Abstract
Background
Non-Hodgkin Lymphoma (NHL) is the fifth most common cancer among men and women. Patients with aggressive NHL receive intense medical treatments that can significantly compromise health-related quality of life (HRQOL). However, knowledge of HRQOL and its correlates among aggressive NHL survivors is limited.
Methods
Self-reported data on HRQOL (physical and mental function, anxiety, depression, and fatigue) were analyzed for 319 survivors of aggressive NHL. Survivors, 2–5 years after diagnosis, were selected from the Los Angeles County Cancer Registry. Bivariate and multivariable methods were used to assess the influence of sociodemographic, clinical, and cognitive health appraisal factors on survivors’ HRQOL.
Results
After accounting for other covariates, marital status was associated with all HRQOL outcomes (p<0.05). Younger survivors reported worse mental function and higher levels of depression, anxiety, and fatigue (p<0.01). Survivors who had more comorbid conditions or lacked private health insurance reported worse physical and mental function and higher levels of depression and fatigue (p<0.05). Survivors who experienced a recurrence reported worse physical function and higher levels of depression and fatigue (p<0.05). With the exception of a nonsignificant association between perceived control and physical function, greater perceptions of personal control and health competence were significantly associated with more positive HRQOL outcomes (p<0.01).
Conclusion
Survivors of aggressive NHL who are younger, are non-married, lack private insurance, or experience greater illness burden may be at risk for poorer HRQOL. Cognitive health appraisal factors were strongly related to HRQOL, suggesting potential benefits of interventions focused on these mutable factors for this population.
INTRODUCTION
Non-Hodgkin Lymphoma (NHL) is the fifth most common cancer for both men and women,1 and accounts for 3.8% of the 11.9 million cancer survivors in the U.S.2 Adult NHL cases are classified based on histology into two groups that have approximately similar rates of incidence: indolent lymphomas (low grade), which grow slowly, and aggressive lymphomas (intermediate and high-grade), which grow more quickly and can be fatal if not treated aggressively. Treatment options for aggressive NHL include multi-agent chemotherapy, radiation, and bone marrow or stem cell transplant, all of which can significantly compromise patients’ health-related quality of life (HRQOL). While negative changes in HRQOL have been identified after NHL diagnosis and during treatment,3,4 limited research has been conducted on the impact of a diagnosis of aggressive NHL and its related treatments on HRQOL outcomes among survivors who have completed active treatment.
The majority of HRQOL studies in cancer survivorship have examined breast, colorectal, or prostate cancer5–7 or mixed cancer survivor populations,8,9 showing increased rates of psychological effects, such as depression and anxiety.10–12 The few studies of NHL survivors have found that they experienced lower physical and mental HRQOL13,14 and increased rates of psychosocial distress.15 While many of the survivorship studies have focused on the association of patient sociodemographic and clinical characteristics with HRQOL, relatively few have examined more intervenable determinants of HRQOL. Existing literature does suggest that deficits in HRQOL may be reduced by enhancing patients’ cognitive health appraisal, such as their perception of personal control over their illness and perceptions of health competence and self-efficacy.16–19 However, the association between cognitive health appraisal and HRQOL has not been studied during post-treatment cancer survivorship, especially among NHL survivors.
To further add to the very limited research on NHL survivors, we conducted a population-based study of HRQOL among survivors of aggressive NHL, diagnosed 2–5 years prior to the study. This period represents an important point in cancer care for this population, as patients transition to follow-up surveillance and may still experience treatment-related HRQOL deficits. We examined survivors’ HRQOL using multiple outcome measures, including physical function, mental function, anxiety, depression, and fatigue. Furthermore, we evaluated the association of several patient sociodemographic, clinical, and cognitive health appraisal factors with NHL survivors’ HRQOL outcomes.
METHODS
Participants and Procedures
We analyzed data from the Experience of Care and Health Outcomes of Survivors of Non-Hodgkin Lymphoma (ECHOS-NHL) study. The cases were adult survivors of aggressive NHL selected in 2003 from the Cancer Surveillance Program (CSP), the Surveillance Epidemiology and End Results (SEER) registry for Los Angeles County, who were diagnosed 2–5 years prior to the study (between June 1, 1998 and August 31, 2001). The selection criteria included individuals aged 20 or older with a first-time diagnosis of either intermediate or high-grade NHL. Survivors were excluded if they were diagnosed with another cancer within a year prior to their NHL diagnosis or subsequently.
