Abstract
Several lines of evidence indicate the instability of CD4+FoxP3+ regulatory T cells (Tregs). We have therefore investigated means of promoting the stability of Tregs. In this study, we found that the proportion of Tregs in mouse strains deficient in TNFR2 or its ligands was reduced in the thymus and peripheral lymphoid tissues, suggesting a potential role of TNFR2 in promoting the sustained expression of FoxP3. We observed that upon in vitro activation with plate-bound anti-CD3 Ab and soluble anti-CD28 Ab, FoxP3 expression by highly purified mouse Tregs was markedly down-regulated. Importantly, TNF partially abrogated this effect of TCR stimulation and stabilized FoxP3 expression. This effect of TNF was blocked by anti-TNFR2 Ab, but not by anti-TNFR1 Ab. Furthermore, TNF was not able to maintain FoxP3 expression by TNFR2-deficient Tregs. In mouse colitis model induced by transfer of naïve CD4 cells into Rag1−/− mice, the disease could be inhibited by co-transfer of WT Tregs, but not by co-transfer of TNFR2-deficient Tregs. Furthermore, in the lamina propria of the colitis model, the majority of WT Tregs maintained FoxP3 expression. In contrast, increased number of TNFR2-deficient Tregs lost FoxP3 expression. Thus, our data clearly show that TNFR2 is critical for the phenotypic and functional stability of Treg in the inflammatory environment. This effect of TNF should be taken into account when designing future therapy of autoimmunity and GVHD by using TNF inhibitors.
Keywords: T cell, cytokine receptor, tolerance/suppression/anergy
INTRODUCTION
CD4+FoxP3+ regulatory T cells (Tregs) play a nonredundant role in the maintenance of immunological homeostasis and in the prevention of autoimmune disorders, and they also represent a major cellular mechanism in immune evasion by tumors (1–2). It is now well established that the transcription factor FoxP3 (forkhead box P3) is a unique marker specific for the Treg lineage, which determines their phenotype and immunosuppressive function (3). Abrogation of Foxp3 gene function leads to the development of lethal multi-organ autoimmune disorders in mice and humans (4–5). In contrast, forced expression of Foxp3 in CD4+CD25− effector T cells (Teffs) is sufficient to convert them into functional Tregs (6–7).
Contrary to the previous notion that Tregs were stable and terminally differentiated cells, recent evidence revealed the phenotypic and functional plasticity of Tregs in response to inflammatory stimulation. For example, IL-1β and IL-6 each re-programmed Treg cells and induced them to express IL-17 (8–10). However, there is compelling contrasting evidence that the number of highly suppressive FoxP3-expressing cells actually increased in various inflammatory sites (11). Therefore, the inflammatory environment seems to have the capacity to restrain the plasticity and promote the phenotypic and functional stability of Tregs. Clarification of the molecular basis of such an effect may be therapeutically beneficial and can further improve our understanding of Treg biology.
TNF is a pleiotropic cytokine and up-regulation of TNF expression is a benchmark of inflammatory responses. The biological functions of TNF are mediated by two structurally related, but functionally distinct receptors, TNFR1 (tumor necrosis factor receptor type I or p55) and TNFR2 (tumor necrosis factor receptor type II or p75) (12). With a death domain (DD) in its cytoplasmic tail, TNFR1 is the primary signaling receptor on most cell types and accounts for the majority of the proinflammatory, cytotoxic and apoptotic effects classically attributed to TNF (13–14). In contrast, TNFR2 lacks an intracellular death domain and predominantly mediates signals promoting lymphocyte activation and proliferation (15–16). Although counterintuitive, the accumulated evidence indicates that TNF by signaling through TNFR2 promotes Treg activity. For example, we reported that TNF by activating TNFR2 is able to activate and expand Tregs (17), and TNFR2 expression identifies the maximal suppressive (18) and replicating Tregs (19) in mice and helps identify functional Tregs in human PBMCs (20). Furthermore, TNF preferentially up-regulates TNFR2 expression on Tregs (21). In confirmation of our observation, Housley and Clark et al have shown that TNFR2 is critical for the in vivo immunosuppressive function of naturally occurring Tregs (22). Grinber-Bleyer and Salomon et al showed that pathogenic Teffs stimulated the activation of Tregs in vivo, at least partially through TNF-TNFR2 interaction (23). Mougiakakos and Kiessling et al found that TNF-TNFR2 interaction enhanced thioredoxin-1 expression on human Tregs, but not on Teffs, and therefore preferentially promoted Treg survival and activity within inflammatory milieu (24). However, it remains elusive whether the replicating Tregs driven by TNF-TNFR2 interaction retain phenotypic and functional attributes of naturally occurring Tregs, especially in the chronic inflammatory condition. This question is clinically relevant now that TNF blockage therapy has become an important tool in the treatment of autoimmune disorders.
In this study, we observed that the frequency of Tregs in the thymus of mouse strains genetically deficient in TNFR2 or its ligands is reduced. Expression of Foxp3 is induced in developing thymocytes upon self-reactive TCR engagement (25). Thus, stimulation of TNFR2 may stabilize FoxP3 expression in response to activation of TCR of Treg cells in immune or inflammatory responses, as shown by our in vitro and in vivo studies.
MATERIALS AND METHODS
Mice
Wild-type C57BL/6 mice, congenic Ly5.2 C57BL/6 mice, Rag1−/− mice, TNFR2−/− mice, TNF−/− mice, TNN/LTα/LTβ−/− mice were provided by the Animal Production Area of the NCI (Frederick, MD). FoxP3/gfp KI (knock in) mice were kindly provided by Dr. Yasmine Belkaid at Laboratory of Parasitic Diseases, NIAID, NIH, and maintained in the Animal Production Area of the NCI (Frederick, MD). Frederick National Laboratory for Cancer Research is accredited by AAALAC International and follows the Public Health Service Policy for the Care and Use of Laboratory Animals. Animal care was provided in accordance with the procedures outlined in the "Guide for Care and Use of Laboratory Animals" (National Research Council; 1996; National Academy Press; Washington, D.C.). Anti-mouse antibodies (Abs) were purchased from BD Biosciences (San Diego, CA) consisted of anti-mouse CD3 (145-2C11), CD4 (GK1.5), CD25 (PC61), CD45, TNFR2 (TR75 –89), Ki-67 (B56), INFγ (XMG1.2) and IL-17A (TC11-18H10). Leukocyte Activation Cocktail was also purchased from BD Biosciences. Functional grade purified anti-mouse CD3e (eBio500A2), CD28 (37.51) and IL-4 (11B11) Abs, Foxp3 Staining Set (FJK-16s) and anti-mouse TCRβ Ab (H57–597) were purchased from eBioscience (San Diego, CA). Functional grade anti-mouse TNFR1 (55R-170), TNFR2 (TR75-32.4) Ab and Ham IgG were purchased from Biolegend (San Diego, CA). Murine IL-6, IL-12 and TNF were purchased from PeproTech (Rocky Hill, NJ). Human rTGFβ1 was from R&D Systems (Minneapolis, MN).
T cell transfer model of colitis
Naive CD4+CD25−CD45RBhi T cells were isolated from WT congenic B6 (CD45.1+) mice and injected i.p. into Rag1−/− immunodeficient recipients (4 × 105 cells/mouse) alone, or co-transferred with WT or TNFR2−/− CD4+CD25+CD45RBlo Treg cells (1.6 × 105/mouse, CD45.2+). In Tregs transfer experiments, CD4+CD25+CD45RBlo Treg cells flow-sorted from WT congenic B6 mice (CD45.1+) and TNFR2−/− mice (CD45.2+) were mixed at 1:1 ratio and i.p. injected into Rag1−/− mice (1.2 × 105/mouse, each). Mice were monitored weekly for the clinical symptoms of colitis such as rectal bleeding, loose feces/diarrhea, rough/hunched posture and body weight by animal facility staff. Any mice losing >20% of its starting body weight or showing severe signs of disease were euthanized.
