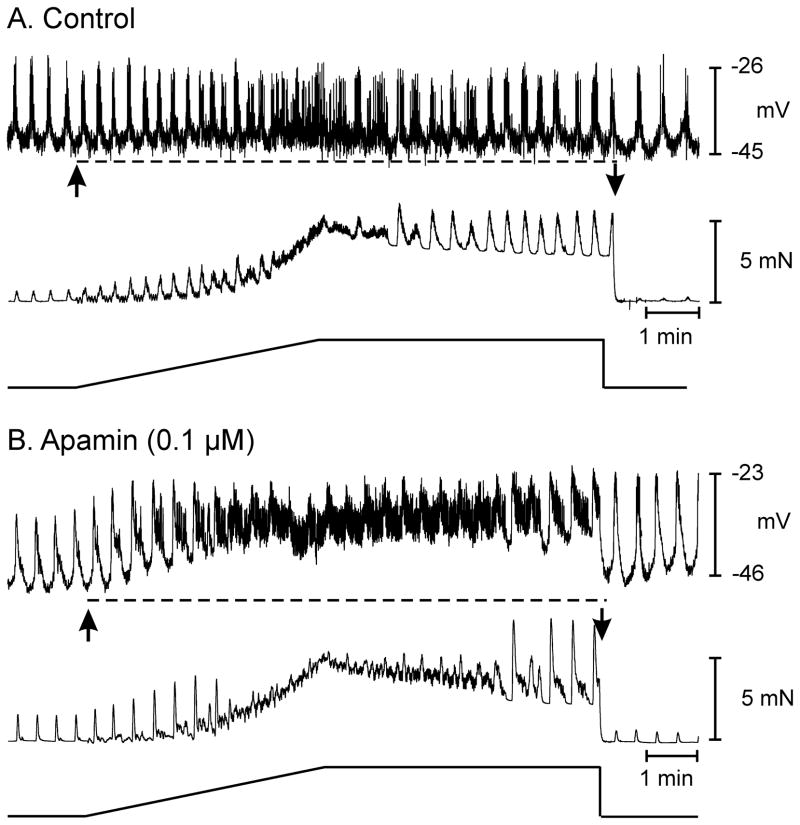
Purines also mediate part of the enteric inhibition colonic muscles via post-junctional P2Y1 receptors and small-conductance Ca2+ activated K+ (SK) channels. 21–34 SK channels are inhibited by apamin, so experiments to assess contributions from this pathway to stretch responses were performed. In these experiments, cholinergic and nitrergic responses were blocked with atropine and L-NNA. Apamin (0.1μM) depolarized cells from −46±1 mV to −41±2 mV (n=6; P<0.05) and increased the amplitude of phasic contractions. Apamin did not affect the frequency of AP complexes or the number of APs per complex (i.e. 2.8±0.2 before and 2.9±0.2 cycles min−1 after apamin; P>0.05). Stretch in the presence of apamin did not produce significant changes in membrane potential (i.e. from −41±2 to −37±2 mV; n=6; P=0.19) but greatly increased the occurrence of APs, making it difficult to distinguish AP complexes. The number of APs increased from 21.5±3.4 before to 35±4 min−1 during the ramp protocol; P<0.05; n=6). Associated with the increase in APs was an increase in the amplitude and frequency of phasic contractions (Fig. 4A, B). Apamin also decreased the compliance of the muscle by 15 ±2 seconds to reach 5 mN force development.
Figure 4.
An apamin sensitive ion conductance contributes to stretch-dependent responses in the proximal colon. (A) Shows electrical activity and associated isometric force in response to a 5 mN tension ramp under control conditions, i.e. atropine (1 μM) and L-NNA (100 μM) to remove any contribution of cholinergic or nitrergic neural inputs. Stretch-dependent membrane hyperpolarization (dashed line in A) was absent in the presence of L-NNA and atropine. (B) Shows the response of the same muscle preparation after the addition of apamin (0.1 μM). Active change in muscle length caused depolarization in membrane potential and caused a fusion of AP complexes into a continuous discharge of APs (dashed line in B) that was associated with an increase in the frequency of phasic mechanical contractions but a decrease in maximal force production. Maintaining the stretch at 5 mN force caused a gradual return in phasic electrical and mechanical activity that immediately returned to pre-stretch control activity following release of the tension ramp.
Excitatory neural pathways involved in stretch dependent responses
The contributions of cholinergic motor neurons were evaluated by applying stretch before and during exposure to atropine. Atropine (1μM), which blocks cholinergic responses in the murine colon,25 did not affect resting membrane potential (i.e. −51±1.0 before and −52±2.0 mV in the presence of atropine; n=8; P>0.05). Atropine also did not affect the frequency of AP complexes (1.4±0.4 before and 1.0±0.4 cycles min−1 in atropine; n=8; P>0.05). Stretch in atropine caused membrane hyperpolarization averaging 6.0±2 mV (i.e. from −52±2 to −58±2 mV; P<0.05; n=8) and inhibition of AP complexes and contractions. Atropine increased the compliance of the circular muscle layer. Under control conditions 313±7s length ramps were required to reach 5mN of stress; after atropine ramps of 348±10 s reached 5mN (P<0.01; n=8).
L-NNA (100μM) and apamin (0.1μM) produced membrane depolarization, as above (i.e. from −54±2 to −42±3 mV; n=3). Nearly continuous discharge of APs was observed in the presence of L-NNA and apamin. Stretch responses were tested before and after addition of atropine and the NK1 and NK2 antagonists GR82334 and SR48968. Stretch of the circular layer in L-NNA and apamin caused membrane depolarization (from −42±3 to −33±2 mV; P<0.01; n=6), increased the frequency of APs from 44±5 to 65±4 per min, and increased the frequency of phasic contractions. Large amplitude phasic contractions interposed with smaller rapid phasic contractions characterized contractile responses under these conditions. Maintaining stretch on muscles after application of ramps led to sustained depolarization, but partial recovery of the mechanical activity.
