Abstract
Background
Drinking to alleviate the symptoms of acute withdrawal is included in diagnostic criteria for alcoholism, but the contribution of acute withdrawal relief to high alcohol intake has been difficult to model in animals.
Methods
Ethanol dependence was induced by passive intragastric ethanol infusions in C57BL/6J (B6) and DBA/2J (D2) mice; non-dependent controls received water infusions. Mice were then allowed to self-administer ethanol or water intragastrically.
Results
The time course of acute withdrawal was similar to that produced by chronic ethanol vapor exposure in mice, reaching a peak at 7-9 h and returning to baseline within 24 h; withdrawal severity was greater in D2 than in B6 mice (Exp. 1). Post-withdrawal delays in initial ethanol access (1, 3 or 5 days) reduced the enhancement in later ethanol intake normally seen in D2 (but not B6) mice allowed to self-infuse ethanol during acute withdrawal (Exp. 2). The post-withdrawal enhancement of ethanol intake persisted over a 5-d abstinence period in D2 mice (Exp. 3). D2 mice allowed to drink ethanol during acute withdrawal drank more ethanol and self-infused more ethanol than non-dependent mice (Exp. 4).
Conclusions
Alcohol access during acute withdrawal increased later alcohol intake in a time-dependent manner, an effect that may be related to a genetic difference in sensitivity to acute withdrawal. This promising model of negative reinforcement encourages additional research on the mechanisms underlying acute withdrawal relief and its role in determining risk for alcoholism.
Keywords: alcohol, dependence, withdrawal, self-administration, negative reinforcement, inbred mice
Introduction
Alcoholics often drink to relieve or avoid the increasingly unpleasant symptoms of withdrawal that are experienced after reduction or termination of a prolonged period of heavy drinking [1, 2]. Indeed, drinking to produce withdrawal-relief is included among the diagnostic criteria for distinguishing between alcohol abuse and alcohol dependence [3]. Two different stages of alcohol withdrawal have been delineated, a period of acute withdrawal lasting 2-3 days and a more protracted period of withdrawal that can last 3 or more months [3, 4]. As defined by DSM-IV-TR (Section 291.81), acute withdrawal is characterized by physical disturbances that include autonomic hyperactivity, hand tremor, insomnia, nausea or vomiting, psychomotor agitation and seizures [3]. Symptoms of protracted withdrawal may include autonomic dysfunction, insomnia, anxiety and other affective disturbances such as anhedonia and dysphoria [3, 4].
Since acute withdrawal symptoms are thought to be of greater intensity than protracted withdrawal symptoms, one might predict that alleviation of the physical symptoms of acute withdrawal would play a more important role in drinking behavior than alleviation of the more affective symptoms of protracted withdrawal. An early report based on structured interviews in alcoholics supported this idea, concluding that, “...the alcoholic drank in response to a wide range of symptoms, but he was more likely to drink if he had the particular constellation of symptoms collectively designated as the physical disturbance withdrawal syndrome ([2], p. 969).” Although animal models have shown increased ethanol self-administration during acute withdrawal, the literature offers little evidence that acute withdrawal relief by ethanol has a greater impact on subsequent ethanol intake than protracted withdrawal relief, casting doubt on the significance of acute withdrawal relief for understanding alcohol and drug addiction [4-6].
An adequate demonstration of the importance of acute withdrawal-relief would presumably show greater intake when ethanol access begins during the first day of withdrawal than when access is delayed for several days. Although the literature offers several examples of enhanced ethanol intake during the first day of withdrawal in rats [7-11], strong evidence that this effect is reduced when access is delayed for several days (after acute withdrawal subsides) is lacking. The only experiments that have directly compared groups of rats initially given ethanol at different times during withdrawal found no differences in ethanol intake [10, 12], but all of these groups were probably in acute withdrawal at the time of initial access (≤ 36 h after withdrawal). On the basis of cross-experiment comparisons, other reports have suggested that rats self-administer less when initial ethanol access is delayed for 2 weeks after withdrawal compared to access within the first 12 h [9, 11], but no single experiment has directly compared ethanol intakes between post-dependent rats given initial ethanol access during acute withdrawal and post-dependent rats given initial ethanol access after acute withdrawal has dissipated.
Current mouse models provide mixed evidence on the importance of ethanol access during acute withdrawal. C57BL/6J (B6) mice made dependent by passive ethanol vapor exposure have shown increased ethanol drinking when access begins 24-80 h after onset of acute withdrawal [13-18], but develop a “strong and persistent aversion” when initially given ethanol 8-24 h after withdrawal [13]. In an operant procedure, repeated access to ethanol during acute withdrawal failed to alter ethanol responding in B6 mice, although responding was increased during later tests after a 1-week abstinence period [19]. In contrast, recent studies revealed significant increases in intragastric alcohol consumption (IGAC) in four different mouse genotypes (including B6) given ethanol access during the first 24 h of acute withdrawal after induction of dependence by passive ethanol infusions [20, 21], consistent with the withdrawal-relief hypothesis.
Here, we used the IGAC procedure (Figure 1) to directly test whether voluntary ethanol intake is greater when mice receive initial ethanol access during the first day of acute withdrawal than when initial access is delayed for one or more days (Exp. 2). This study used the ethanol-preferring C57BL/6J (B6) mouse strain and the ethanol-avoiding DBA/2J (D2) strain. These strains were selected, in part, because they differ in the severity of acute withdrawal (D2 > B6) induced by passive exposure to ethanol vapor [22] or IG ethanol infusion [21], a difference that we confirmed in Experiment 1. Experiment 3 examined the persistence of the withdrawal-induced increase in ethanol intake across several days of abstinence in D2 mice that previously received ethanol access during acute withdrawal. Finally, Experiment 4 tested whether acute withdrawal relief would increase oral ethanol intake in the ethanol-avoiding D2 strain.
