Abstract
The white-throated sparrow is rapidly becoming an important model in the genetics of social behavior because of a chromosomal rearrangement that segregates with a behavioral phenotype. Within a population, 50 % of individuals are heterozygous for a rearranged chromosome 2 (ZAL2m). These birds sing more and are more aggressive than the other 50 %, who lack the rearrangement. A disassortative mating system, in which heterozygotes almost never interbreed, ensures that ZAL2m/2m homozygotes are extremely rare. Here, we provide the first systematic characterization of such a homozygote, a hatch-year female. Her plumage was atypical of her age and sex, resembling that of an adult male. She was extremely vocal and aggressive, dominating her opponents in behavioral tests. Her phenotype was thus an exaggerated version of a typical ZAL2/2m heterozygote, supporting the hypothesis that alleles inside the ZAL2m rearrangement confer high aggression and further emphasizing this species’ value as a model of social behavior.
Keywords: Aggression, Alternative phenotypes, Chromosomal inversion, Life history strategies, Polymorphism, White-throated sparrow
Introduction
The white-throated sparrow (Zonotrichia albicollis) is a common North American songbird that exhibits a plumage polymorphism (Lowther 1961; Piper and Wiley 1989a; Watt 1986a; see Fig. 1). Individuals of the “white-striped” (WS) morph have primarily white and primarily black feathers in the median and lateral crown stripes, respectively, bright yellow lores, and a pure white throat patch. Individuals of the “tan-striped” (TS) morph have primarily tan and brown crown stripes, duller lores, and streaking in the throat patch. The species has received a lot of interest from behavioral biologists because the plumage polymorphism segregates with a behavioral phenotype. WS birds of both sexes sing more and are more aggressive than their TS counterparts, who invest more time in parental behavior (reviewed by Falls and Kopachena 2010; Maney 2008). WS males engage in more mate-seeking, whereas TS males spend more time mate-guarding (Formica and Tuttle 2009; Tuttle 2003). The morphs thus represent two ends of a classic life history tradeoff, with investment in mating opportunities at one end and parental care at the other (Trivers 1972). This species is therefore an excellent model for studying the evolution of alternative life history strategies.
Fig. 1.

Plumage polymorphism in white-throated sparrows. (a) Individuals of the white-striped (WS) morph have alternating black and white stripes on the crown, bright yellow lores, and a clear white throat patch. (b) Individuals of the tan-striped (TS) morph have alternating brown and tan stripes on the crown, duller yellow lores, and dark bars within the white throat patch. Photos by Christopher Gurguis. Reprinted from Horton et al. (2012)
The plumage polymorphism in this species has a clear genetic basis (Thorneycroft 1975). TS birds have two copies of the standard arrangement of chromosome 2 (ZAL2), whereas WS birds are heterozygous for a rearrangement (ZAL2m) that contains two nested pericentric inversions spanning 100 MB (Thomas et al. 2008). The phenotypes are fixed, in that adult individuals do not switch from one to the other, and the white stripe is inherited in a Mendelian fashion as a dominant trait linked to ZAL2m (Thorneycroft 1975). Within a population, approximately half of the birds are WS (ZAL2/2m), whereas the other half are TS (ZAL2/2; Lowther 1961; Thorneycroft 1975). The ZAL2m arrangement is maintained at a frequency of 50 % because of a disassortative mating system; nearly all of the breeding pairs consist of one WS and one TS individual. Thus, half of the offspring are ZAL2/2 and are TS, and the rest are ZAL2/2m and are WS. A ZAL2m/2m homozygote can be produced only from a WS × WS mating, but fewer than 2.5 % of all breeding pairs are WS × WS (Falls and Kopachena 2010; BMH, unpublished data). Extra-pair matings between two WS birds are also thought to be infrequent because WS females tend to act aggressively toward WS males and because TS males effectively guard their WS mates (Formica and Tuttle 2009; Houtman and Falls 1994; Tuttle 2003). Thorneycroft (1975) hypothesized that the rearranged ZAL2m chromosome contains deleterious mutations that may reduce the viability of ZAL2m/2m homozygotes, thus driving the evolution of the disassortative mating system.
In previous studies we proposed and then validated the hypothesis that recombination between the ZAL2 and ZAL2m is profoundly suppressed within the inversions (Huynh et al. 2011; Thomas et al. 2008). However, although the ZAL2m is in a near-constant state of heterozygosity and presumably has limited opportunity to recombine, we did not observe an accumulation of deleterious mutations on the ZAL2m or other signatures of degeneration expected for a non-recombining chromosome (Davis et al. 2011; Huynh et al. 2011). The lack of genetic degeneration suggests that recombination between ZAL2m chromosomes has occurred, even if infrequently, in viable and fertile ZAL2m/2m homozygotes. These individuals, though rare, therefore likely serve as a critical genetic refuge that protects the ZAL2m from a fate similar to the non-recombining segments of sex chromosomes (Charlesworth and Charlesworth 2000; Graves 2006).
Only two ZAL2m/2m homozygotes have been reported in the literature (reviewed by Falls and Kopachena 2010). Both were anecdotally described as aggressive and having plumage typical of the WS morph. In neither case, however, were plumage or behavior systematically studied. Here, we report the first systematic characterization of a ZAL2m/2m homozygote. This bird, a hatch-year female (hereafter referred to as “Bird 1128”), was captured in a mist net on the campus of Emory University during Fall 2011 among 50 other birds collected and genotyped that year from the same site. She is the only ZAL2m/2m homozygote in 602 birds genotyped in our lab over a period of 9 years, and the 3rd in a total combined sample of 1,556 birds (Falls and Kopachena 2010; Michopoulos et al. 2007; Romanov et al. 2009; Thorneycroft 1975; DLM, unpublished data). Here, we document her genotype definitively via polymerase chain reaction, karyotyping, and fluorescence in situ hybridization (FISH) mapping, describe her plumage coloration, and characterize her aggressive behavior in both non-breeding and breeding conditions.
Methods
Animals
All research was conducted in accordance with NIH principles of animal care, federal and state laws, and university guidelines. The bird described here was one of 50 migrating white-throated sparrows collected on the campus of Emory University between November 15 and December 2, 2011. Sex and morph were determined by PCR analysis of a small blood sample (Griffiths et al. 1998; Michopoulos et al. 2007; see below). Age was determined by the shape of the primary coverts and outer rectrices and the degree of skull ossification according to Pyle (1997). Birds were housed in walk-in flight cages (6′l × 4′w × 7′h), 12–15 birds per cage, in the Emory animal care facility and supplied with ad libitum food and water. The light cycle was set to 8.5L:15.5D, a short-day photoperiod under which ovarian development does not occur (Shank 1959), for at least 2 months before the start of the behavioral experiment.
