Abstract
Neutrophil extracellular traps (NETs) represent an important defense mechanism against microorganisms. Clearance of NETs is impaired in a subset of patients with systemic lupus erythematosus (SLE), while NETosis is increased in neutrophils and, particularly, in low-density granulocytes derived from lupus patients. NETs are toxic to the endothelium, expose immunostimulatory molecules, activate plasmacytoid dendritic cells and may participate in organ damage through incompletely characterized pathways. In order to better understand the role of NETs in fostering dysregulated inflammation, we examined inflammasome activation in response to NETs or to LL-37, an antibacterial protein externalized on the NETs. Both NETs and LL-37 activate caspase-1, the central enzyme of the inflammasome, in both human and murine macrophages, resulting in release of active IL-1β and IL-18. LL-37 activation of the NLRP3 inflammasome utilizes P2×7 receptor-mediated potassium efflux. NET and LL-37-mediated activation of the inflammasome is enhanced in macrophages derived from lupus patients. In turn, IL-18 is able to stimulate NETosis in human neutrophils. These results suggest that enhanced formation of NETs in lupus patients can lead to increased inflammasome activation in adjacent macrophages. This leads to release of inflammatory cytokines which further stimulate NETosis, resulting in a feed-forward inflammatory loop that could potentially lead to disease flares and/or organ damage.
Introduction
Neutrophil extracellular traps (NETs) are a meshwork of chromatin fibers decorated with granule-derived antimicrobial peptides and enzymes that appear to play an important role in host defense(1). Recent evidence also places NETs at the center of various pathologic states. Indeed, various groups have suggested the participation of these structures in the development of autoimmune diseases, such as anti-neutrophil cytoplasmic antibody-associated vasculitis and systemic lupus erythematosus (SLE) (2-4).
SLE is a systemic autoimmune syndrome typified by autoantibodies against DNA, chromatin, and DNA-associated proteins, including NET components. Experimental evidence from our group and others suggests that SLE is characterized by an imbalance between NET formation and NET clearance, which may promote breaking of tolerance and tissue damage. (5-7). In particular, a distinct subset of proinflammatory low density granulocytes (LDGs) present in the circulation of patients with SLE, has a much enhanced capacity to form NETs and externalize various immunostimulatory proteins(7, 8). Furthermore, SLE NETs are able to activate plasmacytoid dendritic cells to produce Interferon alpha (IFN-α), a phenomenon that may be key in disease pathogenesis(4, 6, 7). However, it remains unclear if isolated exposure to NETs is sufficient to break tolerance. It is quite likely that specific genetic factors and the inflammatory milieu may be key in triggering sustained autoimmunity(9). Further, which inflammatory factors are key in potentiating the effects of dysregulated NETosis remains unclear.
Among the various antimicrobial peptides externalized in the NETs, the cathelicidin LL-37 is a cationic peptide synthesized by neutrophils, monocytes, keratinocytes and macrophages (MØ), with activity against a wide range of pathogens. LL-37 represents the proteolytic product of human cationic antimicrobial protein of 18 kDa (hCAP-18), and appears to play a myriad of important roles in innate immune processes. Among them, LL-37 can stimulate immune cell chemotaxis via the FPRL1 receptor(10), promote M1 MØ differentiation(11) and enhance TLR3 signaling in response to viral dsRNA(12). Further, LL-37 may contribute to the initiation of inflammatory responses in various skin diseases(13) and, when complexed with double-stranded DNA, appears to be a strong trigger for type I IFN synthesis by pDCs(4). The externalization of LL-37 on the NETs has been described in normal-density lupus neutrophils(6) and in lupus LDGs(7). As LDG NETs have pathogenic effects on endothelial cells and possibly other organs(7), we speculated that LL-37 present in the NETs may have additional pathogenic effects and promote organ damage.
LL-37 was previously described to induce processing and release of IL-1β from freshly isolated monocytes, through activation of the P2×7 receptor (P2×7R)(14). However, monocytes may be primed for activation(15) and are not typically localized in tissues where exposure to LL-37 may have pathogenic relevance. As activation of the inflammasome machinery and IL-1β and IL-18 modulation may have important pathogenic effects on lupus-related cardiovascular disease(16, 17), and in the development of renal (18, 19) and cutaneous pathology(20) in lupus, we characterized the putative role of NETs and LL-37 in activation of the inflammasome machinery in MØ. We also compared how control and SLE MØ respond to NETs and NET-associated proteins, and how inflammasome components may in turn promote the formation of these traps.
Materials and Methods
Human subjects
The University of Michigan institutional review board approved this study. Subjects gave informed consent in accordance with the Declaration of Helsinki. To be enrolled in this study, lupus patients fulfilled the revised American College of Rheumatology criteria for SLE(21) and were enrolled from the University of Michigan outpatient Rheumatology clinic. Age- and gender-matched healthy controls were recruited by advertisement.
Mice
All animal protocols were reviewed and approved by the University of Michigan’s Committee on Use and Care of Animals. Caspase-1 −/−, apoptosis-associated speck-like protein containing a CARD (ASC) −/−, NOD-like receptor (NLR) family pyrin domain-containing 3 (NLRP 3) −/− and P2×7R −/− mice, all of the C57BL/6 background, were a generous gift from Dr. Gabriel Nuñez at the University of Michigan. C57BL/6 mice were purchased from Jackson Laboratories. All mice were housed in a specific pathogen free facility at the University of Michigan until euthanasia.
Isolation of NETs
LDGs and control neutrophils were isolated as previously described(8). Briefly, for LDG isolation, PBMCs were obtained by Ficoll/Hypaque gradient and cell pellets were incubated with a cocktail of equal volumes of biotinylated Abs recognizing human CD3, CD7, CD19, CD79b, CD56, MHC class II, CD86, and CD235a (Ancell, Bayport, MN)), followed by anti-biotin MACS beads (Miltenyi Biotech, Auburn, CA). Cells were applied to a MACS-LS column and nonimmobilized cells were recovered by negative selection, washed once with PBS and then plated for NET isolation. NETs were isolated as previously published(22). Briefly, 1×106 cells were seeded in 6-well plates in serum-free RPMI. LDGs were allowed to spontaneously release NETs for 1 h at 37°C, followed by addition of 10 U/ml micrococcal nuclease (Thermo Scientific) for 20 min at 37°C. After aspiration of NETs, cells were removed by spinning at 300xg for 5 min. Remaining debris and neutrophil granules were removed by centrifugation at 12,000xg for 10 min. To collect NETs from control neutrophils, cells were stimulated with 1 μg/ml LPS (E coli 026:B6, Sigma), 20 nM PMA (Sigma) for 1 h or 25 ng/ml of recombinant IL-18 (Southern Biotech, Birmingham, AL) followed by similar harvesting protocols. Specificity of NET induction to IL-18 was checked by stimulating control neutrophils or LDGs in the presence or absence of neutralizing antibodies to IL-18 (MBL International, Woburn, MA), the IL-18R (Biolegend, San Diego, CA) or to the IL-17R (R&D Systems, Minneapolis, MN).