Muscle stretch in the presence of L-NNA and apamin revealed significant excitatory responses consisting of membrane depolarization and increased AP frequency. Atropine (1μM) did not produce a significant change in membrane potential under basal conditions, but reduced stretch-dependent depolarization by 5±1.5 mV (i.e. from −33±2 to −37±1 mV; n=3; P<0.05). Atropine did not significantly affect the increase in the frequency of APs or the increase in phasic contractions in response to stretch (Fig. 5A, B).
Figure 5.
In the presence of L-NNA and apamin, stretch-dependent excitatory responses are revealed. (A) Shows electric, mechanical and length ramp, respectively in the presence of L-NNA (100 μM) and apamin (0.3 μM), application of length ramps produced membrane depolarization and increase in phasic motor activity. The depolarization (dashed line in A) and increase in phasic mechanical activity in response to stretch was partially inhibited by atropine (B), suggesting that cholinergic motor input contributed to part of the excitatory response observed in the presence of L-NNA and apamin. Neurokinin release from enteric motor nerves contributes to depolarization response. (C) Shows depolarization in membrane potential (dashed line) and increase in electrical activity in response to an active stretch ramp, observed in the presence of L-NNA (100 μM), apamin (0.1 μM) and atropine (1 μM). (D) Shows membrane depolarization (dashed line) in response to stretch was significantly attenuated by the NK1 and NK2 receptor antagonists GR 82334 (1 μM) and SR 48968 (1 μM).
The stretch-dependent response remaining after atropine, L-NNA and apamin were blocked by neurokinin receptor antagonists. Stretch in atropine, L-NNA and apamin resulted in depolarization from −44±1 to −39±1 mV (P<0.05) and a continuous discharge of APs (47±4 min−1; n=6), as described above. In some cases re-formation of AP complexes occurred after sustained stretch (Fig. 5C). GR82334 (1μM) and SR48968 (1μM) did not affect basal membrane potential or action potential discharge, but inhibited membrane depolarization and the increase in APs in response to stretch (Fig. 5D; n=3). The NK2 antagonist (SR48968) alone did not inhibit stretch-dependent responses (n=3), suggesting that a portion of the stretch-dependent excitatory response, unmasked after block of inhibitory neural pathways, was mediated by NK1 receptors.
Channels responsible for dominant inhibitory response to stretch
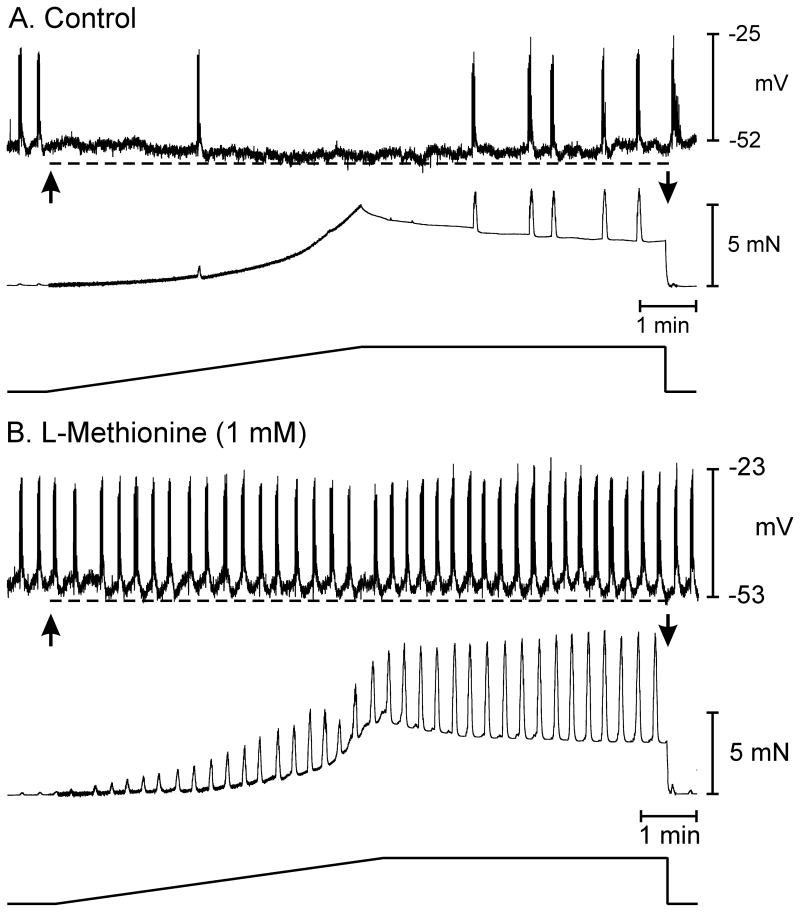
The response to stretch in colonic muscles is complex, involving both inhibitory and excitatory pathways. The predominant, localized response in the region of muscle being stretched is hyperpolarization and stabilization of membrane potential. Previous studies have shown that stretch-dependent K+ (SDK) channels are expressed in colonic smooth muscle cells and ICC that could potentially be activated during cell elongation.10,26 SDK channels are relatively voltage insensitive but activated by NO,18 so these channels may participate in stretch-dependent hyperpolarization and stabilization of membrane potential. Previous studies have shown that SDK channels are blocked by sulfur-containing amino acids, such as L-methionine, and this compound has also been shown to inhibit the SDK channels activated during nitrergic stimulation in murine colonic muscles.12 We tested the effects of L-methionine on stretch-dependent responses. In these experiments stretch caused hyperpolarization averaging 5±1 mV and reduced the frequency of AP complexes from 2.7±0.2 to 0.9±0.3 min−1 under control conditions (n=4; P<0.05). L-methionine (1mM) depolarized the circular muscle cells by 7±2 mV, enhanced AP complex frequency from 2.3±0.2 to 3.1±0.3 cycles min−1 and increased phasic contractions. Stretch in the presence of L-methionine did not cause hyperpolarization (i.e. −48±1 mV before and −49±1 mV after stretch) nor decrease the frequency of AP complexes (3.1±0.3 before and 2.9±0.5 cycles min−1 with L-methionine; Fig.6A, B). A decrease in muscle compliance occurred during stretch in the presence of L-methionine, taking 40±4 seconds to reach 5 mN of force.
