Abstract
The translocator protein (18-kDa) TSPO is an ubiquitous high affinity cholesterol-binding protein reported to be present in the endothelial and smooth muscle cells of the blood vessels; its expression dramatically increased in macrophages found in atherosclerotic plaques. A domain in the carboxy-terminus of TSPO was identified and characterized as the cholesterol recognition/interaction amino acid consensus (CRAC). The ability of the CRAC domain to bind to cholesterol led us to hypothesize that this peptide could be used as an hypocholesterolemic, with potential anti-atherogenic properties, agent. We report herein the therapeutic benefit that resulted for the administration of the VLNYYVWR human CRAC sequence to guinea pigs fed with a high cholesterol diet and ApoE knock-out B6.129P2-Apoetm1Unc/J mice. CRAC treatment (3 and 30 mg/kg once daily for 6 weeks) resulted in reduced circulating cholesterol levels in guinea pigs fed with 2% high cholesterol diet and ApoE knock-out B6.129P2-Apoetm1Unc/J mice. In high cholesterol fed guinea pigs, CRAC treatment administered once daily induced an increase in circulating HDL, decreased total, free and LDL cholesterol, and removed atheroma deposits in the aorta in a dose-dependent manner. The treatment also prevented the high cholesterol diet-induced increase in serum creatine kinase, total and isoforms, markers of neurological, cardiac and muscular damage. No toxicity was observed. Taken together these results support a role of TSPO in lipid homeostasis and atherosclerosis and indicate that CRAC may constitute a novel and safe treatment of hypercholesterolemia and atherosclerosis.
Keywords: TSPO, cholesterol, CRAC domain, LDL, atheroma, Drug development
Introduction
Steroidogenesis begins with the transport of the substrate cholesterol from intracellular sources into mitochondria [1], a process mediated by the translocator protein (18-kDa) TSPO [2]. Although highly expressed in steroid synthesizing cells, where its function has been extensively studied, TSPO is an ubiquitous high affinity cholesterol- and drug-binding protein [3;4]. Among the various tissues studied TSPO levels were reported in the cardiovascular system where radioligand binding studies demonstrated elevated levels of TSPO in heart ventricles and its presence in the endothelial and smooth muscle cells of the blood vessels [5]. TSPO was also found in phagocyte populations, including macrophages, monocytes and mast cells and its levels were dramatically induced in the activated cells and inflammatory disease states [5–7]. In recent studies, autoradiography and positron emission tomography, using radiolabeled high affinity TSPO drug ligands, and immunohistochemistry, demonstrated increased TSPO levels in human carotid atherosclerotic plaques at the sites of macrophage infiltration [8–11]. Based on these data, TSPO imaging as a tool to monitor inflammation in atherosclerotic plaques in vivo was proposed [8–10].
Since we first identified the cholesterol-binding domain of TSPO, CRAC [12–14], this motif has been used to predict proteins that bind cholesterol and partition into cholesterol-rich regions of a membrane [15–18]. The ability of the CRAC domain to bind cholesterol led us to hypothesize that this peptide could be used as an intercalating agent to remove cholesterol from lipoproteins and areas of depot (atheromas) and thus a potential hypocholesterolemic and anti-atherogenic agent acting through a new mechanism of action distinct to the mechanisms mediating the effects of fibrates, statins and ezitimibe. In this report, we describe the use of the 8 amino acid human CRAC sequence (VLNYYVWR) to lower cholesterol in two animal models of hypercholesterolemia, the high cholesterol diet fed guinea pig and the ApoE knock-out B6.129P2-Apoetm1Unc/J mice, and the impact of the treatment on atherosclerosis.
Materials and Methods
In vivo studies
Hartley male guinea pigs weighing 400 g were fed with standard diet (Harlan Teklad® Global Guinea Pig Diet 2040) or 2% cholesterol enriched diet (Harlan Teklad® Global Guinea Pig Diet 2040 with 2% cholesterol) for 14 weeks. The 8 amino acid human CRAC sequence (VLNYYVWR) was synthesized by BioSynthesis (Lewisville, TX). CRAC treatment started at the end of week 8 until the end of the experiment. Guinea pigs received one i.p. injection of CRAC solution at 3 and 30 mg/kg (2.5 ml/kg) or vehicle (glucose 5%) every other day. Blood was withdrawn from the right jugular vein under anesthesia (isoflurane 3%) at the study initiation and at the end of week 8 and week 14 to determine total cholesterol, free cholesterol, LDL and HDL levels. Creatine kinase (CK) was measured at weeks 8 and 14 and its isoforms (MM, MB and BB) were measured at the end of week 14. The blood samples were collected in gold-capped BD Vacutainer® tubes containing clot activator and gel for serum separation. At the end of week 14, aortas were collected for histology purposes and the gallbladder content for cholesterol measurement. The weight of the animals was monitored at day 1, at week 8 when the CRAC treatment started, and every week from week 9 to week 14. The growth curve was then established.
ApoE knock-out B6.129P2-Apoetm1Unc/J mice (Jackson Laboratory, Bar Harbor, MN) weighing 18–22 g received one daily intraperitoneal (i.p.) injection of CRAC solution at 10, 30 and 100 mg/kg (0.15 ml/kg) or vehicle for 14 days. At the end of the treatment period blood was withdrawn from the mice by cardiac puncture.
All experimental protocols were approved by the Georgetown University Animal Care and Use Committee.
Clinical chemistry
Total and free cholesterol was measured in serum and bile salts using Amplex® Red Cholesterol Assay Kit (Molecular Probes, Eugene, OR). Guinea pig HDL, LDL, CK, total and isoforms were outsourced to and measured by Antech Inc (Morrisville, NC).
Histology & immunohistochemistry
Frozen sections
Freshly dissected aorta and liver were cut into blocks and frozen as follows. Acetone slurry was prepared on dry ice. Small aluminum trays were placed into the cold acetone slurry and Tissue-Tek® CRYO-OCT (Optimum Cutting Temperature) was added to the aluminum trays. Tissues were mounted and covered with OCT, which was allowed to harden before cutting or stored at −80°C for future use. Twenty μm sections were cut and placed on coated slides. Slides were air dried before use.
Oil Red O Staining
Oil Red O (Sudan Red VB) is a lipophilic dye used to stain lipids. Sections were rinsed with absolute propylene glycol for 5 minutes and stained with 0.7% Oil Red O in propylene glycol for 10 minutes. Slides were rinsed with 85% propylene glycol for 5 minutes followed by distilled water, counterstained with Mayers hematoxylin for 30 seconds and washed under running tap water for 5 minutes. The sections were cover slipped with aqueous mounting medium.
Immunofluorescence
Antigen retrieval was performed on air dried frozen sections using 1%SDS/PBS for 5 minutes. Sections were blocked with 10% normal goat serum (NGS) in PBS for 30 minutes and incubated with antibodies raised against nitrotyrosine (Chemicon, Temecula, CA) at dilution of 1:100 in 1% NGS/PBS for 30 minutes. Sections were washed in PBS three times for 5 minutes each then incubated with Alexa Fluor 488 conjugated anti-mouse secondary antibody (DyLight®488; Jackson Immunoresearch, Westgrove, PA) at a dilution of 1:200 in 1% NGS/PBS for 60 minutes. Sections were mounted with a hard medium mount (Vectorlabs, Burlingame, CA) and analyzed by confocal microscopy (FV500/BX61, Olympus, Center Valley, PA).
