Abstract
Sites within the hippocampus, amygdala and prefrontal cortex may regulate how responses maintained by cues associated with cocaine are extinguished. To test the role of various brain sites in the consolidation of cocaine-cue extinction learning, the dorsal subiculum (dSUB), rostral basolateral amygdala (rBLA) and infralimbic prefrontal cortex (IL) were manipulated in rats. Following cocaine self-administration training (cues present, cocaine available), responding was assessed during 1 hr extinction tests (cues present, no cocaine available). To study extinction consolidation specifically, the protein synthesis inhibitor anisomycin or vehicle was infused bilaterally into the dSUB, rBLA or IL either immediately following or 6 hr after the first two of three extinction training sessions. With manipulations made immediately after extinction sessions, infusions of anisomycin into the dSUB or the rBLA deterred extinction. Rats maintained elevated levels of cocaine seeking relative to vehicle despite the absence of cocaine delivery. Manipulations of IL had no effect. Control studies showed that bilateral protein synthesis inhibition in dSUB and rBLA 6 hr after the extinction sessions ended was unable to deter extinction. Rats reduced cocaine seeking in the usual manner in the absence of cocaine delivery. Collectively, these findings suggest that the dSUB and rBLA are neural substrates important for consolidation of cocaine-cue extinction learning and have time-dependent roles. Understanding the contribution of individual neural substrates for cocaine-cue extinction consolidation may help guide treatment strategies aimed at enhancing cue exposure therapy in cocaine-dependent people.
Keywords: Anisomycin, Basolateral amygdala, Dorsal subiculum, Infralimbic prefrontal cortex
Introduction
Exposure to environmental cues associated with cocaine use can trigger craving that increases risk of relapse in drug addicts (Ehrman et al., 1992). Cue exposure therapy is an intuitive strategy to treat drug abuse and is a form of extinction training whereby addicts are exposed to cues previously paired with drug to reduce their salience and diminish risk of relapse (O’Brien et al., 1990). However, cue exposure therapy is largely ineffective as a standalone treatment modality for drug relapse prevention (Conklin & Tiffany, 2002). Drug-cue extinction serves as a laboratory approximation to cue exposure therapy by incorporating cues into instrumental response extinction procedures (Szalay et al., 2011). Drug-cue extinction is operationally defined as attenuated instrumental responding over time that is maintained by drug-paired cues in the absence of drug. Identification of neural substrates that underlie drug-cue extinction may lead to new strategies aimed at enhancing cue exposure therapy in drug-dependent people. Recently, it was reported that inactivation of the dorsal subiculum (dSUB) and rostral basolateral amygdala (rBLA), bilaterally and asymmetrically, deterred cocaine-cue extinction learning in rats (Szalay et al., 2011). These findings suggest that neural activity within the hippocampus and amygdala is necessary for acquisition of extinction learning. However, new learning to reduce saliency of cocaine-paired cues must be stabilized to ensure extinction memories are retained.
Stabilization of memory is thought to engage a post-learning neural process called consolidation that involves patterns of activity such as long-term potentiation and de novo protein synthesis (Abraham & Williams, 2008). In fear conditioning studies, infusion of a protein synthesis inhibitor into the BLA immediately following acquisition sessions impairs storage of long-term memory while leaving short-term memory intact (Duvarci et al., 2008, Schafe & LeDoux, 2000). It also was shown that infusion of a protein synthesis inhibitor into the BLA 6-hr following acquisition had no effect on retention (Schafe & LeDoux, 2000). Retention of hippocampal dependent contextual memories can also be influenced by post-acquisition activity in the dorsal hippocampus (DH). Inhibition of protein synthesis by infusion of anisomycin into the DH following extinction of inhibitory avoidance blocked retention the following day (Vianna et al., 2001). Therefore, consolidation processes involving the amygdala and hippocampus are time-dependent and occur within a critical window of time following acquisition, outside of which neuronal manipulation is unable to alter long-term memory.
Research suggests that sites within the hippocampus and amygdala are components of a circuit that interact with the prefrontal cortex (PFC) to regulate memory consolidation in a time-dependent manner (Floresco et al., 1997; Jones & Wilson, 2005; Vazdarjanova & McGaugh, 1999; Laurent & Westbrook, 2008). The infralimbic (IL) region of the PFC is specifically involved in consolidation of fear extinction (Quirk & Mueller, 2008). Notably, when anisomycin is infused into the IL prior to extinction testing, the retention of extinguished fear is hindered (Santini et al., 2004; Akirav et al., 2006). These results establish that de novo protein synthesis within the IL is necessary for fear extinction consolidation. With these considerations in mind, protein synthesis inhibition within the dSUB, rBLA and IL was investigated for its effects on cocaine-cue extinction consolidation, and whether these effects, if any, were time-dependent.
Materials and methods
Subjects
Male Wistar rats [Crl(WI)BR rats; Charles River Laboratories, Portage, MI, USA], weighing approximately 276–300 g upon arrival, were maintained at 90% of a free-feeding body weight while adjusting for growth throughout the duration of the study by providing 16–20 g of food per day. Between experimental sessions, rats were allowed unlimited access to water in their home cages. Rats were individually housed in clear plastic cages (43 × 22 × 20 cm) in a temperature-controlled (21–23 °C) and light-controlled (08:00 h on, 20:00 h off) vivarium. Policies and procedures set forth in the NIH Guide for Care and Use of Laboratory Animals (National Academy of Sciences) were followed, as well as specific national laws. The Boston University Institutional Animal Care and Use Committee approved all research protocols.
Apparatus
Experimental chambers (model ENV-008CT; Med Associates, St Albans, VT, USA) were each equipped with two response levers positioned 8 cm to the left and right of a center-mounted food receptacle, and 7 cm from the grid floor. Connected to the food receptacle was a pellet dispenser capable of delivering 45 mg food pellets (Dustless Precision Pellets; Bio-Serv, Frenchtown, NJ, USA). A white stimulus light was mounted 7 cm above each lever. Each chamber was outfitted with a single-channel fluid swivel (Instech Solomon, Plymouth Meeting, PA, USA) and a spring leash assembly, which were connected to a counterbalanced arm assembly (Med Associates) that allowed the animal to move freely in the chamber. A sound-attenuating cubicle with ventilation fan (model ENV-108 m; Med Associates) enclosed each chamber that also was equipped with a house light to provide general illumination and an 8-ohm speaker to provide auditory stimuli. Motor-driven syringe pumps (model PHM-100; Med Associates) located inside each cubicle were used for intravenous drug delivery. A standard personal computer programmed in Medstate Notation and connected to an interface (Med Associates) controlled experimental events. An Olympus BX51 microscope (Olympus Optical, Tokyo, Japan), a Nikon DXM 1200 digital camera (Nikon, Tokyo, Japan) and Image Pro Plus software (version 4.5.1; Media Cybernetics, Silver Spring, MD, USA) were used to evaluate the histology.
Drugs and intracranial infusion procedures
The drugs used were cocaine hydrochloride (gift from the National Institute on Drug Abuse, Bethesda, MD, USA), and anisomycin (Sigma-Aldrich, St Louis, MO, USA). Cocaine was dissolved in sterile 0.9% saline containing 3 IU heparin/mL to a final concentration of 2.68 mg/mL. For all self-administration sessions, a 1.0 mg/kg unit infusion dose of cocaine was used and delivered intravenously at a rate of 1.8 mL/min. To attain a dose of 1.0 mg/kg, the infusion volume was adjusted for body weight, resulting in drug delivery times of 1.2 s/100 g body weight in individual rats.