Determining morph by PCR
We routinely genotype all of the white-throated sparrows we collect each year by PCR analysis of one or more informative markers (Michopoulos et al. 2007; Thomas et al. 2008). We first became aware that Bird 1128 may be a homozygote during PCR genotyping at locus DSE, which produces a single band in TS birds and normally two bands in WS birds. Bird 1128’s sample produced only one band, which differed in size from the band seen in TS birds. To confirm that Bird 1128 was homozygous for the ZAL2m chromosome, we conducted PCR analyses of two additional loci: VIP (Michopoulos et al. 2007) and FAM83b (Thomas et al. 2008), both of which contain restriction fragment length polymorphisms (RFLPs) associated with the chromosomal rearrangement.
Behavioral experiment
To assess the aggressive behavior of Bird 1128, we observed dyadic interactions in a series of behavioral trials with other laboratory-housed birds. In most cases the other birds were also tested with each other in order to determine relative dominance ranks. From the 50 birds that were collected that year, we selected 6 TS (ZAL2/2) and 6 WS (ZAL2/2m) females while considering familiarity, age, and size. Birds previously housed together in a flight cage were considered familiar, whereas those captured on different days and housed separately since capture were considered unfamiliar. We balanced the number of familiar and unfamiliar opponents such that for each female in the study, the ratio of unfamiliar opponents to familiar ones was 2:1. Because age is a strong predictor of dominance in this species (Piper and Wiley 1989b), we controlled for age by including only hatch-year birds. Thus, all of the females in this experiment, including Bird 1128, were hatched during the 2011 breeding season and were therefore less than 1 year old. Bird 1128 was smaller than average (wing length 67 mm, tarsus 22.5 mm; see Results) and the other females available for the experiment tended to be larger (wing length range = 67–70.5 mm; tarsus range = 21.7–23.3 mm). In this species, however, body size is not thought to affect dominance relationships (Piper and Wiley 1989b). We assessed the birds’ physical condition throughout the experiment by scoring fat (Helms and Drury 1960) and muscle depots (Horton and Holberton 2009).
The day before the start of the behavioral experiment, all birds were moved into individual cages (15″l × 15″w × 17″h) inside walk-in sound-attenuating booths (Industrial Acoustics, Bronx, NY) that housed 4–6 familiar birds. For the trials, two females at a time were placed together in a medium-sized cage (30″l × 18″w × 18″h) for 3 h. These trials were conducted inside separate sound-attenuating booths so that the two birds engaged in a trial could not see or hear other ongoing trials. After each trial ended, the birds were returned to their individual cages until their next trial. Each bird participated in a maximum of one trial per day.
The overall design of the behavioral experiment is diagrammed in Fig. 2. We first conducted a preliminary set of behavioral trials on short days (Fig. 2a). During these trials, Bird 1128 was sequentially paired with 3 opponents of each morph (total 6 trials), alternating between WS and TS opponents. Meanwhile, all of the WS and TS females were also paired with multiple opponents; in some cases the opponent was Bird 1128, and in all remaining cases was another female of the same morph. We did not pair WS with TS birds in this study because of the risk that the WS birds would dominate those interactions (see Maney 2008; Swett 2007), thus biasing the TS group toward conditioned defeat. In order to ensure that the average percentage of wins was equivalent across morph for Bird 1128’s opponents, all trials that did not include her were between two females of the same morph (Fig. 2).
Fig. 2.
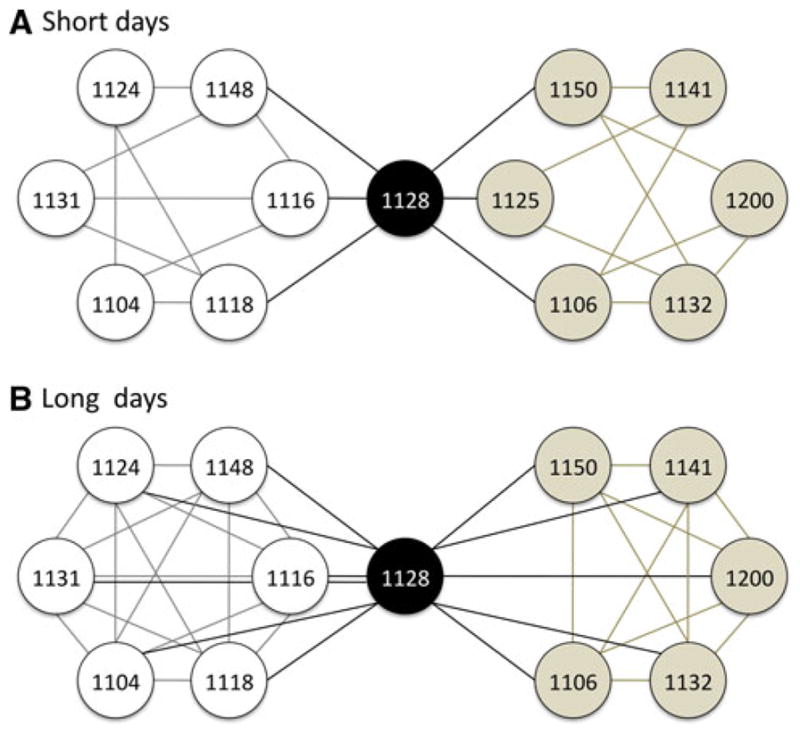
Design of behavioral experiment. Bird 1128 is represented by black circles. White and tan circles represent ZAL2/2m (WS) birds and ZAL2/2 (TS) birds, respectively. Each line connecting two circles represents a single behavioral trial, 3 h in duration, between those two birds. Trials with Bird 1128 are indicated by black lines. WS–WS and TS–TS trials are indicated by gray and tan lines, respectively. Under short days (a), Bird 1128 was paired with 3 WS and 3 TS opponents. Each of the WS and TS birds were sequentially paired with 3–4 opponents, which in some but not all cases included Bird 1128. On long days (b), Bird 1128 was paired with every other bird, and each WS and TS bird was paired with every other same-morph bird. Bird 1125 was unavailable for long-day trials
After the short-day trials were completed, we photostimulated the birds by changing the light cycle to 16L:8D for the duration of the experiment. Until behavioral trials resumed, all birds were housed in individual cages in booths with familiar birds. One TS female was diagnosed with a contagious medical condition during the photostimulation period and was removed from the experiment.
Behavioral trials resumed 6 weeks after the first long day. During the long-day trials (Fig. 2b), Bird 1128 was sequentially paired with each of the other females, alternating between TS and WS opponents (total 11 trials). Meanwhile, each WS and TS female was also paired with each other female of the same morph (total 6 trials for WS birds and 5 trials for TS birds). Thus, approximately half of the long-day trials were a rematch of short-day trials, and the remaining trials were new pairings with either familiar or unfamiliar opponents.