Characterization of NETs
a) Detection of NETs by immunofluorescence microscopy
Control neutrophils or LDGs were isolated as described above, then seeded on polylysine coated (0.001%) coverslips in the presence of 1ug LPS (for control neutrophils) or no stimulus (for LDGs) for 1 h at 37°C. After stimulation, cells were fixed in 4% PFA in PBS overnight at 4°C, washed with PBS, permeabilized for 10 min with 0.2% (wt/vol) Triton X-100 in PBS, and blocked for 30 min with 0.2% (vol/vol) porcine skin gelatin in PBS. Cells were then incubated in a humid chamber for 1 h at 37°C with mouse anti-human cathelicidin or with rabbit anti-human neutrophil elastase (Abcam, Cambridge, MA). Cells were then washed three times with PBS Ca+2/Mg+2 for 5 min at room temperature, and incubated for 30 min at 37°C with Alexa 555-conjugated anti-rabbit IgG or Alexa 488-conjugated anti-mouse IgG secondary Abs (Invitrogen). Samples were washed with PBS with Ca+2/Mg+2 and nuclei were co-stained with Hoechst 33342 (Invitrogen) for 10 min (1:1000). Coverslips were mounted on glass slides using ProLong Gold antifade reagent (Invitrogen). Images were acquired on a Zeiss LSM510 META confocal laser-scanning microscope (Carl Zeiss, Microimaging Inc., Thornwood, NY) with a 63x lens.
b) Quantification of NETs by microscopy
NETs were manually quantified as previously published by our group(7). Briefly, the number of cells double positive for neutrophil elastase and nuclear material (Hoechst) were considered a NET and digitally recorded to prevent multiple counts. The percentage of NETs was calculated as the average of five to six fields (×40) normalized to the total number of cells.
c) Quantification of NETs by plate assay
This was performed as previously described(23) with a few minor modifications. Briefly, neutrophils were resuspended in RPMI without phenol red (Invitrogen) containing 0.2uM of Sytox Green (Invitrogen), to stain extracellular DNA. A total of 1 × 105 neutrophils were incubated in the presence of 1ug/mL LPS, PMA (Sigma) or graded concentrations of recombinant, active IL-18 (BioVision) with or without pre-incubation with 2μg/ml anti-IL-18R antibody, (Biolegend) or anti-IL-17 Receptor (Acris, Herford, Germany) in 96-well black plates for 1 h at 37°C. Fluorescence (excitation 485nm, emission 520 nm) was measured in a Biotek Synergy H1 Hybrid Reader (Biotek VT, USA).
d) Characterization of NET proteins
After isolation, NETs were analyzed by Western Blot and probed for the NET-associated proteins myeloperoxidase (MPO) (DaKo, Carpinteria, CA) and hCAP-18/LL-37 (Abcam, #87701). Concentrations of NET-associated proteins were quantified using the Bio-rad protein assay. An ELISA kit was used to quantify LL-37 concentrations in the NETs (HyCult, Plymouth Meeting, PA).
Human and murine monocyte and MØ purification and isolation
Human control and SLE PBMCs were isolated from blood via centrifugation at 700 × g for 20 min on a Ficoll-Paque (GE Healthcare) gradient. The buffy coat was removed, washed twice with PBS and then subjected to hypotonic lysis to remove RBCs. Cells were plated in tissue culture dishes for 2 h, then washed four times with PBS to remove non-adherent cells. Monocytes were cultured in RPMI 1640 (Invitrogen) supplemented with 10 ng/ml GM-CSF(Peprotech, Rocky Hill, NJ), 10% heat-inactivated fetal bovine serum (Gemini), 2mM penicillin and streptomycin (Invitrogen) and 2mM glutamine (Invitrogen) with media changes every three days. Alternatively, to skew cells toward an M2 phenotype, monocytes were cultured in X-Vivo 15 media (Lonza) supplemented with 10% heat inactivated human serum and 2mM penicillin/streptomycin. Flow cytometric analysis (CyAn, Beckman Coulter Brea, California) was performed to verify M1 and M2 phenotypes. Human MØ cultured in RPMI+GM-CSF were >70% CD86high, MHCclassIIhigh and CD36low consistent with an M1 phenotype as previously reported(24). Human MØ cultured in X-Vivo 15 were >80% CD40low, CD86low, CD36high, consistent with an M2 phenotype. We cannot exclude that our macrophage populations are completely devoid of contaminating dendritic cells.
Murine bone-marrow derived MØ (BMDM) were obtained by isolating bone marrow from tibias and femurs by flushing with PBS, followed by growth in Iscove’s media (Invitrogen), containing 10% heat-inactivated FBS, 20% L-cell conditioned media (as a source of M-CSF), 1% Non-essential amino acids (Invitrogen), 1% sodium pyruvate (Invitrogen), 0.3% β-mercaptoethanol (Gibco) and 2 mM penicillin and streptomycin, for derivation of M2 MØ. For M1-type MØ, isolated BM was cultured in Iscove’s media containing 10% heat-inactivated FBS, 50 ng/ml murine GM-CSF (Peprotech), 1% Non-essential amino acids, 1% sodium pyruvate, 0.3% β-mercaptoethanol and 2mM Penicillin/streptomycin. Cells were cultured for a week with media changes every 3 days. Twenty-four h prior to stimulation, cells were plated at 1×106/well on 12-well tissue-culture plates. Flow cytometric analysis verified M1 and M2 phenotypes. MØ derived in L-Cell conditioned media were Tie-2high, F4/80high and CD11clow, consistent with M2(25). MØ derived in the presence of GM-CSF were CD11chigh and Tie-2low, F4/80low, consistent with an M1 phenotype(25).