Cell isolation
Single cell suspensions from spleen and mLNs (mesenteric LNs) were prepared by filtration through a 70 µm cell strainer (BD Labware, San Jose, CA). Preparation of colon lamina propria (cLP) cells was as previously described (26). Briefly, colons were rinsed in PBS and cut into ~0.3 cm pieces. Intestinal epithelial cells were removed by incubation with Ca- and Mg-free PBS containing 10% FCS and 5 mM EDTA. Colon tissues then were incubated with RPMI 1640 containing 10% FCS and 1 mg/ml collagenase type 4 (Worthington Biochemical Corporation, Lakewood, NJ) for 30 min at 37°C.
In vitro T cell activation and differentiation
Flow-sorted CD4+FoxP3/gfp+ cells or CD4+FoxP3/gfp− cells from FoxP3/gfp KI mice, or CD4+CD25+ cells from WT C57BL/6 mice or TNFR2−/− mice were seeded at 5×104 cells/well in 96-well plate. The cells were stimulated with plate-bound anti-CD3e Ab (10 µg/ml) and soluble anti-CD28 Ab (2 µg/ml) for 5 days. In some experiments, the cells were activated in Th0, Th1 and Th17 polarizing conditions by adding medium alone, or IL-12 (10 ng/ml) plus anti-IL-4 Ab (10 µg/ml), or IL-6 (10 ng/ml) or IL-6 plus TGFβ (1 ng/ml), respectively. In someexperiment, the cells were activated in the presence of TNF (10 ng/ml) and/or IL-6 (10 ng/ml), without or with 10 µg/ml of Ham IgG, or anti-TNFR1 Ab or anti-TNFR2 Ab. Iscove's modified Dulbecco's medium (IMDM, Sigma-Aldrich) was used in Th17 polarizing culture, and RPMI-1640 (Lonza BioWhittaker, Walkersville, MD) was used in all other cultures. The medium was supplemented with 10% fetal bovine serum (FBS, Hyclone, Logan, UT) containing 2 mM glutamine, 100 IU/ml penicillin, and 100 µg/ml streptomycin, 10 mM HEPES, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids and 50 µM 2-ME.
Flow cytometry
After blocking FcR, cells were incubated with appropriately diluted antibodies. Acquisition was performed using a SLRII (BD Biosciences, Mountain View, CA) and data analysis was conducted using FlowJo software (Tree Star Inc., Ashland, OR). For intracellular cytokines staining, cells were re-stimulated with BD Leukocyte Activation Cocktail for 4 h. FACS analysis was gated on the live cells only by using LIVE/DEAD® Fixable Dead Cell Stain Kit.
Statistical analysis
Cumulative incidence of colitis was graphed as survival plot and analyzed with Logrank test, and comparison of other data was analyzed by two-tailed Student’s t test using Graphpad Prism 4.0.
RESULTS
Reduction of thymic and peripheral Tregs in mice deficient in TNFR2 or its ligands
In normal mice, most thymic Tregs express TNFR2 (18). All human thymic CD4+CD25+ Tregs constitutively express TNFR2, while thymic CD4+CD25− cells do not express this receptor (27). TNF is expressed in the thymus of mice and humans, and participates in the development of thymocytes (28). Thus, we investigated the possibility that TNF or LTα (lymphotoxin alpha), the ligands for TNFR2, contribute to the thymic differentiation and generation of Tregs.
We first compared the FoxP3-expressing Tregs in TNFR2−/− and normal WT mice. In adult TNFR2−/− mouse thymus, the proportion of CD4+FoxP3+ Tregs in total thymocytes was reduced by 45%, as compared with WT control B6 mice (Fig 1A, *P<0.05). The proportion of FoxP3+ cells in CD4 SP (single positive) thymocytes was also reduced by ~30% (Fig 1B, p<0.05). It was reported that the cellularity of the thymus of TNFR2−/− mice was greater than that of WT mice, however, the most affected subset of thymocytes were naïve triple negative cells (CD3−CD4−CD8−), while both CD4 and CD8 subsets were not altered (29). Since thymic Tregs were almost exclusively contained in the CD4 SP population, the absolute number of Tregs in the thymus of TNFR2−/− mice was reduced proportionally.
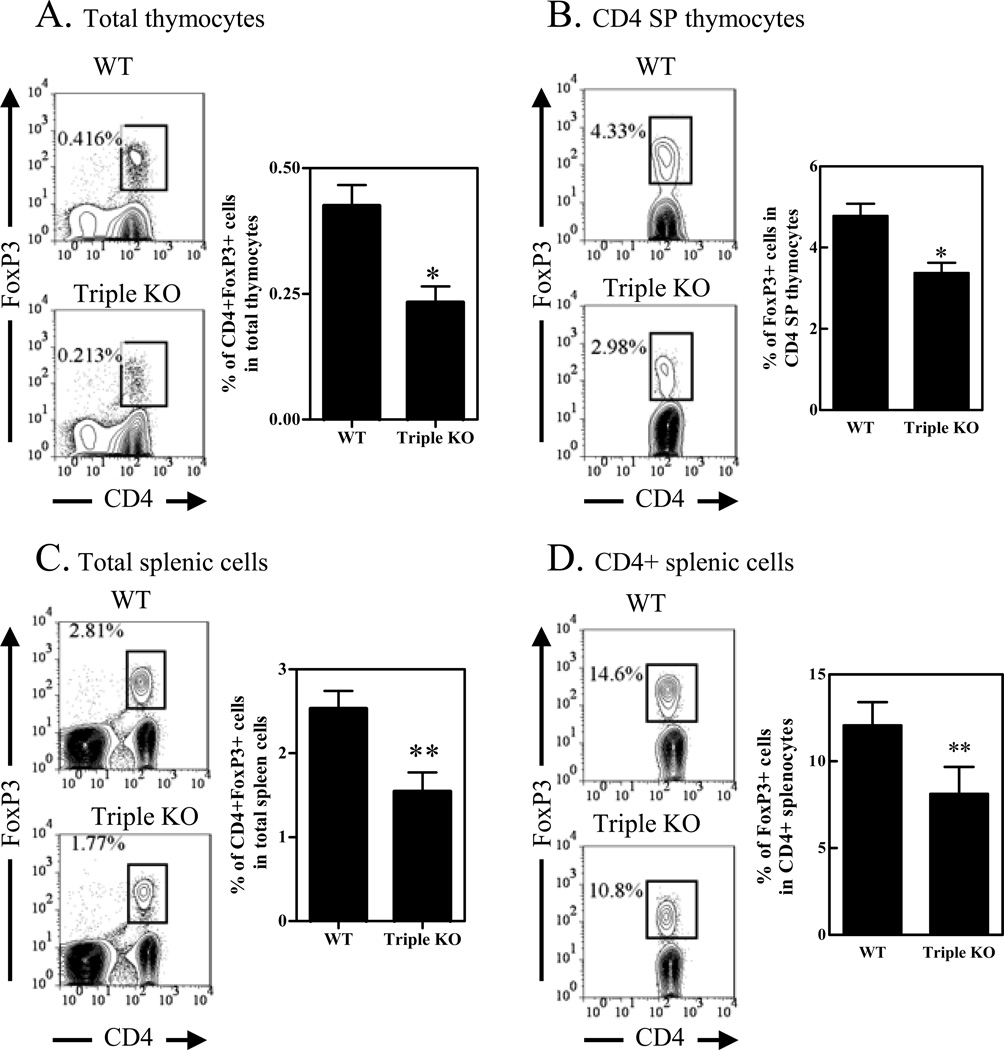
Figure 1.