Anisomycin was dissolved in 12.1 N HCl and brought to a pH of 7.0 with 1.0 N NaOH. Distilled water was added to create a concentration of 200 mg/mL. The pH of 0.9% saline was brought from 5.0 to 7.0 with 1.0 N NaOH so that the pH of all infusions was 7.0. For infusions, 0.5 μL containing either 100 μg anisomycin or vehicle was delivered at a rate of 0.5 μL/min into selected brain sites immediately following the first two extinction test sessions. The 28-gauge stainless steel infusion needle was left in place for 1 min following each infusion.
Surgery and histology
Rats were anesthetized with an intraperitoneal injection of 80 mg/kg ketamine plus 8 mg/kg xylazine. To enable intravenous delivery of cocaine or saline during self-administration sessions, a catheter made of silicon tubing (inner diameter, 0.51 mm; outer diameter, 0.94 mm) was implanted into the right femoral vein. The catheter ran subcutaneously along the back, exited through an incision at the top of the head and was attached to an L-shaped pedestal mount (Plastics One, Roanoke, VA, USA). Subsequent to catheter implantation, 0.1 mL of a solution containing 1.0 mg methohexital sodium (Brevital; King Pharmaceuticals, Bristol, TN, USA) was infused intravenously, as needed, to maintain anesthesia for the remainder of the surgery.
After closing the leg incision with absorbable suture material, the rat was placed into a stereotaxic frame, and bilateral 22-gauge stainless steel guide cannulae (Plastics One) were implanted using bregma coordinates, specified in mm, along the anterior-posterior (AP), medial-lateral (ML) and dorsal-ventral (DV) planes (Swanson, 1992). Guide cannulae were positioned 1 mm above the dSUB (AP −5.7, ML ± 2.5, DV −2.3; n=32) and rBLA (AP −2.0, ML ± 4.5, DV −7.6; n=28) and were positioned 2 mm above the IL (AP +3.0, ML ±1.6, DV −2.9; n=16) to maintain tissue integrity of the prelimbic prefrontal cortex (PL), which is directly dorsal to the IL. Guide cannulae for IL also were placed at a 15° angle on the coronal plane to avoid breach of the medial wall of the cortex (Di Pietro et al., 2006). The guide cannulae, pedestal and three stainless steel anchoring screws were attached to the skull, and permanently imbedded in dental cement. Two 28-gauge stainless steel obturators (Plastics One) were used to occlude guide cannulae between infusions. The head incision was then closed with absorbable suture material. Rats received subcutaneous Buprenex (0.05 mg/kg; Butler Schein, Columbus, OH, USA) as a preemptive analgesic on day of surgery and twice daily for the next 48 hr. Rats also received subcutaneous Meloxicam (0.5 mg/kg, Butler Schein, Columbus, OH, USA) immediately after surgery and one daily for the next 48 hr. The sutured incisions were treated daily with the topical antibiotic powder Polysporin (Johnson and Johnson Consumer Products Company, Skillman NJ, USA) until healed. Intravenous Baytril (5 mg, Bayer Health Supply, Kansas City, KS) was administered for 3 days post-surgery to reduce risk of systemic infection. Catheters were maintained by daily flushing (Monday–Friday) with 0.1 mL of a 0.9% saline solution containing 3 IU of heparin (Baxter Healthcare, Deerfield, IL, USA) and 6.7 mg of Timentin (Glaxo-SmithKline, Research Triangle Park, NC, USA). On Fridays, a locking solution consisting of glycerol and undiluted (1000 IU/mL) heparin (3:1) was used to fill the catheter dead space and minimize blockages. This solution remained in the catheters until Monday, when it was removed and replaced with the heparin/saline solution prior to the start of behavioral sessions. Additionally, catheters were checked for patency on a weekly basis by infusing a 1.0 mg/0.1 mL solution of Brevital intravenously, which produces a rapid temporary loss of muscle tone when catheters are functional. In two cases, a new catheter was implanted into the left femoral vein to replace a leaking or non-functional catheter. Upon completion of the studies, rats were given an overdose of sodium pentobarbital, and then intracardially perfused with saline and 4% paraformaldehyde solution. Brains were extracted, post-fixed in 4% paraformaldehyde for 1 to 4-hr, and then stored in 30% sucrose at 4 °C for 3 days. Forty-micrometer coronal sections were collected using a cryostat. Sections were then mounted on gelatin-coated slides and stained to verify infusion location.
Self-administration training under a second-order schedule
Prior to surgery, rats were trained to press a lever under a fixed ratio (FR) 1 schedule of food pellet delivery. After rats learned to rapidly press the lever for 50 pellets, catheters and guide cannulae were implanted. After 1 week of recovery from surgery, 1 hr cocaine self-administration sessions were started. Rats were trained to self-administer 1.0 mg/kg cocaine, starting from an FR 1 schedule of cocaine delivery and cue light presentation with a 20-sec timeout period (TO) following each infusion. During the TO period, the cue light remained illuminated and the house light was extinguished. The terminal schedule, after incremental training, was a fixed interval (FI)-based second-order schedule (FI 5-min [FR 5 : S]). For this schedule, the cue light was presented under an FR5 contingency during the entire session. The delivery of cocaine co-occurred with cue light presentation upon completion of the first FR 5 after each 5-min fixed interval (FI) elapsed. A 20-s TO followed each infusion. The cue light was illuminated for 2 sec during the 5-min FI and illuminated for the duration of the infusion and 20-sec TO period upon delivery of cocaine. During the 20-s TO, the house light was extinguished. After the 20-s timeout, the house light was illuminated and the FI component was again in effect. During the 1-hr sessions, rats could earn a maximum of 11 infusions. In addition, a 70-db contextual sound cue, continuous white noise, was present for the duration of each session. Thus, cocaine-cues consisted of a response-contingent discrete light cue (stimulus light over the active lever), discrete sound cue (infusion-pump motor) and a response-independent background contextual sound cue (white noise). Responses on the inactive lever were counted separately, but produced no scheduled consequences. Designation of the active and inactive lever was counterbalanced between right and left levers. Rats reached stable baseline levels of responding under the second-order schedule in 25–35 sessions (infusions and responses did not deviate by more than 20% for at least 5 sessions, and the number of responses on the inactive lever was less than 25% of active lever responses).
Cocaine-cue extinction testing
When cocaine self-administration baseline behavior was stable, rats underwent 2 weeks of abstinence prior to extinction tests. As 2 weeks of cocaine and cocaine-cue deprivation make rats more cue reactive (Grimm et al., 2001), rats received 10 1-hr sessions in the operant chambers during the abstinence period for which the levers were retracted, and cocaine and both types of cocaine-cues were omitted. Inclusion of these sessions dampened the association between cocaine and the experimental chamber while leaving the saliency of the cocaine cues intact (Szalay et al., 2011). Following abstinence, rats received three extinction sessions on three consecutive days. During extinction, the FI 5-min [FR 5 : S] second-order schedule was used whereby all contingencies described above (background white noise, response-contingent cue light presentation and pump noise, TO period, and house light onset and offset) were in effect except for the delivery of cocaine. Saline was not delivered in place of cocaine. Thus, extinction sessions allowed for the evaluation of lever pressing patterns in the absence of cocaine, but in the presence of discrete and contextual cues previously associated with cocaine. Following the first and second extinction session, rats received infusion of vehicle or anisomycin into the dSUB of both hemispheres immediately after sessions (dSUB 0-hr); infusion of vehicle or anisomycin into the rBLA of both hemispheres immediately after sessions (rBLA 0-hr); infusion of vehicle or anisomycin into the IL of both hemispheres immediately after sessions (IL 0-hr); infusion of vehicle or anisomycin into the dSUB of both hemispheres six-hours after sessions (dSUB 6-hr); or infusion of vehicle or anisomycin into the rBLA of both hemispheres six-hours after sessions (rBLA 6-hr).
