Abstract
Objective
To investigate the associations between obstructive sleep apnea (OSA) and maternal and neonatal morbidities in a cohort of obese gravid women.
Methods
Participants were enrolled in a prospective observational study designed to screen for OSA and describe the possible risk factors for and outcomes of OSA among obese (BMI 30 kg/m2 or higher) pregnant women. Women underwent an overnight sleep study using a portable home monitor. Studies were manually scored by a central masked Sleep Reading Center using American Academy of Sleep Medicine diagnostic criteria. An apnea hypopnea index of 5 or greater was considered diagnostic of OSA. Perinatal outcomes were compared between women with and without OSA.
Results
Among 175 women, OSA prevalence was 15.4% (13 mild, 9 moderate, 5 severe). Compared with no-OSA (AHI<5), the OSA group had a higher BMI (46.8 ±12.2 vs. 38.1± 7.5 kg/m2, p=0.002) and more chronic hypertension (55.6 vs. 32.4%, p=0.02). Maternal complications included: maternal death (n=1, amniotic fluid embolus [no-OSA group]) and cardiac arrest (n=1, intraoperative at cesarean delivery [OSA group]). One previable birth and two stillbirths occurred in the no-OSA group. Among live births, OSA was associated with more frequent cesarean delivery (65.4 vs. 32.8%, p=0.003), preeclampsia (42.3 vs. 16.9, p=0.005), and NICU admission (46.1 vs. 17.8, p=0.002). After controlling for BMI, maternal age, and diabetes, OSA (OR 3.55 [1.1–11.3]), prior preeclampsia (OR 2.79 [1.09–7.19]), and hypertension (4.25 [1.67–10.77]) were associated with developing preeclampsia.
Conclusion
OSA among obese pregnant women is associated with more frequent preeclampsia, neonatal intensive care unit admissions, and cesarean delivery.
In 2009–2010, 36% of adult women and 16% of children and adolescents in the United States were reported to have a body mass index (BMI) in the obese range (≥30 kg/m2). This number is projected to increase to 42% in adults by the year 2030.(1–3) With rates of both adult and adolescent obesity increasing, the prevalence of obesity among women of childbearing age (aged 15–44 y) can also be expected to increase. Population and cohort studies consistently indicate that obesity is associated with an increased risk of comorbid conditions during pregnancy, cesarean delivery, anesthesia complications, venous thromboembolism and maternal mortality.(4–6) Obesity also has implications for the fetus, which may suffer short term consequences resulting from medically indicated preterm birth and macrosomia, and also long-term implications related to fetal in utero programming.(7) These sober facts have led to increased focus on the mitigation of obesity and its pregnancy related morbidities.
One area of increased investigation in pregnancy has been related to obstructive sleep apnea (OSA). Obstructive sleep apnea, a condition characterized by recurrent cycles of upper airway obstruction, nocturnal hypoxemia, reoxygenation, and sleep fragmentation, is predominately an obesity related comorbid condition.(8, 9) Obesity and weight gain are associated with the development and worsening OSA.
Depending on the definition used, OSA prevalence among reproductive age women has been estimated to be 0.7–5%. But OSA remains underdiagnosed and understudied among reproductive age women.(10, 11) Despite consistent data on the adverse cardiovascular effects of the disease in the nonpregnant population, data regarding the significance of the disease and its effects on reproduction remain scarce. Emerging data indicates an increased risk of gestational diabetes, preeclampsia and small for gestational age neonates in these women, but much of the currently available data is limited to case reports, or studies that lack appropriate control groups, objective testing for OSA, or lack adjustment for obesity.(12, 13) It has become evident that obesity is an important confounding factor and the independent role of OSA is unclear. Therefore, we sought to examine the independent effects of OSA on maternal pregnancy outcomes and neonatal morbidities in a cohort of obese women. We hypothesized that women with OSA would have an increased risk of perinatal morbidities when compared with comparable weight women without OSA.