Quantification of inflammasome Activation
Where indicated, MØ were primed with 100ng/ml of LPS (Sigma) for 4 h prior to stimulation. Media was then removed and replaced with phenol-red-free, serum-free RPMI prior to treatment with 5 mM ATP (Sigma) or various concentrations of recombinant LL-37 (AnaSpec, Fremont, CA) or inactive scrambled peptide (GenScript, Piscataway, NJ) as a control. For experiments using DNA complexes, genomic DNA was isolated using the Wizard genomic isolation kit (Promega, Madison, WI), followed by generation of DNA/LL-37 complexes as previously described(4). Briefly, DNA was mixed with LL-37 at a ratio of 2:1 followed by incubation at room temperature for 1 h prior to addition to the cell culture. Following treatment, media was collected for quantification of IL-1β or IL-18 by commercially-available ELISA (eBioscience, Vienna, Austria), according to manufacturer’s instructions. To control for non-specific effects of treatment in elevated potassium media, quantification of IL-6 by ELISA (R&D Systems) was completed.
For evaluation of active caspase-1 by Western blot, cells were stimulated as above then lysed in 1x SDS sample buffer. Extracellular media was collected, subjected to trichloroacetic acid precipitation and then dissolved in 1xSDS sample buffer. Anti-murine caspase-1 p20 was a generous gift from Dr. Gabriel Nuñez, University of Michigan. Anti-human caspase-1 p20 was purchased from Cell Signaling (Beverly, MA). Pro and mature forms of IL-1β were detected using anti-IL-1β monoclonal antibody (generous gift of the BRB preclinical repository, NCI). Densitometry of imaged blots was completed using ImageJ software.
To detect activated caspase-1 by fluorescent microscopy, MØ were cultured as above, then primed for 4 h with LPS and stimulated with ATP, LL-37 or NETs for 1 h, followed by addition of carboxyfluorescein-tagged YVAD-FMK (ImmunoChemistry Technologies, Bloomington, MN), which binds to active (cleaved) caspase-1. Following an additional hour-long incubation, cells were additionally stained with Hoechst 33342, washed and then directly imaged using fluorescent microscopy. The numeric aperture for the objective lens of the fluorescent microscope was 0.3. Images were acquired with an Olympus DP30BW camera (Olympus Corporation, Tokyo, Japan) using the acquisition software Olympus-BSW (Olympus). Final magnification was 100×. To calculate the percentage of activated cells, images were analyzed using the CellC program (http://www.cs.tut.fi/sgn/csb/cellc/) and the percentage was determined by calculating the number of double positive cells divided by the total number of cells (Hoechst positive) in the image. Percentages were normalized to background stimulation with LPS alone.
Results
NETs stimulate IL-1β and IL-18 release by primary MØ
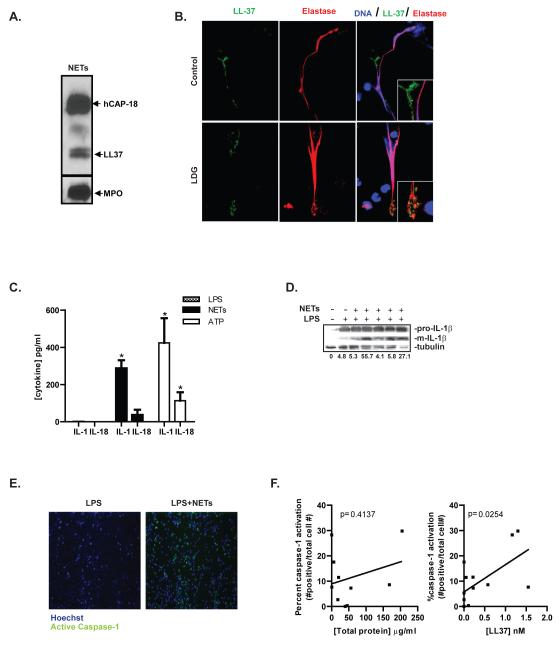
To determine if NETs can promote release of IL-1β and IL-18, similar to other known stimuli of the inflammasome, LPS-primed primary human MØ were exposed to control or lupus NETs. Both control and LDG-isolated NETs induced MØ secretion of IL-1β and IL-18 at levels comparable to those observed after ATP exposure (Figure 1C), and Western analysis confirmed the presence of the mature, active form of IL-1β following NET exposure (Figure 1D). Both lupus and control NETs were able to stimulate cytokine release and no differences between them were noted, and results are from pooled analysis from both types of cells. No detectable levels of IL-1β or IL-18 were detected by ELISA in isolated NETs, indicating that these lattices did not represent a source of the cytokines (not shown).
Figure 1. NETs activate caspase-1 and IL-1β and IL-18 release.
A. Purified NETs were examined by Western analysis to confirm the presence of known NET-associated proteins. Myeloperoxidase, the LL-37 precursor h-CAP-18, and cleaved LL-37 are detected. B. NET formation was assessed in LPS-treated-control neutrophils and in unstimulated LDGs. Expression of LL-37 (green) in the NETs was assessed. Neutrophil elastase is shown in red. Results are representative of three separate experiments. Total magnification is 630×. C. Healthy control MØ (n=4) were primed with 100 ng/ml LPS for 4 h, followed by media change and a 2 h exposure to NETs derived from LPS-treated control neutrophils or from unstimulated lupus LDGs. Exposure to 5mM ATP for 2 h was used as a positive control. IL-1β and IL-18 were quantified by ELISA. Results are mean pg/mL + SEM of seven separate experiments completed in duplicate. D. Western analysis of combined whole-cell lysate and extracellular media following culture with LPS or LPS+NETs as in Figure 1C. Exposure to NET fractions results in increased mature (active) IL-1β. Percent of mature (m)IL-1β versus the house-keeping protein tubulin are shown below the graph. E. Human LPS-primed-control MØ were exposed to NETs as in figure 1C, for 1 h. The caspase-1-specific carboxyfluorescein tagged YVAD-FMK (green) was added to the culture to detect activated caspase-1. Cells were counterstained with Hoechst (blue) and imaged by fluorescent microscopy. Images are representative of 4 independent experiments, each performed in triplicate. Total magnification is 100×. F. Analysis of caspase-1 activation, determined as in Figure 1E, compared to total protein concentration (left) or concentration of LL-37 (right) present in the NETs. LDGs=Low Density Granulocytes. *=p<0.05.
We then determined if NETs can activate caspase-1, the central enzyme of the inflammasome that activates IL-1β and IL-18. Using a fluorescent probe specific for caspase-1 activation, caspase-1 activity was detected in LPS-treated MØ following NET exposure, while LPS alone did not trigger activation(Figure 1E). The ability of NETs to activate caspase-1 positively correlated with the concentration of LL-37, but not to concentration of total isolated proteins present on the NETs (Figure 1F). These results indicate that NETs can activate the inflammasome machinery in human MØ and that this activity may be dependent on the presence and concentration of LL-37 in these traps.