Reduced number of Tregs in TNFR2 deficient mice. Cells from thymus, spleen and LNs in WT mice (C57BL/6) and TNFR2−/− mice were stained with CD3, CD4, CD8, TNFR2, and FoxP3. The expression of FoxP3 was analyzed by FACS, gating on CD3+CD4+ cells or CD3+CD4+CD8− cells (CD4 single positive cells, e.g., CD4 SP cells). (A) Proportion of CD4+FoxP3+ cells in the total thymocytes derived from WT or TNFR2−/− mice. (B) Expression of FoxP3 and TNFR2 on CD4 SP thymocytes from WT or TNFR2−/− mice. (C~D) Proportion of CD4+FoxP3+ cells in the total splenic and LN cells from WT or TNFR2−/− mice. In (A and B), left shows the typical FACS plots, and right shows summary (N=3). (C) shows the typical FACS plots and (D) shows the summary (N=3). (E –F) Expression of FoxP3 and TNFR2 on CD4+ T cells in the spleen and LNs from WT or TNFR2−/− mice. (E) Shows the typical FACS plots and (F) shows the summary (N=3). (G) Number of CD4+FoxP3+ Tregs in the spleen from WT or TNFR2−/− mouse. Number in the FACS plot shows the proportion of positive cells in the indicated gating or respective quadrants. Comparison between two indicated groups: * p<0.05; ** p<0.01. Data shown are representatives of at least 3 separate experiments with same results.
In the periphery, the percent of CD4+FoxP3+ cells in total splenic cells and LN cells was decreased by 42% and 21%, respectively (P<0.01~0.05, Fig 1C~D). The proportion of FoxP3+ cells in the CD4+ splenic cells and CD4+ LN cells was decreased by 36% and 22%, respectively (p<0.05, Fig 1E~F). The absolute number of splenic Tregs in TNFR2−/−mouse was reduced by ~50% (p<0.05, Fig 1G). These data suggest that TNFR2 may participate in the development of Tregs in the thymus. Although TNFR2−/− mouse does not spontaneously develop apparent autoimmune disorders, this strain of mouse nevertheless shows more severe inflammation upon induction of autoimmune disease (30), presumably attributed by the reduced number of Tregs.
The development of FoxP3+ Tregs in mice with depletion of TNFR2 ligands was also investigated. Both TNF−/− mice or LTα/β−/− mice did not exhibit any deficiency in Tregs in the spleen (data not shown). However, the proportion of Tregs in the periphery and thymus of TNF/LTα/LTβ−/− (triple KO) mice was decreased. Despite a profound defect of peripheral lymphoid organs of triple KO mice, this strain of mouse had no change in the major thymocyte populations and T/B ratio in the spleen (31). In the thymus, the proportion of CD4+FoxP3+ Tregs in total thymocytes was reduced by ~50% (p<0.05, Fig 2A). The proportion of FoxP3+ Tregs in CD4 SP thymocytes was reduced by 31% (p<0.05, Fig 2B). In the spleen, the proportion of CD4+FoxP3+ Tregs in total splenic cells was reduced by 37% (p<0.01, Fig 2C). The proportion of FoxP3+ Tregs in CD4+ splenic cells was reduced by 26% (p<0.01, Fig 2D). TNF treatment in vitro largely restored the proportion of FoxP3+ Tregs in CD4 cells derived from triple KO mice (data not shown). Therefore, genetic ablation of TNFR2 or its ligands resulted in a reduction of Tregs in both thymus and peripheral lymphoid tissue.
Figure 2.
Reduced number of Tregs in TNFR2 ligands deficient mice. Cells from thymus, spleen and LNs in WT mice (C57BL/6) or TNF/LTα/LTβ triple KO mice were stained with CD3, CD4, CD8, TNFR2, and FoxP3. The expression of FoxP3 was analyzed by FACS, gating on CD3+CD4+ cells or CD3+CD4+CD8− cells. (A) Proportion of CD4+FoxP3+ cells in total thymocytes from WT or Triple KO mice. (B) Proportion of FoxP3+ cells in the CD4+CD8− thymocytes from WT or Triple KO mouse. In (A and B), left shows the typical FACS plots, and right shows summary (N=3~6). (C) Proportion of CD4+FoxP3+ cells in the spleen from WT or Triple KO mice. (D) Proportion of FoxP3+ cells in CD4+ splenic cells from WT or Triple KO mice. Number in the FACS data shows the proportion of cells in the indicated gating. Comparison between two indicated groups: * p<0.05; ** p<0.01. Data shown are representative of at least 3 separate experiments with same results.
TNF stabilizes FoxP3 expression by TCR-stimulated Tregs in vitro
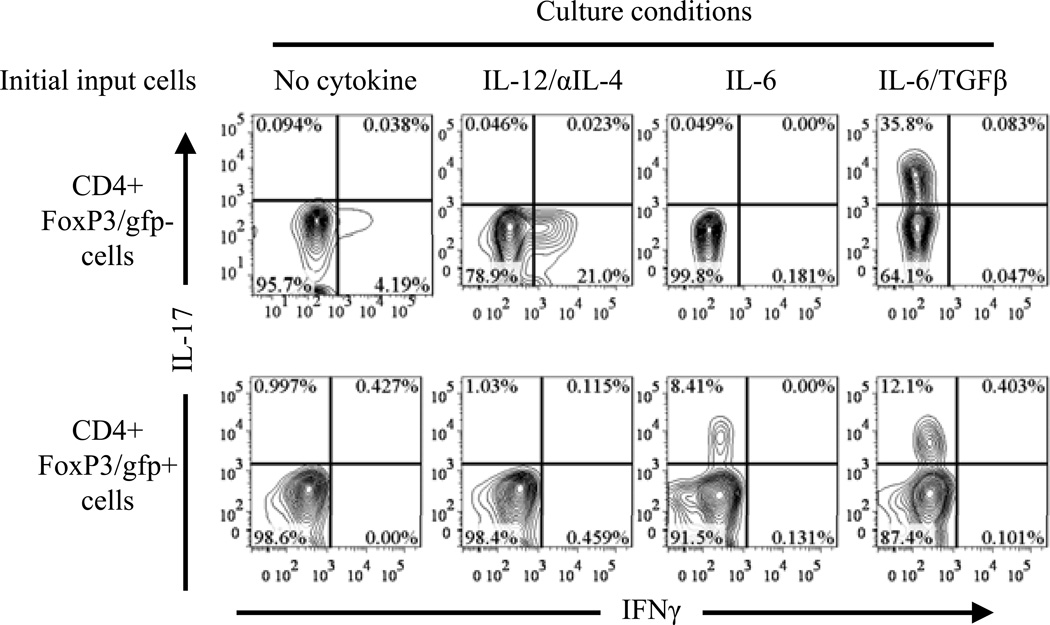
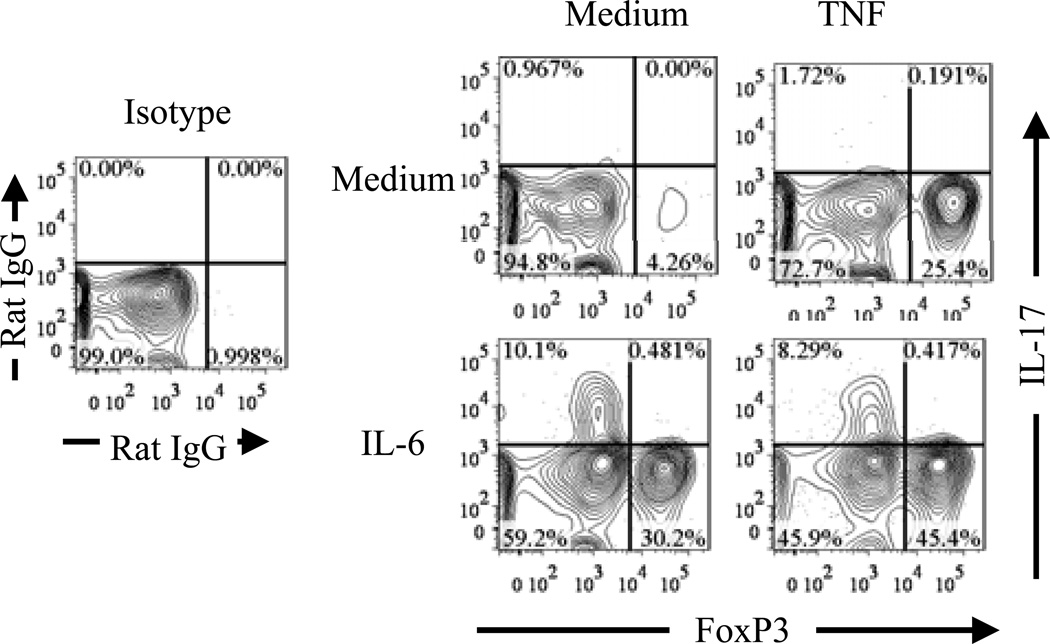