Data analyses
Three dependent measures were calculated: (i) active lever responses; (ii) inactive lever responses; and (iii) infusions earned during self-administration sessions. Data from the last five cocaine self-administration sessions were averaged to establish a stable baseline. To determine the time course of changes, the total number of responses was recorded for each 1-hr session as well as in 15-min bins for each session on Day 1, Day 2 and Day 3 of testing. Because Day 1 testing occurred prior to anisomycin or vehicle treatment, measures obtained on Days 2 and 3 were analyzed separately from measures obtained on Day 1 to reveal potential post-treatment group differences. Dependent measures were analyzed by t-test, one-factor ANOVA or two-factor mixed model repeated measures ANOVA, as appropriate. The Tukey procedure was used for post-hoc testing following significant ANOVA analyses. Data from nine rats whose cannulae placements were outside the targeted sites were not included in the analyses (see below). In addition, self-administration or extinction responses of two rats were greater than three standard deviations from the mean in their respective treatment and cannulae placement groups; hence these rats were excluded from the experiment. Final group sizes for each condition were: dSUB 0-hr Veh (n=8); dSUB 0-hr Ani (n = 9); rBLA 0-hr Veh (n = 7); rBLA 0-hr Ani (n = 8); IL 0-hr Veh (n = 7); IL 0-hr Ani (n = 9); dSUB 6-hr Veh (n = 7); dSUB 6-hr Ani (n = 7); rBLA 6-hr Veh (n = 6); and rBLA 6-hr Ani (n = 7).
Results
Histology
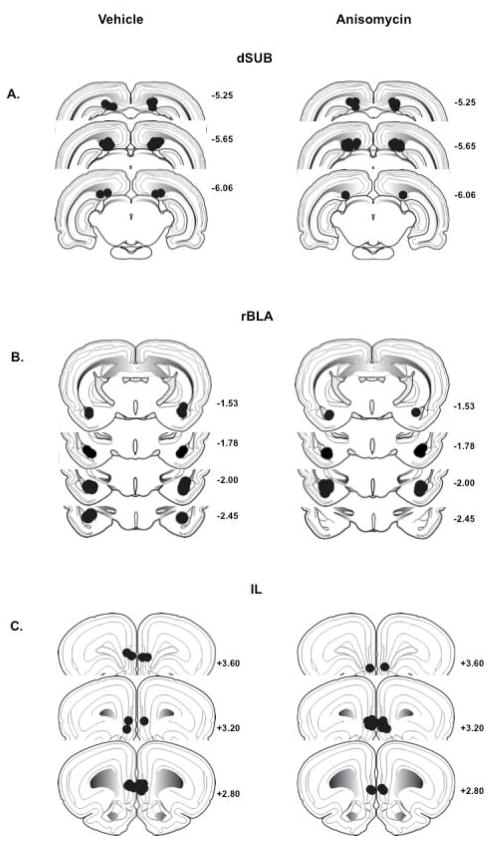
For rats used in the 0-hr manipulation studies, placements were confirmed in 17 of 18 rats with cannulae aimed at the dSUB, in 15 of 17 rats with cannulae aimed at the rBLA, and in 16 of 18 rats with cannulae aimed at the IL. Placements were confirmed in 14 of 16 rats used in the dSUB 6-hr manipulation study, and in 13 of 15 rats used in the rBLA 6-hr manipulation study. Placements were within 0.5– 0.6 mm of the intended position in the AP plane, and within the dSUB, rBLA and IL anatomical ranges (Swanson, 1992). Histological reconstruction of all dSUB, rBLA and IL infusion sites is depicted in Figure 1 A-C. Microscopic examination failed to reveal mechanical damage in the dSUB, rBLA or IL, other than that generated by insertion of the guide and infusion cannulae.
Figure 1.
Schematic drawings representing coronal sections of the (A) dorsal subiculum (dSUB), (B) rostral basolateral amygdala (rBLA) and (C) infralimbic prefrontal cortex (IL) in all experimental groups. Circles indicate point of infusion for vehicle (left) and anisomycin (right). All drawings are based on the atlas of Swanson (1992), with the anterior–posterior references measured from bregma. Each placement is shown at the midpoint of its anterior–posterior extent.
Baseline cocaine self-administration
Prior to cocaine-cue extinction testing, the 10 groups of rats had similar baseline cocaine self-administration behavior (Table 1). Separate analysis of active lever responses (F [9,65] = 1.4, P ≤ 0.22), inactive lever responses (F [9,65] = 1.0, P ≤ 0.59) and infusions earned (F [9,65] = 1.2, P ≤ 0.29) during baseline sessions showed no significant group differences, as determined by one-factor ANOVA.
Table 1.
Cocaine self-administration behavior at baseline (mean ± SEM.)
| Group | Active Responses | Inactive Responses | Infusions |
|---|---|---|---|
| dSUB 0-hr Vehicle | 399.1 ± 53.8 | 17.8 ± 3.8 | 9.4 ± 0.4 |
| dSUB 0-hr Anisomycin | 415.3 ± 54.2 | 36.8 ± 15.0 | 9.6 ± 0.2 |
| rBLA 0-hr Vehicle | 476.1 ± 62.8 | 108.6 ± 59.6 | 9.9 ± 0.1 |
| rBLA 0-hr Anisomycin | 498.0 ± 79.9 | 35.0 ± 11.4 | 9.9 ± 0.1 |
| IL 0-hr Vehicle | 405.3 ± 123.9 | 32.4 ± 18.1 | 9.2 ±0.4 |
| IL 0-hr Anisomycin | 408.0 ± 57.5 | 63.9 ± 46.6 | 9.6 ± 0.2 |
| dSUB 6-hr Vehicle | 426.4 ± 90.2 | 33.2 ± 16.6 | 9.7 ± 0.1 |
| dSUB 6-hr Anisomycin | 374.5 ± 56.4 | 10.0 ± 2.7 | 9.5 ± 0.2 |
| rBLA 6-hr Vehicle | 245.3 ± 37.2 | 20.6 ± 2.9 | 9.1 ± 0.3 |
| rBLA 6-hr Anisomycin | 243.9 ± 29.2 | 14.4 ± 4.2 | 9.3 ± 0.2 |
Cocaine-cue extinction
0-hr manipulations of the dSUB
In rats that received bilateral dSUB manipulations immediately following extinction test sessions (dSUB 0-hr), analysis of active lever responses/1 hr (Figure 2, insert) on Day 1 of extinction prior to treatment initiation showed no differences between treatment groups (t [1,15] = 1.5, P < 0.15). Analysis of active lever responses/1 hr on Days 2 and 3 revealed significant main effects of extinction day (F [1,15] = 5.3, P ≤ 0.04) and treatment (F [1,15] = 7.7, P ≤ 0.01). These analyses indicate that although active lever responses/1 hr were significantly lower, overall, on Day 3 compared to Day 2, responding was significantly higher after anisomycin compared to vehicle treatment over both days of extinction testing. This latter effect is supported by a lack of a significant treatment x day interaction (F [1,15] = 1.1, P ≤ 0.30).
Figure 2.