Figure 6.
L-Methionine blocks membrane hyperpolarization and reduction in AP complexes in response to stretch. (A) Under control conditions, application of the stretch ramp led to hyperpolarization in membrane potential (dashed line) and loss of spontaneous AP complexes and phasic contractions. (B) In the presence of L-methionine (1 mM), AP complexes and associated phasic contractions were more pronounced (B). Application of the 5 mN stretch ramp in the presence of L-methionine blocked membrane hyperpolarization (dashed line) and did not lead to loss of spontaneous contractions.
As shown above, TTX blocked stretch-dependent hyperpolarization and stabilization of membrane response. TTX caused membrane depolarization and increased AP complex frequency and contractions. In the presence of TTX, L-methionine (1mM) had no significant effect on membrane potential (−52±3 before and −49±3 mV in the presence of L-methionine; n=4; P=0.4) or the frequency of AP complexes (i.e. 2.4±0.3 min−1 before and 2.7±0.2 min−1 in the presence of L-methionine; P=0.8; n=4). Stretch in the presence of TTX and L-methionine did not affect membrane potential or increase the frequency of AP complexes (n=4).
Stretch-dependent response is sensitized by nitric oxide donors
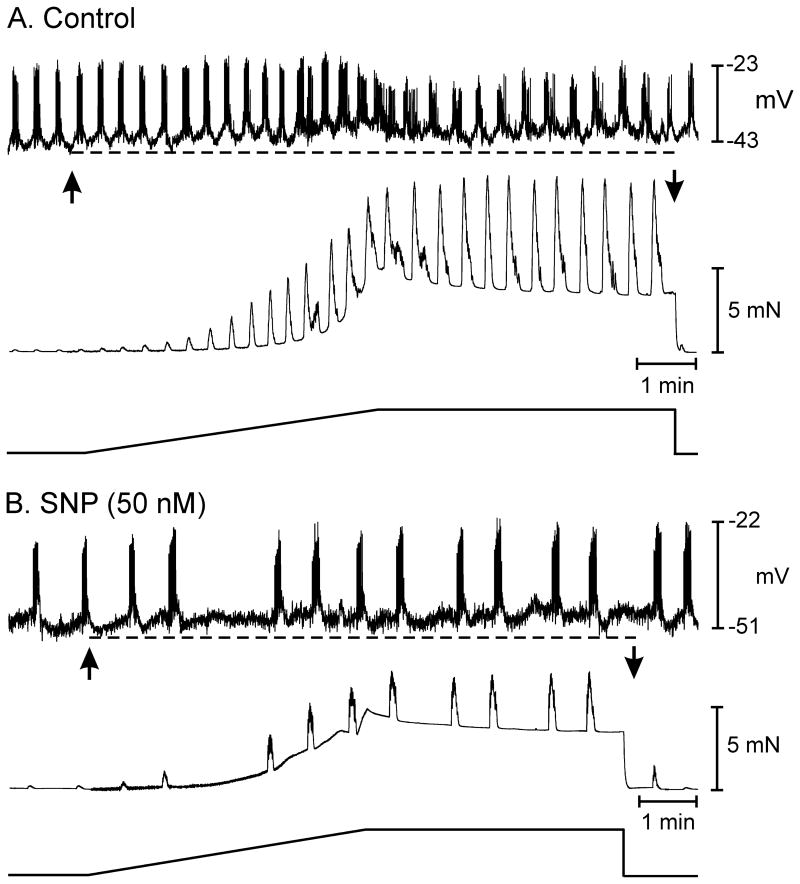
The effects of L-methionine on membrane potential and responses to stretch implicate a role for SDK channels in tonic inhibitory drive imposed on colonic muscles. It is possible these channels are the final mediators in stretch-dependent stabilization of membrane potential and their open probability increases in response to NO released from enteric inhibitory neurons during stretch. An alternative possibility is that stretch of colonic muscles sensitizes SDK channels to activation by NO. We examined the effects of stretch in the presence of L-NNA and atropine before and after sodium nitorprusside (SNP) to test the latter hypothesis. The level of SNP was titrated (50–100nM) to just reverse the depolarization and increase in APs caused by L-NNA (see Fig. 3). Thus, we attempted to restore, approximately, the level of NO lost when muscles were treated with L-NNA.
In these experiments membrane potential and electrical activity after L-NNA (100μM) and atropine (1μM) were as above (i.e. RMP averaged −43±1 mV and AP complexes with an average AP amplitude of 15±2 mV occurred at a frequency of 3.5±0.1 min−1 (n=5). Length ramps in the presence of L-NNA and atropine caused depolarization to −37±1 mV (P<0.05) and increased AP discharge to 10±1.2 per AP complex. AP complexes could not be resolved in some muscles because activity developed into a continuous discharge of APs. In the continued presence of L-NNA and atropine, SNP (50–100 nM) caused hyperpolarization (to −48±2.5 mV) and decreased the frequency of APs or inhibited spike activity (n=5). Application of the length ramp under these conditions caused membrane hyperpolarization to −52±3 mV compared to membrane depolarization during the stretch ramp before SNP (Fig. 7 A, B; P<0.05). AP complexes were reduced or inhibited during the length ramp. In two experiments where APs were inhibited by SNP, membrane hyperpolarization occurred during the length ramp. Thus, stereotypic responses to stretch occurred in the presence of exogenous NO with intrinsic release of neurotransmitter NO inhibited.