The first 2 h of each trial were videotaped to quantify behaviors. All recordings were scored by an observer who was unfamiliar with the natural history of this species and who was blind to the hypothesis. We scored four vocalizations used by females of this species during aggressive encounters: songs, chip (or pink) calls, chip-up calls, and trills (described by Falls and Kopachena 2010). In the photostimulated birds, the trill was sometimes accompanied by a wing quiver and tail-up posture typical of an E2-dependent display that is most often used in a courtship context but can occur during agonistic encounters (Falls and Kopachena 2010) or spontaneously (Maney et al., 2009). Because the function of these displays during female–female interactions is unclear, we did not include those trills among the unambiguously aggressive ones. Finally, we counted a number of physical aggressive behaviors, consisting of attacks, chases, displacements (supplantations), and hold-offs (thwarted displacements), which are used to express dominance in this species (Watt et al. 1984; Wiley et al. 1999). The physical behaviors were used to assess the dominance relationship for each dyad. An individual was considered the winner of a trial if it prevailed in >50 % of the combined interactions, and vice versa for the loser (Watt et al. 1984). Dominance matrices (Watt et al. 1984) were constructed for long-day trials only. Separate matrices were constructed for TS females and for WS females, both of which included Bird 1128.
Statistics
Behavioral data from the short-day and long-day trials were treated separately. For each of the 8 behaviors scored (attacks, chases, displacements, hold-offs, songs, chips, chip-ups, and trills), we calculated the rate at which they were expressed per hour by each female in each photoperiodic condition. To compare aggression more generally, we also used principal components analyses (PCA) to construct a composite aggression score (PC1 score) from attacks, chases, displacements, songs, chip-up and chip calls (Moore et al. 2004; Parker et al. 2010). Hold-offs and trills were excluded from the PCA because of infrequency. For each day length, one PCA was conducted for the trials that involved WS birds and another for the TS birds. Because 1128 was tested with birds of both morph, she was represented in both PCAs.
Because Bird 1128 engaged in 17 trials (6 short-day and 11 long-day) and the other birds engaged in only 8–10 each (3 or 4 short-day and 5 or 6 long-day), we needed to address the possibility that Bird 1128’s greater experience with trials explained her success on long days. We performed two analyses to test for effects of experience. First, we did linear regressions to test whether Bird 1128’s aggression (PC1) score increased with the number of trials on either short days or long days. Second, for the other birds we used Wilcoxon Ranked-Sum tests (χ2 approximation) to determine whether the number of trials on short days (three, n = 5; or four, n = 6) affected the percentage of trials won or aggression scores on long days.
Hormone analysis
After the behavioral experiments were complete (at 9 weeks of photostimulation), we obtained a small blood sample (~250 μl) from the brachial vein. Plasma was harvested and stored at −20 °C until assayed for testosterone (T), 5-alpha dihydrotestosterone (DHT), and estradiol (E2) by B.M.H. and I.T.M. according to the procedures of Stevenson et al. (2012). Briefly, plasma samples were fractionated by column chromatography to separate gonadal steroids and then analyzed by radioimmunoassay. All samples were run in a single assay, and hormone concentrations were corrected for individual extraction efficiencies. The lower limit of detectability was 0.09 ng/mL for T, 0.14 ng/mL for DHT and 0.06 ng/mL for E2.
Tissue collection
All of the birds in this study were sacrificed by isoflurane overdose after 9–14 weeks of photostimulation. All ovaries were inspected to confirm breeding condition and the diameter of the largest ovarian follicle was recorded. Bird 1128 was sacrificed at about 10 weeks of photostimulation and prepared as a museum specimen (Smithsonian USNM 627866). The kidneys from Bird 1128, one TS bird, and one WS bird were excised and placed into cold MEM media (Invitrogen/Gibco, Carlsbad, CA) for cell culture (see “fluorescence in situ hybridization”, below). The brains from the same 3 birds were removed and frozen in powdered dry ice. Brain transcriptomes are being assembled as part of a large-scale study that includes many other birds. Pituitaries, ovaries, and liver samples were removed and stored in RNA Later (Ambion, Austin, TX). These tissues and the rest of Bird 1128’s carcass are stored at Emory in a −80 °C freezer.
G-banding analysis
Fibroblast cell cultures were established from a tissue homogenate produced by manual and enzymatic digestion (Itoh and Arnold 2005). Kidney tissue was washed in 5 ml complete media consisting of MEM enhanced with 0.6 % glucose, 10 % heat-inactivated fetal bovine serum (Irvine Scientific, Santa Ana, CA), 5000 units/ml penicillin and 5 mg/ml streptomycin (Invitrogen/Gibco), and 10 % chicken serum (Sigma-Aldrich, St. Louis, MO). It was then manually minced and resuspended in 0.5 ml PBS (Invitrogen/Gibco). Cells were incubated with collagenase Type II S for 15–30 min at 37 °C. To ensure complete homogenization, the tissue suspension was further digested by mixing through a Pasteur pipette. The digested tissue was placed in 10 ml of complete media and cultures were incubated at 37 °C.
When the cultures reached 80 % confluency, Karyo-MAX colcemid (30 ng; Invitrogen/Gibco) was added and the cells were incubated overnight at 37 °C for 12–16 h. Additional colcemid (0.5 μg) was added, and the cells were further incubated for 3–4 h. The cells were trypsinized from the surface of the flask using TrypLE Express (Invitrogen/Gibco) for 15 min at 37 °C. Cells were rinsed with 1.5 ml of media and centrifuged, and the pellet was suspended in KCl:sodium citrate (60:40; 0.075:0.27 m) hypotonic solution and incubated for 20 min at 37 °C. The cells were then treated with 1 ml of methanol:glacial acetic acid (3:1; 100 %:17.4 n) fixative, centrifuged, and resuspended in 10 ml fixative. This final step was repeated two times before metaphase slide preparation. For karyotype analysis, metaphase slides were prepared and stained by G-banding following standard cytogenetic procedures.
