Abstract
Sepsis is a leading cause of intensive care unit admissions with high mortality and morbidity. Although outcomes have improved with better supportive care, specific therapies are limited. Endothelial activation and oxidant injury are key events in the pathogenesis of sepsis-induced lung injury. The signaling pathways leading to these events remain poorly defined and need to be studied. We sought to determine the role of MAP kinase kinase 3 (MKK3), a kinase of the p38 group in the pathogenesis of sepsis. We used a murine intraperitoneal lipopolysaccharide (LPS) model of systemic inflammation to mimic sepsis. Lung injury parameters were assessed in lung tissue and bronchoalveolar lavage. Primary lung endothelial cells were cultured and assessed for mediators of inflammation and injury such as ICAM-1, AP-1, NF-κB and mitochondrial ROS. Our studies demonstrate that MKK3 deficiency confers virtually complete protection against organ injury after intraperitoneal LPS. Specifically, MKK3 −/− mice were protected against acute lung injury, as assessed by reduced inflammation, mitochondrial reactive oxygen species (ROS) generation, endothelial injury and ICAM-1 expression after LPS. Our results show that endothelial MKK3 is required for inflammatory cell recruitment to the lungs, mitochondrial oxidant-mediated AP-1, NF-κB activation and ICAM-1 expression during LPS challenge. Collectively, these studies identify a novel role for MKK3 in lethal LPS responses and provide new therapeutic targets against sepsis and acute lung injury.
Introduction
Sepsis is the leading cause of acute lung injury and death in critically ill patients in the United States. It is estimated that sepsis develops in 750,000 people, of which more than 210,000 die every year (1). There is currently no curative therapy except for supportive care. Furthermore, the incidence of sepsis is predicted to increase with the aging of our expanding population. There is an urgent need to develop targeted biologic therapies. Our study offers new insights into the critical role of endothelial MKK3, a component of the p38 mitogen-activated protein kinase (MAPK) pathway, in lethal sepsis.
Mitogen-activated protein kinase (MAPK) pathways are core components of signal transduction in the cell. They are intracellular signaling pathways that mediate cell survival/death, proliferation and differentiation in response to a wide variety of signals such as cytokines, growth factors, ultraviolet light, osmotic stress and LPS. There are three distinct MAPK subfamilies: extracellular related kinase (ERK), c-Jun N terminal kinase (JNK), and p38. The p38 MAPK has been shown to be the major tyrosine-phosphorylated protein induced by LPS and plays an essential role in production of pro-inflammatory cytokines and vascular adhesion molecules in response to LPS (2, 3). Upstream kinases of p38 include MKK3 and MKK6 (4). MKK3 activates specifically p38 but not JNK or ERK in response to stress (5). A targeted disruption in the MKK3 gene causes a selective defect in p38 activation and TNFα induction of cytokine gene expression (6). MKK3-deficient macrophages have fundamental defects in inflammatory response as shown by reduced p38 phosphorylation and production of inflammatory cytokines such as IL1α and IL1β in response to LPS (7). MKK3 deficient macrophages also have lower levels of TNFα after LPS exposure (8). However, a functional role for MKK3 in sepsis has not been reported.
Our lab has shown previously that MKK3 mediates anti-apoptotic effects in ischemia reperfusion-induced lung injury (9, 10). MKK3 also has protective effects in hyperoxia-induced lung injury (11). In contrast, MKK3 mediates susceptibility to ventilator-induced lung injury (12). MKK3 has also been shown to mediate renal injury in ischemia-reperfusion, unilateral ureteric obstruction and diabetic models (13–15). MKK3 signaling has been reported to play an essential role in pancreatic injury due to low-dose streptozotocin (16). Therefore, it appears that the MKK3 pathway has distinct roles depending on the type of injury and organ or cell type. A role for MKK3 in innate immune responses elicited by LPS has not been explored. In this study we show that MKK3 deficient (MKK3−/−) mice are protected from lung injury when challenged with systemic LPS, a model of systemic inflammation. The mechanism of this protection is through reduced endothelial mitochondrial ROS-mediated AP-1, NF-κB activation and ICAM-1 expression leading to decreased inflammatory cell recruitment, endothelial and tissue injury. Collectively our data identifies MKK3 as an important upstream mediator of critical processes in sepsis and may be a potential therapeutic target.
Methods
Generation of MKK3 −/− mice
MKK3 −/− mice were generated by deletion of exons 8 and 9, which encode amino acids 217–221 of the murine MKK3 protein, as previously described (7). MKK3 −/− mice expressed normal levels of MKK6, MKK4, JNK and p38 MAP kinases and, therefore, there were no compensatory changes in the expression of these other kinases as consequence of MKK3 deficiency. The MKK3 −/− mice were provided by R. Davis and R. Flavell and have been backcrossed onto a C57BL6 background for greater than 15 generations.
LPS exposure
For survival and injury studies the mice were given 40mg/kg and 5 mg/kg IP LPS respectively (Escherichia coli055:B5 –Sigma Aldrich). Body-surface temperature was measured using Infrascan infrared thermometer (LaCrosse Technologies). Bronchoalveolar lavage (BAL) was performed by tracheal cannulation and whole-lung lavage was performed twice with a total volume of 1.8 ml ice-cold PBS. BAL was centrifuged at 3000 g, and the protein concentration of the supernatant was determined using the BCA Protein Assay Reagent (Pierce Labs). Mouse serum tests for transaminases (AST, ALT), blood urea nitrogen (BUN) and creatinine were performed by Antech Diagnostics. Mouse serum troponin I was measured using an ELISA kit (Life Diagnostics) according to the manufacturer's protocol.
Irradiation and bone marrow transplantation
Whole-body irradiation of recipient mice and harvesting of donor bone marrow was performed as described previously (17). Briefly, donor bone marrow was flushed from the femurs, tibias, and humeri of mice. Cells were pelleted at 300 g for 10 minutes at 4°C before counting. Recipient mice at 6 weeks of age underwent whole-body irradiation (1,000 cGy) followed by intravenous injection of whole bone marrow cells (9 × 106 cells in 0.2 ml PBS). After bone marrow transplantation, mice were maintained until 3 months of age under specific pathogen–free conditions at the Yale University School of Medicine animal facility and fed acidic water.