Self-reactive TCR signaling plays a central role in the thymic generation of Tregs (25). Reduced thymic Tregs in mice deficient in TNFR2 and its ligands suggest that TNF-TNFR2 signaling may play a role in stabilizing FoxP3 expression by Tregs in response to TCR stimulation. We therefore examined the effect of exogenous TNF on FoxP3 expression by highly purified Tregs, which were stimulated in vitro with plate bound anti-CD3 Ab and soluble anti-CD28 Ab. It was reported that, in the presence of proinflammatory cytokine, in vitro TCR stimulation could change the phenotype of Tregs (32). We confirmed this result and found that IL-6+TGFβ induced IL-17 expression by both Tregs and Teffs (effector T cells), IL-6 alone only induced IL-17 expression by TCR-stimulated Tregs, but not by Teffs. In contrast, Th1 polarizing culture condition only induced IFNγ expression by TCR-stimulated Teffs, but not by Tregs (Fig 3). Unexpectedly, TCR-stimulation alone markedly down-regulated FoxP3 expression by Tregs (Fig 4). This was not caused by activation induced cell death (AICD), since FACS analysis only gated on the live cells by using LIVE/DEAD® Fixable Dead Cell Stain Kit described in Methods and Materials. Although IL-6 induced IL-17 expression by a substantial proportion of initial Tregs, which all down-regulated their FoxP3 expression, this proinflammatory cytokine paradoxically supported the persistent expression of FoxP3 by 30% of input cells (Fig 4). In contrast, TNF by itself consistently maintained high levels of FoxP3 expression by 20~30% of initial input Tregs, and can cooperate with IL-6 to further enhanced the proportion of FoxP3-expressing cells without further increasing IL-17-producing cells (Fig 4). Therefore, in addition to activate and expand Tregs (17), TNF can also stabilize FoxP3 expression on Tregs.
Figure 3.
Plasticity of Tregs under proinflammatory stimulation in vitro. Splenic and LN cells from FoxP3/gfp KI mice were stained with CD4. FoxP3/gfp+ or FoxP3/gfp− CD4 cells were flow-sorted. The cells were activated in vitro with plate-bound anti-CD3 and soluble anti-CD28 Abs, in Th0 (no cytokine added) or in Th1 (IL-12 plus anti-IL-4 Ab) or Th17 (IL-6, or IL-6 plus TGFβ) polarizing culture condition. After 5 days, the cells were re-stimulated with BD Leukocyte Activation Cocktail and intracellular IFNγ and IL-17A were analyzed by FACS. Numbers in FACS plots indicate the percentage of cells in the respective quadrant. The data shown are representative of at least three separate experiments with the same results.
Figure 4.
TNF stabilizes FoxP3 expression on Tregs in vitro. Flow-sorted CD4+FoxP3/gfp+ cells were stimulated as described in Fig 3, with medium alone or with IL-6, in the presence of TNF or not. After re-activation, intracellular expression of FoxP3 and IL-17A was analyzed with FACS. Numbers indicate the percentage of cells in the respective quadrant. The data shown are representative of at least three separate experiments with the same results.
TNFR2 mediates the effect of TNF in maintenance of FoxP3 expression in vitro
To further determine which TNF receptor was responsible for stabilizing FoxP3 expression, anti-TNFR1 or anti-TNFR2 neutralizing antibodies were added to the TNF-treated Tregs. The anti-TNFR2 Ab, but not anti-TNFR1 Ab, markedly reduced the proportion of FoxP3-expressing cells (p<0.05, Fig 5A). We further clarified this issue by comparing the response of Tregs from WT mice or TNFR2−/− mice. Although TNFR2−/− mice had reduced number of Tregs, the residual Tregs nevertheless expressed the same levels of FoxP3 as WT Tregs (Fig 5B). As shown in Fig 5C, TCR stimulation reduced FoxP3 expression by TNFR2−/− Tregs and this was not reversed by TNF. In contrast, FoxP3 expression by TNFR2−/− Tregs was partially sustained by IL-6 (data not shown). Thus, our data clearly show that TNF activation of TNFR2 is able to stabilize FoxP3 expression by Tregs upon potent TCR stimulation.
Figure 5.
TNFR2 mediates the effect of TNF in maintaining FoxP3 expression in vitro. (A) Flow-sorted CD4+FoxP3/gfp+ cells were stimulated as in Fig 3, in the presence of TNF (10 ng/mL), and 10 µg/mL of isotype control Ham IgG, or anti-TNFR1 Ab or anti-TNFR2 Ab. FoxP3 expression was analyzed by FACS. Typical histograms and summary (N=3) are shown. (B) Tregs were flow-sorted from WT and TNFR2−/− mice, based on surface expression of CD4+CD25+. The expression of FoxP3 was analyzed by FACS. (C) CD4+ CD25+ cells from TNFR2 KO mice were activated as described in Fig 3, in the presence of TNF (10 ng/mL) or not. The expression of FoxP3 was analyzed by FACS. Typical FACS plot and summary (N=3) are shown. Numbers represent the percentage of cells within the indicated gate. The data shown are representative of at least three separate experiments with the same results. Compared with indicated group, * p<0.05; **p<0.01.