FISH analysis
Zebra finch BAC clones (TG-Ba05K13 and TG-Ba55A1) that hybridize to informative locations on the ZAL2 and ZAL2m were identified as per Thomas et al. (2008). BAC DNA was isolated from overnight cultures with the appropriate antibiotic using an alkaline lysis procedure or an automated extraction system (Autogen, Holliston, MA). Nucleotides labeled with spectrum orange or spectrum green (Abbott Molecular, Des Plaines, IL) were incorporated into the BAC DNA using a standard nick translation or random priming reaction. Metaphase slides (see above under “G-banding”) were baked at 73 °C for proper aging, washed in 2× SSC at 37°for 30 min, and dehydrated sequentially in 70, 80, and 95 % ice-cold ethanol. Chromosomes were denatured in 70 % formamide/2× SSC at 75° for 30 s and then dehydrated as above. Prior to hybridization, probes were denatured at 75 °C for 7 min and reannealed at 45° for 1–10 min. Probes were hybridized to metaphase chromosome spreads for 36 h at 37 °C. Slides were washed in 0.4× SSC/0.3 % NP-40 at 75 °C for 2 min, washed in 0.2× SSC/0.1 % NP-40 at room temperature for 30 s, and counter-stained with DAPI for 3 min. Slides were mounted in VectaShield antifade solution (Vector Laboratories, Burlingame, CA) and analyzed using digital imaging with a CCD camera and software (SmartCapture 2, Digital Scientific, Cambridge, UK).
Results
Genetic analyses
PCR analysis showed that at the loci DSE, FAM83b and VIP, Bird 1128 exhibited only ZAL2m alleles. G-banding analysis (Fig. 3) showed that the TS bird had two copies of the standard submetacentric chromosome 2 (ZAL2) whereas the WS bird had one copy of ZAL2 and one copy of the metacentric arrangement typical of ZAL2m (Thorneycroft 1975). In contrast, Bird 1128 had two copies of the metacentric ZAL2m. The FISH analysis (Fig. 4) showed once again two copies of ZAL2 in the TS bird, one copy of ZAL2 and one of ZAL2m in the WS bird, and two copies of ZAL2m in Bird 1128.
Fig. 3.

The first 12 pairs of chromosomes in a tan-striped female (a), a white-striped female (b), and Bird 1128 (c). Chromosome numbers are listed below the G-banded chromosomes. The TS female has two copies of the ZAL2 (submetacentric), the WS female has one ZAL2 and one ZAL2m (metacentric), and Bird 1128 has two copies of the ZAL2m arrangement
Fig. 4.
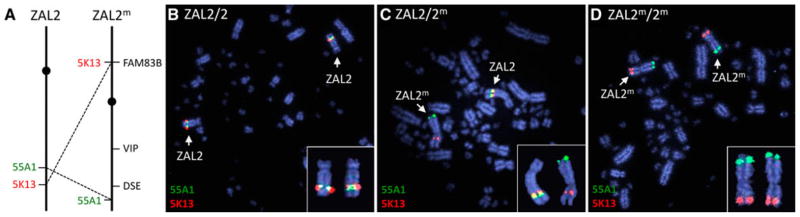
A schematic diagram and results from fluorescence in situ hybridization showing the locations of zebra finch BAC clones 5K13 (red) and 55A1 (green) on ZAL2 and ZAL2m. The two clones hybridize close together on the long arm of ZAL2, but because of an inversion, they map to opposite arms of ZAL2m (a). Tan-striped (TS) birds have two copies of ZAL2 (b) and white-striped (WS) birds have one copy of ZAL2 and one of ZAL2m (c). Bird 1128 clearly shows the ZAL2m hybridization pattern on two chromosomes (d). The chromosome map in (a) shows the locations of the three markers used to genotype Bird 1128 via PCR
Morphology
Bird 1128 was a bit smaller than an average female, but not unusually so. Her wing length was 67 mm and her tarsus length 22.5 mm, compared with a wing length mean and range of 69, 65–73 mm (n = 411) and tarsus 22.9, 21.1–24.4 mm (n = 120) for wintering females at our study site. By comparison, males tend to be larger with a mean wing length of 73, range 70–79 mm (n = 223), and tarsus 23.4, 21.5–26.0 mm (n = 72). Other than her plumage coloration (see below), she had no remarkable physical characteristics. Upon dissection we noted no obvious abnormalities.
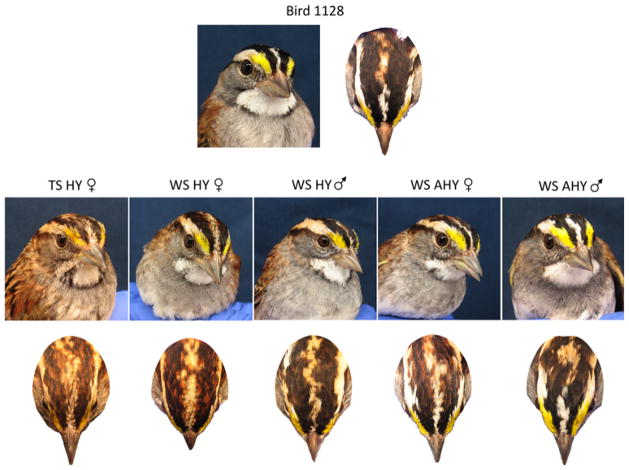
In Zonotrichia sparrows, older birds have brighter plumage than younger birds (Colwell 1999; Emlen 1938; Mailliard 1932; Piper and Wiley 1989a) and males are brighter than females (Colwell 1999; Fugle and Rothstein 1985; Piper and Wiley 1989a; see also Fig. 5). Bird 1128’s plumage was very bright and thus atypical for a hatch-year WS female; it resembled instead that of an adult male (Fig. 5). Her superciliary and median crown stripes contained a much larger percentage of white feathers, and her lateral crown stripes more black feathers, than those of her same-age peers (compare Bird 1128 with other hatch-year birds in Fig. 5). Her throat patch was also brighter white than is typical for her age, and it completely lacked the malar stripes exhibited by TS birds and many young or female WS birds (Lowther 1961). Finally, her lores (the feathers of the rostral superciliary stripe) were the bright canary yellow typical of adults rather than the duller ochre that is normally seen in hatch-year females (Lowther 1961). She was most likely in first basic plumage and we saw no evidence of molt. The timing of prenuptial (prealternate) molt in this species can vary in captivity; data published by Miller and Weise (1978) suggest that the birds in this study were unlikely to molt before May, by which time this study was completed. We cannot, however, rule out the possibility that genes on the ZAL2m may affect the timing of molt and that Bird 1128 may have molted into nuptial plumage before she was captured.
Fig. 5.