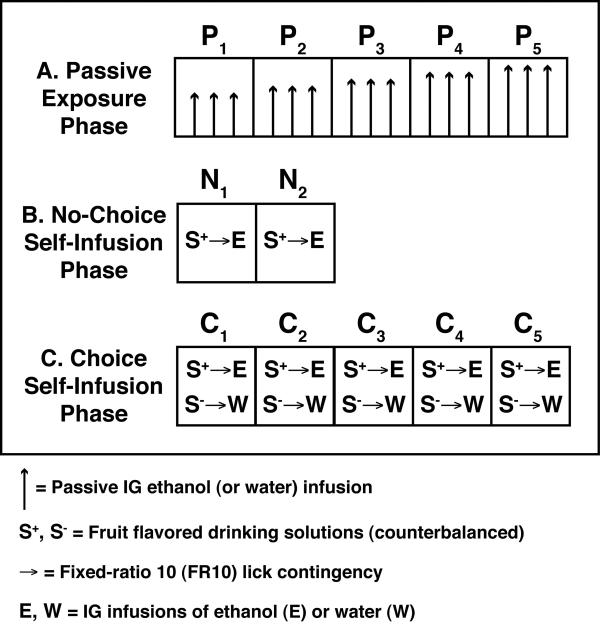
Figure 1.
Overview of the intragastric alcohol consumption (IGAC) procedure. After recovery from surgical implantation of chronic IG catheters, mice are housed in the test chamber for ~23 h/d for the rest of the experiment. (A) On each of 5 d during the Passive Exposure phase (P1-P5), mice automatically receive three passive IG infusions of ethanol or water at 5.7-h intervals. Ethanol dose is 3 g/kg/infusion on the first day and the dose increases by 0.5 g/kg/infusion across days (yielding daily doses of 9.0, 10.5, 12.0, 13.5 and 15 g/kg). (B) During the 2 No-Choice self-infusion days (N1-N2), mice receive access to a single drinking tube (S+) that contains grape or cherry Kool-Aid (0.05% w/v) sweetened with saccharin (0.2% w/v) in tap water. Low-dose ethanol infusions (about 0.05 – 0.07 g/kg/infusion, 20% v/v) are delivered for every 10th lick on the S+ drinking tube (i.e. fixed-ratio 10, FR10). (C) The final 5-d Choice phase C1-C5) is identical to the no-choice phase except for the availability of a second drinking tube (S-) that contains the other flavored Kool-Aid solution (counterbalanced); licks on the S-tube are paired with IG water infusions on an FR10 schedule.
Methods and Materials
Subjects and Apparatus
Male DBA/2J and C57BL/6J mice were shipped at 8-9 weeks of age from the Jackson Laboratory (Sacramento CA). All mice were surgically implanted with a chronic IG catheter and allowed to recover. Experimental chambers were equipped with a fluid swivel, two syringe pumps and two retractable drinking tubes connected to lickometers. Additional details about the subjects, surgery and apparatus are provided in the Supplemental Information.
Procedure
Mice were housed in the apparatus during all experiments, except for about 1 h per day. In most experiments, mice were exposed to all of the following procedures: (1) surgery, (2) recovery (6-10 days), (3) habituation (3 days), (4) passive ethanol or water infusions (5 days), (5) no-choice ethanol self-infusion (2 days), and (6) choice ethanol self-infusion (5 days). However, the final two phases were omitted in Exp. 1.
Habituation
Mice were attached to the swivel and given access to food and two bottles of 0.2% w/v saccharin (Sigma-Aldrich, St. Louis MO) in water. Each tube was available during alternate 30-min periods. All mice were treated identically during these 23-h sessions.
Passive Infusion Phase
Mice had continuous access to food and two water bottles during this phase. In all experiments, mice were exposed to three passive infusions (0.031 ml/min) of ethanol (10% v/v in sterile water) or sterile water (for Control groups) on each of 5 consecutive days. Ethanol dose was 3 g/kg/infusion on the first day (dose was controlled by varying infusion duration). The dose (or equivalent water volume) increased by 0.5 g/kg/infusion across days, yielding daily doses of 9.0, 10.5, 12.0, 13.5 and 15 g/kg. Infusions occurred about 5.7 h apart, with the first occurring near the onset of the dark cycle and the third occurring near the end of the dark cycle. Previous research has shown that this schedule of ethanol exposure produces blood ethanol concentrations of 2.0-2.2 mg/ml after the third infusion on the first day and 3.1-3.3 mg/ml after the third infusion on the fifth day in B6 and D2 mice [21].
Level of intoxication and withdrawal severity were assessed once daily, using procedures that have been described elsewhere [21]. These data are not reported here because scores were generally similar to those reported previously [21].
Experiment 1: Withdrawal Time Course
The purpose of this experiment was to characterize the time course of ethanol withdrawal in B6 and D2 mice after termination of the passive infusion phase. Withdrawal severity was visually scored on a scale from 0 (no convulsion) to 7 by rating handling induced convulsions (HICs) [23]. A baseline HIC measurement was taken at the end of the habituation phase, just before the start of the passive infusion phase. All mice in both strains were then exposed to 5 consecutive days of passive ethanol infusions. HICs were scored hourly for 12 h, beginning 1h after the last infusion began on the fifth day. HICs were also scored again at 24 and 25 h.