We used a mailed questionnaire with telephone follow-up for non-responders to the mailing, and also conducted a medical record abstraction to augment clinical data available from the SEER registry. Of the 744 eligible NHL survivors identified and mailed a questionnaire, 408 completed it, for a response rate of 55%. Among these 408 survivors, 319 completed the full mailed survey and 89 completed a briefer version by telephone. Since the brief survey did not include HRQOL measures, analyses in this study are based only on the 319 full-survey respondents. Survivors who completed the brief telephone survey did not differ from those who responded to the full survey on any of the sociodeomographic or clinical variables. The only exception was that telephone responders were more likely to have been diagnosed earlier (3.8 versus 3.5 years, p<0.01). The study protocol was approved by the Institutional Review Board (IRB) at the University of Southern California. Further information about the population, study design, data collection procedures, differences between responders and non-responders, and conceptual framework used to guide dependent and independent variable selection for this study has been previously reported in detail.20
Measures
HRQOL Outcomes
We measured NHL survivors’ HRQOL using the Short Form-36 (SF-36® v2) health survey,21,22 a standardized measure that has been used extensively in both general and disease-specific populations, including NHL.13 The SF-36 has 8 subscales: physical function, role limitations due to physical health, mental health, role limitations due to emotional problems, social function, bodily pain, vitality, and general health perceptions. Scores from these subscales were used to calculate two summary health scores, the Physical Component Summary (PCS) and the Mental Component Summary (MCS). Based on norms from the 1999 U.S. general population, we standardized all scores on a T-score metric such that a score of 50 represented the average score in the U.S. general population with a standard deviation of 10; higher scores reflect better HRQOL. We evaluated anxiety and depression using the Hospital Anxiety and Depression Scale (HADS),23 a 14-item self-report scale that has shown good reliability and validity in cancer populations.24 Seven items each assessed anxiety and depression on a 4-point, 0–3 response format. As suggested by the authors of this scale, we created separate indicators for anxiety and depression ranging from 0 to 21 (a score from 0–7 is considered normal, 8–10 mild anxiety/depression, 11–14 moderate, and 15–21 severe anxiety/depression).
We evaluated fatigue using the 7-item fatigue interference subscale of the Fatigue Symptom Inventory (FSI), which has been validated across different cancer patient populations.25,26 These 7 items ask respondents to indicate the extent to which fatigue interfered during the past week with their general activity, ability to bathe and dress, normal work activity, ability to concentrate, relations with others, enjoyment of life, and mood. Items are scored from 0–10 with “0” representing no interference and “10” representing extreme interference. An overall score was created by taking the average of the scores on the 7 items; the raw score was then linearly transformed to a 0–100 scale to facilitate interpretation, with a higher score representing greater fatigue interference.
Cognitive Health Appraisal
We measured NHL survivors’ perception of their self-efficacy in taking care of their health using a 4-item short form of the Perceived Health Competence Scale (PHCS),27 which has been used in prior cancer studies.19 The PHCS asks responders, on a 5-point “strongly agree” to “strongly disagree” response scale, their agreement/disagreement with the following items: “No matter how hard I try, my health doesn’t turn out the way I would like”; “I am usually unable to find effective solutions for my health problems”; “My efforts to change things about my health are usually ineffective”; and “Typically, my plans for my health don’t work out well.”
We measured NHL survivors’ perception of personal control using a 4-item Perceived Personal Control (PPC) scale that was developed in a prior study on follow-up care experiences of cancer survivors.28 The 4 PPC items assess, on a 5-point “no control at all” to “complete control” response scale, survivors’ perception of control over their emotional responses to their cancer, the physical side effects of their cancer and its treatment, the kind of follow-up care they receive, and the course of their cancer. Exact wordings of items are published elsewhere.28
Scale scores were created for PHCS and PPC by taking an average of all the item scores. Raw scale scores were linearly transformed to a 0–100 scale; higher scores represented greater perceptions of health competence and control. For this study, we divided PHCS and PPC scores into three approximately equal groups labeled as low, medium, and high to facilitate interpretation.
Patient-Level Variables
We collected data on several patient sociodemographic variables (age at time of study, gender, race/ethnicity, education, household income, insurance, and marital status) and clinical characteristics (type of treatment, NHL grade, recency of treatment, number of comorbid health conditions, time since diagnosis, and experience of recurrence or disease progression). Information on NHL grade was obtained from the SEER registry, and all other variables were collected as part of the survey. Survivors’ self-reports of recurrence/disease progression and treatment were validated against medical record data.20 Comorbid health conditions were based on a self-reported count of being diagnosed with one or more of the following 12 medical conditions used in a prior cancer study: congestive heart failure, heart attack/myocardial infarction, chest pain or angina, high blood pressure, blood clots in the veins of legs or in the lungs, stroke or brain hemorrhage, chronic lung disease or bronchitis or emphysema, liver disease or cirrhosis, inflammatory bowel disease or colitis or Crohn’s disease, diabetes, osteoporosis or brittle bones, and arthritis or rheumatism.29
Analytic Methods
We computed descriptive statistics for all independent variables and outcome measures. To limit the number of outcome variables in our analytical models, we only included the two summary scores from the SF-36 (PCS, MCS), along with the HADS depression and anxiety subscales, and the FSI. We conducted bivariate analyses using t-tests to evaluate the association of the various patient-level variables (sociodemographic and clinical characteristics) with the outcome variables. Patient variables that were associated with at least one of the five HRQOL outcomes at p≤0.1 were included in the multivariable models. NHL grade, time since diagnosis, and treatment type were not associated with any of the outcomes (p>0.1) and were not considered for the multivariable models. We included insurance status in the multivariable models but excluded income because the two variables were significantly correlated (ρ=0.5, p<0.0001), they both had similar associations with the outcome variables, and income had a relatively higher percentage of missing data compared to insurance (9% versus 5%).. Based on findings from the bivariate analyses, we examined the adjusted associations of patient sociodemographic, clinical, and cognitive health appraisal factors with NHL survivors’ HRQOL by estimating two sets of hierarchically built analysis of covariance (ANCOVA) models. The first set (one model for each of the five outcome variables) examined the association of patient sociodemographic and clinical variables (age, gender, race/ethnicity, marital status, education, insurance status, comorbidities, recurrence, and recency of treatment) with each HRQOL outcome. The second set of ANCOVA models examined the association of the two cognitive health appraisal variables (perceived health competency and personal control) with each HRQOL outcome, adjusting for the sociodemographic and clinical variables included in the first set of models. All analyses were conducted using the GLM procedure in SPSS version 14.0 (SPSS, Inc., Chicago IL). A p-value of ≤0.05 was considered statistically significant. In addition to statistical significance, we also highlighted subgroup differences in HRQOL that could be clinically meaningful. HRQOL studies often utilize Cohen’s criteria of effect sizes (small effect: 0.2SD, medium: 0.5SD, large: 0.8SD) to characterize minimally important differences.30,31 For example, a difference of 3–5 points on the SF-36 scale (i.e., 0.3–0.5SD, between small to medium effect size) has been shown to be clinically meaningful.32 In this study, we take a more conservative approach and consider subgroup differences that are ≥0.5SD (i.e., a medium effect size or greater) to be clinically meaningful.