Bird 1128 compared with five other birds collected during Fall 2011. Bird 1128 was a hatch-year (HY) female, but her plumage was not typical of either tan-striped (TS) or white-striped (WS) HY females. Neither was it comparable to that of a WS HY male. Her plumage was more typical of an after hatch-year (AHY) WS female, or even male, in that the median and superciliary crown stripes contained many white feathers, the lateral crown stripes many black feathers, and the lores many yellow feathers
Aggression and dominance behavior
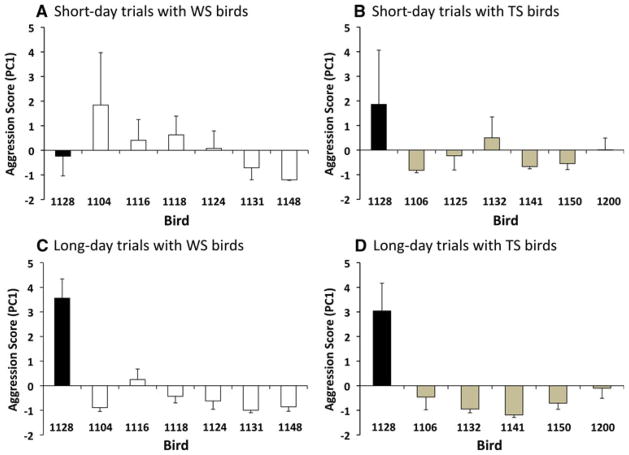
The results of the PCA analyses are shown in Fig. 6, and the means and ranges for the individual behaviors scored are given in Table 1. The composite aggression score (PC1) explained 44 and 47 % of the variation in aggressive behaviors during TS and WS short-day trials, respectively. For the long-day trials, PC1 explained 59 and 51 % of the variation. According to these aggression scores, Bird 1128 was not more aggressive overall than her TS and WS opponents under short-day conditions (Fig. 6). Neither was she particularly high-ranking, losing 33 % of her short-day trials. Under long-day conditions, however, Bird 1128 was the most aggressive and highest-ranking female in the study; she had the highest aggression scores (Fig. 6) and won all 11 of her trials (6 WS, 5 TS opponents; Table 2). Bird 1128 was extraordinarily vocal during long-day trials, singing at a much higher rate than any other female, and more than she did on short days (Table 1).
Fig. 6.
Composite aggression scores (PC1) for Bird 1128 and her opponents calculated from principal components analyses (PCAs) of six aggressive behaviors (attacks, chases, displacements, songs, chip-up and chip calls). Data from Bird 1128 are shown in black, and data from WS and TS birds are shown in white and tan, respectively. Separate PCAs were conducted for (a) short-day trials with WS females (b) short-day trials with TS females (c) long-day trials with WS females, and (d) long-day trials with TS females; thus, aggression scores are comparable only within groups. Bird 1128 was clearly the most aggressive female during long-day trials, but was not distinctively aggressive during short-day trials
Table 1.
Number of aggressive behaviors per hour for Bird 1128 compared with the average rates for TS and WS females in this study. Rates during trials with WS females are shown in open columns and those during trials with TS females are shown in shaded columns.
| Short-day trials | Long-day trials | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Trials with WS females | Trials with TS females | Trials with WS females | Trials with TS females | ||||||
| Bird 1128 | WS females | Bird 1128 | TS females | Bird 1128 | WS females | Bird 1128 | TS females | ||
|
|
|||||||||
| Physical behaviors (per hour) | Displacements | 8.3 ± 4.5 (0–16) | 52.4 ± 17.6 (0–252) | 74.3 ± 58.9 (1–189) | 28.3 ± 11.1 (0–162) | 62.3 ± 6.5 (37–77) | 13.8 ± 3.6 (0–94) | 55.8 ± 12.2 (17–93) | 10.0 ± 4.1 (0–77) |
| Chases | 0 | 2.2 ± 0.7 (0–14) | 0.5 ± 0.3 (0–1) | 0.4 ± 0.2 (0–5) | 10.0 ± 1.6 (5–15) | 0.4 ± 0.2 (0–4) | 13.9 ± 5.6 (1–30) | 0.4 ± 0.3 (0–6) | |
| Attacks | 0 | 0.6 ± 0.2 (0–3) | 0.3 ± 0.3 (0–1) | 0.3 ± 0.2 (0–2) | 1.9 ± 1.1 (0–8) | 0.1 ± 0.1 (0–2) | 4.0 ± 1.5 (0–8) | 0.3 ± 0.2 (0–4) | |
| Hold-offs | 0 | 0 | 0 | 0.1 ± 0.1 (0–1) | 0.4 ± 0.3 (0–2) | 0.1 ± 0.1 (0–1) | 0.5 ± 0.5 (0–3) | 0.1 ± 0.1 (0–1) | |
| Vocalizations (per hour) | Songs | 5.3 ± 3.9 (0–13) | 5.0 ± 2.8 (0–57) | 18.3 ± 17.3 (0–53) | 0.3 ± 0.3 (0–5) | 59.3 ± 17.6 (5–124) | 0.3 ± 0.2 (0–8) | 35.1 ± 13.2 (0–78) | 0.2 ± 0.2 (0–4) |
| Chip-ups | 16.3 ± 13.4 (0–43) | 1.0 ± 0.5 (0–10) | 11.3 ± 10.6 (0–33) | 0.2 ± 0.1 (0–2) | 4.3 ± 3.3 (0–21) | 1.3 ± 0.3 (0–5) | 8.5 ± 3.7 (0–18) | 2.3 ± 1.1 (0–21) | |
| Chips | 7.0 ± 5.8 (0–19) | 1.6 ± 0.7 (0–13) | 0.8 ± 0.8 (0–3) | 0.3 ± 0.1 (0–2) | 6.0 ± 2.5 (0–15) | 3.0 ± 0.9 (0–24) | 7.8 ± 3.7 (2–21) | 3.1 ± 1.4 (0–28) | |
| Trills | 0 | 0 | 0.2 ± 0.2 (0–1) | 0 | 0.6 ± 0.4 (0–3) | 0.7 ± 0.5 (0–20) | 0.2 ± 0.2 (0–1) | 0.3 ± 0.2 (0–3) | |
Values are mean ± SEM, with ranges in parentheses
Table 2.
Dominance matrices showing the results of long-day behavioral trials for Bird 1128 and WS females (A), and for Bird 1128 and TS females (B).