LL-37 activates the inflammasome machinery in primary macrophages
Given that NETs are able to activate the inflammasome in a manner that correlates with concentrations of LL-37 in these lattices, we investigated if this molecule(Figure 1A,B)(6, 7) similarly activated this platform. As shown in Figure 2A, both LPS-primed human M1 and M2 MØ displayed significant enhancement in their capacity to release IL-1β and IL-18 following exposure to LL-37, while a scrambled form of this cathelicidin did not trigger this increase. M1 MØ released significantly higher levels of IL-1β and they had a trend to release more IL-18 than M2 MØ upon LL-37 exposure. Similar findings were observed in murine BMDM, where significant IL-1β release was identified upon exposure to CRAMP (the murine equivalent of LL-37) in M1- but not M2-differentiated MØ (Figure 2B).
Figure 2. LL-37 activates IL-1β and IL-18 release, preferentially in M1 versus M2 human and murine MØ, and this is not enhanced by complexing with DNA.
A. Human MØ differentiated in the presence of GM-CSF (M1) or X-Vivo 15 (M2) were primed with LPS for 4 h, then treated with 20μM LL-37, 20μM inactive scrambled peptide (Scr) or 5 mM ATP for 2 h. Quantification of IL-1β and IL-18 in supernatants was performed by ELISA. B. Murine MØ differentiated in the presence of GM-CSF (M1) or M-CSF (M2) were primed with LPS for 4 h, then treated with 20μM mCRAMP or 5 mM ATP for 2 h. IL-1β was quantified in harvested supernatants by ELISA. C. Human M1 MØ were treated for 1 h with the caspase-1-specific carboxyfluorescein tagged YVAD-FMK (green) added after 1 h of various stimuli. Cells were counterstained with Hoechst and imaged by fluorescent microscopy. Percentage of caspase-1 activation (right) was determined by dividing the number of caspase-1 and Hoechst double-positive cells by the total number of Hoechst positive cells D. Human M1 MØ were treated as in 2A. Whole cell lysates (WCL) or extracellular media (ECM) were harvested and active caspase-1 p20 fragment was detected by Western Blot. Densitometric values of p20/p45 are shown below the image. E. Human M1 MØ were treated as in 2A, followed by 2 hour exposure to 20 μM LL-37 or to LL-37 precomplexed with DNA. IL-1β was quantified in supernatants by ELISA. Results represent mean + SEM, n=4-8 for each experiment; samples were run in duplicate. *=p<0.05, **=p<0.01, ***=p<0.001, ns=non-significant. Rx=treatment.
To confirm that release of IL-1β and Il-18 upon LL-37 exposure occurred via caspase-1 activation, a fluorescent probe specific for active caspase-1 was tested. As shown in Figure 2C, LPS-primed human M1 MØ treated with LL-37, but not the scrambled control peptide, bound the caspase-1 probe substantially more than LPS primed MØ. Western Blot analysis confirmed formation of the active p20 fragment of caspase-1 released from M1 MØ (Figure 2D), consistent with previous descriptions of inflammasome and caspase-1 activation(26, 27). As previous reports indicate that LL-37 can drive M1 MØ differentiation (11), it is plausible that, in inflamed tissues where concentrations of this cathelicidin become increased, it will also promote an M1 phenotype, further increasing the sensitivity for MØ to activate the inflammasome machinery and release active IL-1β and IL-18.
LL-37 complexed with dsDNA can activate pDCs and induce type I IFN release(4). Thus, we investigated if activation of the inflammasome machinery by LL-37 was enhanced in MØ when this peptide was coupled to dsDNA. As shown in Figure 2E, no significant differences in the capacity of LL-37 to activate the inflammasome were observed when comparing LL-37 alone versus LL-37 complexed to DNA. Further, treatment of these complexes with DNase did not alter responses upon exposure to LL-37 (data not shown). These observations indicate that LL-37 does not require being complexed to DNA to be able to activate the inflammasome in MØ.
The NLRP3 inflammasome is required for caspase-1 activation by LL-37
To characterize the pathways by which LL-37 stimulates caspase-1 activation, we compared M1 BMDM isolated from wild-type (C57BL/6) or haplotype-matched mice deficient in NLRP3, ASC or caspase-1. As shown in Figure 3, all three proteins are required for activation this complex upon stimulation with murine CRAMP. Densitometric analysis of Western blots in figure 3B demonstrates that LL-37 stimulation resulted in a 30% increase in active caspase-1 p20 fragments, while this band is undetectable in NLRP3, ASC and caspase-1 knockout mice, in agreement with the ELISA data. These observations indicate that LL-37 activates caspase-1 via promotion of assembly of the NLRP3 inflammasome.
Figure 3. The NLRP3 inflammasome is required for mCRAMP activation of caspase-1 and for release of IL-1β.
A. Murine BMDM, isolated from WT or from haplotype-matched mice deficient in NLRP3, ASC or caspase-1 were differentiated in the presence of GM-CSF for 7 days, followed by priming with 100 ng/ml LPS for 4 h. MØ were treated with 5mM ATP or 20μM mCRAMP (CMP) for 2 h and supernatants harvested for quantification by ELISA. B. Supernatants and cell lysates isolated from MØ treated as in A were analyzed for active caspase-1 (p20) content using Western analysis. Results represent mean + SEM for four separate experiments. *=p<0.05, **=p<0.01, ***=p<0.001. Rx=treatment.