Experiment 2: Post-Withdrawal Delay
Experimental Design
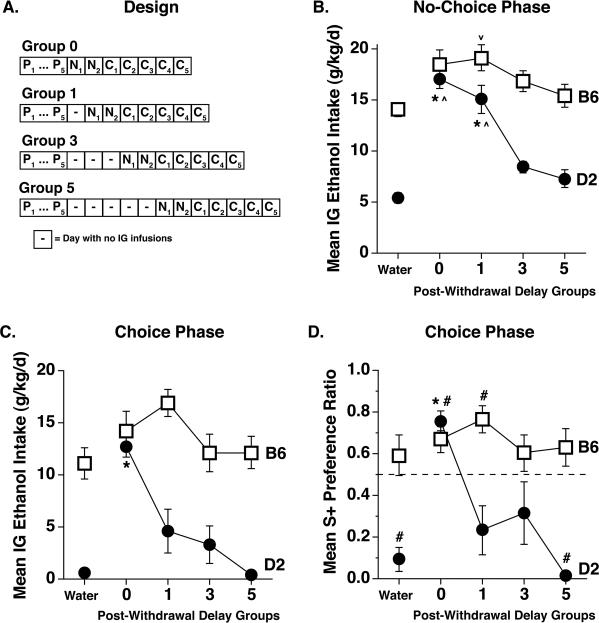
The purpose of this experiment was to examine the effect of acute withdrawal on IGAC by delaying access to self-infused ethanol for different periods of time after the passive exposure phase: 0, 1, 3 or 5 days. More specifically, no-choice self-infusion began about 8 h after the start of the last passive infusion for the 0-day group (i.e., at the time of peak withdrawal), whereas onset of self-infusion was delayed for 32, 80 or 128 h for mice in the 1-, 3- and 5-day groups, respectively (Fig. 3A). D2 and B6 mice were tested in separate experiments (2A and 2B, respectively) using the same general design. Each experiment involved two cohorts of mice that were combined for the final analyses of each strain. Due to initial uncertainty about the range of effective delays, the first cohort of Experiment 2A included mice randomly assigned to the 0- and 3-day groups whereas the second cohort included mice assigned to the 0-, 1- and 5-day groups; both cohorts of Experiment 2B included mice assigned to all four delay groups. Within each cohort, groups were matched for number of days in the apparatus between the last habituation day and the first day of no-choice self infusion by staggering the first day of passive infusions. On days without infusions, mice remained in the chamber with access to food and water. Both experiments included non-dependent control groups passively infused with water. Different subgroups received water infusions on schedules that matched those of the ethanol mice assigned to the shortest and longest delay groups within the same cohort. Statistical analysis showed that these subgroups did not differ during subsequent phases and their data were combined for all analyses.
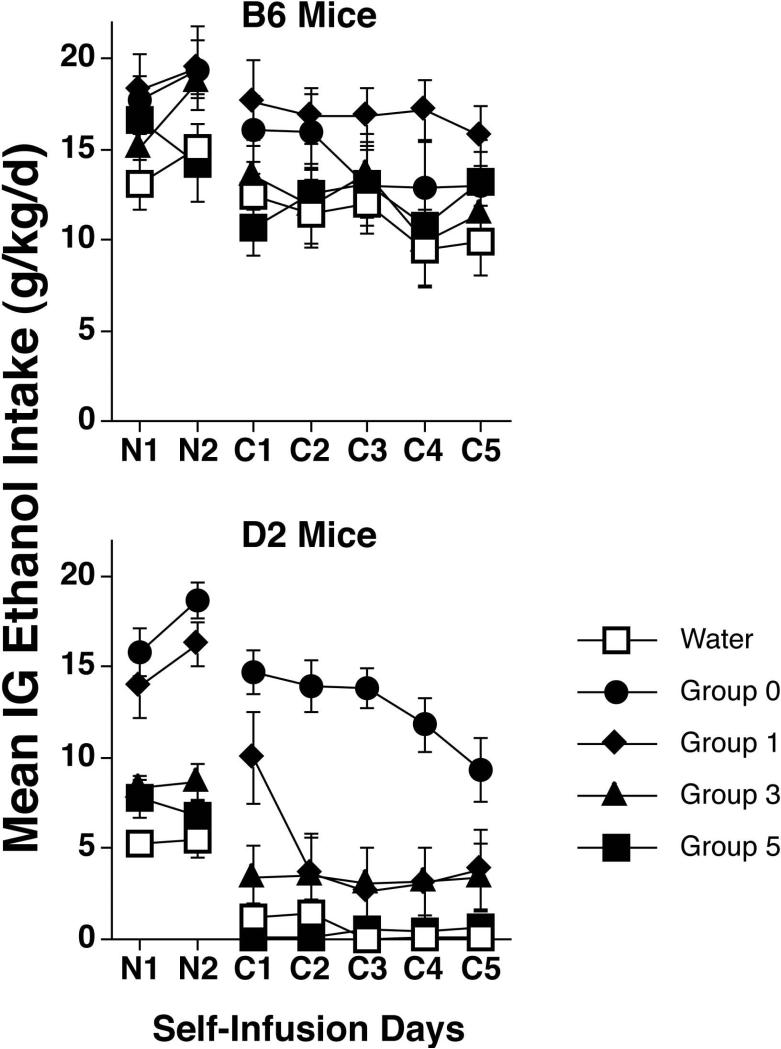
Figure 3.