RESULTS
Sample Characteristics
The sample consisted of equal proportions of men and women, and 42% of survivors were 65 years of age or older, 30% belonged to minority racial/ethnic subgroups, and 65% had private health insurance. Clinically, 16% had experienced a recurrence or progression of their cancer, and 69% were living with one or more comorbid health conditions (Table 1). Of those survivors who reported a recurrence, 46% reported receiving treatment in the past 6 months.
Table 1.
Sociodemographic and Clinical Characteristics (n=319)
| Characteristics | n (%) |
|---|---|
| Sociodemographics | |
| Age | |
| <50 | 84 (26.3) |
| 50–64 | 101 (31.7) |
| 65+ | 134(42.0) |
| Gender | |
| Female | 156 (48.9) |
| Male | 163 (51.1) |
| Race/Ethnicity | |
| Hispanic | 68 (21.3) |
| Non-Hispanic White | 223 (69.9) |
| Other | 28 (8.8) |
| Marital Status | |
| Married/Living as Married | 205 (64.3) |
| Other | 110 (34.5) |
| Missing | 4(1.3) |
| Education | |
| ≤High School | 93 (29.2) |
| Some College | 106 (33.2) |
| College Degree or Higher | 117 (36.7) |
| Missing | 3 (0.9) |
| Household Income | |
| <$20,000 | 65 (20.4) |
| $20,000 – $60,000 | 101 (31.7) |
| $60,000 or more | 125 (39.2) |
| Missing | 28 (8.8) |
| Health Insurance | |
| Private | 208 (65.2) |
| Public or none | 95 (29.8) |
| Missing | 16 (5.0) |
| Clinical Characteristics | |
| NHL Grade | |
| Intermediate | 283 (88.7) |
| High | 36 (11.3) |
| Time Since Diagnosis | |
| Mean Years (Range) | 3.5 (2.1 to 5.5) |
| Recurrence/Disease Progression | |
| Yes | 50 (15.7) |
| No | 264 (82.8) |
| Missing | 5 (1.6) |
| Treatment in past 6 months | |
| Yes | 35 (11.0) |
| No | 279 (87.5) |
| Missing | 5 (1.6) |
| Treatment Type | |
| Chemotherapy and Radiation | 108 (33.9) |
| Chemotherapy only | 156 (48.9) |
| Transplant | 34 (10.7) |
| Missing | 21 (6.6) |
| Comorbid Conditions | |
| None | 99 (31.0) |
| 1 or 2 | 156 (48.9) |
| 3 or more | 63 (19.7) |
| Missing | 1 (0.3) |
HRQOL Outcomes Distribution
Among the SF-36 scales, mean scores on the PCS, physical function, and role limitations due to physical health were at least 5 points lower (>0.5SD) than the mean score of 50 observed in the general U.S. population, which is a clinically meaningful difference. MCS scores were similar to U.S. population norms. (Table 2) The mean HADS scores suggest that the NHL survivors have normal levels of anxiety and depression with respect to U.S. population norms. However, 20% and 16% of survivors did report mild to severe levels of anxiety or depression, respectively (data not shown). The mean score on the fatigue interference scale was 22 (SD=22.8).
Table 2.
Distribution of Outcome Variables
| Outcomes | Mean (SD) | Missing (%) |
|---|---|---|
| Health-Related Quality of Life | ||
| PCS | 44.8 (11.9) | 5.3 |
| MCS | 49.8 (11.0) | 5.3 |
| SF-36 Subscales | ||
| General Health | 46.6 (11.5) | 1.3 |
| Mental Health | 49.6 (10.7) | 1.3 |
| Physical Function | 43.0 (12.4) | 2.2 |
| Role-Emotional | 46.4 (12.9) | 2.8 |
| Role Physical | 44.1 (13.0) | 1.9 |
| Social Function | 46.6 (12.1) | 1.0 |
| Vitality | 49.5 (11.4) | 1.3 |
| Bodily Pain | 49.2 (11.3) | 1.6 |
| Anxiety | 4.7 (3.8) | 1.9 |
| Depression | 3.9 (3.7) | 1.9 |
| Fatigue | 21.6 (22.8) | 3.1 |
Patient Characteristics and HRQOL
Age, marital status, health insurance, number of comorbidities, and NHL recurrence were significantly associated with several of the HRQOL outcomes after adjustment for all other patient characteristics. (Table 3.) With the exception of PCS, younger survivors reported significantly lower HRQOL compared to older survivors (p<0.01). Differences in MCS, depression, anxiety, and fatigue scores among survivors who were younger than 50 years of age and those who were older than 65 years were all ≥0.5SD, suggesting clinically relevant differences. Being married or living as a married couple was associated with better HRQOL outcomes across all domains (p≤0.05).