Immunohistochemistry
Affinity purified (0.307 mg/ml), ELISA titer >200×103 dilution) anti-CRAC peptide antiserum was raised against the 11 amino acid (CCKVLNYYVWR-NH2) domain of human CRAC (Anaspec, Inc. San Jose, CA). Immunohistochemistry was performed using the Ventana BenchMark fully automated machine according to the protocols and instructions provided by Ventana Medical System Inc (Phoenix, AZ). The fully automated processing of bar code labelled slides included baking of the slides, solvent free deparaffinization, and CC1 (Tris/EDTA buffer ph 8.0) antigen retrieval. Slides were incubated with CRAC peptide antibody (dilution 1:2; for 30 min at 37°C, followed by application of biotinylated secondary antibody (8 min, at 37°C), then an avidin/streptavidin enzyme conjugate complex (8 min, at 37°C). The affinity purified antibody provided by Anaspec Inc. had a very low titer when used in immunohistochemistry studies which resulted in a 1:2 dilution regimen. The antibody was detected by Fast Red chromogenic substrate and counterstained with hematoxylin.
Statistical analysis
Data were analyzed by ANOVA followed by Dunnett’s test or ANOVA followed by Student’s t test. Results are presented as means ± SD.
Results
Serum cholesterol level measurement in Hartley male guinea pigs
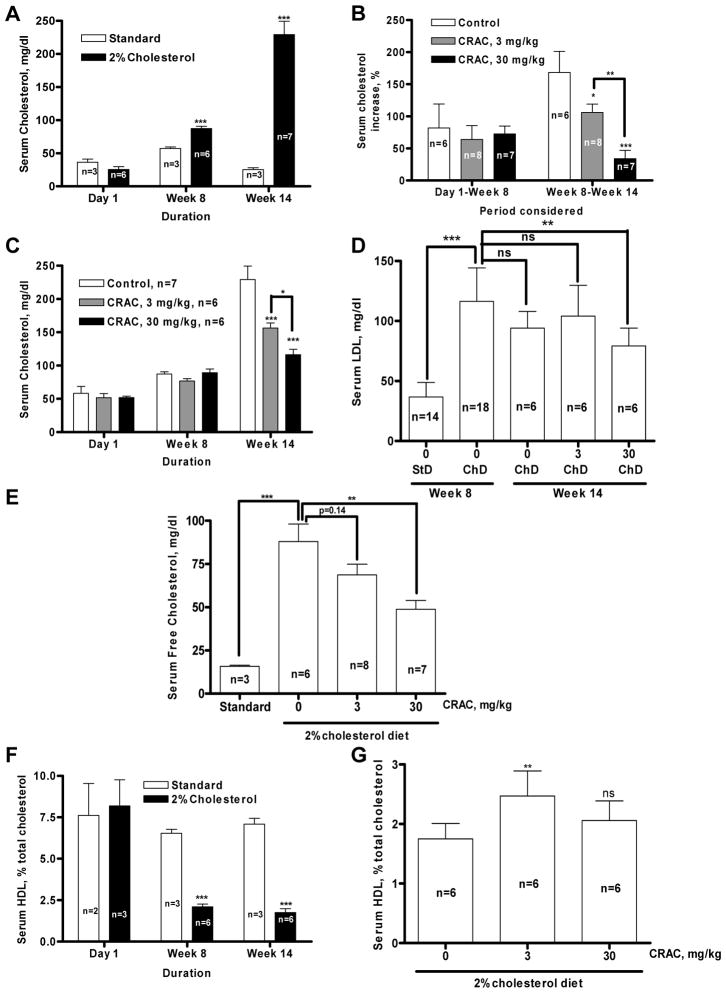
A diet containing 2% cholesterol induced a significant increase of serum cholesterol levels in guinea pigs, measured at week 8, compared to the guinea pigs fed with standard diet (87.31±8.37, n=6, versus 57.17±3.90 mg/dl, n=3, p<0.001); this difference was even more pronounced after 14 weeks (229.2±53.88, n=7, versus 25.33±4.74 mg/dl, n=3, p<0.001) (Fig. 1A). The observed increase between day 1 and week 8 was comparable between the three groups fed with the 2% cholesterol diet (60–80%, Fig. 1B). CRAC treatment reduced in a dose-dependent manner the diet-induced total cholesterol increase measured at week 14 (Fig. 1C). Compared with the average of individual variation of total cholesterol levels between weeks 8 and 14, the increase observed in the control group was 168.51% (n=6) (Fig. 1B). This cholesterol level increase was significantly lowered by 3 mg/kg (105.97±37.24%, n=8, p<0.05) and 30 mg/kg CRAC (34.01±32.56%, n=7, p<0.001). The 2% cholesterol enriched diet also resulted in a threefold increase of LDL cholesterol (Fig. 1D, p<0.001) that what reduced by 37.1% (Fig. 1C, p<0.01) by the CRAC treatment at the dose of 30 mg/kg. The dose of 3 mg/kg did not exert any effect on the diet-induced LDL cholesterol increase.
Figure 1.
A–D. Effect of CRAC treatment on serum total and LDL cholesterol levels in guinea pigs fed with high cholesterol diet. Hartley male guinea pigs were fed with standard or 2% cholesterol enriched diet for 14 weeks. CRAC treatment started at the end of week 8 until the end of the experiment. Guinea pig received one i.p. injection of CRAC solution at 3 and 30 mg/kg (2.5 ml/kg) or vehicle every other day. Blood was withdrawn under anesthesia at the study initiation, at the end of week 8 and week 14 for total cholesterol measurement as described under Materials and Methods. A. Evolution of total cholesterol concentration measured at day 1, week 8 and week 14 in guinea pigs fed with standard- or 2% cholesterol diet. B. Change in total cholesterol concentration from day 1 to week 8 and from week 8 to week 14. Data shown are means ± SD (n, as indicated in the panels). Results were analyzed by ANOVA followed by Dunnett’s test or Student’s t test. * p<0.05, ** p<0.01, *** p<0.001. C. Total cholesterol concentration measured at day 1, week 8 and week 14 in guinea pigs fed with 2% cholesterol diet and treated with CRAC 3, 30 mg/kg or its vehicle. D. LDL cholesterol concentration measured at week 8 in guinea pigs fed with standard (StD)- or 2% cholesterol diet (ChD) and week 14 in guinea pigs fed with 2% cholesterol diet and treated with CRAC at 0, 3 or 30 mg/kg. E. Effect of CRAC treatment on serum free cholesterol levels in guinea pigs fed with high cholesterol diet. Data shown are means ± SD. ** p<0.01, *** p<0.001 (n, as indicated in the panels). Results were analyzed by ANOVA followed by Dunnett’s test. -F–G. Effect of CRAC treatment on serum HDL in guinea pigs fed with high cholesterol diet. F. Evolution of HDL cholesterol concentration measured at day 1, week 8 and week 14 in guinea pigs fed with standard or 2% cholesterol diet. G. Evolution of HDL cholesterol concentration measured at week 14 in guinea pigs fed with 2% cholesterol diet and treated with CRAC 3, 30 mg/kg or its vehicle. Data shown are means ± SD (n, as indicated in the panels). Results were analyzed by ANOVA or followed by Student’s t test. ** p<0.01, *** p<0.001.