METHODS
The patients in this study were enrolled in the Sleep Apnea in Pregnancy Screening Study (SAPSS), a prospective observational cohort study in which obese pregnant women were screened for sleep related breathing disorders. The primary objective of this study was to develop a screening tool to detect OSA among obese pregnant women. The data presented in this manuscript are the results of the preplanned secondary objective which was to identify the risk factors for OSA and to describe the pregnancy outcome among these women. The site for enrollment was a single urban tertiary care center which serves as a regional perinatal referral center. Recruitment occurred between September 2008 and August 2011. Approval from MetroHealth Medical Center’s IRB was obtained prior to the start of the study and each participating subject provided informed consent. Subjects were offered enrollment if they were obese (pre-pregnancy BMI ≥30 kg/m2), age ≥18 years and were willing to be adherent with the study protocol. All gestational ages were included. Exclusion criteria were; chronic use of narcotic or other drugs affecting the central nervous system, and inability to maintain sleep beyond 2 hours. As this was a study that relied on the use of a device, women with a documented history of non-adherence (missing >3 clinic visits) were also excluded. (Figure 1.) In order to limit selection bias, subjects were recruited from both general care and high risk obstetrics clinics.
Figure 1.
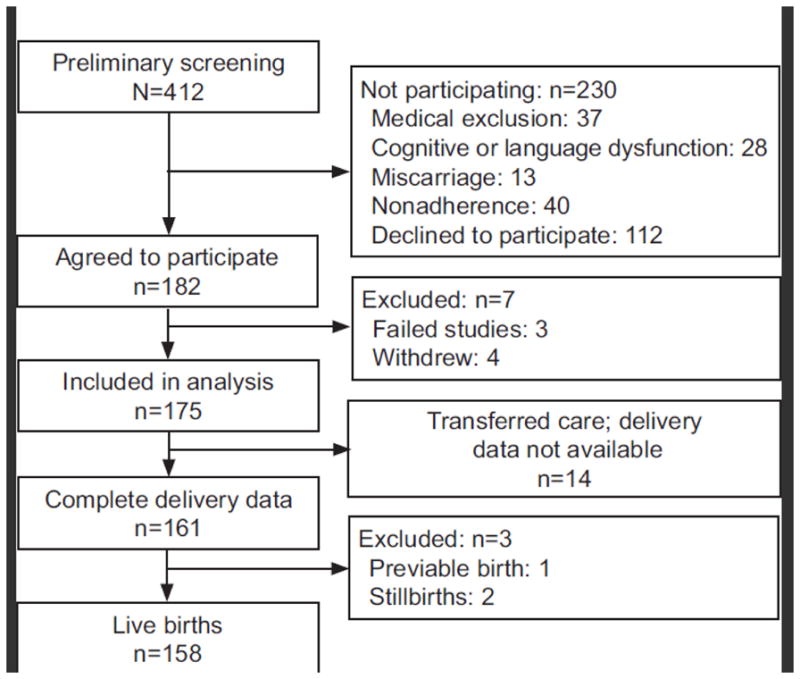
Enrollment.
Objective testing for OSA was performed using in-home portable polysomnography with the ARES Unicorder 5.2 (B-Alert, Carlsbad, CA), a self-applied wireless physiological recorder worn on the forehead. The device measures airflow using a nasal pressure cannula, blood hemoglobin oxygen saturation (SpO2) and pulse rate using reflectance oximetry, head movement and head position using actigraphy, and snoring levels via an acoustic microphone. After the patient had finished the study, the raw data were uploaded, the files transferred via secure server to an independent blinded Sleep Reading Center for manual scoring of the data. Apnea was defined as cessation of airflow for ≥10 seconds. Hypopnea was defined as a ≥50% reduction in airflow with an associated ≥3% decrease in oxygen saturation. Sleep time was estimated from artifact free recording times. The Apnea Hypopnea Index (AHI) was calculated as the sum of all apnea plus hypopnea events divided by estimated sleep time.(14)
An AHI of <5/hour was considered as “no-OSA” and an AHI ≥ 5/hour was considered “OSA”. In addition, severity was described as: 5–15/hour (mild), 16–29/hour (moderate), and ≥30/hour (severe).(15) Subjects with an AHI≥5/hour were referred for an in laboratory overnight polysomnogram, for evaluation by a Sleep Medicine specialist, and their primary obstetric provider was notified. While CPAP was recommended to all who tested positive for OSA, only one subject started CPAP during the pregnancy; at 34 weeks’ gestation. All the other subjects were untreated.
A randomly selected subset of 19 women underwent concurrent ARES Unicorder monitoring and in laboratory overnight full montage PSG. After the studies were conducted, the in-home ARES data was downloaded and transmitted to the Sleep Reading Center. The in-laboratory PSG was scored by the team of clinical sleep technicians using the same criteria as the sleep laboratory. The two teams were masked to the scoring result of the opposing team.