TNFR2 is required for the in vivo immunosuppressive function of Tregs
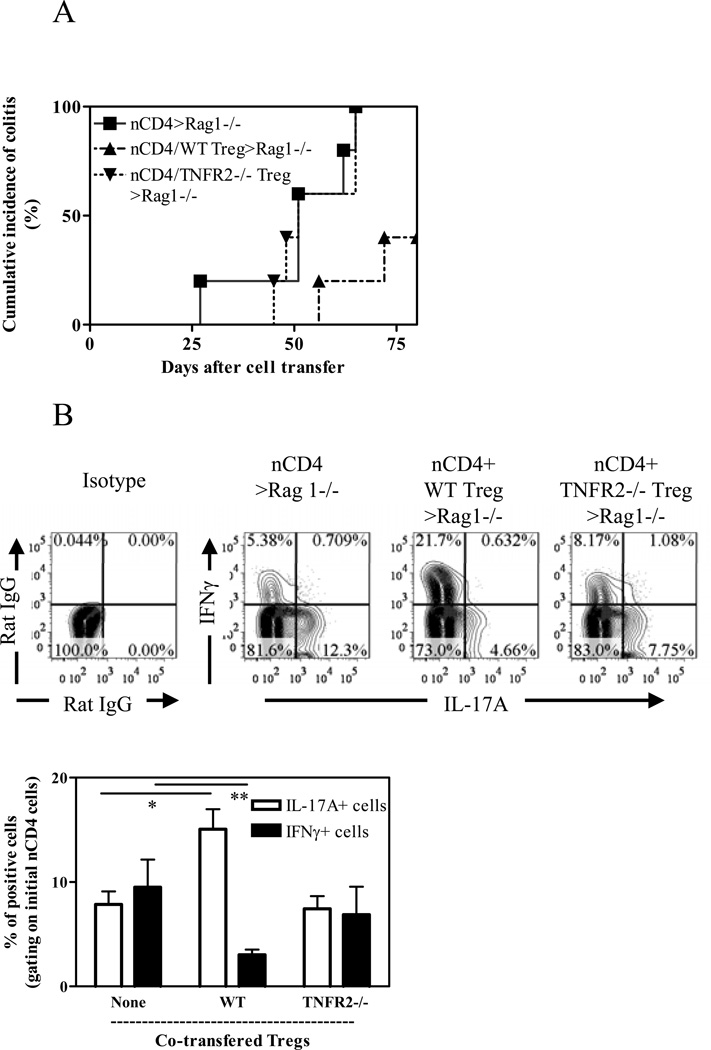
The critical role of functional Tregs in immune homeostasis can be demonstrated in a mouse colitis model induced by transfer of naïve CD4 T cells into Rag1−/− mice (26). In a preliminary experiment, we found that eight weeks after transfer, TNF can be expressed by both host cells and adoptively transferred cells in the cLP (data not shown), while TNFR2 expression was up-regulated by both co-transferred initial naïve CD4 cells and Tregs (data not shown). Thus, this model is appropriate to determine the role of interaction of TNF-TNFR2 on the function of Tregs. We confirmed that TNFR2 expression on Tregs was required for the suppression of colitis induced by transfer of naïve CD4 cells (22) (Fig 6A, p<0.05). Furthermore, we compared the effect of WT orTNFR2-deficient Tregs on the development of Th1 or Th17 responses by co-transferred naïve CD4 cells. As shown in Fig 6B, naïve CD4 cells transferred alone (without Tregs) into Rag1−/− mice could develop into both Th1 and Th17 cells in the colon, as indicated by their expression of IFNγ and IL-17A. Consistent with a previous report (22), WT Tregs markedly inhibited a proportion of IFNγ-producing cells (p<0.01), while TNFR2−/− Tregs failed to do so. It has been shown that, in mouse colitis model induced by transfer of naïve CD4 cells, Th17 cells had paradoxically tissue protective and immunosuppressive effect and consequently suppressed colon inflammation, by inhibiting pathogenic Th1 responses (33) or by enhancing barrier function of intestinal epithelial cells (34). In agreement with a number of recent studies (35–36), we also found that transfer of WT Tregs resulted in greater than a 2-fold increase in the proportion of IL-17A-producing cells developed from the initial naïve CD4 cells, as compared with naïve CD4 cells transfer alone (p<0.01). In contrast, co-transfer of TNFR2-deficient Tregs failed to promote the generation of Th17 cells (p>0.05, Fig 6B). Therefore, TNFR2 is required for Tregs to suppress pathogenic Th1 response in this model.
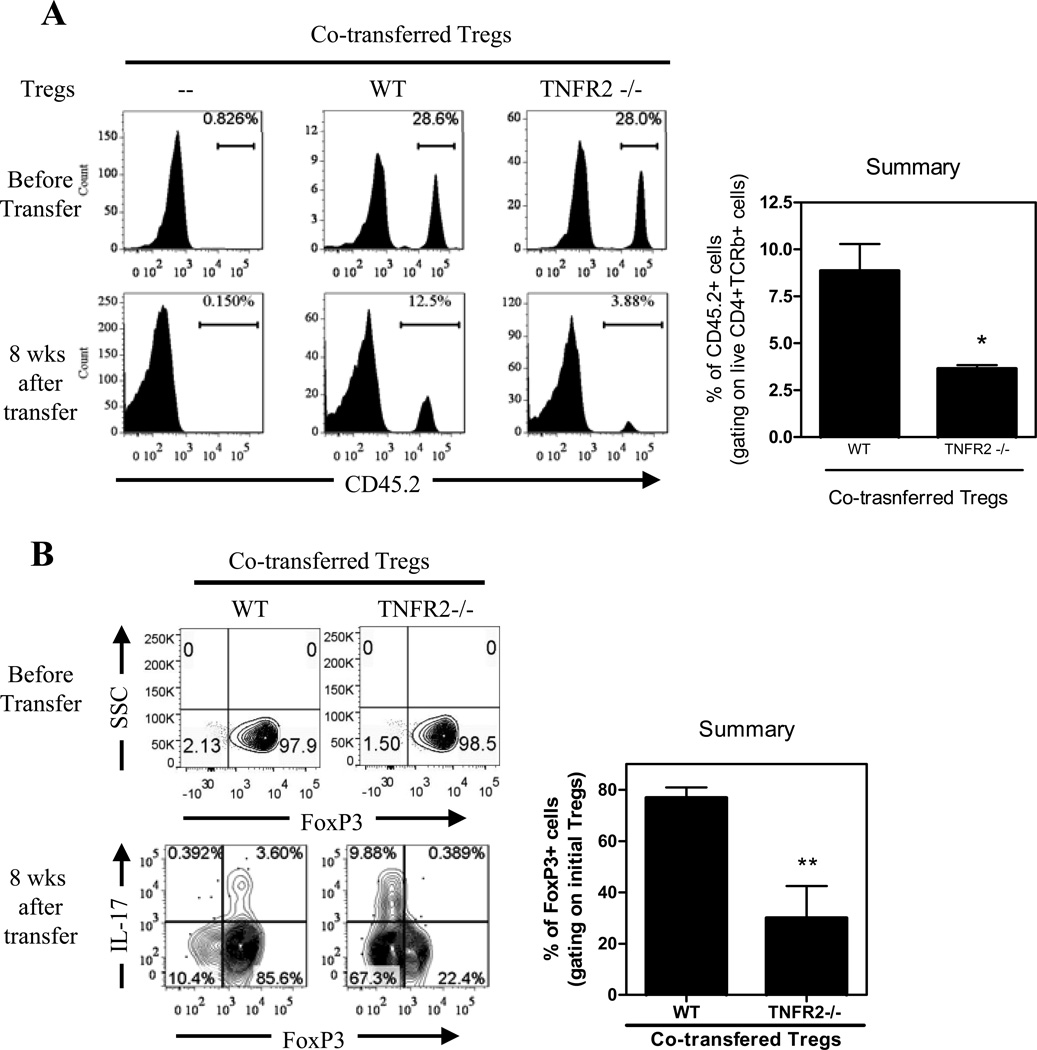
Figure 6.
TNFR2 is required for the immunosuppressive function of Tregs in vivo. CD45.1+ CD4+CD25 –CD45RBhi naïve T (nCD4) cells were transferred alone or co-transferred with CD45.2+ WT or TNFR2−/− Treg cells into Rag1−/− mice. A. Cumulative incidence of colitis. Incidence of colitis in mice co-transferred with naïve CD4 cells and WT Tregs was markedly decreased, by comparison with mice transferred with naïve CD4 cells alone or co-transferred with naïve CD4 cells and TNFR2−/− Tregs (p=0.0132 and 0.0163, respectively). (B) After eight weeks, cLP cells were isolated. The intraceullar expression of IFNγ and IL-17A by initial transferred naïve WT CD4 cells was analyzed by FACS, gating on CD45.1+ cells. Typical FACS plot and summary (N=3~5) are shown. Data shown are representative of three separate experiments with similar results. * p<0.05; ** p<0.01.
TNFR2 is required for maintenance of FoxP3 expression on Tregs in vivo
The accumulation of Tregs and their FoxP3 expression were examined when full-fledged colitis was developed which occurred typically 6 to 8 weeks after cell transfer. As shown in Fig 7A, the proportion of WT Tregs present in the total numbers of transferred cells was reduced from 28.6% to 12.5%, which may be based on the homeostatic restoration of Tregs to the normal 10~15% range in total CD4 pool. However, the proportion ofFoxP3+TNFR2−/− Tregs was reduced to 3.88%, which is markedly lower than 12.5% WT Tregs (p<0.05).