The effect of CRAC treatment on free (unesterified) cholesterol paralleled the effect of the treatment on total (chylomicron+HDL+IDL+LDL+VDL) cholesterol. The 2% cholesterol diet induced a dramatic increase of free cholesterol in guinea pig serum compared to the standard diet (87.96±24.93 mg/dl, n=6, versus 15.81±1.07 mg/dl, n=3, p<0.001) (Fig. 1E). CRAC treatment reduced in a dose-dependent manner the diet-induced cholesterol increase with the 3 mg/kg dose effect close to significance (p=0.14) and the 30 mg/kg dose effect reaching significance (p<0.01) (F Ratio = 9.819, Prob > F = 0.0013). Cholesterol diet induced a decrease of HDL cholesterol compared to the animals fed with the standard diet and this decrease was detectable as early as week 8 (Fig. 1F, p<0.001). Guinea pigs treated with both 3 and 30 mg/kg CRAC doses showed higher HDL cholesterol levels at week 14 compared to the untreated animals (Fig. 1G) by 41% and 18% respectively, although only the 3 mg/kg dose had a significant effect (p<0.01) (F Ratio = 6.576, Prob > F = 0.0089).
Effect of CRAC peptide on the cholesterol-induced atheroma formation and associated-oxidative stress in guinea pig aorta
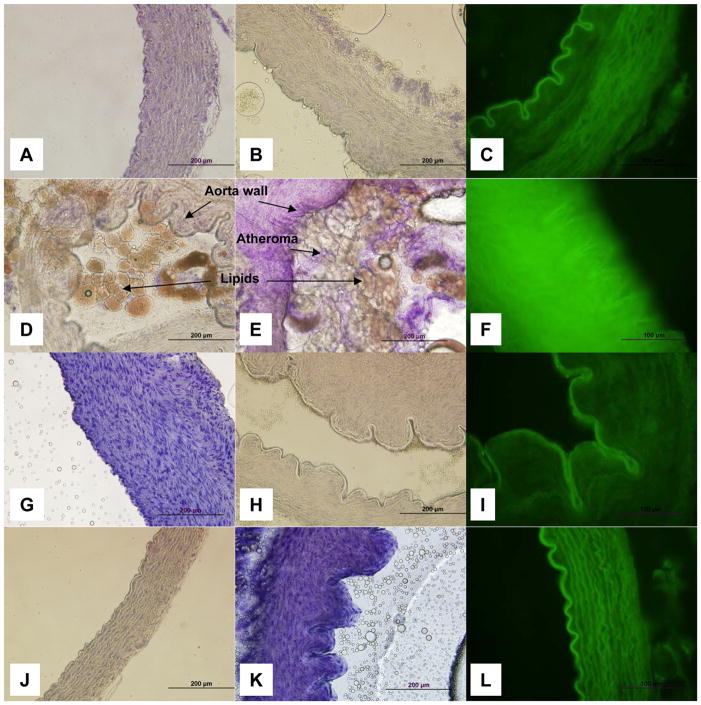
Fourteen weeks of a cholesterol-enriched diet induced the formation of atheroma and lipid deposition in guinea pig aortas (Fig. 2D and 2E) as compared to animals fed with a standard diet (Fig. 2A and 2B). Three animals from each group were used. Oil red O staining revealed that the atheroma plaques formed contain lipids. The aorta seen in Fig. 2D and 2E is almost completely obstructed by the lipid aggregates. CRAC at the doses used, 3 (Fig. 2G and 2H) and 30 mg/kg (Fig. 2J and 2K), removed the formed atheromas and prevented the formation of new atheroma deposits. In CRAC-treated guinea pigs, the aortas were similar to the animals fed with a standard diet and no lipid-containing elements were visible. The morphology, structure and cytological features of the aortas from treated animals also did not differ from the control group. The nitrotyrosine immunostaining was dramatically increased in the aorta of the cholesterol-enriched diet fed guinea pigs revealing the presence of an important oxidative stress (Fig. 3F) as compared to the standard diet fed animals (Fig. 2C). Treatment with either CRAC doses dramatically reduced the nitrotyrosine labeling (Fig. 2I and 2L) and brought it back to the levels seen in controls (Fig. 2C).
Figure 2.
Effect of CRAC treatment on aortic atheroma formation and associated oxidative stress in guinea pigs fed with high cholesterol diet. Hartley male guinea pigs were fed with standard or 2% cholesterol enriched diet for 14 weeks. CRAC treatment started at the end of week 8 until the end of the experiment. Guinea pig received one i.p. injection of CRAC solution at 3 and 30 mg/kg (2.5 ml/kg) or vehicle every other day. At the end of week 14, aortas were collected for histology, lipid histochemistry and for nitrosylated proteins immunofluorescence. This subset of experiments has been conducted on transverse section of aorta, 3 animals per group, 5 sections were analyzed per animal, and the most representative was used for illustration purpose. A–C. Standard diet. D–F. 2% cholesterol diet. G–I. 2% cholesterol diet treated with CRAC 3 mg/kg. J–L. 2% cholesterol diet treated with CRAC 30 mg/kg. A–B, D–E, G–H, J–K. Oil Red O staining and Mayer’s hematoxylin counterstaining. C, F, I, L. Primary antibody: anti-nitrotyrosine; secondary antibody: Alexa Fluor 488 conjugated anti-mouse.
Figure 3.
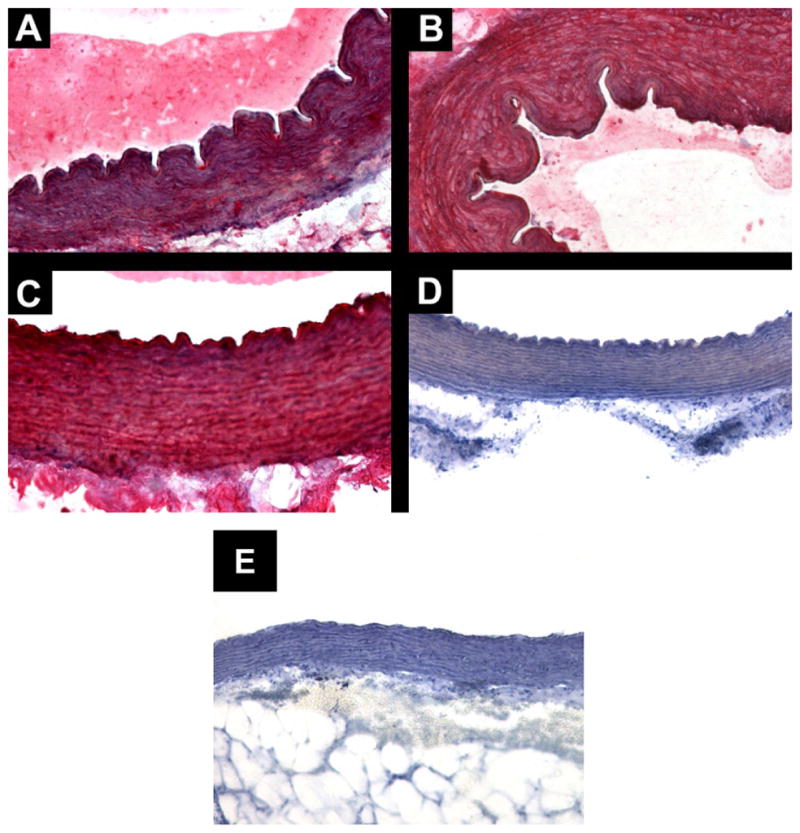
Immunolocalization of CRAC in guinea pig aortas. Transverse cut of guinea pig aortas, showing intense and diffuse immunostaining in all layers of the vessel wall in three different animals. The antibody labeling was detected by Fast Rd chromogenic substrate and tissue counterstained with hematoxylin. A. Group 3 mg/kg, anti-CRAC, magnification 200X. B. Group 30 mg/kg, anti-CRAC, magnification 200X. C: Group 30 mg/kg, anti-CRAC, magnification 400X. D. CRAC peptide pre-adsorbed antiserum, magnification X200. E. Standard diet group aorta immunostained with anti-CRAC antiserum, magnification 200X.