All subjects received obstetrical care by their physician or nurse practitioner. No alteration of obstetrical care was recommended or mandated for study participants. Women with OSA were monitored in Labor and Delivery for 24 to 48 hours post-delivery if they demonstrated intrapartum hypoxemia.(16) The medical records of all enrolled women and their infants were reviewed after their discharge from the hospital, and information regarding antepartum course, delivery, and post-delivery complications (up to 6 weeks) was recorded. Chart abstraction was performed by an individual masked to study OSA status. Obtained maternal data included; age, race, marital status, medical insurance, obstetric history, gestational age at enrollment, pre-pregnancy weight and BMI, total weight gain during pregnancy, parity, and co-morbidities which included hypertension, pre-gestational diabetes mellitus, gestational diabetes, asthma, and tobacco use.
American College of Obstetricians and Gynecologists clinical definitions used for diagnosis of the co-morbidities mentioned above include: Chronic hypertension (blood pressure ≥140 mmHg systolic or ≥90 mmHg diastolic based upon the average of two or more readings at each of two or more visits before 20 weeks’ or use of antihypertensive medication prior to pregnancy), pre-gestational diabetes mellitus (any of the following at the initial prenatal visit: fasting plasma glucose ≥ 126 mg/dL, Hemoglobin-A1c ≥ 6.5%, or random plasma glucose ≥ 200 mg/dl), gestational diabetes (at least one abnormal result on a two hour 75 gram oral glucose tolerance test, or at least 2 abnormal values on a three hour 100gram oral glucose tolerance test during pregnancy).(17) Preeclampsia was defined as; new onset hypertension (systolic BP≥140 mmHg or diastolic BP≥90 mmHg) occurring at or after 20 weeks of gestation in a woman with previously normal blood pressure with documented proteinuria. Proteinuria was defined as total protein excretion of 300 mg or more in a 24-hour urine sample.(18) Preterm delivery and severe preterm delivery were defined as delivery before 37 weeks and 0 days and before 32 weeks and 0 days of gestation, respectively, by best obstetrical estimate.
Clinical outcomes for neonatal respiratory disorders were determined as documented in the medical record by NICU clinicians. Respiratory distress syndrome (RDS) was typically defined as respiratory symptoms (e.g., grunting, flaring, tachypnea, and retractions) with need for supplemental oxygen and NICU admission for further respiratory support, and with the diagnosis verified by chest radiograph findings of a reticulogranular pattern and air bronchograms. Other respiratory outcomes included transient tachypnea of the newborn, pneumonia (chest radiograph verification required), pneumothorax, meconium aspiration, pulmonary hypoplasia, and respiratory failure.
The sample size was determined by the primary objective of the Sleep Apnea in Pregnancy Study which was to develop a tool to screen obese pregnant women for OSA. Assuming a 60% detection rate using the currently available screening questionnaires and an 80% detection rate using our newly developed questionnaire, the SAPSS study would need 182 subjects (α=0.05, power=0.80) to detect a difference. An apriori power analysis was not performed for the secondary aim of the study and therefore, this analysis may be underpowered for the secondary outcomes. Study data were collected and managed using REDCap electronic data capture tools hosted at MetroHealth Medical Center.(19) The agreement between ARES and Full Polysomnogram monitoring was measured using the Lin concordance correlation coefficient (rhoc) and Bradley-Blackwood test, which simultaneously compares the means and variances of two measurements. ‘Good’ agreement was defined as rhoc in (0.6–0.74) range and a non-significant Bradley-Blackwood test.(20–22)
Nominal data were analyzed with χ2 or Fisher’s exact test. Student’s t-test was used for continuous data, and Wilcoxon rank sum test was used for ordinal or non-normally distributed data. Normality was assessed using the Shapiro-Wilk test. Data analysis of outcome variables was limited to subjects with a complete data set. Outcome variables were adjusted for age, race and BMI as these variables are predicted of both OSA and perinatal morbidity. Multivariable logistic regression model was performed to study the association between preeclampsia and OSA after adjusting for the effect of potential confounders. The model goodness-of-fit was established using the Hosmer-Lemeshow’s test. We also sought to control and test for the logit model misspecification using linktest (absence of a linear relationship between the logit of the outcome and predictor variables) and multicollinearity among the predictor variables by assessing the tolerance levels for each variable. All analyses were performed with Stata 11.2
This study is registered in the NIH Clinical Trials registry (NCT01585844).