Table 3.
Adjusted Mean Scores of Survivor Health Outcomes by Sociodemographics and Clinical Characteristics*
| Variable | PCS | p-value | MCS | p-value | Anxiety | p-value | Depression | p-value | Fatigue | p-value |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.28 | <0.001 | <0.001 | <0.01 | <0.001 | |||||
|
| ||||||||||
| <50 | 42.21 | 41.95 | 6.56 | 5.65 | 37.69 | |||||
| 50–64 | 39.51 | 46.07 | 5.27 | 5.12 | 27.52 | |||||
| 65+ | 39.93 | 50.57 | 3.96 | 3.87 | 20.93 | |||||
|
| ||||||||||
| Gender | 0.12 | 0.58 | 0.29 | 0.99 | 0.32 | |||||
|
| ||||||||||
| Female | 39.55 | 46.55 | 5.51 | 4.88 | 30.06 | |||||
| Male | 41.55 | 45.85 | 5.02 | 4.88 | 27.36 | |||||
|
| ||||||||||
| Race/Ethnicity | 0.15 | 0.87 | 0.17 | 0.48 | 0.24 | |||||
|
| ||||||||||
| Hispanic/Other | 41.68 | 46.07 | 5.65 | 4.70 | 26.77 | |||||
| Non-Hispanic White | 39.41 | 46.33 | 4.88 | 5.06 | 30.66 | |||||
|
| ||||||||||
| Marital Status | <0.01 | <0.05 | 0.05 | <0.01 | <0.05 | |||||
|
| ||||||||||
| Married/Living as Married | 42.71 | 48.01 | 4.77 | 4.18 | 24.87 | |||||
| Other | 38.38 | 44.39 | 5.76 | 5.58 | 32.56 | |||||
|
| ||||||||||
| Education | 0.26 | 0.71 | 0.93 | 0.61 | 0.82 | |||||
|
| ||||||||||
| ≤High School | 39.65 | 46.93 | 5.39 | 5.04 | 27.64 | |||||
| Some College | 39.94 | 45.54 | 5.23 | 5.03 | 29.83 | |||||
| College Degree or Higher | 42.05 | 46.12 | 5.17 | 4.57 | 28.67 | |||||
|
| ||||||||||
| Health Insurance | <0.05 | <0.01 | 0.64 | <0.05 | <0.05 | |||||
|
| ||||||||||
| Private | 42.52 | 48.55 | 5.13 | 4.26 | 24.81 | |||||
| Public or none | 38.58 | 43.84 | 5.40 | 5.49 | 32.62 | |||||
|
| ||||||||||
| Recurrence/Progression | <0.01 | 0.10 | 0.53 | 0.05 | <0.05 | |||||
|
| ||||||||||
| Yes | 37.62 | 44.53 | 5.49 | 5.54 | 33.04 | |||||
| No | 43.47 | 47.86 | 5.04 | 4.22 | 24.39 | |||||
|
| ||||||||||
| Treatment in Past 6 Months | 0.47 | 0.87 | 0.30 | 0.85 | 0.84 | |||||
|
| ||||||||||
| Yes | 39.73 | 46.39 | 4.84 | 4.80 | 28.22 | |||||
| No | 41.37 | 46.01 | 5.69 | 4.95 | 29.21 | |||||
|
| ||||||||||
| Comorbid Conditions | <0.001 | <0.05 | 0.13 | <0.01 | <0.01 | |||||
|
| ||||||||||
| None | 46.24 | 49.27 | 4.57 | 3.60 | 20.78 | |||||
| 1 or 2 | 40.09 | 45.79 | 5.21 | 4.97 | 28.84 | |||||
| 3 or more | 35.32 | 43.54 | 6.01 | 6.06 | 36.51 | |||||
|
| ||||||||||
| Adjusted R Squared | 0.28 | 0.12 | 0.08 | 0.11 | 012 | |||||
Controlling for Age, Gender, Race/Ethnicity, Marital Status, Education, Health Insurance, Recurrence/Disease Progression, Treatment in Past 6 Months, and Number of Comorbid Conditions
Survivors with private health insurance reported higher PCS (p<0.05) and MCS (p<0.01) scores compared to survivors with public or no insurance, even after adjusting for other factors such as education, race, and age that are known to be associated with insurance status. The difference in depression and fatigue scores between the two insurance groups was also statistically significant (p<0.05).
Living with any comorbid health condition in addition to cancer was associated with significantly lower HRQOL outcomes for PCS (p<0.001), MCS (p<0.05), depression (p<0.01), and fatigue (p<0.01). Differences in HRQOL among survivors with no comorbidity and those who had 3 or more comorbid health conditions ranged from 0.6SD to 1.1SD, suggesting a clinically meaningful difference between these subgroups. Differences in PCS among survivors with no comorbidity and those who had 1–2 comorbidities was also clinically meaningful (6 points, 0.6SD). NHL survivors who experienced a cancer recurrence also reported lower PCS score (p<0.01) and higher levels of fatigue (p<0.05). Score difference on the PCS was 5.9 points (0.6SD), suggesting clinically meaningful deficits in physical health among survivors whose NHL had recurred compared to those who did not have a recurrence.