Immunodetection of the CRAC peptide in guinea pig aorta wall
Immunohistochemistry of sections of guinea pig aortas using an affinity-purified anti-CRAC antibody showed intense and diffuse labeling of CRAC in all layers of the blood vessel and at all CRAC peptide doses used to treat the animals fed with the cholesterol-enriched diet (Fig. 3A–C). The specificity of CRAC immunostaining was validated by showing no positive staining in the aorta of the control group (Fig. 3E) and pre-adsorbed antibody (Fig. 3D), as discussed in more detail in the discussion.
Effect of CRAC peptide on the liver weight and hepatocytes lipid content
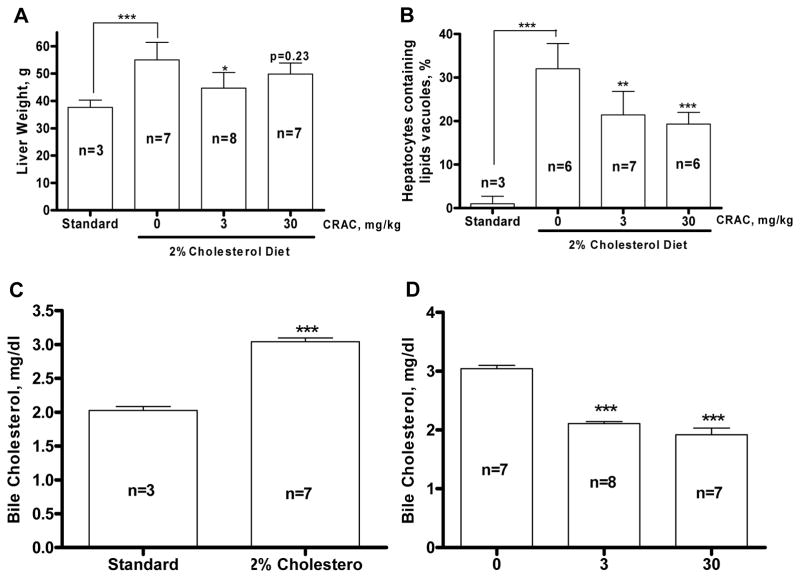
The 2% cholesterol enriched diet induced a 46% increase of the liver weight (p<0.001, Fig. 4A) compared to the control guinea pig. Concomitantly, CRAC at 3 mg/kg resulted in a 20% decrease of the liver weight compared to the vehicle treated guinea pigs (p<0.05, Fig. 4A) whereas the weight decrease observed in the animals treated with the 30 mg/kg dose was not significant at the time measured. On microscopic examination of specimens stained with Oil Red O and counterstained with hematoxylin/eosin, the scoring showed that 32±5.83% of the hepatocytes from the animals fed with the 2% cholesterol enriched diet contained lipid droplets compared to 1±1.73% for the guinea pigs fed with the standard diet (p<0.001, Fig. 4B). The CRAC treatment reduced in a dose-dependent manner the proportion of lipid droplets containing hepatocytes (21.43±5.38%, p<0.01 and 19.33±2.66%, p<0.001 respectively). Use of the 2% cholesterol diet induced a 30% increase of the bile cholesterol content compared to the standard diet fed guinea pigs (Fig. 4C). This increase was completely abolished by the two doses of CRAC (3 and 30 mg/kg, p<0.001, Fig. 4D).
Figure 4.
CRAC treatment decreases the 2% cholesterol diet-induced liver weight increase (A), liver lipid droplet content (B) and biliary cholesterol (C, D). Hartley male guinea pigs were fed with standard or 2% cholesterol enriched diet for 14 weeks. CRAC treatment started at the end of week 8 until the end of the experiment. Guinea pig received one i.p. injection of CRAC solution at 3 and 30 mg/kg (2.5 ml/kg) or vehicle every other day. Tissue and bile were collected at the end of the fourteenth week. To determine the percentage of hepatocytes that contain lipid droplets, 200 cells were counted per liver slice, every 10 slices, 5 slices per animal, stained with oil red O and counterstained with hematoxylin/eosin (B). Effect of 2% cholesterol diet on cholesterol concentration in bile (C) and effect of CRAC treatment on bile cholesterol increase in animals fed with 2% cholesterol diet (D). Statistical analyses were performed by ANOVA followed by Dunnett’s test. * p<0.05, ** p<0.01, *** p<0.001.
CRAC peptide treatment reduced cholesterol diet-induced serum creatine kinase total and isoforms level increase
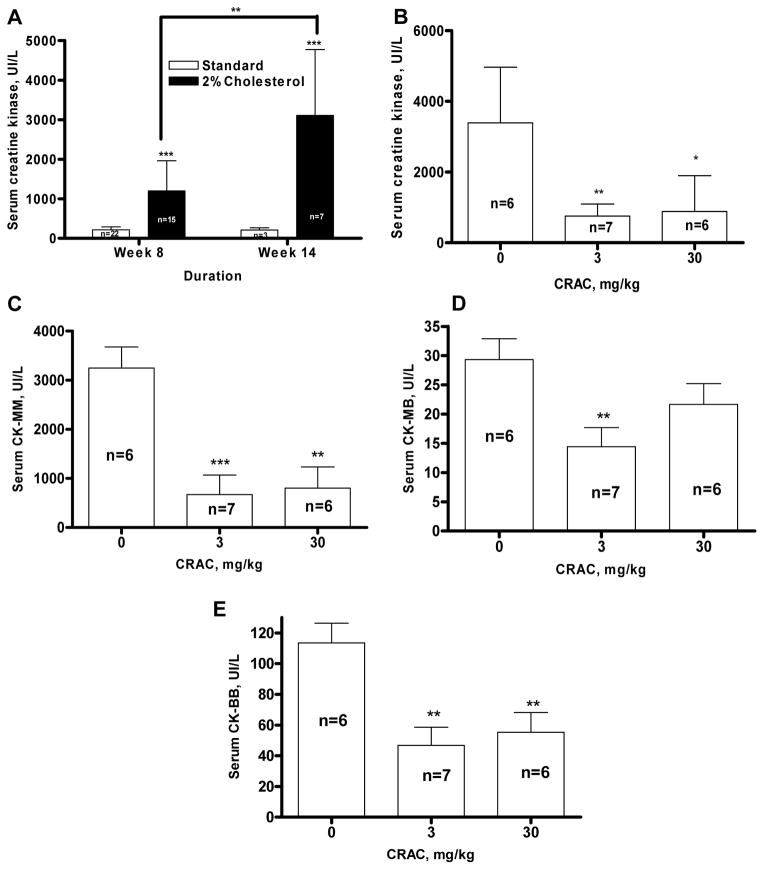
Serum creatine kinase has been used as a marker of cardiac and muscular damage. Cholesterol diet induced a progressive and sustained increase of CK levels in guinea pig serum compared to the control. This increase was detectable at week 8 (1199±762 UI/L, n=15, versus 216±75 UI/L, n=22, p<0.001) and was even more pronounced at week 14 (3105±1168 UI/L, n=7, versus 212±54 UI/L, n=3, p<0.001) (Fig. 5A). Both 3 and 30 mg/kg CRAC doses dramatically reduced the CK increase observed in the serum of guinea pigs fed with the cholesterol-enriched diet by 78% (p<0.01) and 74% (p<0.05) respectively (F ratio = 11.95, Prob > F = 0.0007) (Fig. 5B). The CRAC treatment given at the dose of 3 mg/kg decreased by 79% the CK-MM (p<0.001) (Fig. 5C), by 49% the CK-MB (p<0.01) (Fig. 5D), and by 59% the CK-BB (p<0.01) (Fig. 5E) showing that this CRAC dose was able to alleviate, in addition to the myocardial damage induced by the 2% cholesterol enriched diet, the neurological and striated muscle damage. CRAC at the dose of 30 mg/kg decreased by 75% the CK-MM (p<0.01) (Fig. 5C), and by 51% the CK-BB (p<0.01) (Fig. 5E) suggesting that it reduced striated muscle tissue and neurological suffering. At this 30 mg/kg dose of CRAC, CK-MB level were also reduced by 25% (Fig. 5C), although the effect did not seem to be significant.