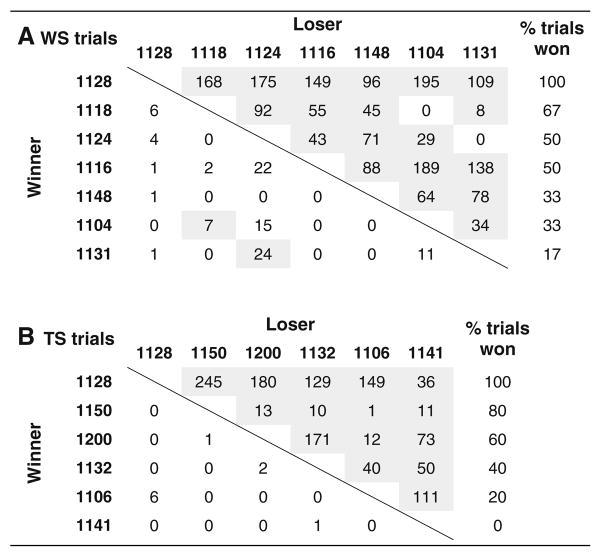
|
Values represent the numbers of aggressive interactions won by the birds listed in the rows (winners) against birds listed in the columns (losers). Shading indicates that the bird listed in that row prevailed overall against the bird listed in that column
There was no evidence that experience with trials affected performance. Bird 1128 lost the third and sixth of six trials on short days, showing that experience with trials did not increase the odds of a win. Second, her aggression scores did not increase with time on short days (R2 = 0.01, p = 0.921) or long days (R2 = 0.04; p = 0.57). Finally, birds that had more short-day trials did not win more long-day trials (WRS, χ2 = 1.21, p = 0.271) or have higher average aggression scores (WRS, χ2 = 1.20, p = 0.273) than birds that had fewer short-day trials.
Ovarian development and gonadal steroid levels
All of the birds in the study showed evidence of ovarian development such as enlarged (>1 mm) or in some cases yolky follicles. Bird 1128, whose largest ovarian follicle was 1.4 mm in diameter, was not an outlier (range 1.0–7.7 mm). Plasma levels of T and DHT in 12 of the 13 females, including 1128, were near or below the lower limit of detectability (T ≤ 0.13 ng/mL; DHT ≤ 0.18 ng/mL). We were able to detect T (0.52 ng/mL) and DHT (0.66 ng/mL) in the plasma of only one bird, a high-ranking WS female. Although we previously demonstrated that photostimulated females in our laboratory have plasma E2 in the range of 0.1–0.5 ng/ml (Lake et al. 2008), we were unable to detect plasma E2 in the birds in this study. We attribute the current result to two factors. First, our current methods differed from those of Lake et al. in that here, we used column chromatography instead of a direct E2 assay. Second, all of the birds in the previous study had been photostimulated for 5–7 weeks, whereas the current birds were photostimulated for 9 weeks at the time of sampling and could have been entering a photorefractory stage (see Dawson et al. 2001). All of the birds in this study, including Bird 1128, had plasma E2 levels near or below the assay’s limit of detectability (all ≤ 0.09 ng/mL). Accurate quantification of E2 positive control standards in the assay (0.5 ng/mL) confirms we would have detected high levels. Thus, we can conclude that Bird 1128 did not have unusually high levels of plasma E2.
Discussion
In this study, we have characterized the plumage and behavior of a white-throated sparrow of the rare genotype ZAL2m/2m. Out of 602 birds genotyped in our lab since 2005, she is the only such individual. The only other published karyotype of a homozygote, a female, appeared 37 years ago in a sample of 397 birds (Thorneycroft 1975). One more homozygote, a male, was anecdotally reported in a sample of 11 by Falls and Kopachena (2010). In a sample of 546 described by Romanov et al. (2009), there were no homozygotes. Thus, the frequency of ZAL2m/2m homozygotes can be estimated at roughly one in 500 birds (3 in 1556). Their existence demonstrates that at least sometimes they are viable, and is consistent with our previous observation that chromosome ZAL2m is not degenerating (Huynh et al. 2011; Davis et al. 2011).
ZAL2m is always present in WS individuals and absent in TS individuals, demonstrating that the diagnostic WS plumage is inherited as a dominant trait linked to ZAL2m (Thorneycroft 1975). Because recombination is strongly suppressed between ZAL2 and ZAL2m (Huynh et al. 2011; Thomas et al. 2008), it is likely that other morph-typical traits are also dominant. The most obvious morph-typical behavioral trait associated with ZAL2m is increased aggression. In free-living populations, WS birds defend their territories more aggressively than do TS birds (Collins and Houtman 1999; Falls and Kopachena 2010; Kopachena and Falls 1993). When in photostimulated groups, WS birds are more aggressive than TS birds and outrank them (Ficken et al. 1978; Harrington 1973; Maney 2008; Watt et al. 1984). Swett (2007) showed that when one WS and one TS bird are housed together, WS birds engage in more displacement behaviors than TS birds. Thus, overall the literature suggests that one or more alleles on the ZAL2m haplotype confer heightened aggression. ZAL2m/2m homozygotes allow us to ask whether an increase in the dosage of these alleles results in a discernibly different phenotype. Under long-day conditions, Bird 1128 dominated 100 % of her opponents (Table 2) and her levels of physical and vocal aggression were far higher than any of the other birds tested (Fig. 6; Table 1). In addition, her crown plumage was remarkably bright and differed from her same-age cohorts (Fig. 5). She thus seemed to exhibit an exaggerated WS phenotype.
Several decades of research indicate that morph differences in aggression depend on endocrine state. In laboratory-housed white-throated sparrows in non-breeding condition, morph is not related to dominance rank or to aggression (reviewed by Maney 2008); however when birds are photostimulated and undergo gonadal recrudescence, WS birds engage in significantly more aggression than their TS cage-mates and tend to outrank them (Maney 2008; Watt et al. 1984). Under short-day conditions, Bird 1128 did not win all of her trials. Under long days, however, she overcame the “social inertia” typical of groups with established relationships (Wiley et al. 1999) and dominated all of her opponents, suggesting further that the expression of ZAL2m genes that contribute toward dominance may interact with endocrine state.
Across Zonotrichia sparrows, older birds have brighter plumage (Colwell 1999; Emlen 1938; Mailliard 1932; Piper and Wiley 1989a) and outrank younger, duller birds (Parsons and Baptista 1980; Piper and Wiley 1989b). Similarly, males are brighter than females (Colwell 1999; Fugle and Rothstein 1985; Piper and Wiley 1989a), and generally outrank them (Parsons and Baptista 1980; Piper and Wiley 1989b). It is thus possible that Bird 1128 dominated her opponents not because she was more aggressive, but because her plumage signaled the dominance status of an older, perhaps even male, bird. In the congeneric Harris sparrow (Z. querula) and white-crowned sparrow (Z. leucophrys), plumage coloration does appear to function as a status signal, affecting how individuals are regarded by conspecifics and therefore possibly how they themselves behave (Fugle et al. 1984; Rohwer 1985; 1977). Status signaling is unlikely to completely explain Bird 1128’s highly aggressive behavior, however. First, there is no evidence that plumage brightness explains dominance relationships in white-throated sparrows (Wiley et al. 1999; Watt 1986b). Second, morph differences in vocal responses to playback persist even when there is no other individual present to assess plumage, suggesting a physiological mechanism (Maney et al., 2009). Finally, the genes that control plumage are physically linked to 1,000 other genes, many of which are known to affect aggression and other social behaviors (Maney 2008; Thomas et al. 2008). We hypothesize that these candidate genes, which include a steroid hormone receptor, a steroid metabolic enzyme, and steroid-sensitive monoamine receptors, lead to morph differences in behavior by affecting whether and how steroids act in the brain. Such a mechanism would explain how behaviors known to be steroid-dependent could vary remarkably between individuals that do not differ in plasma hormone levels.