RESULTS
A total of 182 consenting women provided 175 complete portable PSG data and were further evaluated. The prevalence of OSA was 15.4% (95% CI: 10.4–21.6%) (13 mild, 9 moderate, 5 severe). Women with OSA had a median AHI of 12.9 events per hour of sleep and spent 6.5 ± 2.5% of the night with an SpO2 below 90%. There was good agreement between the ARES AHI and PSG AHI (rhoc =0.70, 95% CI: 0.47–0.92; p < 0.001; Bradley-Blackwood p=0.27).
Fourteen women transferred care from our institution and complete delivery data were not available. These women were excluded from further analysis. Demographic and clinical characteristics of the cohort are presented in Table 1. Compared with controls, the OSA group was older but similar in racial composition and insurance status. Women with OSA had a higher mean BMI (46.8±12.2 vs. 38.1±7.5 kg/m2, p=0.002) and were more likely to be diagnosed with asthma (48.1% vs. 22.9%, p=0.007). They also had more frequent chronic hypertension (55.6% vs. 32.4%, p=0.02), but the rate of pregestational diabetes (18.5% vs. 20.3%, p=0.83) was similar between groups.
Table 1.
Clinical and Demographic Factors by Study Group
| No Obstructive Sleep Apnea (Apnea Hypopnea Index Less Than 5) n=135 |
Obstructive Sleep Apnea (Apnea Hypopnea Index 5 or Greater) n=26 |
P | |
|---|---|---|---|
|
| |||
| Age (years) | 27.3 ± 5.9 | 30 ± 6.44 | 0.04 |
|
| |||
| Race | |||
| Black | 87(64.4) | 16(61.5) | 0.96 |
| Caucasian | 31 (23.0) | 7 (26.9) | |
| Hispanic | 17(12.6) | 3(11.5) | |
|
| |||
| Public Insurance | 113(83.7) | 23 (88.5) | 0.77 |
|
| |||
| Smoking | 33(24.4) | 8(30.8) | 0.11 |
|
| |||
| Prepregnancy BMI (kg/m2) | 39.1 ± 6.2 | 48.3 ± 11.8 | <0.001 |
|
| |||
| Nulliparous | 36(26.7) | 8 (30.8) | 0.53 |
|
| |||
| Chronic hypertension | 45 (33.3) | 15 (57.7) | 0.02 |
|
| |||
| Pregestational diabetes mellitus | 28 (20.7) | 5 (19.2) | 0.83 |
|
| |||
| Gestational diabetes* | 13(10.6) | 4(19) | 0.40 |
|
| |||
| Asthma | 31 (23.0) | 13 (50) | 0.005 |
|
| |||
| Gestational age at diagnosis (weeks’) | 21.1 ±7.6 | 21.2 ±8.5 | 0.95 |
|
| |||
| Gestational weight gain (kg) | 9.5 ± 8 | 7.2 ± 7.89 | 0.17 |
BMI, body mass index.
Data are n (%) or mean ± standard deviation.
Among women without pregestational diabetes mellitus.
Perinatal and newborn outcomes for 158 evaluated subjects with available outcome data are presented in Table 2. There was one previable birth at 22 weeks’ and 2 stillbirths; all three losses occurred in the control group. The cesarean delivery rate was 38% for the overall cohort. Among the 63 cesarean deliveries, indications included; elective repeat cesarean (40%), arrest of labor (25%), abnormal fetal heart rate tracing (19%), fetal malpresentation (10%), suspected macrosomia (3%) and prior shoulder dystocia (3%). Women with OSA were more likely to have a cesarean delivery than the control group (65.4 vs. 32.8%, p = 0.003) and more likely to develop preeclampsia (42.3 vs. 16.9%, p=0.005), but had similar rates of preterm birth (17.6 vs. 18.5%, p=0.91).
Table 2.