Figure 5.
CRAC treatment decreases serum creatine kinase levels in guinea pigs fed with high cholesterol diet. Hartley male guinea pigs were fed with standard or 2% cholesterol enriched diet for 14 weeks. CRAC treatment started at the end of week 8 until the end of the experiment. Guinea pigs received one i.p. injection of CRAC solution at 3 and 30 mg/kg (2.5 ml/kg) or vehicle every other day. Serum total creatine kinase was measured at weeks 8 and 14 in guinea pigs fed with standard and 2% cholesterol diet (A) and at week 14 in 2% cholesterol diet fed guinea pigs treated or not with increasing doses of CRAC (B). The CK isoforms CK-MM (C), CK-MB (D) and CK-BB (E) were measured at weeks 14. Data shown are means ± SD (n, as indicated in the panels). Results were analyzed by ANOVA or Student’s t test. * p<0.05, ** p<0.01, *** p<0.001.
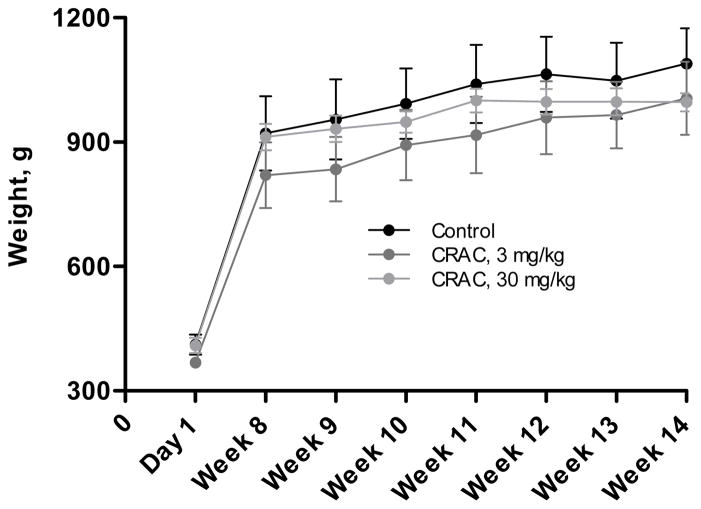
Effect of CRAC treatment on the animal weight
Monitoring the growth curve of the guinea pigs fed with the standard diet and treated with CRAC 3 or 30 mg/kg showed that, even at the highest concentration, the CRAC treatment did not have any impact on the weight gain (Fig. 6). In addition, detailed monitoring of the weight gained each week of the treatment period did not permit to detect any alteration of the growth of the treated animals.
Figure 6.
Effect of the CRAC treatment on the growth curve of guinea pigs fed with the standard diet. The weight of the guinea pigs was monitored at the beginning of the experimental protocol (Day 1), and every week until week 14. The weight was expressed in absolute value. Data shown are means ± SD (n, as indicated in the panels). Results were analyzed by ANOVA and Student’s t test.
Serum cholesterol level measurement in ApoE knock-out B6.129P2-Apoetm1Unc/J mice
CRAC peptide treatment decreased in a dose-dependent manner circulating cholesterol levels in ApoE knock-out B6.129P2-Apoetm1Unc/J mice (Fig. 7). The doses of 10, 30 and 100 mg/kg decreased the cholesterol serum level by 10% (p=0.154, n=12), 17% (p<0.01, n=11) and 21% (p<0.001, n=11) respectively compared to the control (n=11) (F ratio 5.896, Prob > F 0.001).
Figure 7.
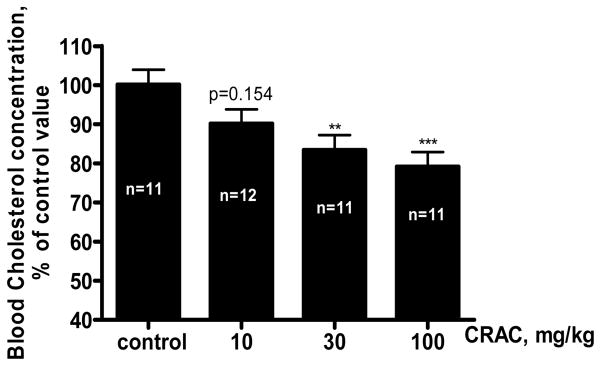
CRAC treatment decreased serum cholesterol levels in hypercholesterolemic ApoE knock-out B6.129P2-Apoetm1Unc/J mice. ApoE knock-out B6.129P2-Apoetm1Unc/J mice weighing 18–22 g at the study initiation received one daily intraperitoneal (i.p.) injection of CRAC solution at 10, 30 and 100 mg/kg (0.15 ml/kg) or vehicle for 14 days. At the end of the treatment period blood was withdrawn by cardiac puncture and total cholesterol measured as described under Materials and Methods. Data shown are means ± SD (n=11–12 as indicated in the figure). Results were analyzed by one way ANOVA and Dunnet’s test. ** p<0.01, *** p<0.001.
Discussion
Hypercholesterolemia is a major concern in western countries as a main etiology for atherosclerosis and coronary heart disease (CHD). Cholesterol lowering drugs are extensively used and in particular statins have been proven to be efficient in reducing the morbidity and mortality of these two high cholesterol related conditions. Although the statins overall safety profile is very good [19], some concerns have been raised regarding the nature of the side-effects [20;21], i.e. rhabdomyolysis, essentially because they seem completely linked to the mechanism of action of this class of drugs. Although these side-effects are fortunately rare, the application of the new recommendations for more aggressive treatment of hypercholesterolemia raises the question of their incidence in the near future. In addition, statins do not work for all patients [22]. Therefore there is a need for drugs that would not target an enzymatic pathway, as statins do, or will not induce global changes of gene expression, as fibrates do, but instead directly target cholesterol present in the blood flow. In this report, we present a proof-of-concept study suggesting that an eight amino acids sequence of the CRAC domain of the cholesterol-binding protein TSPO represents a novel cholesterol-lowering drug candidate.