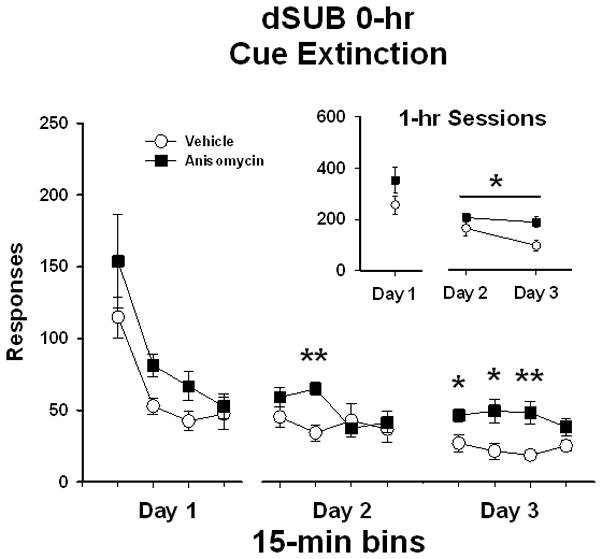
Active lever responses during cocaine-cue extinction tests in rats with bilateral placements within the dorsal subiculum (dSUB) prior to treatment initiation on day 1 and following treatment initiation on days 2 and 3. Rats were treated with either vehicle or anisomycin immediately following (0-hr) extinction tests. Values are the mean ± SEM active lever responses per 1-hr session (insert) and per 15-min bin of each session (main). *P < 0.05; **P < 0.005 compared to vehicle.
To obtain a more refined analysis of the time course of changes between treatments in the dSUB 0-hr experiment, responding was analyzed in 15-min bins. Analysis of active lever responses/15 min (Figure 2) on Day 1 of extinction revealed a significant main effect of bin (F [3,15] = 20.5, P ≤ 0.001). Further Tukey testing indicated that the number of active lever responses in the first 15-min bin was significantly higher (P ≤ 0.05) than the subsequent 15-min bins. On Day 1, there was no difference between treatment groups (F [1,15] = 2.2, P ≤ 0.15), and no treatment x bin interaction (F [3,45] = 0.7, P ≤ 0.58). This analysis indicates that both groups showed a similar within-session pattern of extinction responding prior to any treatment initiation. For the eight 15-min bins collected over Days 2 and 3 of extinction, there was a significant main effect of treatment (F [1,15] = 7.6, P ≤ 0.015) and bins (F [7,15] = 3.1, P ≤ 0.005), as well as a significant treatment x bin interaction (F [7,105] = 2.3, P ≤ 0.03). The main effects of bin and treatment indicate that there was an overall reduction in responding from Day 2 to Day 3, but that responding in anisomycin-treated rats was greater than in vehicle-treated rats. Notably, further Tukey analysis of the interaction revealed that rats treated with anisomycin responded more during the second, fifth, sixth and seventh 15-min bins during post-treatment extinction testing compared to vehicle treatment (P ≤ 0.05). This demonstrates that rats treated with anisomycin in the dSUB did not reduce responding as readily as rats treated with vehicle.
Analysis of inactive lever responses showed that both treatment groups averaged less than 25 responses/1 hr over the course of extinction (data not shown). There were no differences in inactive responses/1 hr between treatment groups prior to treatment initiation on extinction day 1 (t [1,15] = 0.5, P < 0.15). In addition, inactive lever responses/1 hr on Day 2 and Day 3 revealed no significant main effect of extinction day (F [1,15] = 0.54, P ≤ 0.48) or treatment (F [1,15] = 0.51, P ≤ 0.70), and no treatment x day interaction (F [1,15] = 3.0, P ≤ 0.10).
0-hr manipulations of the rBLA
Similarly, in rats that received bilateral rBLA manipulations immediately following extinction test sessions (rBLA 0-hr), analysis of active lever responses/1 hr (Figure 3 insert) on Day 1 of extinction prior to treatment initiation showed no differences between treatment groups (t [1,13] = 1.7, P < 0.11). Analysis of active lever responses/1 hr on Day 2 and Day 3 revealed no significant main effect of extinction day (F [1,13] = 2.5, P ≤ 0.14) or treatment (F [1,13] = 4.0, P ≤ 0.07), and no treatment x day interaction (F [1,13] = 2.9, P ≤ 0.11).
Figure 3.
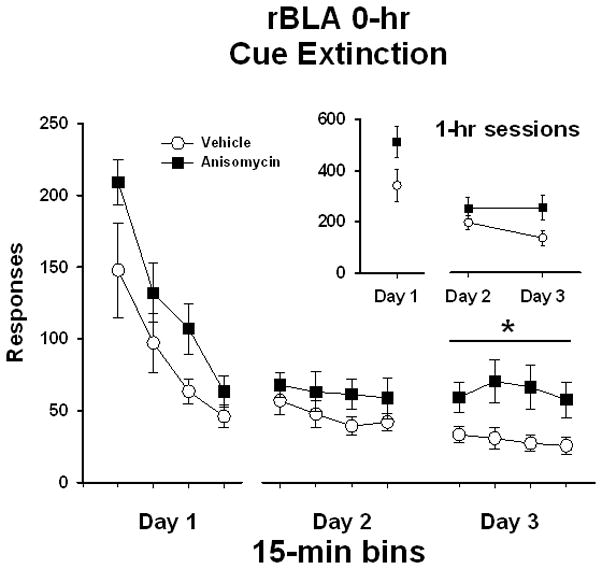
Active lever responses during cocaine-cue extinction tests in rats with bilateral placements within the rostral basolateral amygdala (rBLA) prior to treatment initiation on day 1 and following treatment initiation on days 2 and 3. Rats were treated with either vehicle or anisomycin immediately following (0-hr) extinction tests. Values are the mean ± SEM active lever responses per 1-hr session (insert) and per 15-min bin of each session (main). *P < 0.03 compared to vehicle.
To refine the time course analysis, data for the rBLA 0-hr experiment also were analyzed in 15-min bins. Analysis of active lever responses/15 min (Figure 3) on Day 1 of extinction revealed a significant main effect of bin (F [3,13] = 33.4, P < 0.001), and further Tukey testing indicated that number of active lever responses in first and second 15-min bins was significantly higher (P ≤ 0.05) than the subsequent 15-min bins. On day 1, there was no difference between treatment groups (F [1,13] = 2.9, P ≤ 0.11), and no treatment x bin interaction (F [3,39] = 1.0, P ≤ 0.40), indicating a similar pattern of within session extinction responding prior to treatment initiation. For the eight 15-min bins collected over Days 2 and 3 of extinction, there were no main effects of treatment (F [1,13] = 4.0, P ≤ 0.07) or bin (F [7,13] = 1.6, P ≤ 0.15), and no treatment x bin interaction (F [7,91] = 1.2, P ≤ 0.31). However, as the treatment main effect was close to being significant for both responses/1 hr and responses/15 min, and an examination of Figure 3 suggests that large treatment differences were apparent on Day 3, a separate analysis of the four 15-min bins on Day 3 of extinction was conducted. The analysis revealed no main effect of bin (F [3,13] = 0.7, P ≤ 0.56), but did reveal a significant main effect of treatment (F [1,13] = 6.1, P ≤ 0.028). This analysis indicates that rats previously treated with anisomycin responded more during each bin of Day 3 than rats previously treated with vehicle. This latter effect is supported by a lack of a significant treatment x bin interaction (F [3,39] = 0.6, P ≤ 0.65) for the Day 3 analysis. These findings suggest that anisomycin administration into the rBLA did not alter extinction retention following one day of treatment, but did influence extinction retention following two days of treatment.