Figure 7.
The nitric oxide donor SNP sensitizes stretch-dependent inhibitory responses in the murine colon. (A) Shows excitatory electrical and mechanical responses in the presence of L-NNA and atropine to an increase in circular muscle length. In L-NNA and atropine there was membrane depolarization (dashed line) and an increase in AP complexes. (B) Shows the response of the same tissue in the continued presence of L-NNA and atropine and the addition of sodium nitroprusside (50 nM). In the presence of SNP the excitatory response observed in L-NNA and atropine was changed to an inhibitory one characterized by inhibition of AP complexes.
Cells responsible for the stretch-dependent responses
TREK-1 channels appear to be involved in stretch-dependent responses and been shown to be expressed in smooth muscle cells and ICC.26 Inhibitory motor nerves in the circular layer are closely associated with intramuscular ICC (ICC-IM),27–30 and ICC-IM express soluble guanylate cyclase and signaling molecules required for transduction of nitrergic inhibitory responses.31–33 Animals lacking ICC-IM have suggested that these cells mediate a portion of the nitrergic responses.34–36 Thus, we hypothesized that ICC-IM might mediate stretch-dependent inhibitory responses in colonic muscle.
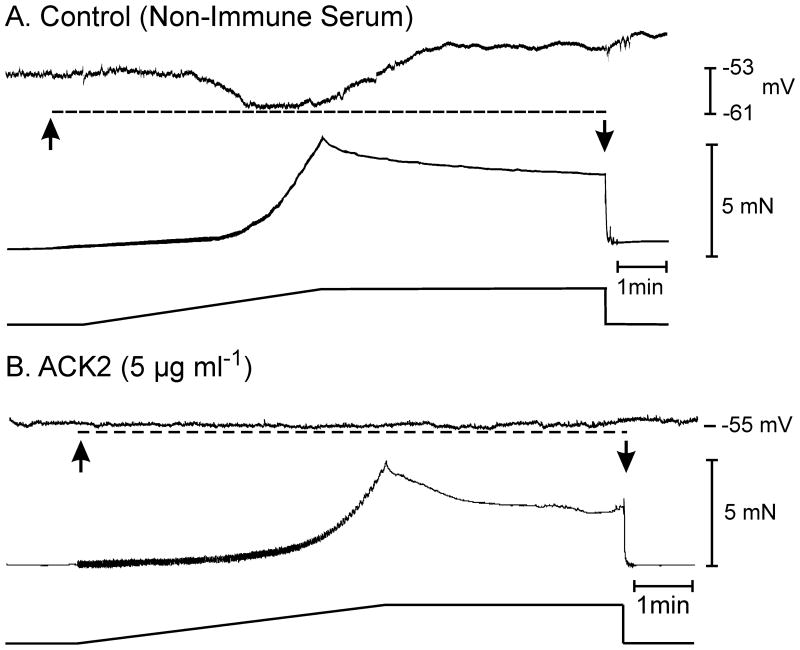
Experiments were performed after treatment of muscles with a neutralizing antibody for Kit (ACK2), which we have shown causes loss of ICC in the colons.13 Control muscles (P10) had resting membrane potentials of −53±2 mV and infrequent AP complexes, as reported previously.37 Application of length ramps caused hyperpolarization (−53±2 mV to −62±3 mV; Fig. 8; n=5, P<0.05). After ACK2 (5 injections; 100μg in 100μl saline; between P0–P10), membrane potentials averaged −56±1 mV. Occasional APs were observed, but not organized into complexes. Application of length ramps caused no significant change in membrane potential (from −56±1 mV to −58±2 mV; Fig. 8; n=7, P>0.05). In the presence of L-NNA, stretching muscles after ACK2 did not cause depolarization (n=4). These data demonstrate that part of the membrane stabilization responses in the colon is likely to be mediated via ICC.
Figure 8.
Blocking the development and maintenance of interstitial cells of Cajal in the murine colon with Kit neutralizing antibody attenuates the stretch-dependent inhibitory responses of the colon. (A) Membrane hyperpolarization (dashed line) and mechanical activity in response to an increase in circular muscle length (arrows) in a control, non-immune serum treated P10 animal. Spontaneous electrical and mechanical activities were intermittent in P10 animals as previously reported.37 (B) In the colons of P10 animals injected with the neutralizing antibody ACK2 (5 μg ml−1, spontaneous electrical and mechanical activities were absent and stretch-dependent responses were greatly reduced or absent (dashed line) is response to a stretch ramp.
A summary of the changes in membrane potential and circular muscle compliance that occurs in response to stretch in the presence and absence of specific antagonists is provided in Supplement table 1.
DISCUSSION
Mechanosensitive neural reflexes modulate the contractile behavior and movement of luminal contents in the GI tract.7,38,39 Mechanosensitive neurons exist in both the small and large intestines,4,6 and neural reflexes have been studied in vivo and in vitro.40 Enteric inhibitory neurons are also active tonically in many regions of the gut, imposing inhibitory drive and limiting spontaneous contractile activity. Neurotoxins, receptor antagonists, or blockade of post-junctional ion channels mediating inhibitory responses depolarize GI muscles, increase action potentials and augment contractions.24,41 Our data show that responses of colonic muscle strips to stretch are superimposed upon ionic mechanisms activated by inhibitory neurotransmitters. Stretch responses require tonic release of NO, but replacement of NO synthesized by neurons is capable of supporting stretch responses. We interpret these findings to mean that mechanosensitive ion channels in post-junctional cells, such as ICC-IM, modulate the gain on enteric neural inhibitory input to the colon.