Isolation of primary lung endothelial cells
Endothelial cells were isolated as described by Kuhlencordt et al (18), with some modifications. Briefly, lungs were extracted, minced, and digested for 1 hour with 0.1% collagenase (Roche Diagnostics). The digest was passed through a 100μm cell strainer and pelleted at 200 g for 5 minutes; resuspended in endothelial medium containing 20% FBS, 40% DMEM, and 40% F12 with 100 U/ml penicillin G and 100 μg/ml streptomycin; and plated onto 0.1% gelatin-coated T75 flasks. Cells were cultured for 2–4 days and resuspended in 2% FBS containing 10μl biotin-labeled rat anti-mouse CD31 (PECAM-1) antibody (BD Biosciences — Pharmingen). After incubation on ice for 30 minutes, the cells were washed with streptavidin magnetic beads (New England Biolabs Inc). Cells were washed with 2% FBS, resuspended in 5 ml of 2% FBS, and incubated on ice for 30 minutes. The cells were then placed on the magnet for 5 minutes; unbound cells were removed, while bound cells were resuspended in medium and plated onto a 0.1% gelatin-coated T25 flask. We confirmed with CD31 staining and flow cytometry that greater than 95% of the cells were endothelial cells.
siRNA knockdown of MKK3
ON-TARGETplus SMARTpool siRNA against MKK3 and scrambled siRNA were obtained from Thermo Scientific (formerly Dharmacon RNAi technologies). Endothelial cells were seeded onto 6-well plates 1 day prior to transfection using 40% DMEM and 40% F12 tissue culture medium supplemented with 20% FBS, without antibiotics. At the time of transfection with the specific siRNA, the cells were 50%–60% confluent. Lipofectamine 2000 Reagent (Invitrogen) was used as the transfection agent. After 48 hours' incubation, the cells were collected and subjected to assays.
Terminal deoxynucleotidyltransferase dUTP nick-end labeling (TUNEL) assay
We used the in situ cell death detection kit according to the manufacturer's protocol (Roche Applied Science). Sections of formalin-fixed, paraffin-embedded lung tissue were deparaffinized and rehydrated, rinsed with PBS, and digested with proteinase K (Roche Applied Science) at a concentration of 20 μg/ml for 20 min. After PBS washes, sections were incubated with TUNEL reaction mixture at 37°C for 1 h, then incubated with antifluorescein conjugated with alkaline phosphatase at 37°C for 30 min. Sections were washed twice with PBS and stained with nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate substrate solution before counterstaining with nuclear fast-red. Apoptotic and normal cells were observed under a light microscope. Normal cells exhibited red nuclear staining, whereas TUNEL positive cells, indicating cell death/apoptosis, exhibited purple nuclear staining. Five hundred cells were counted for each sample, and the number of apoptotic cells is expressed as a percentage of the total counted.
Myeloperoxidase assay
Myeloperoxidase (MPO) levels were assessed as follows. Lung tissue was homogenized in 50mM phosphate buffer (pH 6.0). Then after centrifugation at 10,000g for 15 min, the pellet was resuspended in 50 mM hexadecyltrimethylammonium bromide (Sigma Chemical Co, St Louis, MO) in 50 mmol/L potassium phosphate buffer, pH 6.0, before sonication for 20 seconds in an ice bath. The samples were freeze-thawed 3 times, after which sonication was repeated. Suspensions were then centrifuged at 10,000g for 10 min. Myeloperoxidase activity was assayed spectrophotometrically by mixing 0.1 mL of supernatant with 2.9 mL of 50 mmol/L phosphate buffer, pH 6.0, containing 0.167 mg/mL o-dianisidine dihydrochloride (Sigma Aldrich) and 0.0005% hydrogen peroxide (Sigma Aldrich). The change in absorbance at 460 nm was measured using a spectrophotometer (SmartSpec300, BioRad) periodically for 3 minutes. MPO activity was then derived from the observed change in absorbance per minute.
Western Blot analysis
Protein was extracted from cells using RIPA buffer, electrotransferred, and immunoblotted with primary antibodies. Detection was performed with a horseradish peroxidase Western detection system (Cell Signaling Technology). Equivalent sample loading was confirmed by stripping membranes with blot Restore membrane rejuvenation solution (Thermo Scientific) and probing for actin, α-tubulin or lamin A/C. All of the antibodies were obtained from Santa Cruz biotechnology, CA except p- IKKα/β (Cell Signaling Technology). Nucleus and cytoplasm fractions of endothelial cells were obtained using NE-PER kit (Thermo Scientific) as per instructions. Western blots of nucleus and cytoplasm fraction were then performed as detailed before.
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared by using a NE-PER nuclear and cytoplasmic extraction reagent kit (Pierce, USA) according to the manufacturuer's protocol. The AP-1 site was synthesized as complementary oligodeoxyribonucleotide strands. The sequence of AP-1 consensus oligonucleotides was 5′-CGC TTG ATG ACT CAG CCG GAA-3′ (Sigma-Aldrich). The DNA binding ability of AP-1 in the nuclear extracts was assessed by electrophoretic mobility shift assay (EMSA) with biotin-labeled, double-stranded AP-1. EMSA was carried out by using the Lightshift chemiluminescent EMSA kit (Pierce). Specific binding was confirmed by using a 200-fold excess of an unlabeled probe as a specific competitor. Protein-DNA complexes were separated by using a 6% non-denaturing acrylamide gel electrophoresis and then transferred to positively-charged nylon membranes and cross-linked by UV irradiation. Gel shifts were visualized with streptavidin horseradish peroxidase according to standard protocols.
Oxidant assays
Malondialdehyde (MDA) was measured using Lipid Peroxidation Assay kit (Calbiochem; EMD Biosciences) according to the manufacturer's instructions. CM-H2DCFDA and Mitosox red (Invitrogen), was used to determine levels of ROS in endothelial cells. Cells were seeded onto 6 well non tissue culture plates 1 day before experiment. On the next day cells were stimulated with LPS (1 μg/ml, 6 hrs). Cells were washed and exposed to CM-H2DCFDA (5μM) or Mitosox red (2.5μM) in regular media and kept at 37° C for 25 min. The cells were then washed with PBS and then detached gently with 0.4M EDTA and analyzed on BD FACSCaliber machine. CMH2DCFDA was detected in FL-1 channel and Mitosox red was detected in FL-1 channel. Rotenone was purchased from Sigma Sigma Aldrich and Mito-TEMPO was purchased from Santa Cruz Biotechnology.
Real-time RT-PCR
Total RNA was extracted from one-half of one lung or cells using Trizol reagent according to the manufacturer's protocol (Gibco BRL, Carlsbad, CA, USA). First-strand cDNA was synthesized using Superscript II Reverse Transcriptase (Invitrogen) with random hexamers; conditions were 10 min at 25°C, 30 min at 48°C, and 5 min at 95°C. Real-time RT-PCR reactions were carried out in Power SYBR Green PCR Master Mix (Applied Biosystems) and an ABI Prism 7000 Sequence Detection System (Applied Biosystems). GAPDH was amplified as a control. Real-time PCR conditions were 95°C for 10 min, 40 cycles of: 95°C for 15 s, followed by 60°C for 1 min. The relative quantification values for these gene expressions were calculated from the accurate threshold cycle (CT), which is the PCR cycle at which an increase in reporter fluorescence from SYBR green dye can first be detected above a baseline signal. The CT values for GAPDH were subtracted from the CT values for ICAM-1, MKK3 in each well to calculate ΔCT. The ΔCT values for each sample were averaged. To calculate the fold induction over controls (ΔΔCT), the average ΔCT values calculated for WT animals/cells were subtracted from ΔCT values calculated for MKK3 −/− animals/cells. Next, the fold induction for each well was calculated using the 2–(ΔΔCT) formula. The fold induction values for replicate wells were averaged, and data were presented as the mean ± SEM of triplicate wells.