Cognitive Health Appraisal and HRQOL
After adjusting for key patient characteristics, we observed significant increases in HRQOL scores with increasing levels of health competence groups across all 5 HRQOL domains (p<0.001, Figures 1–3). HRQOL differences among NHL survivors who reported low levels of health competence and those who reported high levels of competence ranged from 0.8SD to 1.0SD, far exceeding the threshold of clinically meaningful differences. Meaningful differences were also identified between survivors with low and medium health competence across all HRQOL domains except MCS.
Figure 1. PCS and MCS by Cognitive Health Appraisal Level.
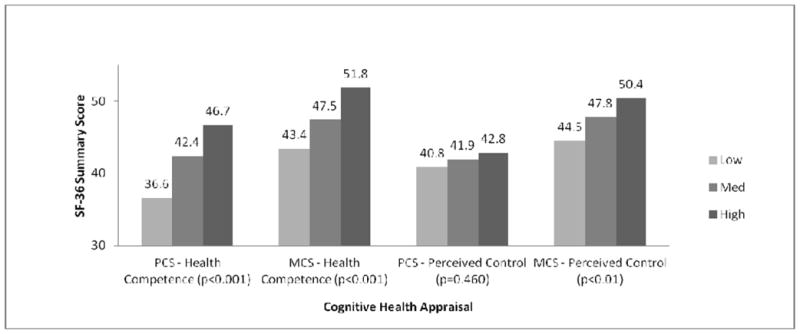
Model controls for: Age, Gender, Race/Ethnicity, Marital Status, Education, Health Insurance, Recurrence/Disease Progression, Treatment in Past 6 Months, and Number of Comorbid Conditions Adjusted R Squared: 0.39 (PCS), 0.29 (MCS)
Figure 3. Fatigue by Cognitive Health Appraisal Level.
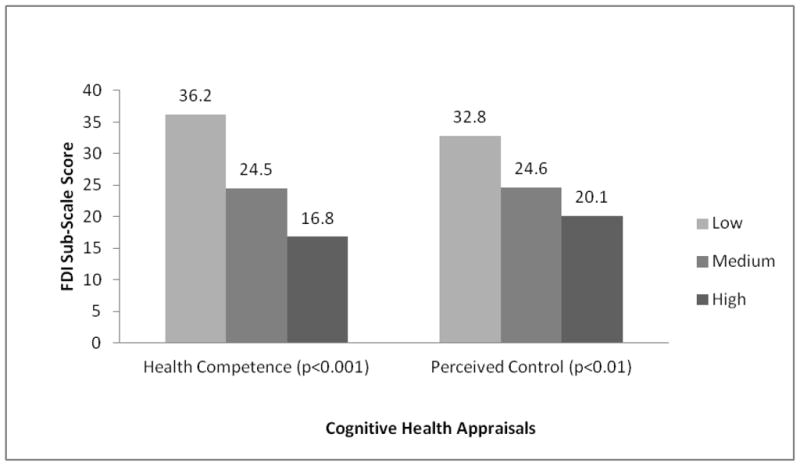
Model controls for: Age, Gender, Race/Ethnicity, Marital Status, Education, Health Insurance, Recurrence/Disease Progression, Treatment in Past 6 Months, and Number of Comorbid Conditions. Adjusted R Squared: 0.30 (Fatigue)
With the exception of PCS, greater perceptions of personal control were also associated with significantly better HRQOL outcomes (p<0.01, Figures 1–3). Differences in the range of medium to large effect sizes (≥0.5SD) were identified between survivors with low and high levels of control.
Sensitivity Analyses
Given that NHL survivors who are disease free are conceptually and clinically distinct from those whose cancer has recurred, we conducted sensitivity analyses where we estimated our regression models for only those survivors whose cancer did not recur and who were in remission at the time of the study. Overall, we observed a similar pattern of association for all sociodemographic, clinical, and cognitive appraisal variables. The only exception was that in our disease free sample, gender was also significantly associated with PCS, with men reporting significantly better physical functioning than women (p=0.01).
DISCUSSION
Our findings provide a snapshot of HRQOL for survivors of aggressive NHL 2–5 years post-diagnosis. While NHL survivors reported MCS scores similar to those of the U.S. general population, their PCS scores were significantly lower, suggesting that many in this survivor population report deficits in physical functioning. Similar findings have been reported in another recent study of NHL survivors that examined all NHL types across a much larger window of time since diagnosis (mean 11 years).14,33 These findings suggest that persistent disease or treatment-related adverse outcomes related to physical function may be present for aggressive NHL survivors during this time period.
This study also provides further details on NHL survivors’ HRQOL by reporting rates of depression, anxiety, and fatigue. While the anxiety and depression scores suggest that, on average, these are healthy survivors who report normal mental health, approximately 1 in 5 survivors reported scores above the clinical threshold for depression or an anxiety disorder. The overall rates of anxiety and depression in this NHL survivor cohort were slightly higher than those reported by the general U.S. population,34 and were consistent with the rates previously reported among lymphoma survivors.35–37 One recent study found a higher rate of anxiety (37%) than we did among aggressive NHL survivors in a clinical setting.38 Fatigue levels were higher than the scores reported (using the same scale we used) for both the general population26 and for cancer survivors more than one year post-treatment.25 This suggests that fatigue is higher among survivors of aggressive NHL than among other cancer survivors, but further research is necessary to determine the clinical relevance of this difference.