We first considered that stretch-dependent inhibitory responses in the colon were due to a neural reflex because the responses were blocked by TTX and L-NNA. Stretching muscles may elicit AP-dependent nitrergic motor neurotransmission via activation of stretch receptors in an afferent pathway or within the myenteric plexus. However, we found little or no evidence for a stretch-dependent neural reflex in the small strips of muscle utilized in our experiments. Hexamethonium, which blocks nicotinic receptors and a majority of inter/motor neuron neurotransmission,42 depolarized membrane potential but did not block stretch-induced membrane hyperpolarization.
Tonic inhibitory drive in murine colon is mediated by at least 2 inhibitory neurotransmitters (NO and a purine, possibly β-NAD).24 NO and a purine are co-transmitters likely released from the same enteric motor neurons, and in our experiments L-NNA or apamin caused additive depolarization and increased action potential discharge. Stretch responses were blocked by L-NNA or L-methionine, suggesting that NO-dependent mechanisms are primarily responsible. If reflex firing of inhibitory motor neurons was responsible for stretch-dependent responses, then purine-dependent inhibitory effects, which produce greater hyperpolarization than NO,22 would be manifest in addition to nitrergic effects. However, blockade of NO synthesis totally blocked stretch-dependent hyperpolarization and cessation of APs. When NO was restored to approximately the levels achieved by tonic firing of enteric motor neurons by titrating exogenous SNP to levels in which electrical activity matched control activity, stretch-dependent responses were rescued. It also appears that responses to stretch were mediated by ICC because loss of these cells abolished stretch responses. Taken together our data suggest that local response to stretch may not depend upon a neural reflex per se. Instead stretch enhances the sensitivity of muscles to NO, possibly by a synergistic action on SDK channels.
Colonic smooth muscle cells10 and ICC (unpublished observations) express SDK channels with a unitary conductance of 95 pS. Activation of these channels could mediate stabilization of membrane potential, as observed when muscles were stretched. Normally, SDK channels have low open probabilities, but when membranes are stretched (negative pressure in patch pipettes or elongation of cells) the open probably of these channels increases dramatically.10 SDK channels are likely to be encoded by TREK-1 and TREK-2, members of the two-pore K+ channel family26,44 that are expressed in colonic cells.10 SDK and homologously expressed TREK-1 channels are insensitive to classical K+ channel blockers,10,43 but these channels are blocked by sulphur-containing amino acids, such as L-cysteine, L-methionine or DL-homocysteine.12 L-methionine is the most selective drug, and it displayed little or no effect on other K+ conductances expressed in colonic myocytes.12
Exogenous NO, like NO released from inhibitory motor neurons, activates TREK (SDK) channels via cGMP-dependent protein kinase (PKG) and phosphorylation of channels at Ser-351 (S351A).10 We propose that NO, released tonically from enteric inhibitory nerve terminals, causes c-GMP-dependent phosphorylation and increases the open probability of TREK channels (causing the net hyperpolarizing drive on colonic muscles revealed by TTX, L-NNA or L-methionine blocking spontaneous APs in motor neurons, synthesis of NO or TREK channels). Stretch of cells with pre-phosphorylated SDK channels further increases the open probability of these channels, leading to hyperpolarization and stabilization of membrane potential. It is possible that pre-phosphorylation and stretch produce synergistic effects on the activation of TREK channels, and this is why stretch responses are only apparent in the presence of NO (either released from neurons or added to the bath), but not observed when NO synthesis is blocked.
It should be noted that colonic smooth muscle cells express ion channels in addition to TREK that respond to stretch. L-type Ca2+ channels and voltage-gated Na+ channels have been reported to be activated by stretch or shear forces in GI muscles.8,44 The Ca2+ channels are nifedipine sensitive and identified in the human small intestine as CaV1.2.45 Neither of these cation channels could generate the stretch responses we observed since their activation would yield inward currents, depolarization, and increased APs. Although nifedipine increased muscle compliance, as reported previously,16,46 stretch-dependent hyperpolarization was unaffected by nifedipine.
Hyperpolarization and stabilization of membrane potential were the dominant responses to stretch, but a 2nd stretch-dependent response was revealed when L-NNA and apamin were present. In this case the response to stretch changed to a significant excitatory response characterized by depolarization and an increase in action potential frequency. This excitatory response is similar to the stretch response observed in the gastric antrum.9 It should also be noted when continuous APs were generated, discrete phasic contractions that are normally associated with AP complexes, were abolished and a large increase in tone was observed. The excitatory response to stretch may be equivalent to the well-described ‘myogenic response’ in smooth muscle,1 however, we have not yet investigated the nature of this response.
The role of intramuscular ICC (ICC-IM) in stretch-dependent responses in the stomach was tested using W/WV mutant animals that lack this class of ICC.9,27–29 The role of ICC-IM in stretch-dependent responses in the colon, however, is more difficult to test because ICC-IM are reduced but not absent in colons of W/WV animals.47 Kit is essential for development and maintenance of ICC phenotype,48 so we used Kit neutralizing antibodies to lesion ICC in the colon.13 In animals treated with Kit antibodies, stretch responses were significantly decreased in colonic muscles, suggesting that the post-junctional mechano-receptor mechanism is located predominantly in ICC. ICC are the cells in which guanylyl cyclase, the receptor for NO, is expressed dominantly in GI muscles.49 Another possible role of ICC in stretch-dependent responses was demonstrated by showing that Ca2+ transients in ICC are potentiated and synchronized during migrating motor complexes in the mouse colon (CMMC).50
In summary, in addition to the well-described neural reflexes that respond to stretch and tone in the GI tract, we have observed a novel type of mechano-regulation in colonic muscles. In the colon, where reservoir function is important for storage of fecal material and recovery of water, stretch of the tunica muscularis results in hyperpolarization and stabilization of membrane potential. This reflex facilitates filling of the colonic lumen without initiation (or enhancement) of contractile activity. The stretch responses we observed depend upon the tonic release of NO from enteric motor neurons, but do not require an increase in motor neuron firing as part of a sensory reflex to stretch. NO increases open probability of TREK channels in colonic muscles, and stretch further activates these channels, possibly in a synergistic manner with cGMP-dependent phosphorylation. It appears that the stretch response is housed in ICC, because it was abolished in animals with reduced ICC populations. This mechanism may also be present in other regions of the GI tract or in other organs, such as bladder and uterus, where TREK channels regulate excitability of smooth muscle cells.