Primers (5' → 3') used for RT-PCR were as follows. ICAM-1 Forward TCACCAGGAATGTGTACCTGA, Reverse ATCACGAGGCCCACAATGAC. VCAM-1 Forward CCCGGATGCGCTTGAC, Reverse CCGATTTGAGCGATCGTTTT. Selectin E Forward GAACCAAAGACTCGGGCATGT Reverse TGACCACTGCAGGATGCATT. CXCL-1 Forward CTGGATTCACCTCAAGAACATC, Reverse CAGGGTCAAGGCAAGCCTC. CXCL-2 Forward GCGCCCAGACAGAAGTCATAG, Reverse AGCCTTGCCTTTGTTCAGTATC. MKK3 Forward GTAGAGAAAGTGCGGCATGCT, Reverse CCCGGATGCGCTTGAC. GADPH Forward TGTGTCCGTCGTGGATCTGA, Reverse CCTGCTTCACCACCTTCTTGAT
Results
MKK3 −/− mice are resistant to lung and organ injury after systemic LPS
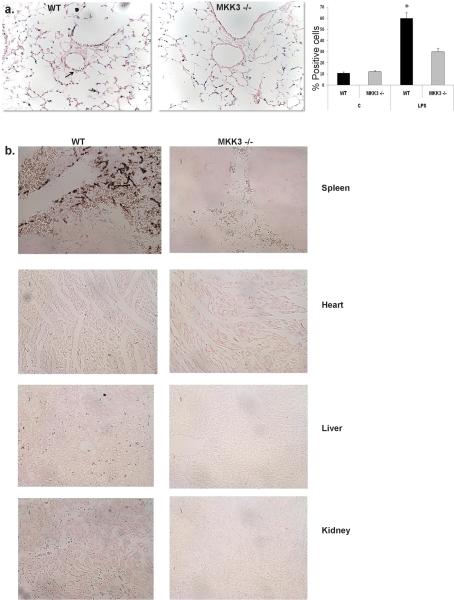
We initially sought to determine if MKK3 deficient mice are resistant to organ injury after LPS exposure. We observed lower levels of cell death in the lungs and vasculature of MKK3 −/− mice, as shown by TUNEL staining (Fig. 1a). Other organs such as kidney, spleen, liver and heart also showed substantially less TUNEL positive cells in MKK3 −/− mice (Fig. 1b). Bronchoalveolar lavage (BAL) cell counts were not elevated in WT and MKK3 −/− mice, indicating absence of inflammatory cell influx into the alveolar space, typical of IP LPS, as reported by others (19). However, parenchymal lung inflammation was markedly increased in WT mice, as shown in representative histopathologic sections of the lung (Fig. 2a). BAL protein levels, a measure of lung endothelial barrier disruption, were significantly elevated in WT mice compared to MKK3 −/− mice (Fig. 2b). Lung myeloperoxidase (MPO) levels, a measure of neutrophil recruitment were significantly decreased in MKK3 −/− mice compared to WT mice after IP LPS. We found similar differences in MPO levels in kidney and liver, key target organs of systemic LPS (Fig. 2c). We measured serum markers of organ injury in mice given LPS. We found significantly higher levels of transaminases (AST, ALT) and blood urea nitrogen (BUN) in WT mice given LPS compared to MKK3 −/− indicating higher liver and kidney damage respectively. We also checked levels of creatinine, another marker of kidney injury and troponin I, a marker of myocardial injury (Fig. 2d). Although there was a trend toward higher creatinine and troponin I levels in septic WT mice the differences did not reach statistical significance (data not shown). Collectively, these data showed that MKK3 deficient mice are resistant to lung and systemic organ injury after LPS exposure.
Fig. 1. MKK3 −/− mice have less cell death after systemic LPS.
a) WT and MKK3 −/− mice were given IP LPS (40 mg/kg) and TUNEL staining was performed on lung sections. Arrows point to TUNEL-positive cells. The number of TUNEL-positive cells were quantified and expressed as a percentage of the total number of lung cells counted on each section (Mean +/− SEM, n=3; *p<0.05)
b) WT and MKK3 −/− mice were given IP LPS (40 mg/kg), and TUNEL staining was performed on organ sections.
Fig. 2. MKK3 −/− mice are resistant to lung injury after systemic LPS.
a) Representative lung histopathology of WT and MKK3 −/− mice after IP LPS (40 mg/kg). 100× original magnification.
b) BAL protein levels in WT and MKK3 −/− mice after IP LPS (40 mg/kg) (Mean +/− SEM, n=5, p*< 0.01)
c) WT and MKK3 −/− mice were given IP LPS (40 mg/kg) and myeloperoxidase levels were measured in organ lysates. (Mean +/− SEM, n=5, p<0.05)
d) WT and MKK3 −/− mice were given IP LPS (40 mg/kg). Serum levels of ALT and AST (marker for hepatic damage) and BUN (measurement of renal function) were measured. (Mean +/− SEM, n=7–8, p<0.05)
Non-hematopoietic cells are important in MKK3 mediated endotoxemia
Next, we determined whether decreased organ injury in MKK3 −/− mice correlated with changes in survival and the relative contribution of MKK3 in cells of hematopoietic versus non-hematopoietic lineages to the responses. Survival studies showed that MKK3 −/− mice transplanted with WT bone marrow were still protected against lethal LPS, suggesting that the loss of MKK3 in non-hematopoietic cells is sufficient for improved survival (Fig. 2a). WT mice transplanted with MKK3 −/− bone marrow appeared to have a trend towards improved survival after IP LPS compared to WT mice transplanted with WT bone marrow, but this trend was not statistically significant (Fig. 3a). For the purposes of this manuscript, we focused on the role of endothelial MKK3 based on the significant survival advantage that MKK3 deficiency in non-hematopoietic lineages provided. Body temperature of mice is an accurate marker of survival in various model of sepsis (20). We found that MKK3 −/− mice transplanted with WT bone marrow exhibited body temperatures similar to that of MKK3 −/− mice transplanted with MKK3 −/− bone marrow after LPS, indicating that MKK3 deficiency on non-hematopoietic cells plays a more important role in protection than MKK3 deficiency on hematopoietic cells. WT mice transplanted with MKK3 −/− bone marrow did not have a statistically significant recovery of body temperatures 6 hours after LPS, consistent with the survival data (Fig. 3b). Of note, the basal body temperatures were similar in all groups. These data suggest that the loss of MKK3 in primarily non-hematopoietic cells has sufficient protective effects against lethal LPS to override any deleterious effects that MKK3 reconstitution, at least in bone marrow, may have. Based on these data and the known critical role of endothelium in sepsis, we chose to focus our subsequent in vitro studies on lung endothelial cells.