LL-37 activation of the NLRP3 inflammasome utilizes P2×7 receptor-mediated potassium efflux
The P2×7 receptor, a ligand-gated ion channel, was previously reported to be important for caspase-1 activation by LL-37 in monocytes(14). However, these studies only utilized chemical inhibitors. Like other mechanisms of NLRP3 activation, the activation of the inflammasome through P2×7 receptor requires induction of potassium efflux through the ion channel (28). To confirm if activation of the inflammasome in MØ by LL-37 required the P2×7R, we differentiated BMDM from wild-type or P2×7R-deficient mice. As shown in Figure 4A and B, activation of caspase-1 in response to ATP or mCRAMP was significantly decreased (by 97% and 96.9% respectively) in P2×7R deficient BMDM. This suggests that the P2×7R plays an important role in activation of the inflammasome in response to LL-37 stimulation. Further, stimulation with recombinant LL-37 resulted in enhanced intracellular LDH release (11±0.3%) when compared to LPS alone (0.3±0.01%), as previously reported with stimulation of the P2×7R(29). Because activation of this receptor results in potassium efflux, an important trigger for inflammasome stimulation(28), we tested if this efflux was required for the activation of caspase-1 in response to LL-37. LPS-primed human MØ were stimulated with LL-37 in the presence or absence of high potassium media, to inhibit potassium efflux. As shown in Figure 4C, high extracellular potassium media inhibits the ability of LL-37 and ATP to induce IL-1β and IL-18 release; however, the macrophages were able to continually secrete IL-6, suggesting that inhibition of potassium release did not impact the capacity to secrete other inflammatory cytokines. These results indicate that potassium efflux, via P2×7R activation, is required for the activation of the inflammasome by LL-37 activation.
Figure 4. LL-37 activation of the inflammasome requires P2×7R-mediated potassium efflux.
A. Murine BMDM isolated from WT or haplotype-matched mice deficient in the P2×7R were differentiated in the presence of GM-CSF for 7 days, followed by priming with 100 ng/ml LPS for 4 h. MØ were then treated with 20μM mCRAMP (CMP) or 5mM ATP for 2 h before harvesting supernatants. B. Similarly-treated MØ were used to detect active caspase-1 by Western Blot. C. Human MØ (n=4) were differentiated for 7 days in the presence of GM-CSF, followed by stimulation with 5mM ATP or 20μM LL-37 in serum-free media with or without 100 mM KCl for 2 h. Release of IL-1β, IL-6 and IL-18 was assessed by ELISA. Results represent mean + SEM. *=p<0.05. ***=p<0.001. Rx=treatment.
SLE MØ have a lower threshold for activation by LL-37 and enhanced inflammasome activation in response to NETs
IL-18 levels are increased in SLE patients(17, 30), but the mechanisms implicated in this phenomenon remain to be well characterized. Given that LL-37 and NETs can activate the inflammasome machinery, and that NET formation is increased in SLE neutrophils, we compared responses in control and SLE MØ upon exposure to LL-37 or NETs. As shown in Figure 5A, SLE MØ have increased IL-1β release at baseline when compared to controls. Importantly, the dose-response for LL-37 is significantly shifted in SLE versus control MØ, as significantly lower concentrations of LL-37 were required for processing and release of IL-1β or IL-18 by the lupus cells. Further, when exposed to identical preparations of control or LDG NETs, SLE MØ displayed increased caspase-1 activation (Figure 5B) and elevated release of IL-1β and IL-18 (Figure 5C). No differences were detected when comparing control LPS-stimulated NETs versus NETs isolated from LDGs, in their ability to stimulate caspase-1 activation (not shown). These observations indicate that LL-37 externalized on NETs can activate the inflammasome and contribute to dysregulated inflammation and increased IL-18 levels in SLE.
Figure 5. Activation of caspase-1 by LL-37 and NETs is enhanced in SLE MØ.
A. Equal number of monocytes isolated from healthy controls (n=7) or from individuals with SLE (n=8) were differentiated for 7 days in GM-CSF, primed with LPS for 4 h, followed by stimulation with various concentrations of LL-37 for 2 h. Cytokine concentrations of the supernatants were measured by ELISA. B. MØ from healthy controls (n=5) or from individuals with SLE (n=5) were similarly differentiated, followed by LPS-priming for 4 h. MØ were then treated for 2 h with NETs isolated from LPS-stimulated control neutrophils (n=4) or from unstimulated LDGs (n=5). Caspase-1 activation was detected via incubation with FLICA-YVAD-fmk during the last hour after NETs treatment. Cells were stained with Hoechst and imaged via fluorescent microscopy (right-representative images). Percentage of caspase-1 activation was determined as in figure 2C after normalization to baseline activation of LPS alone (left). Results from LDG and control NETs were pooled for analysis. C. Released IL-1β and IL-18 from macrophages treated as in B was determined by ELISA. *=p<0.05. **=p<0.01.
IL-18 promotes NETosis
Stimulation of NETosis serves an antimicrobial function and occurs rapidly upon exposure to bacteria, fungi or their products, such as LPS. Facilitating this function, inflammatory cytokines such as TNF and IL-1β have been reported to stimulate NET formation(2, 31). As IL-18 may have important roles in lupus pathology(20, 30, 32), we examined whether this cytokine can also stimulate NET formation. As shown in Figure 6A, recombinant human IL-18 was an effective stimulus of NET release in control neutrophils, as detected by increased binding of the cell-impermeable nucleic acid stain, Sytox Green, in the cultures. The effect of IL-18 was equivalent to what has been reported for two well-characterized NETosis stimuli, PMA and LPS using similar assays (33-35). Further, fluorescence microscopy confirms the presence of NETs following exposure to IL-18 (Figure 6B). This effect is specific to IL-18 signaling, as blocking antibodies to IL-18, and to the IL-18R, but not isotype-matched anti-IL-17 receptor antibodies, abrogate NETosis induced by recombinant IL-18 (Figure 6C). Similarly to NETs induced by LPS or PMA, NETs released after IL-18 stimulation significantly increase caspase-1 activation in primed macrophages (Figure 6D). These observations suggest that NET formation may promote a feed-forward loop, triggering synthesis of activated IL-1β and IL-18 by tissue MØ, which in turn further activates extracellular trap formation in recruited neutrophils (Figure 6E).
Figure 6. IL-18 induces NET formation.
A. Neutrophils isolated from healthy controls were plated in the presence of the cell-impermeable dye Sytox Green, followed by exposure to PMA, LPS or graded concentrations of recombinant active human IL-18. NET release was detected via fluorescence emission. B. Neutrophils isolated from healthy controls were seeded onto polylysine-coated coverslips followed by exposure to LPS or graded concentrations of recombinant human IL-18. NETs were detected via staining for DNA (blue) and elastase (red). NETs were determined by co-localization of DNA and elastase and compared to the total number of neutrophils present to determine the percent of NETting neutrophils. *=p<0.05. C. Neutrophils isolated from healthy controls or LDGs isolated from lupus patients were plated in the presence of Sytox Green with or without a blocking antibody to the IL-18R (2μg/ml), IL-18 (3μg/ml) or an isotype-matched anti-IL-17R antibody (1μg/ml). Cells were then treated with IL-18 and NET release was detected via fluorescence emission. D. Healthy control macrophages were primed with LPS and treated with 25 ng/ml IL-18 or NETs isolated from IL-18-treated neutrophils (n=3). Percent of caspase-1 activation was detected and calculated as in figure 5B. E. Proposed pathway suggesting that NETs containing LL-37 can activate caspase-1 via the P2×7R. The subsequent processing and release of IL-1β and IL-18 further stimulate NETosis, leading to a self-perpetuating cycle of NET production and inflammasome activation. *=p<0.05. **=p<0.01, ***=p<0.001.