Figure 7.
Critical role of TNFR2 in stabilizing FoxP3 expression in vivo. CD45.1+ CD4+CD25 –CD45RBhi naïve T cells were transferred alone or co-transferred with CD45.2+ WT or TNFR2−/− Treg cells into Rag1−/− mice. After eight weeks, cLP cells were analyzed by FACS. (A) Reduced relative number of co-transferred TNFR2−/− Tregs. Proportion of CD45.2+ initial Treg cells in total transferred CD4 cells was determined. For comparison, pre-transferred cells were shown in the upper panel. (B) Reduced FoxP3 expression by initial Tregs from TNFR2−/− mice in cLP. Expression of IL-17A and FoxP3 was analyzed by FACS, by gating on CD45.2+ initial Tregs. Numbers in the FACS plots represent the percentage of cells in the indicated gate or quadrant. Typical FACS plot and summary (N=3~5) are shown. Data shown are representative of three separate experiments with similar results. * p<0.05; ** p<0.01.
Flow-sorted Tregs from WT and TNFR2−/− mice expressed the same high levels of FoxP3 (Fig 7B). By 8 weeks after transfer, the majority of WT Tregs (>80%) maintained their FoxP3 expression in cLP. However, FoxP3 expression by TNFR2-deficient Tregs was markedly reduced (~40%), as compared with WT Tregs (p<0.01, Fig 7B). The reduction of FoxP3 by TNFR2−/− Tregs was more profound in the cLP, while there was not a significant change in the spleen and mesenteric lymph nodes (data not shown), suggesting a role of proinflammatory response in the colon. Therefore, TNFR2 expression is critical for the phenotypic stability of Tregs in the inflammatory environment and for the capacity of Tregs to compete efficiently with colitogenic T cells. The latter effect may be attributable to the lack of suppressive function of TNFR2-deficient Tregs in vivo found in our study and others (22, 37).
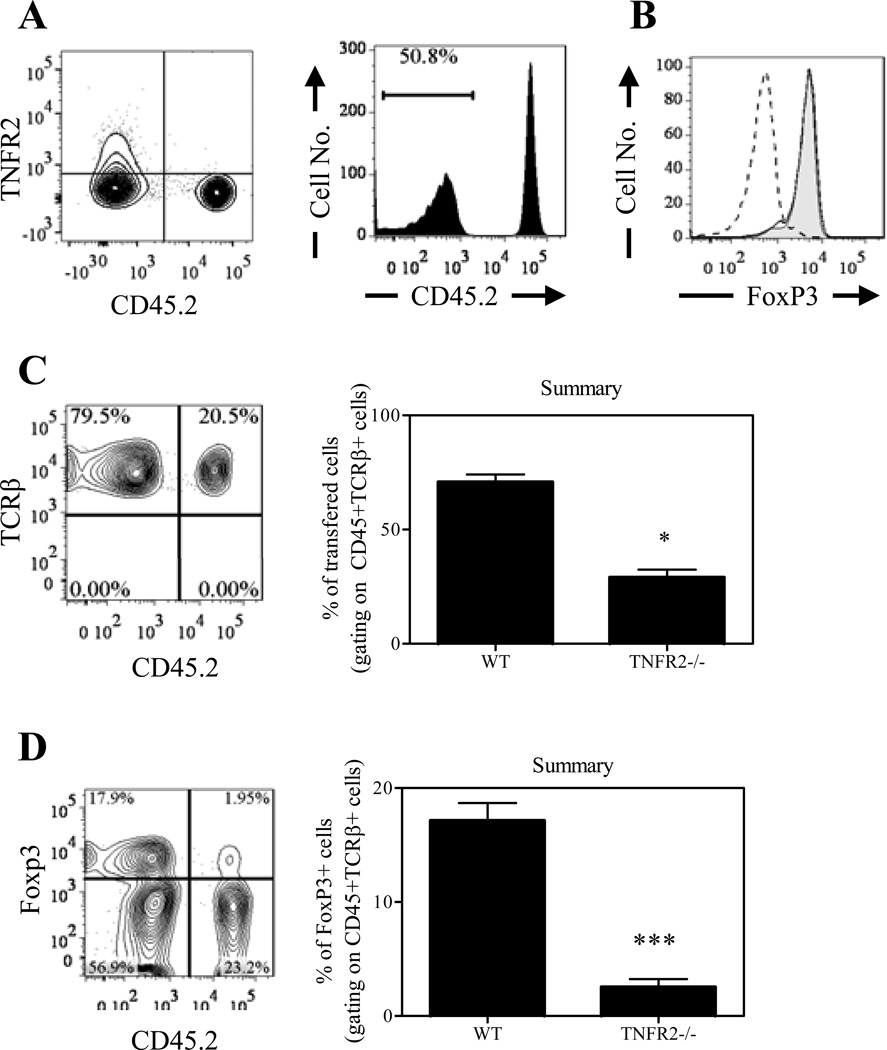
TNFR2 expression is critical for the expansion of Tregs in competitive environment
The magnitude of inflammation was greater in Rag1−/− mice co-transferred with WT naïve CD4 cells containing TNFR2−/− Tregs, which might be attributable to the profound loss of FoxP3 expression in TNFR2−/− Tregs. To clarify this issue, we injected a mixture of WT and TNFR2−/− Tregs into Rag1−/− mice, in order to compare phenotypic stability of WT and TNFR2−/− Tregs in vivo in the identical environment. Tregs were flow-sorted from CD45.1+ WT and CD45.2+ TNFR2−/− mice and transferred at a 1:1 ratio into Rag1−/− mice (Fig 8A). Purified Tregs from these two strains of mice expressed the same high levels of FoxP3 (Fig 8B). Ten weeks after transfer, the proportion of WT Tregs to TNFR2−/− Tregswas changed from 1:1 to roughly 4:1 (Fig 8C, p<0.05), indicating a greater capacity of WT Tregs than TNFR2−/− Tregs to re-constitute the lymphopenic environment (Fig 8C). The majority of WT Tregs lost their FoxP3 expression and <40% expressed FoxP3 (Fig 8D), which is much lower than Tregs co-transferred with naïve CD4 cells (Fig 7B). These data are consistent with previous studies showing that a majority of transferred FoxP3/gfp+ Tregs in cLP of recipient lymphopenic mice lose their FoxP3 expression, while co-transfer of Teffs resulted in maintaining FoxP3 expression by Tregs (38 –39), presumably by producing cytokines such as IL-2 and/or TNF. The reduction in FoxP3 expression by TNFR2-deficient Tregs was considerably greater than by WT Tregs, and more than 95% of initial Tregs derived from TNFR2−/− mice lost their FoxP3 expression (Fig 8D). Consequently, less than 10% of FoxP3-expressing cells in cLP were derived from TNFR2−/− Tregs which was markedly lower than those from WT mice (p<0.0005, Fig 8D). These data clearly show that TNFR2 per se is critical to sustain FoxP3 expression by Tregs in this model. In our experimental system, after transfer into Rag1−/− mice (CD45.2+), TNFR2−/− Tregs isolated from CD45.2+ mice were outnumbered by WT Tregs derived from either CD45.2+ mice (Fig 7) or CD45.1+ mice (Fig 8), mitigating the potential impact of different congenic marker expressed by WT Tregs and TNFR2−/− Tregs.
Figure 8.