Specific patient-level characteristics were found to be associated with HRQOL. Younger survivors, those with limited access to healthcare, those without a partner, and those with greater illness burden reported poorer HRQOL. Subgroup differences often exceeded 0.5SD, suggesting that these HRQOL deficits are likely to be clinically meaningful. Some patient characteristics were found to be specific to physical and mental health domains (PCS and MCS): younger age was associated with lower mental health, and disease recurrence or progression was associated with lower physical health. Other characteristics, such as having a partner, private insurance, or having a greater comorbidity burden, were associated with both lower physical and mental health domains. This supports previous findings reported in survivors of NHL14,39 and other cancers,40,41 as well as the general U.S. population.42 This study did not replicate some other relationships identified in previous studies. While older age has been shown to be associated with poorer physical health in cancer survivors and the general population, no age difference was found in this study for physical health. Instead, PCS scores reported here were significantly lower across all age subgroups of NHL survivors (8–10 points, 0.8SD–1.0SD) than the U.S. general population mean, suggesting a strong physical health burden across this entire survivor population. Additionally, sociodemographic characteristics (education, race/ethnicity) and clinical variables (NHL grade and treatment history) showed no association with any HRQOL domains. Lack of significant differences may be due to this study’s smaller survivorship window of 2–5 years and our focus on aggressive NHL. Although further research is needed to investigate these differences, our findings nonetheless provide important information about specific subgroups of NHL survivors who might be at greater risk for decrements in physical and mental health during the period of 2–5 years post-diagnosis.
Perceptions of greater health competence and personal control were consistently associated with higher HRQOL. Moreover, differences among survivors with low levels of competence and control and those with high levels of competence and control exceeded thresholds for clinical significance. The one exception was that physical health was positively associated with perceived health competence but not with personal control. This finding illustrates the subtle, distinct impact that these two cognitive factors may have on NHL survivors’ perception of physical and mental aspects of their HRQOL. Additionally, the cognitive appraisal variables explained more variance in MCS, anxiety, depression, and fatigue outcomes than the sociodemographic and clinical factors, thereby showing the distinctive associations that static patient characteristics and more mutable cognitive factors may have with respect to HRQOL for this population. Overall, our findings suggest that different aspects of survivors’ cognitive health appraisal may be associated not only with their perceptions of overall physical or mental health but also with specific long-term symptoms, such as anxiety, depression, and fatigue.
Existing studies report that facilitation of cancer survivors’ information-seeking efforts and facilitation of shared decision-making between clinicians and survivors during follow-up care visits may improve cancer survivors’ perceptions of health competence and control.19,28,43 Our findings suggest that such efforts directed at improving survivors’ cognitive health appraisal may also facilitate better adjustment among survivors of aggressive NHL. However, given the cross-sectional nature of our study, we encourage future longitudinal studies to replicate our finding of positive association of perceived health competence and personal control with HRQOL.
Our findings should be interpreted in light of three potential limitations. First, our response rate was modest at 55% and respondents may have systematically differed from non-responders. As has been published elsewhere,20 we compared study responders and non-responders on a limited number of indicators available from the SEER registry (age, gender, race/ethnicity, NHL grade, and time since diagnosis). Among NHL survivors who we were able to contact, we did not find any significant differences among those who participated in the study and those who refused. Also, as noted earlier, we found little bias in our respondent sample between those who completed the abbreviated telephone interview and those who filled out the mailed questionnaire. Second, this cohort was diagnosed between 1998 and 2001, and medical treatments for aggressive NHL have changed over the past 10 years, especially with the introduction of Rituximab. However, treatment for this type of NHL remains aggressive with potential for similar impact on HRQOL, limiting the possibility of any major differences than what has been reported here. Nevertheless, we encourage additional studies with more recent cohorts of NHL survivors be conducted to replicate our findings and further research examining specific treatment protocols. Finally, NHL survivors from Los Angeles County may not be representative of all U.S. survivors and our findings may not generalize to the experiences of longer term survivors, thereby limiting generalizability of our findings. Despite these limitations, this study adds to the very limited literature on the HRQOL of NHL survivors providing a more comprehensive assessment of HRQOL outcomes in this population than has been previously reported. Recruitment of survivors from a population-based SEER registry, rather than a clinically recruited sample, further adds to the strength and validity of our findings.
CONCLUSION
This study identified key post-treatment HRQOL issues faced by NHL survivors; the largest deficits occurred in physical function. Our study found that younger NHL survivors, those who may lack support from a partner or a spouse, those with limited access to care, and survivors who experience a greater illness burden were more likely to experience poorer HRQOL and may warrant special attention during follow-up care visits from health care providers. Our findings also provide a strong foundation for future longitudinal and intervention research that should explore the potential for improving HRQOL among NHL survivors by enhancing their perceptions of health competence and personal control.
Figure 2. Depression and Anxiety by Cognitive Health Appraisal Level.