Discussion
This is the first demonstration that NETs are effective activators of the inflammasome machinery mediated, at least in part, by externalization of the cathelicidin LL-37. This molecule activates the NLRP3 inflammasome in MØ, primarily the M1 subtype, via the P2×7 receptor. Further, lupus MØ are more prone to activate the inflammasome in response to LL-37 and NETs, when compared to control MØ. In addition, this is the first report that IL-18 significantly enhances NET production. These observations suggest a novel mechanism by which inflammatory responses become amplified through a feed forward loop where NET-induced IL-1β and IL-18 production enhances synthesis of extracellular traps in recruited neutrophils.
Further, our data suggest a mechanism by which inflammatory responses are enhanced in patients with SLE. Lupus MØ may undergo enhanced exposure to NETs because SLE neutrophils are primed to die by NETosis and, in a subset of patients, there is impaired degradation of these lattices(5-7). NETs potentially contribute to lupus pathogenesis through enhancement of organ damage, exposure of autoantigens that contribute to autoantibody formation, complement activation and induction of IFNα synthesis by pDCs(3, 4, 7, 36). As SLE MØ appear to display enhanced sensitivity to activate the inflammasome and IL-1β and IL-18 production in response to NETs and LL-37, this phenomenon may lead to significant inflammatory responses in various compartments and may serve as a source for elevated IL-18 levels in SLE patients. Given that NETosis is triggered via microbial exposure(1), our observations also provide a possible explanation for why infections trigger lupus flares: NETs activate pathways leading to the synthesis of potent MØ proinflammatory cytokines and amplification of neutrophil-mediated tissue damage.
Recent observations suggest that the induction of IFNα synthesis by PDCs upon LL-37 exposure requires its complex with DNA(4). However, with regards to activation of caspase-1, our observations indicate that there is no requirement for LL-37 to be complexed with DNA. Rather, the activation of caspase-1 is triggered by its ability to induce potassium efflux utilizing the P2×7R, agreeing with previous findings in monocytes(14). Traditionally, ATP has been proposed as the classical ligand for activation of this receptor. However, given that LL-37 is present at elevated levels in various inflamed tissues(37) and may be concentrated in areas of high NETosis, this cathelicidin makes a plausible additional ligand for P2×7R-mediated signaling pathways. Further, LL-37 has been reported to amplify IL-1β-inflammatory signaling pathways (38), which may potentiate inflammatory responses.
Our data suggest that lupus MØ are more sensitive to inflammasome activation by NETs and LL-37. It is unknown whether differences in P2×7R expression may contribute to this enhanced activation. The levels of caspase-1 were previously reported to be elevated in both SLE kidney and lupus endothelial progenitor cells, and we previously found that caspase-1 is upregulated by type I IFN exposure(17). This increased expression of caspase-1 could increase the propensity for its activation(39). Further, the SLE inflammasome may be dysregulated by dysfunctional phagocytosis of apoptotic bodies, as proper C1q function, which is impaired in SLE(40), represses inflammasome activation(41). Additionally, it is unknown whether there is alternate regulation of inflammasome inhibitory molecules, such as POP1/ASC2(42) in SLE. Certainly, further investigation into the mechanisms resulting in enhanced inflammasome activation in response to LL-37, NETs and other inflammasome regulators is warranted.
Our data suggest that that LL-37 is able to stimulate caspase-1 activation in lower concentrations (nM range) when presented in the context of NETs versus free soluble peptide (which requires μM concentrations). This may reflect several possibilities. First, the effective concentration of LL-37 present in the NETs may be higher than what is measured via ELISA. Alternatively, it is possible that other intracellular contents, such as ATP, which can activate the P2×7R in high concentrations(28), may be released during NETosis and thus synergize with LL-37 to stimulate inflammasome activation. Finally, it is possible that posttranslational modifications of LL-37, such as citrullination (44), which may occur during NETosis, would render the protein a more potent activator of the P2×7R. Further investigations into mechanisms that enhance LL-37 activation of the P2×7R will be informative to understand how the microenvironment of NETs can modulate inflammatory responses.
LL-37 has both pro- and anti-inflammatory properties, depending on the cellular context. Exposure of monocytes to LL-37 promotes M1 MØ differentiation, leading to a more inflammatory phenotype(11), and LL-37 enhances TLR-mediated synthesis of inflammatory cytokines such as IL-6(12). We propose an additional mechanism by which LL-37 promotes inflammation, by activating the NLRP3 inflammasome, resulting in IL-1β and IL-18 release in primary MØ. This activation is dependent on potassium efflux, which is consistent with other studies that indicate that this movement of ions is a key component for NLRP3 activation (28, 43-46). Further, the ability of LL-37 to form pores does not solely explain the induction of potassium efflux as ours, and other studies, have shown that ability of LL-37 to activate the inflammasome requires the P2×7R(14). Future studies should also focus on identifying whether other NET components, besides LL-37, are effective triggers of the inflammasome machinery. This is of particular importance when establishing future therapeutic targets to inhibit deleterious effects triggered by aberrant NETosis.
In the skin, cytosolic DNA triggers the AIM 2 inflammasome in keratinocytes leading to IL-1β synthesis after TNF and IFN-γ priming. In this system, LL-37 complexed to DNA is internalized in a P2×7 independent manner, and is able to inhibit AIM2 inflammasome activation(47). In contrast, our observations indicate that, in primary MØ, LL-37 is proinflammatory, possibly because it utilizes the NLRP3 rather than the AIM2 inflammasome. Future studies should verify this hypothesis.
In summary, we have described a novel mechanism, enhanced in patients with SLE, which leads to activation of the NLRP3 inflammasome machinery in MØ through NETs and the NET-associated protein LL-37. This stimulation likely contributes to a self-perpetuating cycle of enhanced IL-1β and IL-18 production which, in turn, further promote inflammation and NETosis. Our observations provide additional evidence that aberrant NET formation characteristic of SLE and other autoimmune diseases is pathogenic, and promotes perpetuation of inflammatory responses.