TNFR2 expression is critical for the expansion of Tregs in competitive environment. CD45.1+ Tregs and CD45.2+ Tregs were flow-sorted from WT mice and TNFR2−/− mice respectively and co-transferred into Rag1−/− mice at ratio of 1:1. (A) Profile of pre-transferred Tregs. Left shows expression of TNFR2 and CD45.2 and right shows CD45.2 expression alone. (B) Flow-sorted Tregs from WT mice (solid histogram) and TNFR2−/− mice (grey histogram) express comparable levels of FoxP3. Dashed line: isotype control. (C) Ten weeks after transfer, Tregs present in the cLP were analyzed by FACS, gating on CD45+TCRβ+ cells. Left: expression of TCRβ and CD45.2 on cLP Tregs; right: summary of proportion of WT and TNFR2−/− Tregs in total transferred cells present in cLP (N=5). (D) FoxP3 expression on initial Tregs from WT or TNFR2−/− mice co-transferred into Rag1−/− mice (left) and summary of proportion of FoxP3-expressing cells in total transferred cells (right, N=5). The number shown in the FACS plots represent the proportion of cells in the respective quadrant or gate. Data shown are representatives of three separate experiments with similar results. * p<0.05; *** p<0.001.
Discussion
Our data presented in this study clearly show that TNFR2 plays a critical role in sustaining FoxP3 expression, and consequently maintaining the phenotypic and functional stability of Tregs. Therefore, at least in the inflammatory environment, the TNF/TNFR2 pathway is critical for the stabilization of Treg pool that is required to restrain the magnitude and length of an inflammatory immune response and to avoid harmful damage to self tissues.
A previous study reported that, in the same mouse colitis model, the accumulation and FoxP3 expression by TNFR2−/− Tregs in cLP were not changed when cells were harvested two weeks after transfer (22). Since the development of colitis in this model usually starts 5 weeks after transfer of naïve cells, we examined accumulation of Tregs and their FoxP3 expression when full-fledged colitis was developed which occurred typically 8 weeks after cell transfer. In our transfer experiments, both WT and TNFR2−/− Tregs were flow-sorted CD4+CD25+CD45RBlo cells and contained similar high levels of FoxP3+ cells (>95%). The expansion of contaminating FoxP3− Teff cells present in the initial Treg population may partially account for the reduction in FoxP3-expressing cells after transfer into Rag1−/− mice. However, the impact of this contamination population is very limited since it has been shown that the transferred FoxP3+ and FoxP3− T cells were replicating similarly in the lymphopenic mouse. There was no marked outgrowth of an intentionally added 3% FoxP3− Teffs in Tregs after transfer (38–39). Further, TNFR2 is well known for its co-stimulatory effect on Teffs (40), thus it is likely that TNFR2−/− Teffs present in TNFR2−/− Tregs are less proliferative than WT Teffs.
IL-2 produced by Teffs has been proposed to be a paracrine factor to maintain FoxP3 expression and phenotypic as well as functional stability of Tregs (41). There are number of observations challenging the dispensable role of IL-2 on Tregs. 1) Only ~50% reduction of FoxP3+ Tregs in CD4 cells was found in the thymus and peripheral lymphoid tissues of Il2−/− mice or Il2ra−/− mice, and residual Tregs had normal suppressive function (42). 2) A recent study found that the stability of transferred Tregs in the lymphopenic mouse was maintained by Teff cells independent of IL-2, since the administration of IL-2 did not promote the maintenance of FoxP3 expression by Tregs (39). 3) The stimulatory effect of Teffs on Tregs in vivo was not abrogated by neutralization of IL-2, and IL-2 deficient Teffs still had the capacity to stimulate the activation of Tregs (23). TGFβ is able to induce FoxP3 expression (43) and thus may play a role in the persistent expression of FoxP3 on Tregs. Nevertheless, conditional deletion of TGFβ receptor I (T RI) in T cells also only delayed the appearance of FoxP3+ Tregs in neonatal mouse thymus, however, beginning 1 week after birth, the same TβRI-mutant mice showed accelerated expansion of thymic Tregs (44). The proportion of Tregs in mice devoid of TNFR2 or its ligand reduced by ~50% in both thymus and periphery. These data suggest that multiple factors, including TNF-TNFR2 signaling, contribute to the thymic generation and peripheral homeostasis of Tregs. Recently, Cuss and Green reported that thymic Tregs actually were heterogeneic and contained resident Tregs and newly developed Tregs. They proposed that resident Tregs were IL-2 dependent for their homeostasis, whereas newly developed Tregs were not (45). Further study is warranted to determine which subset of thymic Tregs is defective inmouse strains deficient in TNFR2 or its ligands, as well as in Il2−/− or Il2ra−/− and T RI−/− mice.
In conclusion, TNFR2 is a key factor in maintaining sustained FoxP3 expression and function of Tregs contributing to immune regulation in the inflammatory environment, which may explain why anti-TNF therapy fails or even at times exacerbates some autoimmune disorders (46). This should be taken into account when designing future therapy of autoimmunity by using TNF inhibitors.
Acknowledgement
The authors thank Drs. Giorgio Trinchieri and Scott K. Durum for discussion and critical review of the manuscript, Dr. Sergei A. Nedospasov for generating TNF/LTα/LTβ KO mice. Drs. Rosalba Salcedo, Ram Savan and Miranda Hanson for their help in the mouse colitis study. We thank NCI-Frederick Cancer Inflammation Program Fluorescence Cytometry core for expert technical assistance with flow cytometry.
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E. This Research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. X.Q.W. and Q.Z. was supported by China Scholarship Council.
Abbreviations used in this paper
- cLP
colon lamina propria
- Teffs
effector T cells
- FoxP3
forkhead box P3
- KI
knock in
- LTα
lymphotoxin-alpha
- LTβ
lymphotoxin-beta
- mLN
mesenteric lymph nodes
- nCD4
naïve CD4 cells
- SP
single positive
- TNFR2
tumor necrosis factor receptor type II
- Tregs
regulatory T cells
- triple KO
TNF/LTα/LTβ−/−
Footnotes
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government.