Association Between Obstructive Sleep Apnea and Obstetric and Newborn Outcomes
| No Obstructive Sleep Apnea n=135 |
Obstructive Sleep Apnea n=26 |
Unadjusted Odds ratio (95% CI) | Adjusted Odds Ratio (95% CI)* | |
|---|---|---|---|---|
|
| ||||
| Delivery gestational age (weeks) | 38.1±2.9 | 37.5±2.2 | ||
|
| ||||
| Birth weight (grams) | 3139.6±771.7 | 3079.1±535.9 | ||
|
| ||||
| Preterm birth | ||||
| Before 37 weeks | 26 (18.5) | 5 (17.6) | 1.07 (0.37–3.08) | 0.63 (0.18–2.24) |
| Before 32 weeks | 7 (4.7) | 1(3.7) | 0.77 (0.09–6.56) | 0.94 (0.1–8.92) |
|
| ||||
| Cesarean† | 45(32.8) | 17 (65.4) | 3.86 (1.6–9.33) | 3.04 (1.14–8.1) |
|
| ||||
| Preeclampsia† | 23 (16.9) | 11 (42.3) | 3.6 (1.47–8.84) | 3.54 (1.26–9.92) |
|
| ||||
| Wound complications† | 5(3.7) | 4 (15.4) | 4.72 (1.17–18.9) | 3.44 (0.7–16.93) |
|
| ||||
| NICU admission† | 24 (17.8) | 12 (46.1) | 3.96 (1.63–9.63) | 3.39 (1.23–9.32) |
|
| ||||
| Hyperbilirubinemia† | 40(30.3) | 15 (57.6) | 3.14 (1.32–7.43) | 3.63 (1.35–9.76) |
|
| ||||
| Respiratory morbidity | 21 (15.9) | 6 (23.1) | 1.58 (0.57–4.42) | 1.56 (0.5–4.59) |
NICU, neonatal intensive care unit.
Data are n (%) or mean± standard deviation.
Adjusted for age, race and body mass index.
P<0.05.
Severe maternal complications occurred in 6 of 158 women (3.8%), highlighting the high-risk nature of this population. These complications included; Maternal death (N=1, amniotic fluid embolus in the control group) and cardiac arrest (N=1, intraoperative at cesarean delivery in the OSA group). Operative complications included one re-operation for cystotomy and two cases of acute blood loss anemia requiring transfusion in the control group. Postpartum complications included one readmission for pulmonary embolus within 2 weeks after delivery in the OSA group.
Among live births, the two groups had a similar gestational age at delivery and birth weights. There were 6 neonates classified as small for gestational age (1 in the OSA group and 5 in the control group) and 2 neonates classified as large for gestational age (both in the control group). OSA was associated with more frequent NICU admission (46 vs. 17.8%, p=0.002) and hyperbilirubinemia (57.6 vs. 30.3%, p=0.009) but similar rates of respiratory morbidity. (Table 2). The primary indications for NICU admission included respiratory complications (N= 26), prematurity (N=5), malformations (N=3), hypoglycemia (N=1) and observation (N=1).
There was one case of hypoxic ischemic encephalopathy in the OSA group.
Results of the logistic regression examining factors associated with preeclampsia are presented in Table 3. The model provided a good fit (P = 0.90) and was correctly specified (P = 0.80). There was no evidence of multicollinearity (the tolerance was > 79%). After controlling for maternal age, chronic hypertension, prior preeclampsia, BMI, and pregestational diabetes, OSA remained significantly associated with preeclampsia (OR 3.55 [1.12–11.3]).
Table 3.
Factors Associated With Preeclampsia
| Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI) | |
|---|---|---|
| Obstructive sleep apena | 3.60 (1.47, 8.84) | 3.55 (1.12–11.3) |
| Maternal age | 0.99 (0.94, 1.06) | 0.94 (0.87 – 1.03) |
| Prior preeclampsia | 3.28 (1.45, 7.44) | 2.79 (1.09–7.19) |
| BMI | 0.98 (0.94, 1.02) | 0.98 (0.94–1.04) |
| Hypertension | 5.31 (2.36, 11.98) | 4.25 (1.67–10.77) |
| Diabetes | 1.61 (0.66, 3.87) | 1.57 (0.51–4.84) |
CI, confidence interval; BMI, body mass index.
DISCUSSION
We have found a 15.4% prevalence of OSA among obese pregnant women. These women, who suffered frequent obesity related comorbid conditions, had a high rate of cesarean delivery and preeclampsia. Additionally, the NICU admission rate was high in this cohort of women.