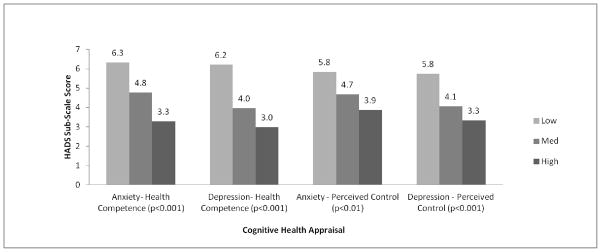
Model controls for: Age, Gender, Race/Ethnicity, Marital Status, Education, Health Insurance, Recurrence/Disease Progression, Treatment in Past 6 Months, and Number of Comorbid Conditions. Adjusted R Squared: 0.25 (Anxiety), 0.36 (Depression)
Acknowledgments
Funding Source: The collection of cancer incidence data used in this study was supported by the California Department of Health Services (CADHS) as part of the Surveillance, Epidemiology and End Results Program under contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute (PHI); and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the PHI.
This article reflects personal opinions of Drs. Arora, Rowland, and Aziz and does not convey any official position of the National Institutes of Health.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2008. National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER website, 2011. [Google Scholar]
- 2.Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH. Cancer survivors: a booming population. Cancer Epidemiol Biomarkers Prev. 2011;20:1996–2005. doi: 10.1158/1055-9965.EPI-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jerkeman M, Kaasa S, Hjermstad M, Kvaloy S, Cavallin-Stahl E. Health-related quality of life and its potential prognostic implications in patients with aggressive lymphoma: a Nordic Lymphoma Group Trial. Med Oncol. 2001;18:85–94. doi: 10.1385/MO:18:1:85. [DOI] [PubMed] [Google Scholar]
- 4.Reeve BB, Potosky AL, Smith AW, et al. Impact of cancer on health-related quality of life of older Americans. J Natl Cancer Inst. 2009;101:860–868. doi: 10.1093/jnci/djp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yost KJ, Hahn EA, Zaslavsky AM, Ayanian JZ, West DW. Predictors of health-related quality of life in patients with colorectal cancer. Health Qual Life Outcomes. 2008;6:66. doi: 10.1186/1477-7525-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganz PA, Rowland JH, Desmond K, Meyerowitz BE, Wyatt GE. Life after breast cancer: understanding women’s health-related quality of life and sexual functioning. J Clin Oncol. 1998;16:501–514. doi: 10.1200/JCO.1998.16.2.501. [DOI] [PubMed] [Google Scholar]
- 7.Penson DF, Feng Z, Kuniyuki A, et al. General quality of life 2 years following treatment for prostate cancer: what influences outcomes? Results from the prostate cancer outcomes study. J Clin Oncol. 2003;21:1147–1154. doi: 10.1200/JCO.2003.07.139. [DOI] [PubMed] [Google Scholar]
- 8.Richardson LC, Wingo PA, Zack MM, Zahran HS, King JB. Health-related quality of life in cancer survivors between ages 20 and 64 years: population-based estimates from the Behavioral Risk Factor Surveillance System. Cancer. 2008;112:1380–1389. doi: 10.1002/cncr.23291. [DOI] [PubMed] [Google Scholar]
- 9.Schag CA, Ganz PA, Wing DS, Sim MS, Lee JJ. Quality of life in adult survivors of lung, colon and prostate cancer. Qual Life Res. 1994;3:127–141. doi: 10.1007/BF00435256. [DOI] [PubMed] [Google Scholar]
- 10.Burgess C, Cornelius V, Love S, Graham J, Richards M, Ramirez A. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ. 2005;330:702. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vahdaninia M, Omidvari S, Montazeri A. What [does] predict anxiety and depression in breast cancer patients? A follow-up study. Soc Psychiatry Psychiatr Epidemiol. 2010;45:355–361. doi: 10.1007/s00127-009-0068-7. [DOI] [PubMed] [Google Scholar]
- 12.Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112:2577–2592. doi: 10.1002/cncr.23448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith SK, Zimmerman S, Williams CS, Zebrack BJ. Health status and quality of life among non-Hodgkin lymphoma survivors. Cancer. 2009;115:3312–3323. doi: 10.1002/cncr.24391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith SK, Crespi CM, Petersen L, Zimmerman S, Ganz PA. The impact of cancer and quality of life for post-treatment non-Hodgkin lymphoma survivors. Psychooncology. 2010;19:1259–1267. doi: 10.1002/pon.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith SK, Zimmerman S, Williams CS, Preisser JS, Clipp EC. Post-traumatic stress outcomes in non-Hodgkin’s lymphoma survivors. J Clin Oncol. 2008;26:934–941. doi: 10.1200/JCO.2007.12.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ranchor AV, Wardle J, Steptoe A, Henselmans I, Ormel J, Sanderman R. The adaptive role of perceived control before and after cancer diagnosis: A prospective study. Soc Sci Med. 2010;70:1825–1831. doi: 10.1016/j.socscimed.2009.10.069. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham AJ, Lockwood GA, Cunningham JA. A relationship between perceived self-efficacy and quality of life in cancer patients. Patient Educ Couns. 1991;17:71–78. doi: 10.1016/0738-3991(91)90052-7. [DOI] [PubMed] [Google Scholar]