Supplementary Material
Repolarization of membrane potential in L-NNA does not restore stretch-dependent inhibitory responses. (A) Stretch under control conditions stretch of circular muscle produces hyperpolarization in membrane potential (dashed line in upper panel) that recovered when stretch was relieved (middle and lower panels). (B) In the presence of L-NNA (100 μM), stretch did not produce membrane hyperpolarization observed under control conditions. (C) In the continued presence of L-NNA, pinacidil (0.1 μM) hyperpolarized membrane potential to control levels. Stretch of the circular muscle in the presence of L-NNA and pinacidil, when membrane potential was restored to control levels did not produce membrane hyperpolarization. These data suggest that membrane depolarization alone did not inhibit stretch-dependent inhibitory responses.
Effect of atropine on stretch-dependent responses in the proximal colon. (A) Shows membrane potential hyperpolarization (dashed line) and isometric force recordings (middle panel) in response to a stretch ramp that was delivered at 6 μm s−1 until a 5 mN isometric force was reached (lower panel). In this muscle preparation the 5 mN force was reached in 307 seconds. (B) Shows changes in membrane potential (dashed line) and isometric force measurement (middle panel) in response to a stretch ramp recorded from the same muscle preparation in the presence of atropine (1 μM). Atropine had no effect on membrane potential but had a slight but not significant decrease in the frequency of AP complexes. Stretch ramps in the presence of atropine caused membrane hyperpolarization and abolished any further AP discharge until the active change in length ramp was ceased. Atropine also increased the compliance of the circular muscle. In the presence of atropine the muscle preparation required 315 seconds to reach 5 mN of isometric force.
Acknowledgments
This work was supported by NIH DK57236 to SMW and DK41315 to KMS and SMW.
Footnotes
CONFLICT OF INTEREST
AUTHOR CONTRIBUTION
K-JW, SMW and KMS designed the research study. K-JW performed the research in the study. K-JW and SMW analyzed the data. K-JW, SMW and KMS wrote the manuscript. SMW and KMS reviewed the paper.
References
- 1.Bulbring E. Smooth muscle potentials recorded in the taenia coli of the guinea pig. J Physiol. 1954;123:55P–56P. [PubMed] [Google Scholar]
- 2.Burnstock G, Prosser CL. Responses of smooth muscles to quick stretch: relation of stretch to conduction. Am J Physiol. 1960;198:921–925. doi: 10.1152/ajplegacy.1960.198.5.921. [DOI] [PubMed] [Google Scholar]
- 3.Johansson B. Myogenic tone and reactivity: definitions based on muscle physiology. J Hypertens Suppl. 1989;7:S5–S9. [PubMed] [Google Scholar]
- 4.Kunze WA, Furness JB, Bertrand PP, et al. Intracellular recording from myenteric neurons of the guinea-pig ileum that respond to stretch. J Physiol. 1998;506:827–842. doi: 10.1111/j.1469-7793.1998.827bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spencer NJ, Hennig GW, Smith TK. Stretch-activated neuronal pathways to longitudinal and circular muscle in guinea pig distal colon. Am J Physiol. 2003;284:G231–G241. doi: 10.1152/ajpgi.00291.2002. [DOI] [PubMed] [Google Scholar]
- 6.Spencer NJ, Smith TK. Mechanosensory S-neurons rather than AH-neurons appear to generate a rhythmic motor pattern in guinea-pig distal colon. J Physiol. 2004;558:577–596. doi: 10.1113/jphysiol.2004.063586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith TK, Spencer NJ, Hennig GW, et al. Recent advances in enteric neurobiology: mechanosensitive interneurons. Neurogastroenterol Motil. 2007;19:869–878. doi: 10.1111/j.1365-2982.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- 8.Strege PR, Ou Y, Sha L, et al. Sodium current in human intestinal interstitial cells of Cajal. Am J Physiol. 2003;285:G1111–G1121. doi: 10.1152/ajpgi.00152.2003. [DOI] [PubMed] [Google Scholar]
- 9.Won K-J, Sanders KM, Ward SM. Stretch induced changes in pacemaker frequency are mediated through ICC in the murine gastric antrum. Proc Natl Acad Sci U S A. 2005;102:14913–14918. doi: 10.1073/pnas.0503628102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koh SD, Sanders KM. Stretch-dependent potassium channels in murine colonic smooth muscle cells. J Physiol. 2001;533:155–163. doi: 10.1111/j.1469-7793.2001.0155b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koh SD, Monaghan K, Sergeant GP, et al. TREK-1 regulation by nitric oxide and cGMP-dependent protein kinase. An essential role in smooth muscle inhibitory neurotransmission. J Biol Chem. 2001;276:44338–44346. doi: 10.1074/jbc.M108125200. [DOI] [PubMed] [Google Scholar]
- 12.Park KJ, Baker SA, Cho SY, et al. Sulfur-containing amino acids block stretch-dependent K+ channels and nitrergic responses in the murine colon. Br J Pharmacol. 2005;144:1126–1137. doi: 10.1038/sj.bjp.0706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torihashi S, Ward SM, Nishikawa S, et al. c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res. 1995;280:97–111. doi: 10.1007/BF00304515. [DOI] [PubMed] [Google Scholar]