CRAC treatment every other day for 7 weeks reduced in a dose-dependent manner the cholesterol enriched diet-induced hypercholesterolemia in guinea pigs. In these studies we observed that the individual cholesterol increase between week 8 and week 14 was 169% for the untreated group whereas it was 106% for the group that received the 3 mg/kg dose and only 34% for the animals treated with the highest dose of CRAC. It is interesting that this effect took place in animals continuously fed with the cholesterol-enriched diet suggesting that in a standard therapeutic procedure where specific low cholesterol diets are prescribed, the CRAC effect could occur at lower doses. In addition CRAC treatment when given at 30 mg/kg reduced the 3-fold increase of LDL cholesterol induced by high cholesterol diet. It is of a major importance the finding that guinea pigs treated with CRAC had higher HDL level than control untreated animals fed with the high cholesterol diet. Indeed, HDL cholesterol serum concentrations were 41% and 18% higher than the control untreated guinea pigs. In comparison, a recent report showed that, at best, statins increase HDL in hypercholesterolemic human by 6% resulting in a 25–30% decrease of the probability to develop CHD [23]. Recent data suggest that increasing the concentrations of HDL cholesterol could be a more relevant therapeutic target than lowering LDL cholesterol in the prevention of CHD and atheroma formation [23–25]. These data are supported by clinical data obtained with the newly developed drug evacetrapib [26;27] and dalcetrapib [28], inhibitors of cholesteryl ester transfer protein that increases HDL cholesterol in hypercholesterolemic patients. In the same line of thought, there are ongoing attempts to develop peptidomimetic analogs of Apo-AI [29]. Apo-AI is the protein constitutive of HDL and it is the current belief that drugs mimicking the 3D structure of HDL cholesterol binding pocket would display HDL-like activity, bind circulating cholesterol and clear it from the blood through the bile [30;31]. ATI5261 and D-4F are two Apo-AI peptidomimetics [32;33], 26 and 18 aminoacids residue respectively, which hypocholesterolemic and anti-atherogenic properties have been validated and proven of therapeutic interest in vitro and in various animal models of dyslipidemia [29;32–35].
In the spontaneously hypercholesterolemic ApoE knock-out B6.129P2-Apoetm1Unc/J mice, CRAC treatment also reduced in a dose-dependent manner the serum total cholesterol levels further demonstrating the cholesterol lowering properties of the CRAC peptide.
The total cholesterol lowering effect of CRAC in guinea pigs was associated to a decrease of serum free cholesterol and to a decrease of the bile total cholesterol. Knocking down the expression Apo-AI did not affect total biliary cholesterol in mice [36;37] suggesting that although preferential contributor, HDL is not the main source of biliary cholesterol. Thus, the decrease of the bile cholesterol we observed could be explained by the reduction of the free and LDL circulating cholesterol resulting from CRAC treatment. However, since the gallbladder is part of the cholesterol entero-hepatic circulation system, we wonder if this circulation could be affected by the CRAC treatment. Indeed, we could legitimately hypothesize that CRAC bound to cholesterol would be eliminated through the bile and that the complex formed would prevent the cholesterol reabsorption by the intestine identically to the bile acid sequestrants [38]. Thus, since 50% to 75% of the cholesterol eliminated through the bile is reabsorbed by the intestine, it would explain, at least in part, the efficacy of CRAC at lowering the diet-induced hypercholesterolemia. However, further studies are required to validate this hypothesis. It is noteworthy that LDL decrease induced by the CRAC treatment overpowered the combined increase of HDL, therefore resulting in a decrease of biliary cholesterol. Indeed, a potential side-effect of HDL increase to be taken seriously is the formation of gallstone consecutive to the bile saturation in cholesterol [39]. Our data suggest that CRAC pharmacodynamics may prevent such a pitfall.
The hypercholesterolemic guinea pigs displayed an important lipid droplet accumulation at microscopic examination of the hepatocytes. CRAC dramatically reduced in a dose-dependent manner the number of hepatocytes that contain lipid droplets. Further experiments are however required with guinea pigs put back on standard diet when treated with CRAC to rule out a possible lower treatment efficacy in animal fed continuously with the 2% cholesterol enriched diet. The lipid accumulation was associated with a liver weight increase that was partially corrected by CRAC given at the dose of 3 mg/kg.
Elevated cholesterol diet led to increased oxidative stress and the development of atheromas in the guinea pig aortas. CRAC at both concentrations used 3 and 30 mg/kg, completely prevented the aortic deposits with the same efficacy and decreased the nitrotyrosine immunostaining back to what we observed in the standard diet fed animals suggesting that CRAC treatment, even at its lowest dose used in this study, suppressed the atheroma-associated oxidative stress. It is interesting that CRAC suppressed the diet-induced oxidative stress. Dimitrova-Shumkovska et al. recently reported that treatment of rats with high fat high cholesterol atherogenic diet was accompanied by a reduction in TSPO binding density in the aorta wondering whether the involvement of TSPO is compensatory or contributory to oxidative stress in the aorta [11]. The finding that CRAC treatment suppresses oxidative stress induced by high cholesterol diet in guinea pig aortas suggests that reduced TSPO levels in the aorta may indeed be contributing to oxidative stress. The mechanism by which CRAC removes aortic atheromas remains to be determined and it may not be related to its action on serum cholesterol, LDL and HDL. It is noteworthy that a direct action of D-4F on cholesterol has been reported to contribute to the anti-atheroma property of this peptidomimetic independently of the increase of circulating HDL [33]. The ability of the peptide to bind cholesterol with high affinity [13;14;40] suggests a direct action on the plaque, removing cholesterol from its depot sites, thus contributing to the effect seen. The presence of the CRAC peptide in the aorta wall of the treated animals supports an on-site action. Indeed, although TSPO is present in the endothelial and smooth muscle cells of the blood vessels [5], its levels are dramatically increased in human carotid atherosclerotic plaques at the sites of macrophage infiltration [8–10] suggesting that CRAC may be competing or interfering with TSPO’s function at these sites. Considering the strength of the CRAC immunostaining seen in the atherosclerotic aortas, the lack of CRAC immunostaining in normal aortas and the fact that in control studies the anti-CRAC antiserum used showed weak crossreactivity with the entire TSPO protein (data not shown) we assume that the immunoreactive material detected represents peptide accumulation. However, at present we cannot exclude the possibility that the antibody used recognizes the native TSPO overexpressed in the atherosclerotic aortas TSPO. Interestingly, CRAC tested on normal animals had no effect suggesting that its action relates to an hyperlipidemic/atherogenic event. Moreover, CRAC administration resulted in the elimination of the oxidative stress seen in the vascular wall.