- 18.Henselmans I, Sanderman R, Baas PC, Smink A, Ranchor AV. Personal control after a breast cancer diagnosis: stability and adaptive value. Psychooncology. 2009;18:104–108. doi: 10.1002/pon.1333. [DOI] [PubMed] [Google Scholar]
- 19.Arora NK, Johnson P, Gustafson DH, McTavish F, Hawkins RP, Pingree S. Barriers to information access, perceived health competence, and psychosocial health outcomes: test of a mediation model in a breast cancer sample. Patient Educ Couns. 2002;47:37–46. doi: 10.1016/s0738-3991(01)00170-7. [DOI] [PubMed] [Google Scholar]
- 20.Arora NK, Hamilton AS, Potosky AL, et al. Population-based survivorship research using cancer registries: a study of non-Hodgkin’s lymphoma survivors. J Cancer Surviv. 2007;1:49–63. doi: 10.1007/s11764-007-0004-3. [DOI] [PubMed] [Google Scholar]
- 21.Ware JE, Jr, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Lincoln, RI: QualityMetric Incorporated; 2000. [Google Scholar]
- 22.Ware JE, Jr, Kosinski M, Dewey JE. How to Score Version Two of the SF-36 Health Survey. Lincoln, RI: QualityMetric Incorporated; 2000. [Google Scholar]
- 23.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 24.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 25.Hann DM, Denniston MM, Baker F. Measurement of fatigue in cancer patients: further validation of the Fatigue Symptom Inventory. Qual Life Res. 2000;9:847–854. doi: 10.1023/a:1008900413113. [DOI] [PubMed] [Google Scholar]
- 26.Hann DM, Jacobsen PB, Azzarello LM, et al. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7:301–310. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- 27.Smith MS, Wallston KA, Smith CA. The development and validation of the Perceived Health Competence Scale. Health Educ Res. 1995;10:51–64. doi: 10.1093/her/10.1.51. [DOI] [PubMed] [Google Scholar]
- 28.Arora NK, Weaver KE, Clayman ML, Oakley-Girvan I, Potosky AL. Physicians’ decision-making style and psychosocial outcomes among cancer survivors. Patient Educ Couns. 2009;77:404–412. doi: 10.1016/j.pec.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potosky AL, Harlan LC, Stanford JL, et al. Prostate cancer practice patterns and quality of life: the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 1999;91:1719–1724. doi: 10.1093/jnci/91.20.1719. [DOI] [PubMed] [Google Scholar]
- 30.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1988. pp. 25–27. [Google Scholar]
- 31.Hays RD, Farivar SS, Liu H. Approaches and recommendations for estimating minimally important differences for health-related quality of life measures. COPD. 2005;2:63–67. doi: 10.1081/copd-200050663. [DOI] [PubMed] [Google Scholar]
- 32.Samsa G, Edelman D, Rothman ML, Williams GR, Lipscomb J, Matchar D. Determining clinically important differences in health status measures: a general approach with illustration to the Health Utilities Index Mark II. Pharmacoeconomics. 1999;15:141–155. doi: 10.2165/00019053-199915020-00003. [DOI] [PubMed] [Google Scholar]
- 33.Crespi CM, Smith SK, Petersen L, Zimmerman S, Ganz PA. Measuring the impact of cancer: a comparison of non-Hodgkin lymphoma and breast cancer survivors. J Cancer Surviv. 2010;4:45–58. doi: 10.1007/s11764-009-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kornblith AB, Herndon JE, Zuckerman E, et al. Comparison of psychosocial adaptation of advanced stage Hodgkin’s disease and acute leukemia survivors. Cancer and Leukemia Group B. Ann Oncol. 1998;9:297–306. doi: 10.1023/a:1008297130258. [DOI] [PubMed] [Google Scholar]
- 36.Loge JH, Abrahamsen AF, Ekeberg O, Hannisdal E, Kaasa S. Psychological distress after cancer cure: a survey of 459 Hodgkin’s disease survivors. Br J Cancer. 1997;76:791–796. doi: 10.1038/bjc.1997.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004:57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- 38.Thompson CA, Charlson ME, Schenkein E, et al. Surveillance CT scans are a source of anxiety and fear of recurrence in long-term lymphoma survivors. Ann Oncol. 2010;21:2262–2266. doi: 10.1093/annonc/mdq215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mols F, Aaronson NK, Vingerhoets AJ, et al. Quality of life among long-term non-Hodgkin lymphoma survivors: a population-based study. Cancer. 2007;109:1659–1667. doi: 10.1002/cncr.22581. [DOI] [PubMed] [Google Scholar]
- 40.Zebrack BJ, Yi J, Petersen L, Ganz PA. The impact of cancer and quality of life for long-term survivors. Psychooncology. 2008;17:891–900. doi: 10.1002/pon.1300. [DOI] [PubMed] [Google Scholar]
- 41.Sammarco A. Quality of life of breast cancer survivors: a comparative study of age cohorts. Cancer Nurs. 2009;32:347–356. doi: 10.1097/NCC.0b013e31819e23b7. [DOI] [PubMed] [Google Scholar]
- 42.Zahran HS, Kobau R, Moriarty DG, et al. Health-related quality of life surveillance--United States, 1993–2002. MMWR Surveill Summ. 2005;54:1–35. [PubMed] [Google Scholar]
- 43.Hesse BW, Arora NK, Burke BE, Finney Rutten LJ. Information support for cancer survivors. Cancer. 2008;112:2529–2540. doi: 10.1002/cncr.23445. [DOI] [PubMed] [Google Scholar]