Acknowledgments
This work was supported by the Alliance for Lupus Research and by the National Institutes of Health (NIH) through Public Health Service Grant HL088419 (both to MJK). This work was also supported in part by the NIH through the University of Michigan’s Rheumatic Disease Core Center (Grant P30 AR48310). Support to JMK and CCR was provided by PHS grant T32-AR-007080and additional support to JMK came through the American College of Rheumatology Rheumatology Research Foundation. CKS received support through PHS grant T32-AI-007413.
Abbreviations
- ECM
extracellular media
- hCAP-18
human cationic antimicrobial protein of 18 kDa
- LDG
low density granulocytes
- Mφ
macrophage
- mIL-1β
mature IL-1β
- NETs
neutrophil extracellular traps
- pDC
plasmacytoid dendritic cell
- SLE
systemic lupus erythematosus
- WCL
whole cell lysate
REFERENCES
- 1.Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol. 2007;5:577–582. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- 2.Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, Grone H-J, Brinkmann V, Jenne DE. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knight JS, Kaplan MJ. Lupus neutrophils: ‘NET’ gain in understanding lupus pathogenesis. Current opinion in rheumatology. 2012;24:441–450. doi: 10.1097/BOR.0b013e3283546703. [DOI] [PubMed] [Google Scholar]
- 4.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, Bassett R, Amuro H, Fukuhara S, Ito T, Liu YJ, Gilliet M. Neutrophils Activate Plasmacytoid Dendritic Cells by Releasing Self-DNA-Peptide Complexes in Systemic Lupus Erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, Herrmann M, Voll RE, Zychlinsky A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010;107:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, Barrat FJ, Banchereau J, Pascual V. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, Rubin CJ, Zhao W, Olsen SH, Klinker M, Shealy D, Denny MF, Plumas J, Chaperot L, Kretzler M, Bruce AT, Kaplan MJ. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187:538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denny M, Yalavarthi S, Zhao W, Thacker S, Anderson M, Sandy A, McCune WJ, Kaplan M. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. The journal of immunology. 2010;184:3284–3297. doi: 10.4049/jimmunol.0902199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu C, Tangsombatvisit S, Rosenberg J, Mandelbaum G, Gillespie E, Gozani O, Alizadeh A, Utz P. Specific post-translational histone modifications of neutrophil extracellular traps as immunogens and potential targets of lupus autoantibodies. Arthritis research & therapy. 2012;14:R25. doi: 10.1186/ar3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Does AM, Beekhuizen H, Ravensbergen B, Vos T, Ottenhoff THM, van Dissel JT, Drijfhout JW, Hiemstra PS, Nibbering PH. LL-37 Directs Macrophage Differentiation toward Macrophages with a Proinflammatory Signature. The journal of immunology. 2010;185:1442–1449. doi: 10.4049/jimmunol.1000376. [DOI] [PubMed] [Google Scholar]
- 12.Lai Y, Adhikarakunnathu S, Bhardwaj K, Ranjith-Kumar CT, Wen Y, Jordan JL, Wu LH, Dragnea B, Mateo LS, Kao CC. LL37 and Cationic Peptides Enhance TLR3 Signaling by Viral Double-stranded RNAs. PLoS One. 2011;6:e26632. doi: 10.1371/journal.pone.0026632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emelianov VU, Bechara FG, Gläser R, Langan EA, Taungjaruwinai WM, Schröder JM, Meyer KC, Paus R. Immunohistological pointers to a possible role for excessive cathelicidin (LL-37) expression by apocrine sweat glands in the pathogenesis of hidradenitis suppurativa/acne inversa. British Journal of Dermatology. 2012;166:1023–1034. doi: 10.1111/j.1365-2133.2011.10765.x. [DOI] [PubMed] [Google Scholar]
- 14.Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J Immunol. 2004;172:4987–4994. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 15.Kahlenberg JM, Dubyak GR. Differing caspase-1 activation states in monocyte versus macrophage models of IL-1beta processing and release. J Leukoc Biol. 2004;76:676–684. doi: 10.1189/jlb.0404221. [DOI] [PubMed] [Google Scholar]
- 16.Thacker S, Berthier C, Mattinzoli D, Rastaldi M, Kretzler M, 2010 M. Kaplan. The detrimental effects of IFN-α on vasculogenesis in lupus are mediated by repression of IL-1 pathways: potential role in atherogenesis and renal vascular rarefaction. The journal of immunology. 185:4457–4469. doi: 10.4049/jimmunol.1001782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahlenberg JM, Thacker SG, Berthier CC, Cohen CD, Kretzler M, Kaplan MJ. Inflammasome activation of IL-18 results in endothelial progenitor cell dysfunction in systemic lupus erythematosus. J Immunol. 2011;187:6143–6156. doi: 10.4049/jimmunol.1101284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu D, Liu X, Chen S, Bao C. Expressions of IL-18 and its binding protein in peripheral blood leukocytes and kidney tissues of lupus nephritis patients. Clin Rheumatol. 2010;29:717–721. doi: 10.1007/s10067-010-1386-6. [DOI] [PubMed] [Google Scholar]
- 19.Kinoshita K, Yamagata T, Nozaki Y, Sugiyama M, Ikoma S, Funauchi M, Kanamaru A. Blockade of IL-18 Receptor Signaling Delays the Onset of Autoimmune Disease in MRL-Faslpr Mice. The journal of immunology. 2004;173:5312–5318. doi: 10.4049/jimmunol.173.8.5312. [DOI] [PubMed] [Google Scholar]
- 20.Wang D, Drenker M, Eiz-Vesper B, Werfel T, Wittmann M. Evidence for a pathogenetic role of interleukin-18 in cutaneous lupus erythematosus. Arthritis & Rheumatism. 2008;58:3205–3215. doi: 10.1002/art.23868. [DOI] [PubMed] [Google Scholar]
- 21.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 22.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parker H, Dragunow M, Hampton MB, Kettle AJ, Winterbourn CC. Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. Journal of leukocyte biology. 2012 doi: 10.1189/jlb.1211601. epublished ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 25.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 26.Kahlenberg JM, Lundberg KC, Kertesy SB, Qu Y, Dubyak GR. Potentiation of caspase-1 activation by the P2X7 receptor is dependent on TLR signals and requires NF-kappaB-driven protein synthesis. J Immunol. 2005;175:7611–7622. doi: 10.4049/jimmunol.175.11.7611. [DOI] [PubMed] [Google Scholar]