Fig. 3. MKK3 deficiency in non-hematopoietic cells, in part, determines survival after lethal LPS.
Adoptive transfer of bone marrow was performed from WT mice to WT mice (WT → WT), MKK3 −/− mice to MKK3 −/− mice (MKK3 −/− → MKK3 −/−), WT mice to MKK3 −/− mice (WT → MKK3 −/−), and MKK3 −/− mice to WT mice (MKK3 −/− → WT). Survival (a) and body temperature in centigrade (C) at 6hrs (b) were measured after IP LPS (40mg/kg). (n= 15,*p< 0.05 compared to WT → WT)
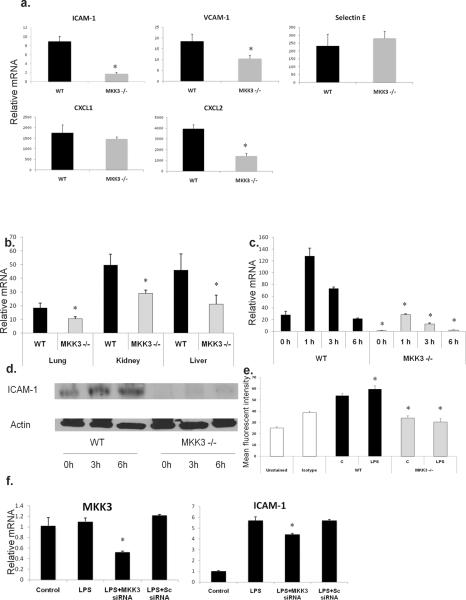
ICAM-1 expression is lower in MKK3 −/− organs and endothelial cells after LPS
MKK3 −/− lungs had decreased inflammatory influx compared to WT lungs after LPS (Fig. 2). Given that endothelial adhesion and chemokine molecules are important in inflammatory cell recruitment, we analyzed MKK3 −/− lungs for ICAM-1, VCAM-1, selectin E, CXCL-1 and CXCL-2. In the molecule profile we analyzed, ICAM-1 expression was notably different in MKK3 −/− lungs after LPS compared to WT lungs (Fig. 4a). Levels of VCAM-1 and CXCL-2 were also lower in MKK3 −/− lungs but we decided to focus on ICAM-1 given the strong association of ICAM1 with septic responses in previous reports (21). ICAM-1 mRNA was decreased in lungs, kidney and liver of MKK3 −/− mice after LPS (Fig. 4b). As ICAM-1 is expressed by endothelial cells and function in inflammatory cell recruitment (22), we proceeded to study ICAM-1 in primary mouse lung endothelial cells. We found that ICAM-1 mRNA and protein levels were decreased in MKK3 −/− cells at baseline and after LPS stimulation (Fig. 4c, 4d). We examined surface expression of ICAM-1 by flow cytometry and consistent with our cell lysate data, we found decreased surface expression of ICAM-1 in MKK3 −/− endothelial cells (Fig. 4e). We confirmed specific regulation of ICAM-1 by MKK3 using silencing RNA (siRNA) against MKK3. A ~50% reduction in MKK3 using siRNA in WT endothelial cells caused a small but significant reduction of ICAM-1 after LPS exposure (Fig. 4f). These studies show for the first time that MKK3 is an upstream regulator ICAM-1 in organs and endothelial cells and a viable therapeutic target.
Fig. 4. ICAM-1 expression is lower in MKK3 −/− organs and endothelial cells after LPS.
a) WT and MKK3 −/− mice were given IP LPS (40 mg/kg) and ICAM-1, VCAM-1, selectin E, CXCL-1 and CXCL-2 mRNA were measured by real-time PCR in lung lysates. The values are expressed as mean fold induction over untreated mice +/− SEM (n=5, p<0.05).
b) WT and MKK3 −/− mice were given IP LPS (40 mg/kg) and ICAM-1 mRNA was measured by real-time PCR in lung, kidney and liver lysates. The values are expressed as mean fold induction over untreated mice +/− SEM (n=5, p<0.05).
c) Primary cultures of lung endothelial cells were exposed to LPS (1μg/ml) and mRNA levels of ICAM-1 were measured at different time points. The values are expressed as mean fold induction over unstimulated cells +/− SEM (*p<0.05). The results are representative of at least 3 independent experiments.
d) Lung endothelial cells were exposed to LPS (1μg/ml) and protein levels of ICAM-1 were assessed at different time points. Actin was detected as a loading control. The results are representative of at least 3 independent experiments.
e) Lung endothelial cells were exposed to LPS (1μg/ml) and surface expression of ICAM-1 was measured using flow cytometry. The values are expressed as mean fluorescent intensity +/− SEM. (p<0.05). The results are representative of at least 3 independent experiments.
f) Knockdown of MKK3 in WT lung endothelial cells was achieved using siRNA and MKK3 or ICAM-1 mRNA expression detected by real-time PCR after LPS stimulation (1μg/ml). The values are expressed as mean fold induction over unstimulated cells +/− SEM (*p<0.05). The results are representative of at least 3 independent experiments.
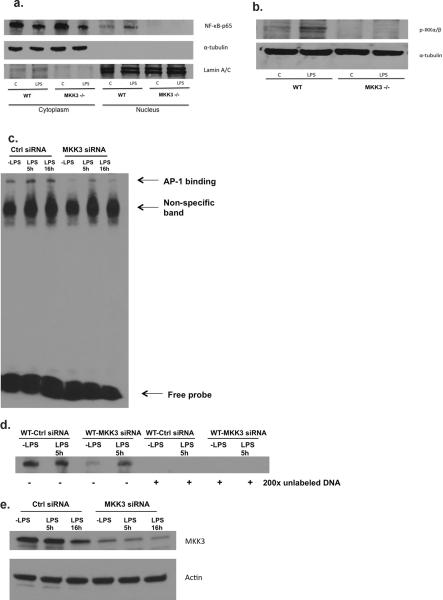
Activation of ICAM-1-related transcription factors was lower in MKK3 −/− endothelial cells after LPS
In order to identify the mechanism whereby MKK3 regulates ICAM-1, we explored the role of NF-κB and its upstream kinase IKKα/β. NF-κB has been shown to regulate ICAM-1 via the IKK pathway (23, 24). We found reduced translocation of NF-κB to the nucleus in MKK3 −/− endothelial cells at baseline and after LPS, as shown by Western blots of NF-κB in nuclear and cytoplasmic fractions (Fig. 5a). The loss of NF-κB nuclear translocation is known to be caused by the inability of the upstream kinase, IKKα/β, to be phosphorylated and subsequently inactivated. Therefore, we determined whether IKKα/β phosphorylation is altered in MKK3 −/− cells. Consistent with our NF-κB Western blots, we found reduced phosphorylation of IKKα/β, in MKK3 −/− endothelial cells compared to WT cells at baseline and in response to LPS (Fig. 5b). ICAM-1 transcription is also dependent on the transcription factor AP-1, which appears to cooperatively interact with NF-κB in ICAM-1 gene regulation (25, 26). Hence we examined if AP-1 activity is altered in MKK3 −/− cells. We found that in electrophoretic mobility shift assays (EMSA) there was less AP-1 binding to the target sequence in MKK3 −/− cells compared to WT at baseline and after LPS (Fig. 5c–e). . These results show that MKK3 regulates both basal and LPS-induced AP-1, NF-κB / IKKα/β activation and that a consequence of MKK3 deficiency is decreased AP-1, NF-κB and ICAM-1 expression. Our studies are the first to identify a regulator of constitutive AP-1, NF-κB and ICAM-1 expression in endothelial cells.