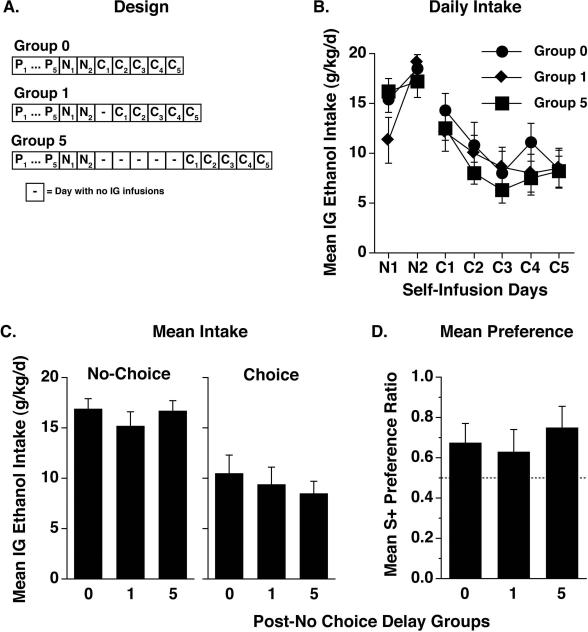
Daily mean ethanol intakes (g/kg/d ± SEM) in each B6 (upper panel) and D2 (lower panel) group of Experiments 2A and 2B during the no-choice (N1-N2) and choice (C1-C5) self-infusion phases. Non-dependent Water control mice previously received passive infusions of water for 5 days, while the other (dependent) groups received passive infusions of ethanol. Delays of 0, 1, 3 or 5 days separated the last passive infusion session from the first no-choice self-infusion session for the ethanol-dependent groups. Data averaged over two no-choice sessions and five choice sessions are shown in Figure 4.
No-Choice Self-Infusion Phase
All mice received access to a single drinking tube (S+) that contained 0.05% w/v grape or cherry Kool-Aid (Kraft Foods, Rye Brook, NY) sweetened with 0.2% w/v saccharin in water. This tube was placed on the side that had been preferred during the habituation and passive phases. Ethanol infusions (20% v/v) were contingent upon drinking the S+ solution. More specifically, every 10th lick was followed by a 4-sec infusion (0.129 ml/min) of ethanol (about 0.05 – 0.07 g/kg/infusion for 20-30 g mice) up to a maximum of 1.5 g/kg/30 min. The dose limit was imposed to minimize the likelihood of conditioning taste aversion to S+. The 2 no-choice days were included to ensure that all mice would encounter the contingency between the S+ and ethanol infusion, providing an opportunity for alleviation of withdrawal. Both sessions were about 23 h long.
Choice Self-Infusion Phase
The 5-day choice phase was the same as the no-choice phase except for the availability of a second drinking tube (S-) that contained the other flavored Kool-Aid solution (counterbalanced). Licks on the S- tube were paired with water infusions using the parameters described for the S+ tube, which remained in the same position. All mice were treated identically during each session, which lasted about 23 h.
Experiment 3: Delay After No-Choice Self-Infusion
Exp. 3 was designed to examine the effect of imposing different delays between the no-choice and choice self-infusion phases. All mice were first exposed to habituation (3 days), passive ethanol infusions (5 days) and no-choice self-infusion (2 days) over 10 consecutive days using procedures described earlier. Groups differed in the delay (0, 1 or 5 days) between the second no-choice day and the first of 5 choice self-infusion sessions. Mice in the 1- and 5-day groups remained in the chamber with access to food and water during the delay. We hypothesized that if the time-related effect shown by D2 mice in Exp. 2 depended specifically on learning the relationship between ethanol intake and alleviation of acute withdrawal during the first 24 h, insertion of time delays after dissipation of acute withdrawal in Exp. 3 should not produce the same precipitous decline in choice ethanol self-infusion observed in Exp. 2. However, a delay-dependent reduction in intake similar to that seen in Exp. 2 would argue against the learning interpretation. Since Exp. 2 showed little effect of post-withdrawal delay in B6 mice, only D2 mice were used in Exp. 3.
Experiment 4: Dependence-Enhanced Oral Ethanol Intake
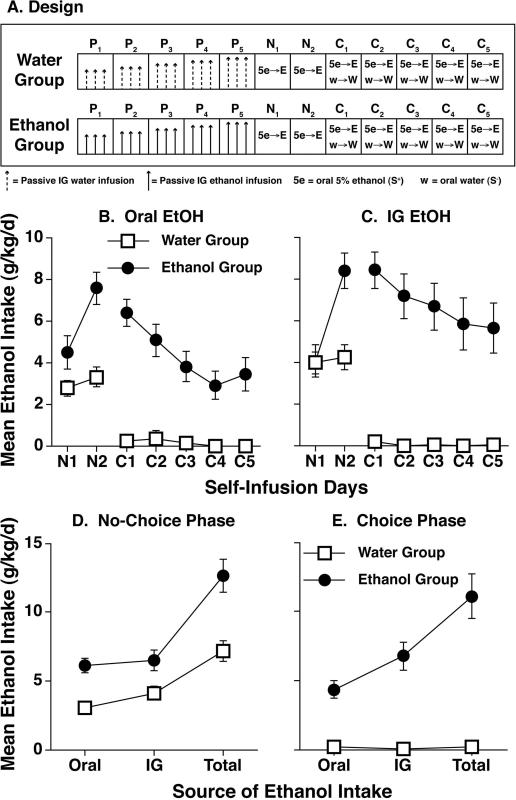
The purpose of this experiment was to determine whether ethanol access during acute withdrawal would enhance oral intake of ethanol in D2 mice, a strain that typically avoids ethanol in drinking studies [e.g., 24]. On consecutive days, mice were exposed to habituation (3 days), passive ethanol or water infusions (5 days), no-choice self-infusion (2 days) and choice self-infusion (5 days). Procedures in all phases were identical to those described above except that sweetened ethanol (5.0% v/v plus 0.2% w/v saccharin in tap water) was used as the S+ solution and water was used as the S- solution during the self-infusion phases (Fig. 6A).