The development of atheromas was associated to a dramatic increase in serum CK levels suggesting that the heart of the exposed animals was under stress and suffering. The fact that both CRAC doses used reduce the CK elevation by more than 60% in a manner that parallels the increase in HDL cholesterol and removal of the atheromas further argues in favor of a restoration of the coronary/heart function. Indeed, determination of various CK isoforms indicated that it reduced striated muscle tissue, neurological and heart suffering. Administration of the CRAC peptide to control animals did not induce any interference with the cholesterol metabolism as total and free cholesterol, LDL, and HDL serum levels measured in standard diet fed guinea pigs treated with CRAC at 3 or 30 mg/kg remained unchanged. In addition, no sign of distress appeared in any of the standard diet fed animals treated by CRAC at either 3 or 30 mg/kg. Gross anatomy and tissue histology did not reveal any organ toxicity in liver, spleen, kidney and testis (data not shown). Furthermore, CRAC treatment did not alter the growth curve of the animals. This data led us to conclude that the CRAC peptide administered intraperitoneally up to 30 mg/kg every other day over 6 weeks is devoid of any toxicity.
Atherosclerosis and CHD are two high cost burden pathologies for which many therapeutic alternatives to fibrates and statins remain unexplored. We presented herein a novel treatment using an eight amino acid CRAC peptide found in the carboxy terminus of TSPO. The CRAC peptide significantly increased the HDL cholesterol, prevented the formation of the aortic formed atheroma and decreased the associated oxidative stress. Although we have a good understanding of the mechanism of action of CRAC to explain its ability to bind to cholesterol and alter the cholesterol-apolipoprotein interaction and remove atheromas, its mechanism of action on the regulation of circulating cholesterol, LDL and HDL levels, is not yet understood. The recent finding that Tspo is a new genomic loci regulating lipid levels [41] may provide some explanation for a TSPO-mediated effect of CRAC on lipid levels. It should be also noted that the data presented also suggest that CRAC might interfere with the cholesterol entero-hepatic circulation. Taken together all these preclinical data suggest that CRAC peptide might represent an interesting and safe prototypical drug to treat dyslipidemia and atherosclerosis and that could be used alone or in association with existing lipid lowering treatments.
Highlights.
The TSPO cholesterol recognition/interaction amino acid consensus (CRAC) was used in vivo
The VLNYYVWR CRAC sequence has hypocholesterolemic properties
The VLNYYVWR CRAC sequence has anti-atherogenic properties
CRAC treatment prevents high cholesterol diet-induced serum creatine kinase increase
The VLNYYVWR CRAC sequence has no short-term toxicity in animal models
Acknowledgments
The authors thank Drs J. Genest and A. Sniderman (Division of Cardiology, Department of Medicine, McGill University Health Centre) for critically reviewing the manuscript.
Funding
This work was supported in part by grants from the National Institutes of Health (HD037031), the Canadian Institutes of Health Research (MOP102647), contracts from Samaritan Pharmaceuticals, Las Vegas, NV, United States and Samaritan Therapeutics, Saint Laurent, Quebec, Canada, a Canada Research Chair in Biochemical Pharmacology to V.P., and a Royal Victoria Hospital Foundation award to L.L. The Research Institute of MUHC was supported by a Center grant from Le Fonds de la recherche du Québec - Santé. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Jefcoate C. High-flux mitochondrial cholesterol trafficking, a specialized function of the adrenal cortex. J Clin Invest. 2002 Oct;110(7):881–90. doi: 10.1172/JCI16771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P, et al. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006 Aug;27(8):402–9. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Lacapere JJ, Papadopoulos V. Peripheral-type benzodiazepine receptor: structure and function of a cholesterol-binding protein in steroid and bile acid biosynthesis. Steroids. 2003 Sep;68(7–8):569–85. doi: 10.1016/s0039-128x(03)00101-6. [DOI] [PubMed] [Google Scholar]
- 4.Papadopoulos V, Mukhin AG, Costa E, Krueger KE. The peripheral-type benzodiazepine receptor is functionally linked to Leydig cell steroidogenesis. J Biol Chem. 1990 Mar 5;265(7):3772–9. [PubMed] [Google Scholar]
- 5.Veenman L, Gavish M. The peripheral-type benzodiazepine receptor and the cardiovascular system. Implications for drug development. Pharmacol Ther. 2006 Jun;110(3):503–24. doi: 10.1016/j.pharmthera.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Chen MK, Guilarte TR. Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacol Ther. 2008 Apr;118(1):1–17. doi: 10.1016/j.pharmthera.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veenman L, Papadopoulos V, Gavish M. Channel-like functions of the 18-kDa translocator protein (TSPO): regulation of apoptosis and steroidogenesis as part of the host-defense response. Curr Pharm Des. 2007;13(23):2385–405. doi: 10.2174/138161207781368710. [DOI] [PubMed] [Google Scholar]
- 8.Bird JL, Izquierdo-Garcia D, Davies JR, Rudd JH, Probst KC, Figg N, et al. Evaluation of translocator protein quantification as a tool for characterising macrophage burden in human carotid atherosclerosis. Atherosclerosis. 2010 Jun;210(2):388–91. doi: 10.1016/j.atherosclerosis.2009.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujimura Y, Hwang PM, Trout IH, Kozloff L, Imaizumi M, Innis RB, et al. Increased peripheral benzodiazepine receptors in arterial plaque of patients with atherosclerosis: an autoradiographic study with [(3)H]PK 11195. Atherosclerosis. 2008 Nov;201(1):108–11. doi: 10.1016/j.atherosclerosis.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 10.Gaemperli O, Shalhoub J, Owen DR, Lamare F, Johansson S, Fouladi N, et al. Imaging intraplaque inflammation in carotid atherosclerosis with 11C-PK11195 positron emission tomography/computed tomography. Eur Heart J. 2011 Sep 19; doi: 10.1093/eurheartj/ehr367. [DOI] [PubMed] [Google Scholar]
- 11.Dimitrova-Shumkovska J, Veenman L, Ristoski T, Leschiner S, Gavish M. Chronic high fat, high cholesterol supplementation decreases 18 kDa Translocator Protein binding capacity in association with increased oxidative stress in rat liver and aorta. Food Chem Toxicol. 2010 Mar;48(3):910–21. doi: 10.1016/j.fct.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Papadopoulos V. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology. 1998 Dec;139(12):4991–7. doi: 10.1210/endo.139.12.6390. [DOI] [PubMed] [Google Scholar]