Analysis of inactive lever responses showed that both treatment groups averaged less than 36 responses/1 hr over the course of extinction (data not shown). There were no differences in inactive responses/1 hr between treatment groups prior to treatment initiation on extinction day 1 (t [1,13] = −0.89, P < 0.39). Inactive lever responding/1 hr on Day 2 and Day 3 revealed no significant main effect of treatment (F [1,13] = 0.65, P ≤ 0.44). However, there was a main effect of extinction day (F [1,13] = 4.6, P ≤ 0.05) and treatment x day interaction (F [1,13] = 7.9, P ≤ 0.02). Follow up testing revealed that rats previously treated with vehicle decreased inactive lever responding from day 2 (35.4 ± 10.7) to day 3 (18.3 ± 8.2) of extinction (P ≤ 0.005), whereas rats previously treated with anisomycin maintained inactive lever responding across days 2 (18.0 ± 4.3) and 3 (20.2 ± 5.1) of extinction.
0-hr manipulations of the IL
In rats that received bilateral IL manipulations immediately following extinction test sessions (IL 0-hr), analysis of active lever responses/1 hr (Figure 4 insert) on Day 1 of extinction prior to treatment initiation showed no differences between treatment groups (t [1,14] = 1.6, P ≤ 0.14). Analysis of active lever responses on Day 2 and Day 3 revealed no significant main effect of extinction day (F [1,14] = 2.7, P ≤ 0.12) or treatment (F [1,14] = 2.0, P ≤ 0.18), and no treatment x day interaction (F [1,14] = 0.07, P ≤ 0.80). As in the 0-hr dSUB and rBLA experiments, analysis of responses/15 min (Figure 4) on extinction Day 1 for the IL 0-hr experiment revealed a significant main effect of bin (F [3,14] = 32.6, P ≤ 0.001). Further Tukey testing indicated that the number of active lever responses during the first and second 15-min bins was significantly higher (P ≤ 0.05) than the subsequent 15-min bins. There was no difference between treatment groups (F [1,14] = 2.5, P ≤ 0.14), and no treatment x bin interaction (F [3,42] = 2.1, P ≤ 0.11) on Day 1. Analysis of the eight 15-min bins collected over Days 2 and 3 of extinction for the IL groups revealed a significant main effect of bin (F [7,14] = 3.3, P ≤ 0.003) and further Tukey testing indicated that the number of active lever responses in the first 15-min bin of day 2 was significantly higher (P ≤ 0.05) than the all of the 15-min bins on day 3. However, there was no main effect of treatment (F [1,14] = 1.9, P ≤ 0.19) and no treatment x bin interaction (F [7,98] = 0.2, P ≤ 0.99). This indicates that both anisomycin- and vehicle-treated rats had similar patterns of extinction responding over the course of testing.
Figure 4.
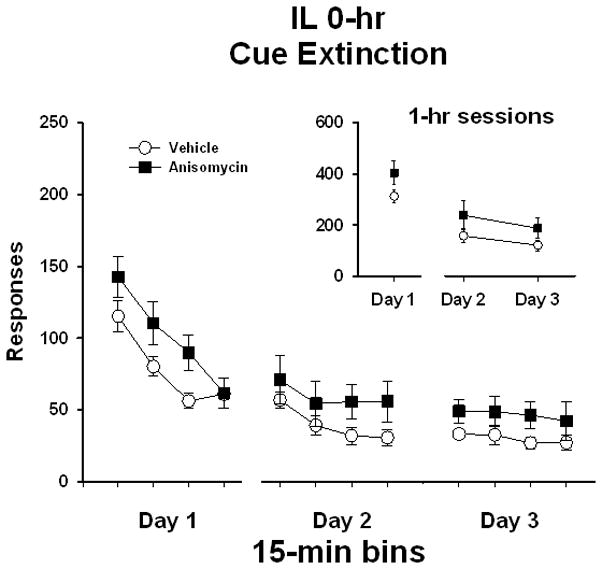
Active lever responses during cocaine-cue extinction tests in rats with bilateral placements within the infralimbic prefrontal cortex (IL) prior to treatment initiation on day 1 and following treatment initiation on days 2 and 3. Rats were treated with either vehicle or anisomycin immediately following (0-hr) extinction tests. Values are the mean ± SEM active lever responses per 1-hr session (insert) and per 15-min bin of each session (main).
Analysis of inactive lever responses showed that both treatment groups averaged less than 40 responses/1 hr over the course of extinction (data not shown). There were no differences in inactive responses/1 hr between treatment groups prior to treatment initiation on extinction day 1 (t [1,14] = 1.3, P < 0.22). Inactive lever responding/1 hr on Day 2 and Day 3 revealed no significant main effect of extinction day (F [1,14] = 0.03, P ≤ 0.87) or treatment (F [1,14] = 2.2, P ≤ 0.70), and no treatment x day interaction (F [1,14] = 0.23, P ≤ 0.64).
6-hr manipulations of the dSUB
In rats that received bilateral dSUB manipulations 6 hr following extinction test sessions (dSUB 6-hr), analysis of active lever responses/1 hr (Figure 5 insert) on Day 1 of extinction prior to treatment initiation showed no differences between treatment groups (t [1,12] = 0.34, P ≤ 0.74). Analysis of active lever responses/1 hr on Day 2 and Day 3 revealed a significant main effect of extinction day (F [1,12] = 9.8, P ≤ 0.01), indicating that responses were significantly lower, overall, on Day 3 compared to Day 2 of extinction testing. However, there was no significant main effect of treatment (F [1,12] = 2.0, P ≤ 0.18) and no treatment x day interaction (F [1,12] = 1.4, P ≤ 0.25). As in the 0-hr experimental groups, analysis of response/15 min (Figure 5) on extinction Day 1 for the dSUB 6-hr experiment revealed a significant main effect of bin (F [3,12] = 35.3, P ≤ 0.001), and further Tukey testing indicated that the number of active lever responses during the first 15-min bin was significantly higher (P ≤ 0.001) than the subsequent 15-min bins. On day 1, there was no difference between treatment groups (F [1,12] = 0.08, P ≤ 0.78), and no treatment x bin interaction (F [3,36] = 0.31, P ≤ 0.82). Analysis of responses/15 min collected over Days 2 and 3 of extinction for the dSUB 6-hr experiment revealed a significant main effect of bin (F [7,12] = 12.9, P ≤ 0.001), and further Tukey testing indicated that the number of active lever responses in the first 15-min bin of day 2 was significantly higher (P ≤ 0.05) than the subsequent 15-min bins. However, there was no main effect of treatment (F [1,12] = 1.4, P ≤ 0.26) and no treatment x bin interaction (F [7,84] = 1.3, P ≤ 0.25). This indicates that both anisomycin- and vehicle-treated rats had similar patterns of extinction responding over the course of testing.
Figure 5.
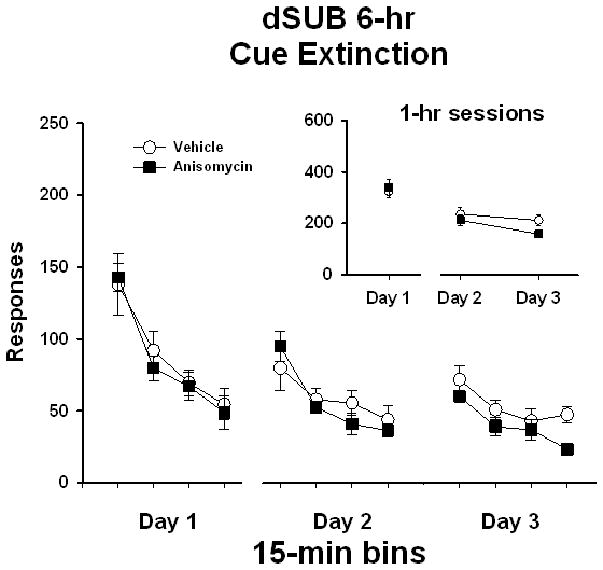
Active lever responses during cocaine-cue extinction tests in rats with bilateral placements within the dorsal subiculum (dSUB) prior to treatment initiation on day 1 and following treatment initiation on days 2 and 3. Rats were treated with either vehicle or anisomycin six-hours following (6-hr) extinction tests. Values are the mean ± SEM active lever responses per 1-hr session (insert) and per 15-min bin of each session (main).