Our findings are consistent with prior published studies in pregnant women. In a case control study of women with PSG confirmed OSA, preeclampsia was more prevalent among those women with OSA compared with normal weight and obese controls (30% vs. 10% and 12%, respectively; P< .01).(13) Another large population based study of 759 Chinese women with confirmed OSA demonstrated an increased risk of gestational hypertension (3.18 [2.14–4.73]) and preeclampsia (1.60 [2.16–11.26]) associated with OSA.(23) Other large studies have relied on symptom-based screening in clinic-based populations and found a similar 2 fold odds of developing preeclampsia in the presence of sleep apnea symptoms (24). However, most prior studies have not considered a comprehensive set of risk factors, such as prior preeclampsia, ethnicity and gestational weight gain in addition to comorbid conditions.(25). Our findings appear to suggest that OSA may have an independent association with preeclampsia after controlling for these major confounding variables.
In the general population obesity and obesity-related comorbid conditions such as chronic hypertension, diabetes, weight gain and older age are well identified risk factors for OSA.(26, 27) Our data appear to confirm the presence of those same risk factors in this younger population. In our cohort, the women with OSA were heavier and had more chronic hypertension, consistent with the reports in the general population. Conversely, we did not find an increased prevalence of pregestational diabetes mellitus or gestational diabetes (GDM), perhaps due to the high prevalence of obesity overall in the USA. This is in contrary to a recently published report of 759 pregnant women not selected on the basis of obesity. In that study, OSA was associated with gestational diabetes (OR 1.6 [1.07–2.8]).(23) However, that population-based study estimated BMI and obesity using ICD-9 diagnosis, which could result in misclassification. In fact, the reported obesity rate in that cohort was just 5%. Other reports of the association between sleep-related breathing disorders and pregnancy related diabetes have relied on symptom-based screening and not objective testing.(1, 24, 28) Our inability to find a difference in the frequency of diabetes among women with OSA may be related to the obesity of our overall cohort and to the small number of subjects. Nonetheless, this highlights the importance of obesity as a significant confounding variable when studying pregnancy outcomes among women with OSA.
We were surprised to find the increased NICU admission rates among the offspring of women with OSA despite similar frequencies of preterm birth between groups. Many of these admissions were secondary to respiratory morbidity in the neonate. The relationship between respiratory morbidity and cesarean delivery has been well described. Epidemiological and observation studies have demonstrated that term neonates with transient tachypnea of the newborn (TTN) have a 4-fold increased odds of being delivered via cesarean.(29) Gestational age also influences neonatal respiratory morbidity. In an analysis of data from a large observational cohort of 13,258 women undergoing elective cesarean delivery at 19 centers across the United States, respiratory morbidity incidence decreased with increasing term gestational age between 37 weeks and 39 weeks. (30) Compared to women delivering at 39 weeks, women who delivered at 38 or 37 weeks’ were 1.5 to 2 times as likely to have a delivery complicated by NICU admission and 1.7 to 2.5 times as likely to have a baby with respiratory morbidity. This reported relationship may further explain our findings. The women with OSA were less likely to be admitted for spontaneous labor and were more likely to undergo induction of labor and had higher cesarean delivery rates. They delivered at term but on average 1 week earlier than the group without OSA. Nonetheless, there may be alternative explanations. As we move forward and study the pathophysiological mechanisms of OSA, it will be important to bear in mind the potential consequences of early term birth.
Another unexpected finding of our study was the occurrence of maternal death and the cardiac arrest in this small group of patients. However it does highlight the risk of pregnancy in the obese woman. Maternal mortality rates have plateaued in recent decades. Obesity is a risk factor for precipitating or exacerbating all of the most common causes of maternal death after live birth in the USA (hemorrhage, hypertensive disorders of pregnancy, cardiovascular conditions, cardiomyopathy, infection and thrombotic pulmonary embolism).(31) Comorbid conditions also play a role. In population-based studies of near-miss morbidity/mortality, the most common comorbidities are hypertensive disorders of pregnancy (34.7%), previous cesarean delivery (15.7%), diabetes mellitus (10.5%) and preexisting hypertension (10.2%).(32)
Our study has several limitations that require consideration. Our cohort was limited to obese patients so we could not comment on the implications of the disease in women who are not obese. Despite limiting ourselves to obese individuals, the women with OSA on average had a BMI that was greater than the control group. Therefore, there may still be an obesity-related effect that we did not fully account for in our analyses. In that case we may be overestimating the effect of OSA. In 86% of the subjects, OSA was diagnosed based on a one night study. Night to night variability in AHI has been described and as a result, there may have been some misclassification of OSA. Some patients transferred their care out of the system and we were unable to obtain data on their deliveries. Those women were mostly in the control group. If they had severe outcomes too, we may be overestimating the observed effect size. Finally, our cohort included only a limited number of women with severe OSA. This, coupled with a relatively small number of observed cases of preeclampsia, NICU admission and hypertension, prevented evaluation of the impact of severity of disease on pregnancy outcomes and suggesting that both the adjusted and unadjusted odds ratios should be interpreted with caution. Nonetheless, these results are an important preliminary investigation of the morbidity to be expected in obese women with OSA. In conclusion, obese women with OSA have high rates of other obesity-related comorbid conditions and poor pregnancy outcomes. An optimal approach to decrease morbidity in women with OSA should be directed at treatment of obesity prior to pregnancy, which might also improve co-morbid OSA. Nonetheless, weight loss is often difficult. Evidence of OSA operating as an independent risk factor for adverse maternal and neonatal outcomes also supports the need to address ways to better screen and treat OSA in pregnancy.