- 14.Maggi CA, Manzini S, Meli A. Contribution of neurogenic and myogenic factors in the response of rat proximal colon to distension. Am J Physiol. 1987;252:G447–G457. doi: 10.1152/ajpgi.1987.252.4.G447. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto Y, Suzuki H. Two types of stretch-activated channel activities in guinea-pig gastric smooth muscle cells. Jap J Physiol. 1996;46:337–345. doi: 10.2170/jjphysiol.46.337. [DOI] [PubMed] [Google Scholar]
- 16.Spencer NJ, Bywater RA, Holman ME, et al. Inhibitory neurotransmission in the circular muscle layer of mouse colon. J Auton Nerv Syst. 1998;70:10–14. doi: 10.1016/s0165-1838(98)00045-9. [DOI] [PubMed] [Google Scholar]
- 17.Mule F, D’Angelo S, Serio R. Tonic inhibitory action by nitric oxide on spontaneous mechanical activity in rat proximal colon: involvement of cyclic GMP and apamin-sensitive K+ channels. Br J Pharmacol. 1999;127:514–520. doi: 10.1038/sj.bjp.0702537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinning PG, Szczesniak M, Cook IJ. Removal of tonic nitrergic inhibition is a potent stimulus for human proximal colonic propagating sequences. Neurogastroenterol Motil. 2006;18:37–44. doi: 10.1111/j.1365-2982.2005.00724.x. [DOI] [PubMed] [Google Scholar]
- 19.Schneider DA, Perrone M, Galligan JJ. Nicotinic acetylcholine receptors at sites of neurotransmitter release to the guinea pig intestinal circular muscle. J Pharmacol Exp Ther. 2000;294:363–369. [PubMed] [Google Scholar]
- 20.Nurgali K, Furness JB, Stebbing MJ. Analysis of purinergic and cholinergic fast synaptic transmission to identified myenteric neurons. Neuroscience. 2003;116:335–347. doi: 10.1016/s0306-4522(02)00749-2. [DOI] [PubMed] [Google Scholar]
- 21.Bayguinov O, Hagen B, Bonev AD, et al. Intracellular calcium events activated by ATP in murine colonic myocytes. Am J Physiol. 2000;279:C126–C135. doi: 10.1152/ajpcell.2000.279.1.C126. [DOI] [PubMed] [Google Scholar]
- 22.Hirst GD, Bywater RA, Teramoto N, et al. An analysis of inhibitory junction potentials in the guinea-pig proximal colon. J Physiol. 2004;558:841–855. doi: 10.1113/jphysiol.2004.065052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallego D, Hernandez P, Clave P, et al. P2Y1 receptors mediate inhibitory purinergic neuromuscular transmission in the human colon. Am J Physiol. 2006;291:G584–G594. doi: 10.1152/ajpgi.00474.2005. [DOI] [PubMed] [Google Scholar]
- 24.Mutafova-Yambolieva VN, Hwang SJ, Hao X, et al. Beta-nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc Natl Acad Sci U S A. 2007;104:16359–16364. doi: 10.1073/pnas.0705510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bywater RA, Small RC, Taylor GS. Neurogenic slow depolarizations and rapid oscillations in the membrane potential of circular muscle of mouse colon. J Physiol. 1989;413:505–519. doi: 10.1113/jphysiol.1989.sp017666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders KM, Koh SD. Two-pore-domain potassium channels in smooth muscles: new components of myogenic regulation. J Physiol. 2006;570:37–43. doi: 10.1113/jphysiol.2005.098897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burns AJ, Lomax AE, Torihashi S, et al. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci U S A. 1996;93:12008–12013. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beckett EA, Horiguchi K, Khoyi M, et al. Loss of enteric motor neurotransmission in the gastric fundus of Sl/Sl(d) mice. J Physiol. 2002;543:871–887. doi: 10.1113/jphysiol.2002.021915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki H, Ward SM, Bayguinov YR, et al. Involvement of intramuscular interstitial cells in nitrergic inhibition in the mouse gastric antrum. J Physiol. 2003;546:751–763. doi: 10.1113/jphysiol.2002.033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward SM, McLaren GJ, Sanders KM. Interstitial cells of Cajal in the deep muscular plexus mediate enteric motor neurotransmission in the mouse small intestine. J Physiol. 2006;573:147–159. doi: 10.1113/jphysiol.2006.105189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shuttleworth CW, Xue C, Ward SM, et al. Immunohistochemical localization of 3′, 5′-cyclic guanosine monophosphate in the canine proximal colon: responses to nitric oxide and electrical stimulation of enteric inhibitory neurons. Neuroscience. 1993;56:513–22. doi: 10.1016/0306-4522(93)90350-o. [DOI] [PubMed] [Google Scholar]
- 32.Young HM, McConalogue K, Furness JB, et al. Nitric oxide targets in the guinea-pig intestine identified by induction of cyclic GMP immunoreactivity. Neuroscience. 1993;55:583–596. doi: 10.1016/0306-4522(93)90526-l. [DOI] [PubMed] [Google Scholar]
- 33.Salmhofer H, Neuhuber WL, Ruth P, et al. Pivotal role of the interstitial cells of Cajal in the nitric oxide signaling pathway of rat small intestine. Morphological evidence. Cell Tissue Res. 2001;305:331–340. doi: 10.1007/s004410100410. [DOI] [PubMed] [Google Scholar]