- 13.Jamin N, Neumann JM, Ostuni MA, Vu TK, Yao ZX, Murail S, et al. Characterization of the cholesterol recognition amino acid consensus sequence of the peripheral-type benzodiazepine receptor. Mol Endocrinol. 2005 Mar;19(3):588–94. doi: 10.1210/me.2004-0308. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Yao Z, Degenhardt B, Teper G, Papadopoulos V. Cholesterol binding at the cholesterol recognition/interaction amino acid consensus (CRAC) of the peripheral-type benzodiazepine receptor and inhibition of steroidogenesis by an HIV TAT-CRAC peptide. Proc Natl Acad Sci U S A. 2001 Jan 30;98(3):1267–72. doi: 10.1073/pnas.031461598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epand RM, Sayer BG, Epand RF. Caveolin scaffolding region and cholesterol-rich domains in membranes. J Mol Biol. 2005 Jan 14;345(2):339–50. doi: 10.1016/j.jmb.2004.10.064. [DOI] [PubMed] [Google Scholar]
- 16.Epand RF, Sayer BG, Epand RM. The tryptophan-rich region of HIV gp41 and the promotion of cholesterol-rich domains. Biochemistry. 2005 Apr 12;44(14):5525–31. doi: 10.1021/bi0500224. [DOI] [PubMed] [Google Scholar]
- 17.Vincent N, Genin C, Malvoisin E. Identification of a conserved domain of the HIV-1 transmembrane protein gp41 which interacts with cholesteryl groups. Biochim Biophys Acta. 2002 Dec 23;1567(1–2):157–64. doi: 10.1016/s0005-2736(02)00611-9. [DOI] [PubMed] [Google Scholar]
- 18.Zheng YH, Plemenitas A, Fielding CJ, Peterlin BM. Nef increases the synthesis of and transports cholesterol to lipid rafts and HIV-1 progeny virions. Proc Natl Acad Sci U S A. 2003 Jul 8;100(14):8460–5. doi: 10.1073/pnas.1437453100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKenney JM, Davidson MH, Jacobson TA, Guyton JR. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006 Apr 17;97(8A):89C–94C. doi: 10.1016/j.amjcard.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 20.Ambrosi P, Gayet JL, Andrejak M. The best of 2001. Clinical pharmacology. Arch Mal Coeur Vaiss. 2002 Jan;95(Spec No 1 (5 Spec 1)):33–8. [PubMed] [Google Scholar]
- 21.Bays H. Statin safety: an overview and assessment of the data--2005. Am J Cardiol. 2006 Apr 17;97(8A):6C–26C. doi: 10.1016/j.amjcard.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Tziomalos K, Athyros VG, Karagiannis A, Mikhailidis DP. Management of statin-intolerant high-risk patients. Curr Vasc Pharmacol. 2010 Sep;8(5):632–7. doi: 10.2174/157016110792006932. [DOI] [PubMed] [Google Scholar]
- 23.Rosenson RS. Low high-density lipoprotein cholesterol and cardiovascular disease: risk reduction with statin therapy. Am Heart J. 2006 Mar;151(3):556–63. doi: 10.1016/j.ahj.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 24.Lee JM, Choudhury RP. Prospects for atherosclerosis regression through increase in high-density lipoprotein and other emerging therapeutic targets. Heart. 2007 May;93(5):559–64. doi: 10.1136/hrt.2005.066050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toth PP. High-density lipoprotein as a therapeutic target: clinical evidence and treatment strategies. Am J Cardiol. 2005 Nov 7;96(9A):50K–8K. doi: 10.1016/j.amjcard.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Cao G, Beyer TP, Zhang Y, Schmidt RJ, Chen YQ, Cockerham SL, et al. Evacetrapib is a novel, potent, and selective inhibitor of cholesteryl ester transfer protein that elevates HDL cholesterol without inducing aldosterone or increasing blood pressure. J Lipid Res. 2011 Dec;52(12):2169–76. doi: 10.1194/jlr.M018069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholls SJ, Brewer HB, Kastelein JJ, Krueger KA, Wang MD, Shao M, et al. Effects of the CETP inhibitor evacetrapib administered as monotherapy or in combination with statins on HDL and LDL cholesterol: a randomized controlled trial. JAMA. 2011 Nov 16;306(19):2099–109. doi: 10.1001/jama.2011.1649. [DOI] [PubMed] [Google Scholar]
- 28.Lim GB. Coronary artery disease: Dalcetrapib safely raises HDL-cholesterol level in the phase IIb dal-PLAQUE trial. Nat Rev Cardiol. 2011;8(11):610. doi: 10.1038/nrcardio.2011.150. [DOI] [PubMed] [Google Scholar]
- 29.Sherman CB, Peterson SJ, Frishman WH. Apolipoprotein A-I mimetic peptides: a potential new therapy for the prevention of atherosclerosis. Cardiol Rev. 2010 May;18(3):141–7. doi: 10.1097/CRD.0b013e3181c4b508. [DOI] [PubMed] [Google Scholar]
- 30.Mendez AJ. The promise of apolipoprotein A-I mimetics. Curr Opin Endocrinol Diabetes Obes. 2010 Apr;17(2):171–6. doi: 10.1097/MED.0b013e3283373cb5. [DOI] [PubMed] [Google Scholar]
- 31.Navab M, Shechter I, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Fogelman AM. Structure and function of HDL mimetics. Arterioscler Thromb Vasc Biol. 2010 Feb;30(2):164–8. doi: 10.1161/ATVBAHA.109.187518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bielicki JK, Zhang H, Cortez Y, Zheng Y, Narayanaswami V, Patel A, et al. A new HDL mimetic peptide that stimulates cellular cholesterol efflux with high efficiency greatly reduces atherosclerosis in mice. J Lipid Res. 2010 Jun;51(6):1496–503. doi: 10.1194/jlr.M003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navab M, Anantharamaiah GM, Hama S, Garber DW, Chaddha M, Hough G, et al. Oral administration of an Apo A-I mimetic Peptide synthesized from D-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation. 2002 Jan 22;105(3):290–2. doi: 10.1161/hc0302.103711. [DOI] [PubMed] [Google Scholar]
- 34.Liu XH, Xiao J, Mo ZC, Yin K, Jiang J, Cui LB, et al. Contribution of D4-F to ABCA1 expression and cholesterol efflux in THP-1 macrophage-derived foam cells. J Cardiovasc Pharmacol. 2010 Sep;56(3):309–19. doi: 10.1097/FJC.0b013e3181edaf69. [DOI] [PubMed] [Google Scholar]
- 35.Song X, Fischer P, Chen X, Burton C, Wang J. An apoA-I mimetic peptide facilitates off-loading cholesterol from HDL to liver cells through scavenger receptor BI. Int J Biol Sci. 2009;5(7):637–46. doi: 10.7150/ijbs.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ji Y, Wang N, Ramakrishnan R, Sehayek E, Huszar D, Breslow JL, et al. Hepatic scavenger receptor BI promotes rapid clearance of high density lipoprotein free cholesterol and its transport into bile. J Biol Chem. 1999 Nov 19;274(47):33398–402. doi: 10.1074/jbc.274.47.33398. [DOI] [PubMed] [Google Scholar]
- 37.Jolley CD, Dietschy JM, Turley SD. Induction of bile acid synthesis by cholesterol and cholestyramine feeding is unimpaired in mice deficient in apolipoprotein AI. Hepatology. 2000 Dec;32(6):1309–16. doi: 10.1053/jhep.2000.19811. [DOI] [PubMed] [Google Scholar]
- 38.Insull W., Jr Clinical utility of bile acid sequestrants in the treatment of dyslipidemia: a scientific review. South Med J. 2006 Mar;99(3):257–73. doi: 10.1097/01.smj.0000208120.73327.db. [DOI] [PubMed] [Google Scholar]
- 39.Dikkers A, Tietge UJ. Biliary cholesterol secretion: more than a simple ABC. World J Gastroenterol. 2010 Dec 21;16(47):5936–45. doi: 10.3748/wjg.v16.i47.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindemann P, Koch A, Degenhardt B, Hause G, Grimm B, Papadopoulos V. A novel Arabidopsis thaliana protein is a functional peripheral-type benzodiazepine receptor. Plant Cell Physiol. 2004 Jun;45(6):723–33. doi: 10.1093/pcp/pch088. [DOI] [PubMed] [Google Scholar]
- 41.Leduc MS, Hageman RS, Verdugo RA, Tsaih SW, Walsh K, Churchill GA, et al. Integration of QTL and bioinformatic tools to identify candidate genes for triglycerides in mice. J Lipid Res. 2011 Sep;52(9):1672–82. doi: 10.1194/jlr.M011130. [DOI] [PMC free article] [PubMed] [Google Scholar]