Figure 6.
Effect of acute withdrawal relief on oral ethanol intake in D2 mice (Experiment 4). (A) D2 mice received 5 days of passive Ethanol (E) or Water (W) infusions (P1-P5), 2 days of no-choice self-infusion (N1-N2) and 5 days of choice self-infusion (C1-C5) using procedures shown in Figure 1 except that sweetened ethanol (5% v/v plus 0.2% w/v saccharin in tap water) was used as the S+ solution and water was used as the S- solution. (B) Daily mean ethanol intakes (g/kg/d) consumed orally during the no-choice (N1-N2) and choice (C1-C5) self-infusion phases. (C) Daily mean ethanol intakes (g/kg/d) consumed intragastrically during the no-choice (N1-N2) and choice (C1-C5) self-infusion phases. (D) Mean ethanol intakes (g/kg/d) were averaged over 2 no-choice sessions. (E) Mean ethanol intakes (g/kg/d) were averaged over 5 choice sessions. Total = Oral + IG. Error bars depict SEMs.
Statistical Analysis
Analysis of variance (ANOVA) was used to analyze data collected during the passive, no-choice and choice phases. The alpha level for all analyses was set at .05 P-values for post-hoc comparisons between strains or groups were Bonferroni-corrected for multiple comparisons.
Results
Data from 20 (9%) of the 222 mice that began these experiments were removed due to various problems, including poor health, catheter leakage, equipment malfunctions and experimenter error. In cases where such problems did not occur until the choice phase, data from earlier phases were included in the analyses. Final group sizes for each phase are reported in the figure captions or text.
Experiment 1: Withdrawal Time Course
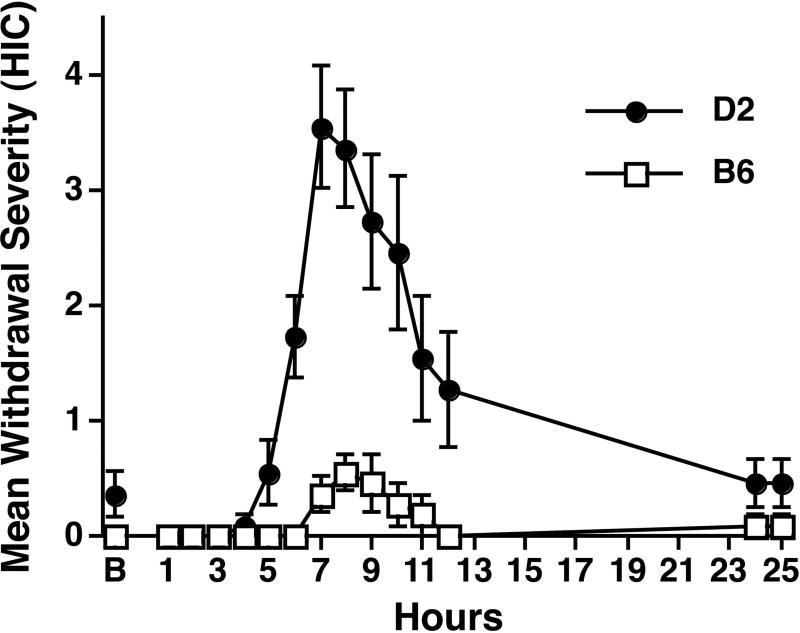
Figure 2 depicts mean HIC scores during baseline (B) and during the first 25 h of withdrawal after the final ethanol infusion for both strains. Withdrawal severity peaked about 7-9 h after onset of the last infusion and returned to baseline by 24 h. Consistent with previous findings [21, 22], D2 mice showed higher HIC scores than B6 mice [F(1,20) = 23.1, p < .0001].
Figure 2.
Time course and severity of acute ethanol withdrawal measured in adult male C57BL/6J (B6) and DBA/2J (D2) mice during the first 25 h after termination of the passive intragastric dosing procedure shown in Figure 1. Withdrawal severity was indexed by handling-induced convulsions (HICs) scored on a scale from 0 (no convulsion) to 7 during the habituation baseline (B) session (before induction of dependence) and at various times after the onset of the last passive ethanol infusion (n = 11/group). Withdrawal severity peaked about 7-9 h after onset of the last infusion, reaching a significantly higher level in D2 mice than in B6 mice. A Strain × Time ANOVA yielded significant main effects of Strain [F(1,20) = 23.1, p < .0001] and Time [F(14,280) = 17.5, p < .0001] as well as a significant interaction [F(14,280) = 10.4, p < .0001]. Error bars depict SEM.