Analysis of inactive lever responses showed that both treatment groups averaged less than 35 responses/1 hr over the course of extinction (data not shown). There were no differences in inactive responses/1 hr between treatment groups prior to treatment initiation on extinction day 1 (t [1,12] = −1.5, P < 0.17). Inactive lever responding/1 hr on Day 2 and Day 3 revealed no significant main effect of extinction day (F [1,12] = 0.60, P ≤ 0.45) or treatment (F [1,12] = 2.6, P ≤ 0.14), and no treatment x day interaction (F [1,12] = 0.53, P ≤ 0.48).
6-hr manipulations of the rBLA
As in all placement groups, rats that received bilateral rBLA manipulations 6 hr following extinction test sessions (rBLA 6-hr) showed no differences in active lever responses/1 hr (Figure 6 insert) on Day 1 of extinction (t [1,11] = 0.75, P ≤ 0.47). Analysis of active lever responses/1 hr on Day 2 and Day 3 revealed no main effect of day (F [1,11] = 1.1, P ≤ 0.32) or treatment (F [1,11] = 1.2, P ≤ 0.30), and no treatment x day interaction (F [1,11] = 0.2, P ≤ 0.83). These findings indicate that both anisomycin- and vehicle-treated rats had similar patterns of responding across extinction tests. Similarly to the dSUB 6-hr experiment, analysis of response/15 min (Figure 6) on extinction Day 1 for the rBLA 6-hr experiment revealed a significant main effect of bin (F [3,11] = 25.3, P ≤ 0.001), and further Tukey testing indicated that the number of active lever responses during the first 15-min bin was significantly higher (P ≤ 0.001) than the subsequent 15-min bins. On Day 1, there was no difference between treatment groups (F [1,11] = 0.55, P ≤ 0.47), and no treatment x bin interaction (F [3,33] = 0.20, P ≤ 0.90) on Day 1. Similar to the IL 0-hr and the dSUB 6-hr experiments, responses/15 min collected over Days 2 and 3 of extinction for the rBLA 6-hr groups revealed a significant main effect of bin (F [7,11] = 9.0, P ≤ 0.001) and Tukey post hoc testing indicated that the number of active lever responses in the first 15-min bin was significantly higher (P ≤ 0.05) than the subsequent 15-min bins. However, there was no main effect of treatment (F [1,12] = 1.2, P ≤ 0.30) and no treatment x bin interaction (F [7,77] = 1.2, P ≤ 0.31). This indicates that both anisomycin- and vehicle-treated rats had similar patterns of extinction responding over the course of testing.
Figure 6.
Active lever responses during cocaine-cue extinction tests in rats with bilateral placements within the rostral basolateral amygdala (rBLA) prior to treatment initiation on day 1 and following treatment initiation on days 2 and 3. Rats were treated with either vehicle or anisomycin six-hours following (6-hr) extinction tests. Values are the mean ± SEM active lever responses per 1-hr session (insert) and per 15-min bin of each session (main).
Analysis of inactive lever responses showed that both treatment groups averaged less than 30 responses/1 hr over the course of extinction (data not shown). There were no differences in inactive responses/1 hr between treatment groups prior to treatment initiation on extinction day 1 (t [1,11] = 0.12, P < 0.91). Inactive lever responses/1 hr on Day 2 and Day 3 revealed no significant main effect of extinction day (F [1,11] = 0.09, P ≤ 0.77) or treatment (F [1,11] = 2.5, P ≤ 0.15), and no treatment x day interaction (F [1,11] = 0.11, P ≤ 0.75).
Discussion
Importance of the hippocampus and amygdala for cocaine-cue extinction consolidation
Disruption of neuronal processing with protein synthesis inhibition after extinction is acquired can reveal which brain regions are involved in the consolidation of cocaine-cue extinction learning. This method of post-training manipulation is analogous to those used to identify the DH, BLA and IL as substrates of fear extinction consolidation in rats (Vianna et al., 2001; Laurent & Westbrook, 2008; Chhatwal et al., 2006; Hugues et al., 2004, 2006; Burgos-Robles el al., 2007). Effects from post-session treatment with anisomycin can be inferred to have an effect through temporary and reversible inhibition of protein synthesis. Anisomycin has been shown to be non-toxic and effective, as it inhibits 95% of protein synthesis in vitro (Grollman, 1967), and maintains 80–90% cerebral protein synthesis inhibition for at least 2-hrs in rodent models of learning and memory following systemic or intracranial administration (Flood et al., 1973; Grecksch & Matthies, 1980; Frey et al., 2001). It maintains efficacy following successive administration within a single day (Flood et al., 1973; Lattal & Abel, 2004), as well as administration over multiple days (Bracha et al., 1998) in preclinical learning and memory tasks.
Bilateral inhibition of protein synthesis in the dSUB immediately following extinction test sessions was shown to disrupt consolidation of cocaine-cue extinction learning. This was demonstrated in anisomycin-treated rats, as they maintained elevated levels of responding compared to vehicle-treated rats on Days 2 and 3, as revealed by 1-hr session and 15-min bin analyses. Bilateral inhibition of protein synthesis in the rBLA immediately following extinction test sessions was shown to disrupt consolidation of cocaine-cue extinction learning as well. This was demonstrated in anisomycin-treated rats, as they maintained elevated levels of responding compared to vehicle-treated rats on Day 3, as revealed by 15-min bin analyses. Notably, there was no significant increase from Day 1 to Day 2 or Day 2 to Day 3 of extinction testing in inactive lever responses in any group. All groups either showed no change or decreased inactive lever responses over the course of extinction testing, indicating that rats were not omitting active lever responses to accommodate pressing the inactive lever as an alternative cocaine-seeking strategy for earning response contingent infusions of cocaine.
Insights from manipulation of the infralimbic prefrontal cortex
Inhibition of protein synthesis in the IL immediately following extinction sessions was unable to influence behavior during the three days of extinction testing. There are several possible explanations for why the IL, which has been established as a neural substrate of consolidation for fear extinction (Hugues et al., 2004, 2006; Burgos-Robles el al., 2007), would not be important for the consolidation of cocaine-cue extinction learning during our 3-day extinction protocol.
One possibility for the lack of effect is the potential inadvertent disruption of the PL in addition to manipulation of the IL. The PL is located directly dorsal to the IL. If the PL were mechanically damaged through the insertion of infusion needles, or pharmacologically disrupted by the spread of anisomycin into the anatomical range of the PL, this would potentially counter the effects initially expected from post-session IL manipulation. As the PL and IL have been shown to have functionally opposite roles in the regulation of fear expression and cocaine-seeking behavior (Corcoran & Quirk, 2007; Vidal-Gonzalez et al., 2006; Peters et al., 2008), damage and/or manipulation to both regions simultaneously would potentially cancel any individual effects that would have been observed with site-specific manipulation. Fear extinction studies have shown that large lesions of the medial prefrontal cortex that encompass the PL and IL do not affect extinction (Farinelli et al., 2006; Garcia et al., 2006; Gewirtz et al., 1997). However, the possibility that PL disruption negated affects in the IL is unlikely for this study because guide cannulae were positioned 2mm dorsal to the IL (1mm dorsal to the PL) for the specific purpose of minimizing tissue damage to the PL. In addition, any cannulae tips that were determined to be out of the anatomical range of the IL were eliminated from the study.