Fig. 5. Activation of ICAM-1 transcription factors was lower in MKK3 −/− endothelial cells after LPS.
a) After WT and MKK3 −/− lung endothelial cells were exposed to LPS (1μg/ml), cell nuclear and cytoplasmic fractions were isolated and analyzed for p65 subunit of NF-kB by Western blots. α-tubulin was detected as cytoplasmic protein loading controls. Lamin A/C was detected as nuclear protein loading controls. The results are representative of at least 3 independent experiments.
b) WT and MKK3 −/− lung endothelial cells were exposed to LPS (1μg/ml) and lysates were analyzed for phosphorylated (p) -IKKα/β by western blots. α-tubulin was detected as protein loading controls. The results are representative of at least 3 independent experiments.
c) Nuclear extract prepared from the control or MLEC cells transfected with MKK3 siRNA and followed by LPS treatment (1μg/ml) was mixed with biotin-labeled oligonucleotide containing AP-1 motif. Bound complexes were analyzed by electrophoresis. The results are representative of at least 3 independent experiments.
d) Competitive inhibition of AP-1 binding with non labeled probe. Nuclear extract prepared from the control or MLEC cells transfected with MKK3 siRNA and followed by LPS treatment (1μg/ml) was mixed with biotin-labeled oligonucleotide containing AP-1 motif along with 200× unlabeled probe. Bound complexes were analyzed by electrophoresis. The results are representative of at least 3 independent experiments.
e) Control or WT MLEC cells were transfected with MKK3 siRNA and treated with LPS (1μg/ml). Cell lysates were analyzed by western blot to determine efficacy of MKK3 inhibition. The results are representative of at least 3 independent experiments.
ROS production was lower in MKK3 −/− mice and endothelial cells after LPS
LPS is known to induce ROS and activate NF-κB in neutrophils (27). To investigate if MKK3 is involved in LPS-induced ROS generation we checked malondialdehyde (MDA) levels in serum of LPS exposed mice. MDA assay measures lipid peroxidation and is a marker of ROS excess. We found that MDA levels were significantly lower in the serum of MKK3 −/− compared to WT mice after LPS (Fig. 6a). We also detected intracellular ROS in endothelial cells by measuring CM-H2DCFDA, an indicator of H2O2, a major component of ROS. We found that CM-H2DCFDA levels were significantly lower in MKK3 −/− endothelial cells at baseline and after LPS exposure (Fig. 6b), indicating that the absence of MKK3 is associated with decreased ROS. The major sources of ROS in cells are either the cytoplasmic families of NADPH oxidases (Nox) or related family of dual oxidases (Duox) or from the mitochondria. In order to determine the source of ROS we initially checked mRNA expression of Nox 1–4 and Duox 1,2 by PCR in lungs and endothelial cells and did not find any difference between WT and MKK3 −/− mice and endothelial cells. Furthermore when we specifically inhibited Nox using diphenylene iodonium (DPI) we did not see any difference in ICAM-1 expression in WT endothelial cells (Data not shown). We then considered the mitochondria as a source and investigated the levels of mitochondrial ROS using Mitosox red, a fluorescent dye that is specific for mitochondrial ROS (28). We found that levels of mitochondrial ROS were lower in MKK3 −/− compared to WT endothelial cells at baseline and in response to LPS (Fig. 6c).
Fig. 6. ROS production was lower in MKK3 −/− mice and endothelial cells after LPS.
a) WT and MKK3 −/− mice were given IP LPS (40mg/ml) and malondialdehyde (MDA) levels were measured in serum (n=5–8, Mean +/− SEM are shown, *p<0,05)
b) WT and MKK3 −/− lung endothelial cells were exposed to LPS (1μg/ml). Cells were stained with CM-H2DCFDA, which detects H2O2, levels were measured by flow cytometry. The values are expressed as mean fluorescent intensity +/− SEM (p<0.05). The results are representative of at least 3 independent experiments. Representative fluorescence histograms of LPS exposed cells are shown.
c) WT and MKK3 −/− lung endothelial cells were exposed to LPS (1μg/ml). Cells were stained with Mitosox red, which detects mitochondrial ROS, levels were measured by flow cytometry. The values are expressed as mean fluorescent intensity +/− SEM (p<0.05). The results are representative of at least 3 independent experiments. Representative fluorescence histograms of LPS exposed cells are shown.
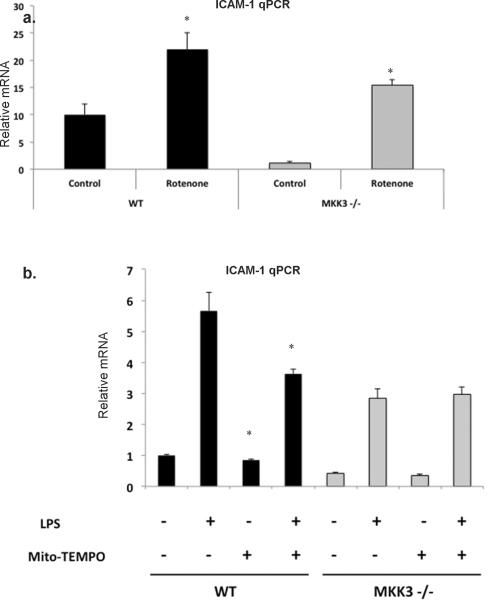
Mitochondrial ROS is upstream of ICAM-1
Next, we determined whether there were causative links between reduced mitochondrial ROS and ICAM-1 expression in MKK3 −/− endothelial cells. We asked if specific induction of mitochondrial ROS can restore ICAM-1 expression in MKK3 −/− endothelial cells. Cells were exposed to rotenone, a mitochondrial respiratory chain inhibitor which induces production of mitochondrial ROS. We found that ICAM-1 mRNA was induced in WT and MKK3 −/− endothelial cells after rotenone exposure suggesting that not only is mitochondrial ROS is sufficient for ICAM-1 upregulation but that depressed mitochondrial ROS can account for decreased ICAM-1 expression in MKK3 −/− endothelial cells (Fig. 7a). To confirm these results, we used Mito-TEMPO, an antioxidant specific to the mitochondria (29), to determine the contribution of mitochondrial ROS to ICAM-1 expression. We found that in WT endothelial cells Mito-TEMPO reduced significantly the expression of ICAM-1 mRNA at baseline and after LPS exposure. In contrast MKK3 −/− endothelial cells showed no difference in ICAM-1 expression after Mito-Tempo exposure (Fig. 7b). The latter was expected because MKK3 −/− endothelial cells already have reduced mitochondrial ROS and there is no added benefit to addition of Mito-TEMPO. In conclusion we show that endothelial MKK3 is an important regulator of mitochondrial redox status with subsequent regulation of AP-1, NF-κB and ICAM-1 during LPS exposure. Collectively, these studies identify a novel role for MKK3 in lethal LPS responses and provide new therapeutic targets against sepsis and acute lung injury.