- 27.Dubyak GR. P2X7 receptor regulation of non-classical secretion from immune effector cells. Cellular microbiology. 2012;14:1697–1706. doi: 10.1111/cmi.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahlenberg JM, Dubyak GR. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am J Physiol Cell Physiol. 2004;286:C1100–1108. doi: 10.1152/ajpcell.00494.2003. [DOI] [PubMed] [Google Scholar]
- 29.Le Feuvre RA, Brough D, Iwakura Y, Takeda K, Rothwell NJ. Priming of Macrophages with Lipopolysaccharide Potentiates P2X7-mediated Cell Death via a Caspase-1-dependent Mechanism, Independently of Cytokine Production. Journal of Biological Chemistry. 2002;277:3210–3218. doi: 10.1074/jbc.M104388200. [DOI] [PubMed] [Google Scholar]
- 30.Calvani N, Richards HB, Tucci M, Pannarale G, Silvestris F. Up-regulation of IL-18 and predominance of a Th1 immune response is a hallmark of lupus nephritis. Clinical and experimental immunology. 2004;138:171–178. doi: 10.1111/j.1365-2249.2004.02588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitroulis I, Kambas K, Chrysanthopoulou A, Skendros P, Apostolidou E, Kourtzelis I, Drosos GI, Boumpas DT, Ritis K. Neutrophil Extracellular Trap Formation Is Associated with IL-1β and Autophagy-Related Signaling in Gout. PLoS One. 2011;6:e29318. doi: 10.1371/journal.pone.0029318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bossu P, Neumann D, Del Giudice E, Ciaramella A, Gloaguen I, Fantuzzi G, Dinarello C, Di Carlo E, Musiani P, Meroni P, Caselli G, Ruggiero P, Boraschi D. IL-18 cDNA vaccination protects mice from spontaneous lupus-like autoimmune disease. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14181–14186. doi: 10.1073/pnas.2336094100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keshari RS, Verma A, Barthwal MK, Dikshit M. Reactive oxygen species-induced activation of ERK and p38 MAPK mediates PMA-induced NETs release from human neutrophils. Journal of Cellular Biochemistry. 2012 doi: 10.1002/jcb.24391. epublished ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Neeli I, Dwivedi N, Khan S, Radic M. Regulation of Extracellular Chromatin Release from Neutrophils. Journal of innate immunity. 2009;1:194–201. doi: 10.1159/000206974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leffler J, Martin M, Gullstrand B, Tyden H, Lood C, Truedsson L, Bengtsson AA, Blom AM. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol. 2012;188:3522–3531. doi: 10.4049/jimmunol.1102404. [DOI] [PubMed] [Google Scholar]
- 37.Gilliet M, Lande R. Antimicrobial peptides and self-DNA in autoimmune skin inflammation. Curr Opin Immunol. 2008;20:401–407. doi: 10.1016/j.coi.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Yu J, Mookherjee N, Wee K, Bowdish DME, Pistolic J, Li Y, Rehaume L, Hancock REW. Host Defense Peptide LL-37, in Synergy with Inflammatory Mediator IL-1β, Augments Immune Responses by Multiple Pathways. The journal of immunology. 2007;179:7684–7691. doi: 10.4049/jimmunol.179.11.7684. [DOI] [PubMed] [Google Scholar]
- 39.Stehlik C, Lee SH, Dorfleutner A, Stassinopoulos A, Sagara J, Reed JC. Apoptosis-Associated Speck-Like Protein Containing a Caspase Recruitment Domain Is a Regulator of Procaspase-1 Activation. The journal of immunology. 2003;171:6154–6163. doi: 10.4049/jimmunol.171.11.6154. [DOI] [PubMed] [Google Scholar]
- 40.Santer DM, Hall BE, George TC, Tangsombatvisit S, Liu CL, Arkwright PD, Elkon KB. C1q deficiency leads to the defective suppression of IFN-alpha in response to nucleoprotein containing immune complexes. J Immunol. 2010;185:4738–4749. doi: 10.4049/jimmunol.1001731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benoit ME, Clarke EV, Morgado P, Fraser DA, Tenner AJ. Complement Protein C1q Directs Macrophage Polarization and Limits Inflammasome Activity during the Uptake of Apoptotic Cells. The journal of immunology. 2012 doi: 10.4049/jimmunol.1103760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stehlik C, Krajewska M, Welsh K, Krajewski S, Godzik A, Reed JC. The PAAD/PYRIN-only protein POP1/ASC2 is a modulator of ASC-mediated nuclear-factor-kappa B and pro-caspase-1 regulation. Biochem. J. 2003;373:101–113. doi: 10.1042/BJ20030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darisipudi MN, Thomasova D, Mulay SR, Brech D, Noessner E, Liapis H, Anders H-J. Uromodulin Triggers IL-1β-Dependent Innate Immunity via the NLRP3 Inflammasome. Journal of the American Society of Nephrology. 2012 doi: 10.1681/ASN.2012040338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang T-T, Ojcius DM, Young JD, Wu Y-H, Ko Y-F, Wong T-Y, Wu C-Y, Lu C-C, Lai H-C. The Anti-Tumorigenic Mushroom Agaricus blazei Murill Enhances IL-1β Production and Activates the NLRP3 Inflammasome in Human Macrophages. PLoS One. 2012;7:e41383. doi: 10.1371/journal.pone.0041383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Segovia J, Sabbah A, Mgbemena V, Tsai S-Y, Chang T-H, Berton MT, Morris IR, Allen IC, Ting JPY, Bose S. TLR2/MyD88/NF-κB Pathway, Reactive Oxygen Species, Potassium Efflux Activates NLRP3/ASC Inflammasome during Respiratory Syncytial Virus Infection. PLoS One. 2012;7:e29695. doi: 10.1371/journal.pone.0029695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Menu P, Mayor A, Zhou R, Tardivel A, Ichijo H, Mori K, Tschopp J. ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell Death Dis. 2012;3:e261. doi: 10.1038/cddis.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dombrowski Y, Peric M, Koglin S, Kammerbauer C, Göß C, Anz D, Simanski M, Gläser R, Harder J, Hornung V, Gallo RL, Ruzicka T, Besch R, Schauber J. Cytosolic DNA Triggers Inflammasome Activation in Keratinocytes in Psoriatic Lesions. Science translational medicine. 2011;3:82ra38. doi: 10.1126/scitranslmed.3002001. [DOI] [PMC free article] [PubMed] [Google Scholar]