Another possibility for why post-session treatment with anisomycin was unable to influence behavior is that cocaine-cue extinction was not fully acquired. There was evidence of acquisition over the first three days, but responding had not completely diminished. In general, the percent of baseline levels of responding on Day 3 in all vehicle-treated groups averaged 39.4 (± 6.2). In the IL 0-hr experiment, in particular, levels of responding on Day 3 were 43.1 (± 10.5) and 50.1 (± 11.5) percent of baseline for the vehicle- and anisomycin-treated groups, respectively. This suggests that responding in the presence of formerly cocaine-associated cues was not under full inhibitory control within 3 days of extinction training. In fear conditioning studies, the acquisition of fear and its subsequent extinction can be accomplished within individual training sessions (Quirk & Mueller, 2008). By contrast, extinction in cocaine self-administration paradigms requires multiple sessions to reach sustained low levels of responding (Fuchs et al., 2006, 2008; Wells et al., 2011). This is compounded by the use of a second-order schedule, which is more resistant to extinction than FR schedules (Arroyo et al., 1998). Under a second-order schedule, not every response-contingent conditioned stimulus presentation is paired with cocaine during self-administration training, and therefore, it would require a greater period of exposure to unpaired conditioned stimuli to extinguish their salience. Evidence from other studies suggests that the IL contributes to extinction consolidation at a later time point after extinction is more fully acquired.
The time course of IL involvement is evident in response extinction models of cocaine-seeking behavior for which cues are omitted during training. Lalumiere and colleagues (2010) showed that the IL was required for extinction consolidation, though this was revealed only following extensive extinction training (five 30-min sessions) coupled with repeated post-session neuronal inactivation and prolonged retention testing (seven 2-hr sessions). These experimental conditions allowed for full acquisition of extinction and a sufficient duration of retention testing for detecting treatment differences in response patterns. There also is a delayed involvement of the IL in consolidation of contextual fear memory (Frankland et al., 2004) and contextual fear extinction (Lebron et al., 2004). Corcoran and Quirk (2007), in their review, state that the IL may be more involved with the recall of extinction than the initial storage. This would suggest that extinction would require a certain level of acquisition that involves other brain regions before it is able to exert the necessary retrieval for extinction retention. Collectively, the above studies suggest that the IL may be involved in long-term retrieval (days-weeks) of drug-related extinction memory, whereas other brain regions, such as the hippocampus, may be involved in short-term (hours-days) storage of drug-related extinction memory (Lalumiere et al., 2010; Corcoran & Quirk, 2007; Quirk & Mueller, 2008; Frankland et al., 2004). One limitation of the current study is that we did not determine a role for the IL in long-term cocaine-cue extinction consolidation, as manipulations were made only using the 3-day extinction protocol.
Time-dependency of consolidation processes in the dSUB and rBLA following extinction training
The processes disrupted by anisomycin administration immediately following extinction sessions were not disrupted by anisomycin administration 6-hrs following extinction sessions. The lack of effect on extinction retention with delayed manipulations shows that de novo protein synthesis in these regions is not necessary for consolidation of extinction learning beyond 6-hrs. This conclusion is consistent with the rationale that there are time-dependent consolidation processes, as put forth by McGaugh (1966; 2000).
The timing of both the test sessions and the anisomycin infusions was carefully considered to ensure that the potential effects of protein synthesis inhibition would be exclusive to consolidation while avoiding conflation with potential effects on reconsolidation. Reconsolidation is a neural process of memory whereby subjects are exposed to contexts or cues previously associated with conditioned behaviors, and these associations are retrieved or “reactivated” to strengthen recall (Suzuki et al., 2004; Alberini, 2005). During this initial reactivation, memory is thought to be labile, and hence experimental manipulations can disrupt or enhance the long-term storage of associative memory. Reconsolidation and extinction, while both involve retrieval, are functionally opposite and have distinct biochemical signatures (Suzuki et al., 2004). Similarly to acquisition and consolidation of new learning such as extinction, the processes of reactivation and reconsolidation are time-dependent (Nader et al., 2000; Alberini, 2008; Inda et al., 2011), as there is a critical window in which memories can be reactivated and reconsolidated. It takes a brief exposure for existing memories to be reactivated and brought to a labile state. In fear and drug conditioning studies, reactivation-reconsolidation happens within the first 30 min of exposure, whereas after 30 min there is extinction learning and consolidation (Suzuki et al., 2004, Fuchs et al., 2009; Wells et al., 2011). Additionally, there is a limited window for reconsolidating memory, which becomes increasingly difficult to modify following sustained incubation of previously conditioned stimuli. It was shown by Alberini and colleagues (2006) that previously conditioned behavior becomes less susceptible to manipulation of reconsolidation as conditioning is prolonged and memory is fully consolidated. Repeated retrieval of consolidated memory transitions from reconsolidation to extinction (Inda et al., 2011).
Studies that use the response extinction model have identified the DH and BLA as substrates of reconsolidation for cocaine-seeking behavior (Fuchs el at., 2009, Wells et al., 2011). Because these regions overlap as substrates for extinction-consolidation and reactivation-reconsolidation, it is imperative that experimental procedures investigate each learning and memory process with specificity. With these considerations in mind, self-administration training spanned 25–35 sessions over 5–7 weeks prior to cue extinction testing, at which point conditioned behavior would be less susceptible to reconsolidation manipulations. Moreover, extinction sessions lasted a full hour, longer than the 30-min window of cue exposure when associations would hypothetically be labile for reconsolidation. The prolonged self-administration training and 1-hr cue extinction tests make it possible to conclude that exposure to the cues would not reactivate their original memory, but would allow the acquisition of extinction.
Conclusions
The dSUB and rBLA are important neural substrates for the initial stages of cocaine-cue extinction consolidation and their contributions are time-dependent. Notably, these sites overlap with those shown to be important for acquisition of cocaine-cue extinction learning (Szalay et al 2011) and have clinical implications. Medications and strategies that enhance extinction-related synaptic plasticity within the hippocampus and amygdala might augment cue exposure therapy for relapse prevention more effectively than cue exposure therapy alone or treatments that target cortical functioning.
Acknowledgments
The authors thank Ms. Lisa Goldberg and Mr. Kyle Barrett for technical assistance. This project was supported by DA024315 from the National Institute on Drug Abuse and by SMA-0835976 from the National Science Foundation. The content is solely the responsibility of the authors, and does not necessarily represent the official views of the National Institute on Drug Abuse or National Science Foundation.
Abbreviations
- Ani
anisomycin
- ANOVA
analysis of variance
- AP
anterior-posterior
- BLA
basolateral amygdala
- DH
dorsal hippocampus
- dSUB
dorsal subiculum
- DV
dorsal-ventral
- FI
fixed interval
- FR
fixed ratio
- IL
infralimbic prefrontal cortex
- ML
medial-lateral
- rBLA
rostral basolateral amygdala
- Veh
vehicle
Footnotes
The authors have no conflicts of interest to disclose.