References
- 1.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 2.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Rudensky AY. Regulatory T cells and Foxp3. Immunol Rev. 2011;241:260–268. doi: 10.1111/j.1600-065X.2011.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 5.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 6.Allan SE, Alstad AN, Merindol N, Crellin NK, Amendola M, Bacchetta R, Naldini L, Roncarolo MG, Soudeyns H, Levings MK. Generation of potent and stable human CD4+ T regulatory cells by activation-independent expression of FOXP3. Mol Ther. 2008;16:194–202. doi: 10.1038/sj.mt.6300341. [DOI] [PubMed] [Google Scholar]
- 7.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 8.Hori S. Developmental plasticity of Foxp3+ regulatory T cells. Curr Opin Immunol. 2010;22:575–582. doi: 10.1016/j.coi.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Beriou G, Costantino CM, Ashley CW, Yang L, Kuchroo VK, Baecher-Allan C, Hafler DA. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113:4240–4249. doi: 10.1182/blood-2008-10-183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng XH, Jetten AM, Dong C. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Oppenheim JJ. TNF-alpha: an activator of CD4+FoxP3+TNFR2+ regulatory T cells. Curr Dir Autoimmun. 2010;11:119–134. doi: 10.1159/000289201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandenabeele P, Declercq W, Beyaert R, Fiers W. Two tumour necrosis factor receptors: structure and function. Trends Cell Biol. 1995;5:392–399. doi: 10.1016/s0962-8924(00)89088-1. [DOI] [PubMed] [Google Scholar]
- 13.Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- 14.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 15.Grell M, Becke FM, Wajant H, Mannel DN, Scheurich P. TNF receptor type 2 mediates thymocyte proliferation independently of TNF receptor type 1. Eur J Immunol. 1998;28:257–263. doi: 10.1002/(SICI)1521-4141(199801)28:01<257::AID-IMMU257>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 16.Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4:1116–1122. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Baumel M, Mannel DN, Howard OM, Oppenheim JJ. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J Immunol. 2007;179:154–161. doi: 10.4049/jimmunol.179.1.154. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Subleski JJ, Kopf H, Howard OM, Mannel DN, Oppenheim JJ. Cutting edge: expression of TNFR2 defines a maximally suppressive subset of mouse CD4+CD25+FoxP3+ T regulatory cells: applicability to tumor-infiltrating T regulatory cells. J Immunol. 2008;180:6467–6471. doi: 10.4049/jimmunol.180.10.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X, Hamano R, Subleski JJ, Hurwitz AA, Howard OM, Oppenheim JJ. Expression of costimulatory TNFR2 induces resistance of CD4+FoxP3− conventional T cells to suppression by CD4+FoxP3+ regulatory T cells. J Immunol. 2010;185:174–182. doi: 10.4049/jimmunol.0903548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X, Subleski JJ, Hamano R, Howard OM, Wiltrout RH, Oppenheim JJ. Co-expression of TNFR2 and CD25 identifies more of the functional CD4+FOXP3+ regulatory T cells in human peripheral blood. Eur J Immunol. 2010;40:1099–1106. doi: 10.1002/eji.200940022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamano R, Huang J, Yoshimura T, Oppenheim JJ, Chen X. TNF optimally activatives regulatory T cells by inducing TNF receptor superfamily members TNFR2,4-1BB and OX40. Eur J Immunol. 2011;41:2010–2020. doi: 10.1002/eji.201041205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Housley WJ, Adams CO, Nichols FC, Puddington L, Lingenheld EG, Zhu L, Rajan TV, Clark RB. Natural but not inducible regulatory T cells require TNF-alpha signaling for in vivo function. J Immunol. 2011;186:6779–6787. doi: 10.4049/jimmunol.1003868. [DOI] [PubMed] [Google Scholar]
- 23.Grinberg-Bleyer Y, Saadoun D, Baeyens A, Billiard F, Goldstein JD, Gregoire S, Martin GH, Elhage R, Derian N, Carpentier W, Marodon G, Klatzmann D, Piaggio E, Salomon BL. Pathogenic T cells have a paradoxical protective effect in murine autoimmune diabetes by boosting Tregs. J Clin Invest. 2010;120:4558–4568. doi: 10.1172/JCI42945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mougiakakos D, Johansson CC, Jitschin R, Bottcher M, Kiessling R. Increased thioredoxin-1 production in human naturally occurring regulatory T cells confers enhanced tolerance to oxidative stress. Blood. 2011;117:857–861. doi: 10.1182/blood-2010-09-307041. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh CS, Lee HM, Lio CW. Selection of regulatory T cells in the thymus. Nat Rev Immunol. 2012;12:157–167. doi: 10.1038/nri3155. [DOI] [PubMed] [Google Scholar]
- 26.Griseri T, Asquith M, Thompson C, Powrie F. OX40 is required for regulatory T cell-mediated control of colitis. J Exp Med. 2010;207:699–709. doi: 10.1084/jem.20091618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Annunziato F, Cosmi L, Liotta F, Lazzeri E, Manetti R, Vanini V, Romagnani P, Maggi E, Romagnani S. Phenotype, localization, and mechanism of suppression of CD4(+)CD25(+) human thymocytes. J Exp Med. 2002;196:379–387. doi: 10.1084/jem.20020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chatzidakis I, Mamalaki C. T cells as sources and targets of TNF: implications for immunity and autoimmunity. Curr Dir Autoimmun. 2010;11:105–118. doi: 10.1159/000289200. [DOI] [PubMed] [Google Scholar]
- 29.Baseta JG, Stutman O. TNF regulates thymocyte production by apoptosis and proliferation of the triple negative (CD3−CD4−CD8−) subset. J Immunol. 2000;165:5621–5630. doi: 10.4049/jimmunol.165.10.5621. [DOI] [PubMed] [Google Scholar]
- 30.Eugster HP, Frei K, Bachmann R, Bluethmann H, Lassmann H, Fontana A. Severity of symptoms and demyelination in MOG-induced EAE depends on TNFR1. Eur J Immunol. 1999 doi: 10.1002/(SICI)1521-4141(199902)29:02<626::AID-IMMU626>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 31.Kuprash DV, Alimzhanov MB, Tumanov AV, Grivennikov SI, Shakhov AN, Drutskaya LN, Marino MW, Turetskaya RL, Anderson AO, Rajewsky K, Pfeffer K, Nedospasov SA. Redundancy in tumor necrosis factor (TNF) and lymphotoxin (LT) signaling in vivo: mice with inactivation of the entire TNF/LT locus versus single-knockout mice. Mol Cell Biol. 2002;22:8626–8634. doi: 10.1128/MCB.22.24.8626-8634.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25−Foxp3− T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 33.O'Connor W, Jr, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, Wang YH, Schluns KS, Broaddus RR, Zhu Z, Dong C. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Haines CJ, Gutcher I, Hochweller K, Blumenschein WM, McClanahan T, Hammerling G, Li MO, Cua DJ, McGeachy MJ. Foxp3(+) regulatory T cells promote T helper 17 cell development in vivo through regulation of interleukin-2. Immunity. 2011;34:409–421. doi: 10.1016/j.immuni.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Sujino T, Kanai T, Ono Y, Mikami Y, Hayashi A, Doi T, Matsuoka K, Hisamatsu T, Takaishi H, Ogata H, Yoshimura A, Littman DR, Hibi T. Regulatory T cells suppress development of colitis, blocking differentiation of T-helper 17 into alternative T-helper 1 cells. Gastroenterology. 2011;141:1014–1023. doi: 10.1053/j.gastro.2011.05.052. [DOI] [PubMed] [Google Scholar]
- 37.van Mierlo GJ, Scherer HU, Hameetman M, Morgan ME, Flierman R, Huizinga TW, Toes RE. Cutting edge: TNFR-shedding by CD4+CD25+ regulatory T cells inhibits the induction of inflammatory mediators. J Immunol. 2008;180:2747–2751. doi: 10.4049/jimmunol.180.5.2747. [DOI] [PubMed] [Google Scholar]
- 38.Duarte JH, Zelenay S, Bergman ML, Martins AC, Demengeot J. Natural Treg cells spontaneously differentiate into pathogenic helper cells in lymphopenic conditions. Eur J Immunol. 2009;39:948–955. doi: 10.1002/eji.200839196. [DOI] [PubMed] [Google Scholar]
- 39.Yurchenko E, Shio MT, Huang TC, Da Silva Martins M, Szyf M, Levings MK, Olivier M, Piccirillo CA. Inflammation-Driven Reprogramming of CD4(+)Foxp3(+) Regulatory T Cells into Pathogenic Th1/Th17 T Effectors Is Abrogated by mTOR Inhibition in vivo. PLoS One. 2012;7:e35572. doi: 10.1371/journal.pone.0035572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim EY, Priatel JJ, Teh SJ, Teh HS. TNF receptor type 2 (p75) functions as a costimulator for antigen-driven T cell responses in vivo. J Immunol. 2006;176:1026–1035. doi: 10.4049/jimmunol.176.2.1026. [DOI] [PubMed] [Google Scholar]
- 41.Malek TR, Yu A, Zhu L, Matsutani T, Adeegbe D, Bayer AL. IL-2 family of cytokines in T regulatory cell development and homeostasis. J Clin Immunol. 2008;28:635–639. doi: 10.1007/s10875-008-9235-y. [DOI] [PubMed] [Google Scholar]
- 42.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 43.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 45.Cuss SM, Green EA. Abrogation of CD40–CD154 signaling impedes the homeostasis of thymic resident regulatory T cells by altering the levels of IL-2, but does not affect regulatory T cell development. J Immunol. 2012;189:1717–1725. doi: 10.4049/jimmunol.1200588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caminero A, Comabella M, Montalban X. Tumor necrosis factor alpha (TNF-alpha), anti-TNF-alpha and demyelination revisited: an ongoing story. J Neuroimmunol. 2011;234:1–6. doi: 10.1016/j.jneuroim.2011.03.004. [DOI] [PubMed] [Google Scholar]