Fig. 7. Mitochondrial ROS is upstream of ICAM-1.
a) WT and MKK3 −/− lung endothelial cells were exposed to rotenone, an inducer of mitochondrial ROS (5 μM, 5 hrs) and cell lysates were checked for ICAM-1 by real-time PCR. The results are representative of at least 3 independent experiments.
b) WT and MKK3 −/− endothelial cells were pretreated with 50 μM Mito-Tempo for 2 hrs and then exposed to LPS (1μg/ml, 2 hr) and cell lysates were checked for ICAM-1 by RT-PCR. The values are expressed as fold induction over unstimulated cells +/− SEM (*p<0.05 compared to samples untreated with Mito-TEMPO). The results are representative of at least 3 independent experiments.
Discussion
Sepsis remains a critical problem with significant mortality and morbidity despite intense efforts to find effective therapies. The prevailing theory of sepsis is that there is an unregulated inflammatory response or cytokine storm that results in organ damage and death (30). However, multiple trials of anti-inflammatory or anti-cytokine therapies have been disappointing failures (31). Most of the unsuccessful therapies try to inhibit mediators that act far downstream to the initial stimulus that set in motion the inflammatory response. By identifying more proximal mediators, we may have a better chance of halting the inflammatory response before deleterious consequences ensue. Our study identifies MKK3, a proximal-activating kinase in the p38 MAP kinase pathway, as a potential therapeutic target. We demonstrate for the first time that MKK3 deficiency leads to protection against LPS-induced lung injury through the reduction of endothelial mitochondrial ROS, AP-1, NF-κB activation and ICAM-1 expression, ultimately reducing tissue inflammation.
Mitochondria are ancient bacterial endosymbionts in eukaryotic cells that are responsible for oxidative phosphorylation and generation of ATP. Mitochondria are also a major source of ROS and inducers of apoptosis. New evidence suggests that mitochondria play a critical role in inflammatory responses (32, 33). Mitochondrial dysfunction is associated with worse severity and outcomes in patients with sepsis and lung injury (34, 35). Here we show that MKK3 deficient mice have lower mitochondrial ROS (at baseline and after LPS) and are protected against endotoxemia and lung injury. ICAM-1 is predominantly transcriptionally regulated, under the control of AP-1 and NF-κB transcription factors, key players in the inflammatory responses of sepsis (36). AP-1 and NF-κB are redox sensitive transcription factors and cooperatively influence the expression of ICAM-1 in endothelial cells (37–40). The mechanism of regulation of AP-1 and NF-κB by oxidant signaling remains undetermined. IKK is considered the major proximal redox modulated regulatory kinase for NF-κB signaling (41). Our studies indicate that MKK3 may influence the phosphorylation of IKK through the mitochondrial redox status in endothelial cells in response to LPS. We show for the first time novel links between MKK3, mitochondrial redox status, AP-1, IKK/ NF-κB signaling and ICAM-1 expression in endotoxemia and lung injury (Fig. 8).
Fig.8. Summary of MKK3 Signaling.
We postulate that endothelial MKK3 is required for LPS-induced mitochondrial ROS generation, IKKα/β phosphorylation, NF-kB and AP-1 activation and ICAM-1 expression, ultimately leading to inflammatory recruitment.
Toll-like receptor 4 (TLR4) is reported as the canonical LPS responsive pathway. Mice that are deficient in TLR4 function are highly resistant to endotoxic shock (42–44). MKK3 appears to be an important part of LPS responses and may function in tandem with TLR4 as part of the innate immune response. The specific contribution of MKK3 in innate immunity has not been well characterized, and we show for the first time that MKK3 mediates critical responses to LPS. We believe that MKK3 −/− mice are not completely deficient in TLR4 responses, as the increase in ICAM-1 in response to LPS was not completely abolished and MKK3 −/− mice retained the ability to induce TLR4-induced cytokines such as IL6 and TNFα (not shown). There was a general reduction in other adhesion and chemokine markers, such as VCAM-1 and CXCL-2, in MKK3-deficient mice. Hence MKK3 likely has both TLR4-dependent and TLR4-independent effects. Future studies will help us delineate the specific contribution of MKK3 to TLR4 function. We show dramatic protection against injury in LPS-challenged MKK3 −/− mice. To our knowledge, apart from TLR4 deficient mice, there are only a few other studies that show substantial protection against endotoxic shock using knock out mice. Mice deficient in IL1-β converting enzyme (Caspase-1), PARP-1 and ICAM-1 were completely protected against death after LPS exposure (21, 45, 46). Notably, we show that MKK3 −/− endothelial cells and mice are deficient in ICAM-1 after LPS exposure, which identifies MKK3 as a critical upstream regulator of molecules that determine survival after LPS. Our identification of MKK3 as a major mediator of LPS-induced injury provides new insights into innate immune pathways and serves as a basis for new therapies against sepsis.
Vascular inflammation is a sentinel event in sepsis. The endothelium is central to the pathogenesis of sepsis through effects on inflammation, leukocyte recruitment, vascular tone, coagulation and thrombosis. Key endothelial changes in sepsis are disruption of the endothelial barrier and promotion of leukocyte adhesion. Leukocyte accumulation is orchestrated by coordinated expression of chemokine and adhesion molecules. ICAM-1 on endothelial cells attaches to β2 integrin receptors on leukocytes, leading to firm binding and transmigration (47). We show that MKK3 deficiency prevents the loss of endothelial barrier function and prevents inflammatory influx by decreasing ICAM-1 expression. MKK3 have not been reported to be involved in ICAM-1 regulation, but our studies point to a pivotal role for MKK3 during LPS challenge. Lung epithelial cells express ICAM-1 in response to LPS (48). Reduced epithelial ICAM-1 expression in MKK3 deficient mice may also contribute to the protection in sepsis, as our bone marrow chimera mice suggest that MKK3 expression in non-hematopoetic cells is important. In future studies we will examine the role of epithelial cell in determining septic responses in MKK3 deficient mice.