- 34.Ward SM, Sanders KM. Interstitial cells of Cajal: primary targets of enteric motor innervation. Anat Rec. 2001;262:125–135. doi: 10.1002/1097-0185(20010101)262:1<125::AID-AR1017>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 35.Ward SM, Sanders KM. Involvement of intramuscular interstitial cells of Cajal in neuroeffector transmission in the gastrointestinal tract. J Physiol. 2006;576:675–682. doi: 10.1113/jphysiol.2006.117390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirst GD, Ward SM. Interstitial cells: involvement in rhythmicity and neural control of gut smooth muscle. J Physiol. 2003;550:337–346. doi: 10.1113/jphysiol.2003.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ward SM, Harney SC, Bayguinov JR, McLaren GJ, Sanders KM, et al. Development of electrical rhythmicity in the murine gastrointestinal tract is specifically encoded in the tunica muscularis. J Physiol. 1997;505:241–258. doi: 10.1111/j.1469-7793.1997.241bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bayliss WM, Starling EH. The movements and innervation of the large intestine. J Physiol. 1990;26:107–118. doi: 10.1113/jphysiol.1900.sp000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dickson EJ, Spencer NJ, Hennig GW, et al. An enteric occult reflex underlies accommodation and slow transit in the distal large bowel. Gastroenterology. 2007;132:1912–1924. doi: 10.1053/j.gastro.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 40.Furness JB. The enteric nervous system. Blackwell Publishing; Oxford, UK: 2006. pp. 122–123. [Google Scholar]
- 41.Keef KD, Anderson U, O’Driscoll K, et al. Electrical activity induced by nitric oxide in canine colonic circular muscle. Am J Physiol. 2002;282:G123–G129. doi: 10.1152/ajpgi.00217.2001. [DOI] [PubMed] [Google Scholar]
- 42.Galligan JJ. Pharmacology of synaptic transmission in the enteric nervous system. Curr Opin Pharmacol. 2002;2:623–629. doi: 10.1016/s1471-4892(02)00212-6. [DOI] [PubMed] [Google Scholar]
- 43.Patel AJ, Honoré E. Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci. 2001;24:339–346. doi: 10.1016/s0166-2236(00)01810-5. [DOI] [PubMed] [Google Scholar]
- 44.Farrugia G, Holm AN, Rich A, et al. A mechanosensitive calcium channel in human intestinal smooth muscle cells. Gastroenterology. 1999;117:900–905. doi: 10.1016/s0016-5085(99)70349-5. [DOI] [PubMed] [Google Scholar]
- 45.Lyford GL, Strege PR, Shepard A, et al. alpha(1C) (Ca(V)1. 2) L-type calcium channel mediates mechanosensitive calcium regulation. Am J Physiol. 2002;283:C1001–C1018. doi: 10.1152/ajpcell.00140.2002. [DOI] [PubMed] [Google Scholar]
- 46.Bogeski G, Lean NP, Kitchener PD, et al. Analysis of factors that determine the compliance of rat jejunum to distension in vivo. Neurogastroenterol Motil. 2003;4:417–25.51. doi: 10.1046/j.1365-2982.2003.00423.x. [DOI] [PubMed] [Google Scholar]
- 47.Sanders KM, Hwang SJ, Ward SM. Neuroeffector apparatus in gastrointestinal smooth muscle organs. J Physiol. 2010;588:4621–4639. doi: 10.1113/jphysiol.2010.196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beckett EA, Ro S, Bayguinov Y, et al. Kit signaling is essential for development and maintenance of interstitial cells of Cajal and electrical rhythmicity in the embryonic gastrointestinal tract. Dev Dyn. 2007;236:60–72. doi: 10.1002/dvdy.20929. [DOI] [PubMed] [Google Scholar]
- 49.Iino S, Horiguchi K, Nojyo Y. Interstitial cells of Cajal are innervated by nitrergic nerves and express nitric oxide-sensitive guanylate cyclase in the guinea-pig gastrointestinal tract. Iino Neuroscience. 2008;152:437–448. doi: 10.1016/j.neuroscience.2007.12.044. [DOI] [PubMed] [Google Scholar]
- 50.Bayguinov PO, Hennig GW, Smith TK. Ca2+ imaging of activity in ICC-MY during local mucosal reflexes and the colonic migrating motor complex in the murine large intestine. J Physiol. 2010;588:4453–4474. doi: 10.1113/jphysiol.2010.196824. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Repolarization of membrane potential in L-NNA does not restore stretch-dependent inhibitory responses. (A) Stretch under control conditions stretch of circular muscle produces hyperpolarization in membrane potential (dashed line in upper panel) that recovered when stretch was relieved (middle and lower panels). (B) In the presence of L-NNA (100 μM), stretch did not produce membrane hyperpolarization observed under control conditions. (C) In the continued presence of L-NNA, pinacidil (0.1 μM) hyperpolarized membrane potential to control levels. Stretch of the circular muscle in the presence of L-NNA and pinacidil, when membrane potential was restored to control levels did not produce membrane hyperpolarization. These data suggest that membrane depolarization alone did not inhibit stretch-dependent inhibitory responses.
Effect of atropine on stretch-dependent responses in the proximal colon. (A) Shows membrane potential hyperpolarization (dashed line) and isometric force recordings (middle panel) in response to a stretch ramp that was delivered at 6 μm s−1 until a 5 mN isometric force was reached (lower panel). In this muscle preparation the 5 mN force was reached in 307 seconds. (B) Shows changes in membrane potential (dashed line) and isometric force measurement (middle panel) in response to a stretch ramp recorded from the same muscle preparation in the presence of atropine (1 μM). Atropine had no effect on membrane potential but had a slight but not significant decrease in the frequency of AP complexes. Stretch ramps in the presence of atropine caused membrane hyperpolarization and abolished any further AP discharge until the active change in length ramp was ceased. Atropine also increased the compliance of the circular muscle. In the presence of atropine the muscle preparation required 315 seconds to reach 5 mN of isometric force.