Experiment 2: Post-Withdrawal Delay
Mean ethanol intakes (g/kg/d) during each no-choice and choice self-infusion day are shown for all groups in Figure 3. To simplify analysis, ethanol intakes were averaged over the 2 no-choice days (Figure 4B) and 5 choice days (Figure 4C). Intakes of mice in the ethanol 0-day and water control groups were generally similar to those previously reported for these strains after the same schedule of passive exposure [20, 21]. That is, D2 ethanol 0-day mice showed a large increase in ethanol intake relative to water controls whereas B6 ethanol 0-day mice did not. Of greater interest, post-withdrawal delay had a greater impact on ethanol intakes in D2 mice than in B6 mice, especially during the choice phase. Since the data for each strain were collected in different experiments, separate analyses were applied to the data for each strain from each phase. These one-way ANOVAs yielded significant effects of group during both the no-choice [F(4,38) = 32.3, p < .0001] and choice [F(4,37) = 18.3, p < .0001] phases for D2 mice, but only during the no-choice phase for B6 mice [F(4,40) = 3.7, p < .05]; post-hoc comparisons are shown in the figure panels.
Figure 4.
Effect of delaying initial access to ethanol self-infusion during withdrawal (Experiment 2). (A) Ethanol-dependent mice received daily passive infusions of ethanol for 5 days (P1-P5) as shown in Figure 1; non-dependent water control mice received matched passive infusions of water (not shown in design). Delays of 0, 1, 3 or 5 days separated the last passive infusion session from the first no-choice self-infusion session for Groups 0, 1, 3 and 5, respectively. (B) Mean ethanol intakes (g/kg/d) during the no-choice self-infusion phase. Data were averaged over 2 no-choice sessions (n = 7-13/group). *Significantly different from all other groups within the same strain, Bonferroni-corrected p's < .005; ∧ significantly different from Groups 3 and 5 within the same strain, Bonferroni-corrected p's < .001; ∨ significantly different from Water group, Bonferroni-corrected p < .05 (C) Mean ethanol intakes (g/kg/d) during the choice self-infusion phase were averaged over 5 sessions (n = 7-12/group). *Significantly different from all other groups within the same strain, Bonferroni-corrected p's < .005. (D) Mean S+ preference ratios (S+ licks divided by total of S+ and S- licks) during the choice self-infusion phase. *Significantly different from all other groups within the same strain, Bonferroni-corrected p's < .005; #significantly different from S+ preference ratio = 0.50 (dashed line, no preference), Bonferroni-corrected p's < .05. Error bars depict SEM.
The daily ratios of licks on the S+ tube to total licks (S+ and S- licks) were averaged over the 5 choice days to determine the mean S+ preference ratio for each mouse (Figure 4D). These data generally mirrored the intake data, showing a greater effect of post-withdrawal delay in D2 mice [Main effect of Group: F(4,37) = 13.9, p < .0001] than in B6 mice [p > .5]. Post-hoc comparisons indicated that the D2 ethanol 0-day group expressed a significantly stronger preference than all other D2 groups (Bonferroni p's < .005), which did not differ. Overall, Experiment 2 suggests that there are time- and genotype-dependent differences in sensitivity to ethanol's negative reinforcing effect during acute ethanol withdrawal.
Experiment 3: Delay After No-Choice Self-Infusion
As expected, ethanol intakes during the no-choice phase (Figure 5) were similar to those of the D2 ethanol 0-day group in Experiment 2A and did not differ among groups [F(2,32) < 1, p > .5], which were treated identically before and during this phase of the experiment. Of greater interest, all three groups continued to show elevated ethanol intakes during the choice phase and there was no effect of imposing various delays between the no-choice and choice phases on either ethanol intake or S+ preference [both F's(2,32) < 1, p > .3]. Overall, these data support the idea that a negative reinforcement learning process engaged by acute-withdrawal relief during the no-choice phase contributed to an increase in ethanol intake that persisted over at least 5 days of abstinence.
Figure 5.
Effect of inserting an abstinence period between the no-choice and choice ethanol self-infusion phases in D2 mice (Experiment 3). (A) Ethanol-dependent mice received daily passive infusions of ethanol for 5 days (P1-P5) as shown in Figure 1. Delays of 0, 1, or 5 days separated the second no-choice self-infusion session from the first choice self-infusion session for Groups 0, 1, and 5, respectively. (B) Daily mean ethanol intakes (g/kg/d) during the no-choice (N1-N2) and choice (C1-C5) self-infusion phases. (C) Mean ethanol intakes (g/kg/d) during the 2 no-choice self-infusion sessions (left panel) and during the 5 choice self-infusion sessions (right panel) (n = 11-13/group). (D) Mean S+ preference ratios (S+ licks divided by total of S+ and S- licks) during the choice self-infusion phase (dashed line = no preference). Error bars depict SEM.
Experiment 4: Dependence-Enhanced Oral Ethanol Intake
Ethanol-dependent (Ethanol Group) mice drank more ethanol and self-infused more ethanol than non-dependent Water control mice during both self-infusion phases (Figure 6). Separate analyses of the mean oral and IG intakes (averaged across days within each phase) yielded significant effects of passive phase treatment during both the no-choice (both F's(1,37) > 5.9, p's < .02) and choice (both F's(1,36) > 29.7, p's < .0001) self-infusion phases. The preference ratio for 5% ethanol (S+) during the choice phase was also significantly higher [F(1,36) = 37.3, p < .0001] in the ethanol group (0.65 ± 0.9) than in the control group (0.03 ± 0.02).