References
- Abraham WC, Williams JM. LTP maintenance and its protein synthesis-dependence. Neurobiol Learn Mem. 2008;89:260–268. doi: 10.1016/j.nlm.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Akirav I, Khatsrinov V, Vouimba RM, Merhav M, Ferreira G, Rosenblum K, Maroun M. Extinction of conditioned taste aversion depends on functional protein synthesis but not on NMDA receptor activation in the ventromedial prefrontal cortex. Learn Mem. 2006;13:254–258. doi: 10.1101/lm.191706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini CM. The role of protein synthesis during the labile phases of memory: Revisiting the skepticism. Neurobiol Learn Mem. 2008;89:234–246. doi: 10.1016/j.nlm.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini CM. Mechanisms of memory stabilization: Are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 2005;28:51–56. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Arroyo M, Markou A, Robbins TW, Everitt BJ. Acquisition, maintenance and reinstatement of intravenous cocaine self-administration under a second-order schedule of reinforcement in rats: Effects of conditioned cues and continuous access to cocaine. Psychopharmacology (Berl) 1998;140:331–344. doi: 10.1007/s002130050774. [DOI] [PubMed] [Google Scholar]
- Bracha V, Irwin KB, Webster ML, Wunderlich DA, Stachowiak MK, Bloedel JR. Microinjections of anisomycin into the intermediate cerebellum during learning affect the acquisition of classically conditioned responses in the rabbit. Brain Res. 1998;788:169–178. doi: 10.1016/s0006-8993(97)01535-7. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat Neurosci. 2006;9:870–872. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Recalling safety: Cooperative functions of the ventromedial prefrontal cortex and the hippocampus in extinction. CNS Spectr. 2007;12:200–206. doi: 10.1017/s1092852900020915. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. Eur J Neurosci. 2006;24:3285–3298. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Nader K, LeDoux JE. De novo mRNA synthesis is required for both consolidation and reconsolidation of fear memories in the amygdala. Learn Mem. 2008;15:747–755. doi: 10.1101/lm.1027208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Farinelli M, Deschaux O, Hugues S, Thevenet A, Garcia R. Hippocampal train stimulation modulates recall of fear extinction independently of prefrontal cortex synaptic plasticity and lesions. Learn Mem. 2006;13:329–334. doi: 10.1101/lm.204806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood JF, Rosenzweig MR, Bennett EL, Orme AE. The influence of duration of protein synthesis inhibition on memory. Physiol Behav. 1973;10:555–562. doi: 10.1016/0031-9384(73)90221-7. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Frey S, Bergado-Rosado J, Seidenbecher T, Pape HC, Frey JU. Reinforcement of early long-term potentiation (early-LTP) in dentate gyrus by stimulation of the basolateral amygdala: Heterosynaptic induction mechanisms of late-LTP. J Neurosci. 2001;21:3697–3703. doi: 10.1523/JNEUROSCI.21-10-03697.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Bell GH, Ramirez DR, Eaddy JL, Su ZI. Basolateral amygdala involvement in memory reconsolidation processes that facilitate drug context-induced cocaine seeking. Eur J Neurosci. 2009;30:889–900. doi: 10.1111/j.1460-9568.2009.06888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Ramirez DR, Bell GH. Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2008;200:545–556. doi: 10.1007/s00213-008-1234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Feltenstein MW, See RE. The role of the basolateral amygdala in stimulus-reward memory and extinction memory consolidation and in subsequent conditioned cued reinstatement of cocaine seeking. Eur J Neurosci. 2006;23:2809–2813. doi: 10.1111/j.1460-9568.2006.04806.x. [DOI] [PubMed] [Google Scholar]
- Garcia R, Chang CH, Maren S. Electrolytic lesions of the medial prefrontal cortex do not interfere with long-term memory of extinction of conditioned fear. Learn Mem. 2006;13:14–17. doi: 10.1101/lm.60406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz JC, Falls WA, Davis M. Normal conditioned inhibition and extinction of freezing and fear-potentiated startle following electrolytic lesions of medical prefrontal cortex in rats. Behav Neurosci. 1997;111:712–726. doi: 10.1037//0735-7044.111.4.712. [DOI] [PubMed] [Google Scholar]
- Grecksch G, Matthies H. Two sensitive periods for the amnesic effect of anisomycin. Pharmacol Biochem Behav. 1980;12:663–665. doi: 10.1016/0091-3057(80)90145-8. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grollman AP. Inhibitors of protein biosynthesis. II. mode of action of anisomycin. J Biol Chem. 1967;242:3226–3233. [PubMed] [Google Scholar]
- Hugues S, Chessel A, Lena I, Marsault R, Garcia R. Prefrontal infusion of PD098059 immediately after fear extinction training blocks extinction-associated prefrontal synaptic plasticity and decreases prefrontal ERK2 phosphorylation. Synapse. 2006;60:280–287. doi: 10.1002/syn.20291. [DOI] [PubMed] [Google Scholar]
- Hugues S, Deschaux O, Garcia R. Postextinction infusion of a mitogen-activated protein kinase inhibitor into the medial prefrontal cortex impairs memory of the extinction of conditioned fear. Learn Mem. 2004;11:540–543. doi: 10.1101/lm.77704. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Saito H, Abe K. Requirement of basolateral amygdala neuron activity for the induction of long-term potentiation in the dentate gyrus in vivo. Brain Res. 1995;671:351–354. doi: 10.1016/0006-8993(94)01403-5. [DOI] [PubMed] [Google Scholar]
- Inda MC, Muravieva EV, Alberini CM. Memory retrieval and the passage of time: From reconsolidation and strengthening to extinction. J Neurosci. 2011;31:1635–1643. doi: 10.1523/JNEUROSCI.4736-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn Mem. 2010;17:168–175. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal KM, Abel T. Behavioral impairments caused by injections of the protein synthesis inhibitor anisomycin after contextual retrieval reverse with time. Proc Natl Acad Sci U S A. 2004;101:4667–4672. doi: 10.1073/pnas.0306546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, Westbrook RF. Distinct contributions of the basolateral amygdala and the medial prefrontal cortex to learning and relearning extinction of context conditioned fear. Learn Mem. 2008;15:657–666. doi: 10.1101/lm.1080108. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Time-dependent processes in memory storage. Science. 1966;153:1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan T, Ehrman R. Integrating systemic cue exposure with standard treatment in recovering drug dependent patients. Addict Behav. 1990;15:355–365. doi: 10.1016/0306-4603(90)90045-y. [DOI] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, LeDoux JE. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. Elsevier; Amsterdam: 1992. [Google Scholar]
- Szalay JJ, Morin ND, Kantak KM. Involvement of the dorsal subiculum and rostral basolateral amygdala in cocaine cue extinction learning in rats. Eur J Neurosci. 2011;33:1299–1307. doi: 10.1111/j.1460-9568.2010.07581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazdarjanova A, McGaugh JL. Basolateral amygdala is involved in modulating consolidation of memory for classical fear conditioning. J Neurosci. 1999;19:6615–6622. doi: 10.1523/JNEUROSCI.19-15-06615.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna MR, Szapiro G, McGaugh JL, Medina JH, Izquierdo I. Retrieval of memory for fear-motivated training initiates extinction requiring protein synthesis in the rat hippocampus. Proc Natl Acad Sci U S A. 2001;98:12251–12254. doi: 10.1073/pnas.211433298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AM, Lasseter HC, Xie X, Cowhey KE, Reittinger AM, Fuchs RA. Interaction between the basolateral amygdala and dorsal hippocampus is critical for cocaine memory reconsolidation and subsequent drug context-induced cocaine-seeking behavior in rats. Learn Mem. 2011;18:693–702. doi: 10.1101/lm.2273111. [DOI] [PMC free article] [PubMed] [Google Scholar]