Acknowledgments
Supported by the Robert Wood Johnson Foundation Physician Faculty Program and the Case Western Reserve University/Cleveland Clinic CTSA Grant Number UL1 RR024989 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Financial Disclosure: Dr. Redline received a grant from ResMed Foundation and research equipment from Resmed Inc and Philips Respironics. The other authors did not report any potential conflicts of interest.
Presented at the 32nd annual meeting of the Society for Maternal-Fetal Medicine, Dallas, TX, Feb. 6–11, 2012.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of Obesity and Trends in the Distribution of Body Mass Index Among US Adults, 1999–2010. JAMA: The Journal of the American Medical Association. 2012 Feb 1;307(5):491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B, et al. Obesity and Severe Obesity Forecasts Through 2030. American Journal of Preventive Medicine June. 2012;42(6) doi: 10.1016/j.amepre.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Obesity and Trends in Body Mass Index Among US Children and Adolescents, 1999–2010. JAMA: The Journal of the American Medical Association. 2012 Feb 1;307(5):483–90. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ovesen P, Rasmussen S, Kesmodel U. Effect of Prepregnancy Maternal Overweight and Obesity on Pregnancy Outcome. Obstetrics & Gynecology. 2011;118(2 Part 1):305–12. doi: 10.1097/AOG.0b013e3182245d49. [DOI] [PubMed] [Google Scholar]
- 5.Vricella LK, Louis JM, Mercer BM, Bolden N. Impact of morbid obesity on epidural anesthesia complications in labor. American Journal of Obstetrics and Gynecology. 2011;205(4):370.e1–e6. doi: 10.1016/j.ajog.2011.06.085. [DOI] [PubMed] [Google Scholar]
- 6.Goffman D, Madden RC, Harrison EA, Merkatz IR, Chazotte C. Predictors of maternal mortality and near-miss maternal morbidity. J Perinatol. 2007;27(10):597–601. doi: 10.1038/sj.jp.7211810. [DOI] [PubMed] [Google Scholar]
- 7.Catalano PM, Ehrenberg HM. Review article: The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG: An International Journal of Obstetrics & Gynaecology. 2006;113(10):1126–33. doi: 10.1111/j.1471-0528.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 8.Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions Between Obesity and Obstructive Sleep Apnea. Chest. 2010 Mar 1;137(3):711–9. doi: 10.1378/chest.09-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA: the journal of the American Medical Association. 2000 Dec 20;284(23):3015–21. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 10.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, et al. Prevalence of sleep-disordered breathing in women: effects of gender. American journal of respiratory and critical care medicine. 2001 Mar;163(3 Pt 1):608–13. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 11.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. The New England journal of medicine. 1993 Apr 29;328(17):1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 12.Venkata C, Venkateshiah SB. Sleep-Disordered Breathing During Pregnancy. J Am Board Fam Med. 2009 Mar 1;22(2):158–68. doi: 10.3122/jabfm.2009.02.080057. [DOI] [PubMed] [Google Scholar]
- 13.Louis JM, Auckley D, Sokol RJ, Mercer BM. Maternal and neonatal morbidities associated with obstructive sleep apnea complicating pregnancy. American Journal of Obstetrics and Gynecology. 2010;202(3):261.e1–e5. doi: 10.1016/j.ajog.2009.10.867. [DOI] [PubMed] [Google Scholar]
- 14.Iber C, Redline S, Kaplan Gilpin A, Quan S, Zhang L, Gottlieb D, et al. Polysomnography performed in the unattended home versus the attended laboratory setting--Sleep Heart Health Study methodology. Sleep. 2004;1(27):536–40. doi: 10.1093/sleep/27.3.536. [DOI] [PubMed] [Google Scholar]