Discussion
These data show a time-dependent enhancement of ethanol intake during withdrawal in D2 mice, but not in B6 mice. More specifically, dependent D2 mice significantly increased ethanol intake (relative to non-dependent mice) when initial ethanol access began during the first 24 h of withdrawal, but not when initial access was delayed for 1, 3 or 5 days after withdrawal (Exp. 2). In contrast, dependent B6 mice showed consistently high ethanol intakes in all conditions, with little enhancement of intake relative to control mice, regardless of when initial access began (Exp. 2). When dependent D2 mice received initial ethanol access during the first 2 days of withdrawal, the enhancement persisted over a 5-day abstinence period (Exp. 3). Moreover, dependent D2 mice showed greater oral intake of ethanol that was paired with IG ethanol infusion during acute withdrawal (Exp. 4).
The time-dependent enhancement in D2 mice provides strong support for the potentially important role played by acute withdrawal relief in the development of excessive ethanol intake and alcoholism. One possible interpretation is that ethanol intake during the first 24 h of withdrawal established a learned preference for S+ based on negative reinforcement produced by alleviation of the aversive physical symptoms of acute withdrawal [2] or by alleviation of a concurrent negative affective state such as anxiety [4, 5]. In the absence of data showing a graded relationship between acute withdrawal severity (Fig. 1) and ethanol intake between 8-25 h post withdrawal, it is difficult to argue that these data uniquely support an interpretation based on alleviation of physical withdrawal symptoms. However, regardless of the underlying mechanism, these data show that ethanol access during the initial 24 h of withdrawal can play a critical role in determining subsequent ethanol intake.
Of particular interest, withdrawal-prone D2 mice showed a greater detrimental effect of delayed ethanol access on ethanol intake than withdrawal-resistant B6 mice, raising the possibility of a positive genetic correlation between sensitivity to withdrawal severity and sensitivity to the intake enhancing effect of ethanol access during acute withdrawal. However, this conclusion must be tempered by several considerations. Most critically, because B6 and D2 are inbred strains, they differ in many different phenotypes that may or may not be related to ethanol intake during withdrawal. It will be necessary to study a larger number of inbred strains (or mouse lines selectively bred for withdrawal sensitivity) in this procedure in order to determine whether the relationship observed here represents a true genetic correlation [25]. Since non-dependent B6 mice self-infused ethanol at relatively high levels, another reason for cautiously interpreting the genetic differences is that lack of increased intake in dependent B6 mice could simply reflect a ceiling effect. Alternatively, the parameters of passive ethanol exposure might not have established a sufficient level of dependence in B6 mice [21]. It is also possible that the predisposition to high drinking in B6 mice is completely unrelated to withdrawal sensitivity. Regardless of the interpretation, the present data underscore the importance of genotype in determining ethanol intake in both dependent and non-dependent mice.
A previous meta-analysis suggested a genetic correlation different from that seen here. That is, withdrawal-prone genotypes have generally been found to drink less ethanol than withdrawal-resistant genotypes [26]. However, a critical difference between our studies and those included in the meta-analysis is that we examined ethanol intake in dependent mice given ethanol access during withdrawal. In contrast, studies included in the meta-analysis examined ethanol intake only in mice that never experienced withdrawal. Indeed, the difference between non-dependent B6 and D2 mice in Exp. 2 is quite consistent with the meta-analysis in showing higher ethanol intake in the withdrawal-resistant B6 strain than in the withdrawal-prone D2 strain. Our finding that ethanol access during acute withdrawal had a relatively greater impact on later ethanol intake in D2 mice than in B6 mice raises the possibility that the genetic relationship between withdrawal sensitivity and ethanol intake might be different when animals receive ethanol access during acute withdrawal.
The persistence of enhanced ethanol intake and preference across a 5-day abstinence period in D2 mice given initial ethanol access during acute withdrawal is consistent with the hypothesized development of a learned Pavlovian preference for S+ based on negative reinforcement during acute withdrawal. Alternatively, it could be explained by negative reinforcement of the instrumental licking response. In either case, the persistence of such learned responses over a period of abstinence could explain relapse to alcohol taking long after the physical symptoms of acute withdrawal have subsided. Since the magnitude of negative reinforcement diminishes after acute withdrawal has ended, learned responses would be expected to gradually extinguish, which might also explain the slow decrease in ethanol intake over the 5 days of choice testing (Fig. 5).
In addition to negative reinforcement, enhanced ethanol intake in D2 mice can be explained, at least in part, by tolerance to aversive post-absorptive ethanol effects that would otherwise produce conditioned taste aversion [20, 21]. Thus, the detrimental effect of delaying initial ethanol access during withdrawal might also reflect a temporal decay in tolerance to those effects. Future studies could address this possibility by directly examining the decay in tolerance to ethanol's aversive effect during the first 5 days of withdrawal to see whether the time courses for loss of tolerance and reduction in ethanol intake are similar. Another alternative to the negative reinforcement interpretation is that passive ethanol exposure enhanced ethanol's rewarding effects in a time-dependent manner in D2 mice. This possibility could be tested using conditioned place preference, although it would be difficult to distinguish experimentally between a transient sensitization-like enhancement of ethanol reward and a transient withdrawal-related increase in ethanol's negative reinforcing effect since both effects could explain increased place preference.