Finally, it's notable that even the baseline levels of mitochondrial ROS, ICAM-1, AP-1 and NF-κB activation in MKK3 −/− lung and endothelial cells are lower than that of WT. It is known that ICAM-1 is expressed constitutively in specific vascular beds, with the highest expression in the lung, and that the up regulation of ICAM-1 expression in the lung is increased after LPS exposure (49). This suggests that the pulmonary vasculature may serve as an active gateway for inflammatory cell recruitment and is primed to adhere and recruit neutrophils in response to a pro-inflammatory stimulus by rapidly up regulating ICAM-1 expression. Similar mechanisms of regulation may be in effect in organs, such as liver and kidney, with high populations of endothelial cells. We show that MKK3 plays an important role in this regulation and demonstrate that MKK3 −/− mice have less ICAM-1 expression and tissue damage in lung, liver and kidney. It is interesting to note that the lung, kidney and liver are the most common organs to fail in sepsis and that multi-organ failure is the major cause of death in sepsis-induced lung injury. Our studies have identified MKK3 as a promising therapeutic target for sepsis-induced multi-organ failure.
Acknowledgements
We thank Susan Ardito for administrative and editorial assistance. We also thank Richard A. Flavell, Yale University and Roger J. Davis, University of Massachusetts Medical School for their kind gift of the MKK3 deficient mice.
PM is supported by American Heart Association grant, AHA 09FTF2090019. PJL is supported by R01 HL090660 and R01 HL071595.
Footnotes
The authors have declared that no conflicts of interest exist
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 3.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 4.Han J, Wang X, Jiang Y, Ulevitch RJ, Lin S. Identification and characterization of a predominant isoform of human MKK3. FEBS Lett. 1997;403:19–22. doi: 10.1016/s0014-5793(97)00021-5. [DOI] [PubMed] [Google Scholar]
- 5.Derijard B, Raingeaud J, Barrett T, Wu IH, Han J, Ulevitch RJ, Davis RJ. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 6.Wysk M, Yang DD, Lu HT, Flavell RA, Davis RJ. Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for tumor necrosis factor-induced cytokine expression. Proc Natl Acad Sci U S A. 1999;96:3763–3768. doi: 10.1073/pnas.96.7.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu HT, Yang DD, Wysk M, Gatti E, Mellman I, Davis RJ, Flavell RA. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. Embo J. 1999;18:1845–1857. doi: 10.1093/emboj/18.7.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pellizzari R, Guidi-Rontani C, Vitale G, Mock M, Montecucco C. Anthrax lethal factor cleaves MKK3 in macrophages and inhibits the LPS/IFNgamma-induced release of NO and TNFalpha. FEBS Lett. 1999;462:199–204. doi: 10.1016/s0014-5793(99)01502-1. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Shan P, Alam J, Davis RJ, Flavell RA, Lee PJ. Carbon monoxide modulates Fas/Fas ligand, caspases, and Bcl-2 family proteins via the p38alpha mitogen-activated protein kinase pathway during ischemia-reperfusion lung injury. J Biol Chem. 2003;278:22061–22070. doi: 10.1074/jbc.M301858200. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Shan P, Alam J, Fu XY, Lee PJ. Carbon monoxide differentially modulates STAT1 and STAT3 and inhibits apoptosis via a phosphatidylinositol 3-kinase/Akt and p38 kinase-dependent STAT3 pathway during anoxia-reoxygenation injury. J Biol Chem. 2005;280:8714–8721. doi: 10.1074/jbc.M408092200. [DOI] [PubMed] [Google Scholar]
- 11.Otterbein LE, Otterbein SL, Ifedigbo E, Liu F, Morse DE, Fearns C, Ulevitch RJ, Knickelbein R, Flavell RA, Choi AM. MKK3 mitogen-activated protein kinase pathway mediates carbon monoxide-induced protection against oxidant-induced lung injury. Am J Pathol. 2003;163:2555–2563. doi: 10.1016/S0002-9440(10)63610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dolinay T, Wu W, Kaminski N, Ifedigbo E, Kaynar AM, Szilasi M, Watkins SC, Ryter SW, Hoetzel A, Choi AM. Mitogen-activated protein kinases regulate susceptibility to ventilator-induced lung injury. PLoS One. 2008;3:e1601. doi: 10.1371/journal.pone.0001601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim AK, Nikolic-Paterson DJ, Ma FY, Ozols E, Thomas MC, Flavell RA, Davis RJ, Tesch GH. Role of MKK3-p38 MAPK signalling in the development of type 2 diabetes and renal injury in obese db/db mice. Diabetologia. 2009;52:347–358. doi: 10.1007/s00125-008-1215-5. [DOI] [PubMed] [Google Scholar]
- 14.Ma FY, Tesch GH, Flavell RA, Davis RJ, Nikolic-Paterson DJ. MKK3-p38 signaling promotes apoptosis and the early inflammatory response in the obstructed mouse kidney. Am J Physiol Renal Physiol. 2007;293:F1556–1563. doi: 10.1152/ajprenal.00010.2007. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Ji HX, Zheng JN, Pei DS, Hu SQ, Qiu SL. Protective effect of selenite on renal ischemia/reperfusion injury through inhibiting ASK1-MKK3-p38 signal pathway. Redox Rep. 2009;14:243–250. doi: 10.1179/135100009X12525712409896. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda K, Tesch GH, Yap FY, Forbes JM, Flavell RA, Davis RJ, Nikolic-Paterson DJ. MKK3 signalling plays an essential role in leukocyte-mediated pancreatic injury in the multiple low-dose streptozotocin model. Lab Invest. 2008;88:398–407. doi: 10.1038/labinvest.2008.10. [DOI] [PubMed] [Google Scholar]
- 17.Cohn L, Herrick C, Niu N, Homer R, Bottomly K. IL-4 promotes airway eosinophilia by suppressing IFN-gamma production: defining a novel role for IFN-gamma in the regulation of allergic airway inflammation. J Immunol. 2001;166:2760–2767. doi: 10.4049/jimmunol.166.4.2760. [DOI] [PubMed] [Google Scholar]
- 18.Kuhlencordt PJ, Rosel E, Gerszten RE, Morales-Ruiz M, Dombkowski D, Atkinson WJ, Han F, Preffer F, Rosenzweig A, Sessa WC, Gimbrone MA, Jr., Ertl G, Huang PL. Role of endothelial nitric oxide synthase in endothelial activation: insights from eNOS knockout endothelial cells. Am J Physiol Cell Physiol. 2004;286:C1195–1202. doi: 10.1152/ajpcell.00546.2002. [DOI] [PubMed] [Google Scholar]