- 15.CI, Ancoli-Israel S, AC, SFQ, editors. Medicine ftAAoS. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, Ill: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 16.Louis J, Auckley D, Bolden N. Management of Obstructive Sleep Apnea in Pregnant Women. Obstetrics & Gynecology. 2012;119(4):864–8. doi: 10.1097/AOG.0b013e31824c0c2f. [DOI] [PubMed] [Google Scholar]
- 17.American College of Obstetricians and Gynecologists. Practice Bulletin No. 30. Gestational Diabetes Obstet Gynecol. 2001;98:525–38. [Google Scholar]
- 18.American College of Obstetricians and Gynecologists. Practice Bulletin No 33: Diagnosis and Management of Preeclampsia and Eclampsia. Obstetrics & Gynecology. 2002;99(1):159–67. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradley EL, Blackwood LG. Comparing Paired Data - a Simultaneous Test for Means and Variances. Am Stat. 1989 Nov;43(4):234–5. [Google Scholar]
- 21.Cicchetti DV. The precision of reliability and validity estimates re-visited: distinguishing between clinical and statistical significance of sample size requirements. J Clin Exp Neuropsychol. 2001 Oct;23(5):695–700. doi: 10.1076/jcen.23.5.695.1249. [DOI] [PubMed] [Google Scholar]
- 22.Lin L, Hedayat AS, Sinha B, Yang M. Statistical methods in assessing agreement: Models, issues, and tools. J Am Stat Assoc. 2002 Mar;97(457):257–70. [Google Scholar]
- 23.Chen Y-H, Kang J-H, Lin C-C, Wang IT, Keller JJ, Lin H-C. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. American Journal of Obstetrics and Gynecology. 2011;(0) doi: 10.1016/j.ajog.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Bourjeily G, Raker CA, Chalhoub M, Miller MA. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology. 2010 Oct;36(4):849–55. doi: 10.1183/09031936.00021810. [DOI] [PubMed] [Google Scholar]
- 25.Schutte JM, Steegers EAP, Schuitemaker NWE, Santema JG, de Boer K, Pel M, et al. Rise in maternal mortality in the Netherlands. BJOG: An International Journal of Obstetrics & Gynaecology. 2010;117(4):399–406. doi: 10.1111/j.1471-0528.2009.02382.x. [DOI] [PubMed] [Google Scholar]
- 26.Young T, Skatrud J, Peppard PE. Risk Factors for Obstructive Sleep Apnea in Adults. JAMA: The Journal of the American Medical Association. 2004 Apr 28;291(16):2013–6. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
- 27.Yaggi HK, Strohl KP. Adult Obstructive Sleep Apnea/Hypopnea Syndrome: Definitions, Risk Factors, and Pathogenesis. Clinics in chest medicine. 2010;31(2):179–86. doi: 10.1016/j.ccm.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 28.CDC. State-Specific Obesity Prevalence Among Adults --- United States, 2009. CDC; Aug 6, 2010. [PubMed] [Google Scholar]
- 29.Tutdibi E, Gries K, Bücheler M, Misselwitz B, Schlosser RL, Gortner L. Impact of Labor on Outcomes in Transient Tachypnea of the Newborn: Population-Based Study. Pediatrics. 2010 Mar 1;125(3):e577–e83. doi: 10.1542/peds.2009-0314. [DOI] [PubMed] [Google Scholar]
- 30.Tita ATN, Landon MB, Spong CY, Lai Y, Leveno KJ, Varner MW, et al. Timing of Elective Repeat Cesarean Delivery at Term and Neonatal Outcomes. New England Journal of Medicine. 2009;360(2):111–20. doi: 10.1056/NEJMoa0803267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-Related Mortality in the United States, 1998 to 2005. Obstetrics & Gynecology. 2010;116(6):1302–9. doi: 10.097/AOG.0b013e3181fdfb11. [DOI] [PubMed] [Google Scholar]
- 32.Hawkins JL, Chang J, Palmer SK, Gibbs CP, Callaghan WM. Anesthesia-Related Maternal Mortality in the United States: 1979–2002. Obstetrics & Gynecology. 2011;117(1):69–74. doi: 10.1097/AOG.0b013e31820093a9. [DOI] [PubMed] [Google Scholar]