Many of the traits that differ between WS and TS white-throated sparrows, such as aggression and parental behavior, are selected along dimensions defined by life history trade-offs (Trivers 1972). The resulting disruptive selection may result in the evolution of two distinct phenotypes (Sinervo and Svensson 1998; 2002; Zera and Harshman 2001). In most vertebrates, including the white-throated sparrow, alternative strategies related to aggression and parenting segregate with sex chromosomes. In the white-throated sparrow, they segregate also with a second pair of heteromorphic chromosomes, ZAL2 and ZAL2m. Bird 1128 showed clearly that increased dosage of ZAL2m alleles results in an exaggerated WS strategy maximizing competition and vocal aggression. This chromosome therefore represents a valuable genetic target for further research on the mechanisms underlying these behaviors and the evolution of alternative phenotypes.
Acknowledgments
The authors would like to thank Demesew Abebe, Jennifer Asher, Erin Baldwin, Anya Grozhik, Christopher Horoszko, Josh Lowman, Lisa Matragrano, Katy Renfro, and Carlos Rodriguez for technical assistance. Christopher Malinsky of the Smithsonian Institution assisted with museum skin preparation. We also thank the Biology Department at Emory University for the use of resources. This work was supported by NIMH 1R01MH082833-01A2 to DLM. JWT was supported by the NHGRI Intramural Research program at the NIH.
Contributor Information
Brent M. Horton, Department of Psychology, Emory University, Atlanta, GA 30322, USA
Yuchen Hu, Department of Psychology, Emory University, Atlanta, GA 30322, USA.
Christa L. Martin, Department of Human Genetics, School of Medicine, Emory University, Atlanta, GA 30322, USA
Brian P. Bunke, Department of Human Genetics, School of Medicine, Emory University, Atlanta, GA 30322, USA
Beth S. Matthews, Department of Human Genetics, School of Medicine, Emory University, Atlanta, GA 30322, USA
Ignacio T. Moore, Department of Biological Sciences, Virginia Tech, Blacksburg, VA 24061, USA
James W. Thomas, NIH Intramural Sequencing Center, National Human Genome Research Institute, NIH, Rockville, MD 20892, USA
Donna L. Maney, Email: dmaney@emory.edu, Department of Psychology, Emory University, Atlanta, GA 30322, USA, O. Wayne Rollins Research Center, 1510 Clifton Road NE, Room 2006, Mail Stop 1940-001-AC, Atlanta, GA 30322, USA
References
- Charlesworth B, Charlesworth D. The degeneration of Y chromosomes. Phil Trans R Soc Lond B. 2000;355:1563–1572. doi: 10.1098/rstb.2000.0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CE, Houtman AM. Tan and white color morphs of white-throated sparrows differ in their non-song vocal responses to territorial intrusion. Condor. 1999;101:842–845. [Google Scholar]
- Colwell RR. Age-specific crown variation in basic-plumaged golden-crowned sparrows. North American Bird Bander. 1999;24:138–142. [Google Scholar]
- Davis JK, Mittel B, Lowman JJ, Thomas PJ, Maney DL, Martin CL, Thomas JW NISC Comparative Sequencing Program. Haplotype-based genomic sequencing of a chromosomal polymorphism in the white-throated sparrow (Zonotrichia albicollis) J Heredity. 2011;102:380–390. doi: 10.1093/jhered/esr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. J Biol Rhythms. 2001;16:365–380. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- Emlen JT. A plucking experiment with white-crowned sparrows. Wilson Bull. 1938;50:57–58. [Google Scholar]
- Falls JB, Kopachena JG. White-throated Sparrow (Zonotrichia albicollis) In: Poole A, editor. The birds of North America online. Cornell Laboratory of Ornithology; Ithaca: 2010. [Google Scholar]
- Ficken RW, Ficken MS, Hailman JP. Differential aggression in genetically different morphs of the white-throated sparrow (Zonotrichia albicollis) Z Tierpsychol. 1978;46:43–57. doi: 10.1111/j.1439-0310.1978.tb01437.x. [DOI] [PubMed] [Google Scholar]
- Formica VA, Tuttle EM. Examining the social landscapes of alternative reproductive strategies. J Evol Biol. 2009;22:2395–2408. doi: 10.1111/j.1420-9101.2009.01855.x. [DOI] [PubMed] [Google Scholar]
- Fugle GN, Rothstein SI. Age- and sex-related variation in size and crown plumage brightness in wintering white-crowned sparrows. J Field Ornith. 1985;56:356–368. [Google Scholar]
- Fugle GN, Rothstein SI, Osenberg CW, McGinley MA. Signals of status in wintering white-crowned sparrows, Zonotrichia leucophrys gambelii. Anim Behav. 1984;32:86–93. [Google Scholar]
- Graves JA. Sex chromosome specialization and degeneration in mammals. Cell. 2006;124:901–914. doi: 10.1016/j.cell.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Griffiths R, Double MC, Orr K, Dawson RJ. A DNA test to sex most birds. Mol Ecol. 1998;7:1071–1075. doi: 10.1046/j.1365-294x.1998.00389.x. [DOI] [PubMed] [Google Scholar]
- Harrington BA. Aggression in winter resident and spring migrant white-throated sparrows in Massachusetts. Bird Banding. 1973;44:314–315. [Google Scholar]
- Helms CW, Drury WH. Winter and migratory weight and fat: field studies on some North American buntings. Bird-Banding. 1960;31:1–40. [Google Scholar]
- Horton BM, Holberton RL. Corticosterone manipulations alter morph-specific nestling provisioning behavior in polymorphic male white-throated sparrows, Zonotrichia albicollis. Horm Behav. 2009;56:510–518. doi: 10.1016/j.yhbeh.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Horton BM, Hauber ME, Maney DL. Morph Matters: aggression bias in a polymorphic sparrow. PLoS ONE. 2012;7:e48705. doi: 10.1371/journal.pone.0048705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtman AM, Falls JB. Negative assortative mating in the whitethroated sparrow, Zonotrichia albicollis: the role of mate choice and intra-sexual competition. Anim Behav. 1994;48:377–383. [Google Scholar]