- 19.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warn PA, Brampton MW, Sharp A, Morrissey G, Steel N, Denning DW, Priest T. Infrared body temperature measurement of mice as an early predictor of death in experimental fungal infections. Lab Anim. 2003;37:126–131. doi: 10.1258/00236770360563769. [DOI] [PubMed] [Google Scholar]
- 21.Xu H, Gonzalo JA, St Pierre Y, Williams IR, Kupper TS, Cotran RS, Springer TA, Gutierrez-Ramos JC. Leukocytosis and resistance to septic shock in intercellular adhesion molecule 1-deficient mice. J Exp Med. 1994;180:95–109. doi: 10.1084/jem.180.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 23.De Plaen IG, Han XB, Liu X, Hsueh W, Ghosh S, May MJ. Lipopolysaccharide induces CXCL2/macrophage inflammatory protein-2 gene expression in enterocytes via NF-kappaB activation: independence from endogenous TNF-alpha and platelet-activating factor. Immunology. 2006;118:153–163. doi: 10.1111/j.1365-2567.2006.02344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kisseleva T, Song L, Vorontchikhina M, Feirt N, Kitajewski J, Schindler C. NF-kappaB regulation of endothelial cell function during LPS-induced toxemia and cancer. J Clin Invest. 2006;116:2955–2963. doi: 10.1172/JCI27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roebuck KA. Oxidant stress regulation of IL-8 and ICAM-1 gene expression: differential activation and binding of the transcription factors AP-1 and NF-kappaB (Review) Int J Mol Med. 1999;4:223–230. doi: 10.3892/ijmm.4.3.223. [DOI] [PubMed] [Google Scholar]
- 26.Fujioka S, Niu J, Schmidt C, Sclabas GM, Peng B, Uwagawa T, Li Z, Evans DB, Abbruzzese JL, Chiao PJ. NF-kappaB and AP-1 connection: mechanism of NF-kappaB-dependent regulation of AP-1 activity. Mol Cell Biol. 2004;24:7806–7819. doi: 10.1128/MCB.24.17.7806-7819.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol. 2004;172:2522–2529. doi: 10.4049/jimmunol.172.4.2522. [DOI] [PubMed] [Google Scholar]
- 28.Mukhopadhyay P, Rajesh M, Yoshihiro K, Haskó G, Pacher P. Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem Biophys Res Commun. 2007;358:203–208. doi: 10.1016/j.bbrc.2007.04.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 31.Deans KJ, Haley M, Natanson C, Eichacker PQ, Minneci PC. Novel therapies for sepsis: a review. J Trauma. 2005;58:867–874. doi: 10.1097/01.ta.0000158244.69179.94. [DOI] [PubMed] [Google Scholar]
- 32.Kepp O, Galluzzi L, Kroemer G. Mitochondrial control of the NLRP3 inflammasome. Nat Immunol. 2011;12:199–200. doi: 10.1038/ni0311-199. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carré JE, Orban JC, Re L, Felsmann K, Iffert W, Bauer M, Suliman HB, Piantadosi CA, Mayhew TM, Breen P, Stotz M, Singer M. Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am J Respir Crit Care Med. 2010;182:745–751. doi: 10.1164/rccm.201003-0326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–223. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 36.Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sen CK, Khanna S, Reznick AZ, Roy S, Packer L. Glutathione regulation of tumor necrosis factor-alpha-induced NF-kappa B activation in skeletal muscle-derived L6 cells. Biochem Biophys Res Commun. 1997;237:645–649. doi: 10.1006/bbrc.1997.7206. [DOI] [PubMed] [Google Scholar]
- 38.Janssen-Heininger YM, Poynter ME, Baeuerle PA. Recent advances towards understanding redox mechanisms in the activation of nuclear factor kappaB. Free Radic Biol Med. 2000;28:1317–1327. doi: 10.1016/s0891-5849(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt KN, Amstad P, Cerutti P, Baeuerle PA. The roles of hydrogen peroxide and superoxide as messengers in the activation of transcription factor NF-kappa B. Chem Biol. 1995;2:13–22. doi: 10.1016/1074-5521(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 40.Fan H, Sun B, Gu Q, Lafond-Walker A, Cao S, Becker LC. Oxygen radicals trigger activation of NF-kappaB and AP-1 and upregulation of ICAM-1 in reperfused canine heart. Am J Physiol Heart Circ Physiol. 2002;282:H1778–1786. doi: 10.1152/ajpheart.00796.2000. [DOI] [PubMed] [Google Scholar]
- 41.Pantano C, Reynaert NL, van der Vliet A, Janssen-Heininger YM. Redox-sensitive kinases of the nuclear factor-kappaB signaling pathway. Antioxid Redox Signal. 2006;8:1791–1806. doi: 10.1089/ars.2006.8.1791. [DOI] [PubMed] [Google Scholar]
- 42.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 43.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 44.Haziot A, Ferrero E, Kontgen F, Hijiya N, Yamamoto S, Silver J, Stewart CL, Goyert SM. Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity. 1996;4:407–414. doi: 10.1016/s1074-7613(00)80254-x. [DOI] [PubMed] [Google Scholar]
- 45.Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 46.Oliver FJ, Menissier-de Murcia J, Nacci C, Decker P, Andriantsitohaina R, Muller S, de la Rubia G, Stoclet JC, de Murcia G. Resistance to endotoxic shock as a consequence of defective NF-kappaB activation in poly (ADP-ribose) polymerase-1 deficient mice. Embo J. 1999;18:4446–4454. doi: 10.1093/emboj/18.16.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rahman A, Fazal F. Hug tightly and say goodbye: role of endothelial ICAM-1 in leukocyte transmigration. Antioxid Redox Signal. 2009;11:823–839. doi: 10.1089/ars.2008.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JH, Del Sorbo L, Uhlig S, Porro GA, Whitehead T, Voglis S, Liu M, Slutsky AS, Zhang H. Intercellular adhesion molecule-1 mediates cellular crosstalk between parenchymal and immune cells after lipopolysaccharide neutralization. J Immunol. 2004;172:608–616. doi: 10.4049/jimmunol.172.1.608. [DOI] [PubMed] [Google Scholar]
- 49.Panes J, Perry MA, Anderson DC, Manning A, Leone B, Cepinskas G, Rosenbloom CL, Miyasaka M, Kvietys PR, Granger DN. Regional differences in constitutive and induced ICAM-1 expression in vivo. Am J Physiol. 1995;269:H1955–1964. doi: 10.1152/ajpheart.1995.269.6.H1955. [DOI] [PubMed] [Google Scholar]