Although our studies have focused on negative reinforcement during acute withdrawal, these findings do not preclude possible contributions by other risk factors, including differences in sensitivity to ethanol's ability to alleviate negative affective states (e.g., anxiety, depression) that may persist during protracted abstinence [4,5]. The possibility that genetically mediated individual differences in sensitivity to acute withdrawal or in ethanol's ability to alleviate acute withdrawal deserves greater attention in the study of the brain mechanisms underlying alcoholism, especially in light of data suggesting that adult children of alcoholics who have not yet developed alcoholism (but are more likely to become alcoholic) experience more severe acute withdrawal symptoms (“hangovers”) than children of non-alcoholics [27]. Such findings raise the possibility that drinking during acute withdrawal might be more strongly reinforced in individuals who are at high risk for developing alcoholism. Finally, these data encourage greater consideration of treatment and relapse prevention pharmacotherapies that target processes influencing negative reinforcement during acute withdrawal.
Supplementary Material
Acknowledgements
This research was supported by a grant from the National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism, U01-AA13479-INIA Project and by funds from the Oregon Health & Science University. Some of the data reported here were previously presented in posters at the 2011 meetings of the Research Society on Alcoholism and the Society for Neuroscience.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information: Additional Methods and Materials
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Edwards G, Gross MM. Alcohol dependence: provisional description of a clinical syndrome. Br Med J. 1976;1:1058–61. doi: 10.1136/bmj.1.6017.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hershon HI. Alcohol withdrawal symptoms and drinking behavior. J Stud Alcohol. 1977;38:953–71. doi: 10.15288/jsa.1977.38.953. [DOI] [PubMed] [Google Scholar]
- 3.American-Psychiatric-Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition, Text Revision American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 4.Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol. 2010;15:169–84. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koob GF. Animal models of drug dependence: motivational perspective. In: Johnson BA, editor. Addiction Medicine: Science and Practice. Vol. 1. Springer; New York: 2011. pp. 333–357. [Google Scholar]
- 6.Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- 7.Fidler TL, Clews TW, Cunningham CL. Reestablishing an intragastric ethanol self-infusion model in rats. Alcohol Clin Exp Res. 2006;30:414–28. doi: 10.1111/j.1530-0277.2006.00046.x. [DOI] [PubMed] [Google Scholar]
- 8.Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–98. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- 9.Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–94. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- 10.O'Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–82. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- 11.Valdez GR, Roberts AJ, Chan K, et al. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- 12.Fidler TL, Oberlin BG, Struthers AM, Cunningham CL. Schedule of passive ethanol exposure affects subsequent intragastric ethanol self-infusion. Alcohol Clin Exp Res. 2009;33:1909–23. doi: 10.1111/j.1530-0277.2009.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–38. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- 14.Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 2005;181:688–96. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- 15.Finn DA, Snelling C, Fretwell AM, et al. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12-41). Alcohol Clin Exp Res. 2007;31:939–49. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- 16.Griffin WC, Lopez MF, Becker HC. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol Clin Exp Res. 2009a;33:1893–900. doi: 10.1111/j.1530-0277.2009.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin WC, Lopez MF, Yanke AB, Middaugh LD, Becker HC. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology (Berl) 2009b;201:569–80. doi: 10.1007/s00213-008-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez MF, Grahame NJ, Becker HC. Development of ethanol withdrawal-related sensitization and relapse drinking in mice selected for high- or low-ethanol preference. Alcohol Clin Exp Res. 2011;35:953–62. doi: 10.1111/j.1530-0277.2010.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacol Biochem Behav. 2007;86:813–21. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fidler TL, Dion AM, Powers MS, et al. Intragastric self-infusion of ethanol in high- and low-drinking mouse genotypes after passive ethanol exposure. Genes Brain Behav. 2011;10:264–75. doi: 10.1111/j.1601-183X.2010.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fidler TL, Powers MS, Ramirez JJ, et al. Dependence induced increases in intragastric alcohol consumption in mice. Addiction Biology. 2012;17:13–32. doi: 10.1111/j.1369-1600.2011.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crabbe JC. Provisional mapping of quantitative trait loci for chronic ethanol withdrawal severity in BXD recombinant inbred mice. Journal of Pharmacology and Experimental Therapeutics. 1998;286:263–271. [PubMed] [Google Scholar]
- 23.Metten P, Crabbe JC. Alcohol withdrawal severity in inbred mouse (Mus musculus) strains. Behav Neurosci. 2005;119:911–25. doi: 10.1037/0735-7044.119.4.911. [DOI] [PubMed] [Google Scholar]
- 24.Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- 25.Crabbe JC, Phillips TJ, Kosobud A, Belknap JK. Estimation of genetic correlation: Interpretation of experiments using selectively bred and inbred animals. Alcoholism: Clinical and Experimental Research. 1990;14:141–151. doi: 10.1111/j.1530-0277.1990.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 26.Metten P, Phillips TJ, Crabbe JC, et al. High genetic susceptibility to ethanol withdrawal predicts low ethanol consumption. Mammalian Genome. 1998;9:983–90. doi: 10.1007/s003359900911. [DOI] [PubMed] [Google Scholar]
- 27.McCaul ME, Turkkan JS, Svikis DS, Bigelow GE. Alcohol and secobarbital effects as a function of familial alcoholism: extended intoxication and increased withdrawal effects. Alcohol Clin Exp Res. 1991;15:94–101. doi: 10.1111/j.1530-0277.1991.tb00524.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.