- Huynh LY, Maney DL, Thomas JW. Chromosome-wide linkage disequilibrium caused by an inversion polymorphism in the white-throated sparrow (Zonotrichia albicollis) Heredity. 2011;106:537–546. doi: 10.1038/hdy.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Arnold AP. Chromosomal polymorphism and comparative painting analysis in the zebra finch. Chromosome Res. 2005;13:47–56. doi: 10.1007/s10577-005-6602-x. [DOI] [PubMed] [Google Scholar]
- Kopachena JG, Falls JB. Aggressive performance as a behavioral correlate of plumage polymorphism in the white-throated sparrow (Zonotrichia albicollis) Behaviour. 1993;124:249–266. [Google Scholar]
- Lake JI, Lange HS, O’Brien S, Sanford SE, Maney DL. Activity of the hypothalamic-pituitary-gonadal axis differs between behavioral phenotypes in female white-throated sparrows (Zonotrichia albicollis) Gen Comp Endocrinol. 2008;156:426–433. doi: 10.1016/j.ygcen.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Lowther JK. Polymorphism in the white-throated sparrow, Zonotrichia albicollis (Gmelin) Can J Zool. 1961;39:281–292. [Google Scholar]
- Mailliard J. Observations on the head markings of the golden-crowned sparrow. Condor. 1932;34:66–70. [Google Scholar]
- Maney DL. Endocrine and genomic architecture of life history trade-offs in an avian model of social behavior. Gen Comp Endocrinol. 2008;157:275–282. doi: 10.1016/j.ygcen.2008.03.023. [DOI] [PubMed] [Google Scholar]
- Maney DL, Lange HS, Raees MQ, Reid AE, Sanford SE. Behavioral phenotypes persist after gonadal steroid manipulation in white-throated sparrows. Horm Behav. 2009;55:113–120. doi: 10.1016/j.yhbeh.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Maney DL, Morehouse CB, Thomas JW. A genotyping assay to determine plumage morph in the white-throated sparrow (Zonotrichia albicollis) Auk. 2007;124:1330–1335. [Google Scholar]
- Moore IT, Wada H, Perfito N, Busch DS, Hahn TP, Wingfield JC. Territoriality and testosterone in an equatorial population of rufous-collared sparrows, Zonotrichia capensis. Anim Behav. 2004;67:411–420. [Google Scholar]
- Parker KA, Hauber ME, Brunton DH. Contemporary cultural evolution of a conspecific recognition signal following serial translocations. Evolution. 2010;64:2431–2441. doi: 10.1111/j.1558-5646.2010.01013.x. [DOI] [PubMed] [Google Scholar]
- Parsons J, Baptista LF. Crown color and dominance in the white-crowned sparrow. Auk. 1980;97:807–815. [Google Scholar]
- Piper WH, Wiley RH. Distinguishing morphs of the white-throated sparrow in basic plumage. J Field Ornith. 1989a;60:73–83. [Google Scholar]
- Piper WH, Wiley RH. Correlates of dominance in wintering white-throated sparrows: age, sex and location. Anim Behav. 1989b;37:298–310. [Google Scholar]
- Pyle P. Identification guide to North American birds. Slate Creek Press; Bolinas CA: 1997. [Google Scholar]
- Rohwer S. Status signalling in Harris Sparrows: some experiments in deception. Behaviour. 1977;61:107–129. [Google Scholar]
- Rohwer S. Dyed birds achieve higher social status than controls in Harris’ sparrows. Anim Behav. 1985;33:1325–1331. [Google Scholar]
- Romanov MN, Tuttle EM, Houck ML, Modi WS, Chemnick LG, Korody ML, et al. The value of avian genomics to the conservation of wildlife. BMC Genomics. 2009;10:S10. doi: 10.1186/1471-2164-10-S2-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank MC. The natural termination of the refractory period in the slate-colored junco and in the white-throated sparrow. Auk. 1959;76:44–54. [Google Scholar]
- Sinervo B, Svensson E. Mechanistic and selective causes of life history trade-offs and plasticity. Oikos. 1998;83:432–442. [Google Scholar]
- Sinervo B, Svensson E. Correlational selection and the evolution of genomic architecture. Heredity. 2002;89:329–338. doi: 10.1038/sj.hdy.6800148. [DOI] [PubMed] [Google Scholar]
- Stevenson TJ, Small TW, Ball GF, Moore IT. Variation in the gonadotrophin-releasing hormone-1 and the song control system in the tropical breeding rufous-collared sparrow (Zonotrichia capensis) is dependent on sex and reproductive state. Gen Comp Endocrinol. 2012;178:1–7. doi: 10.1016/j.ygcen.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swett MB. PhD Dissertation. University of Montana; 2007. Hormonal mediation of a unique behavioral polymorphism in the white-throated sparrow (Zonotrichia albicollis) [Google Scholar]
- Thomas JW, Caceres M, Lowman JJ, Morehouse CB, Short ME, Baldwin EL, Maney DL, Martin CL. The chromosomal polymorphism linked to variation in social behavior in the white-throated sparrow (Zonotrichia albicollis) is a complex rearrangement and suppressor of recombination. Genetics. 2008;179:1455–1468. doi: 10.1534/genetics.108.088229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneycroft HB. A cytogenetic study of the white-throated sparrow, Zonotrichia albicollis. Evolution. 1975;29:611–621. doi: 10.1111/j.1558-5646.1975.tb00855.x. [DOI] [PubMed] [Google Scholar]
- Trivers RL. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man. Aldine; Chicago: 1972. pp. 139–179. [Google Scholar]
- Tuttle EM. Alternative reproductive strategies in the white-throated sparrow: behavioral and genetic evidence. Behav Ecol. 2003;14:425–432. [Google Scholar]
- Watt DJ. Plumage brightness index for white-throated sparrows. J Field Ornithol. 1986a;57:105–113. [Google Scholar]
- Watt DJ. A comparative study of status signalling in sparrows (genus Zonotrichia) Anim Behav. 1986b;34:1–15. [Google Scholar]
- Watt DJ, Ralph CJ, Atkinson CT. The role of plumage polymorphism in dominance relationships of the white-throated sparrow. Auk. 1984;101:110–120. [Google Scholar]
- Wiley RH, Steadman L, Chadwick L, Wollerman L. Social inertia in the white-throated sparrows results from recognition of opponents. Anim Behav. 1999;57:455–463. doi: 10.1006/anbe.1998.0991. [DOI] [PubMed] [Google Scholar]
- Zera AJ, Harshman LG. The physiology of life history tradeoffs in animals. Annu Rev Ecol Syst. 2001;32:95–126. [Google Scholar]