Abstract
Purpose
To investigate the association of psychosocial distress with risk of stroke mortality and incident stroke in older adults.
Methods
Data were from the Chicago Health and Aging Project, a longitudinal population-based study conducted in three contiguous neighborhoods on the south side of Chicago, Illinois. Participants were community-dwelling black and non-Hispanic white adults, age 65 and older (N=4,120 for stroke mortality; N=2,649 for incident stroke). Psychosocial distress was an analytically-derived composite measure of depressive symptoms, perceived stress, neuroticism, and life dissatisfaction. Cox proportional hazards models examined the association of distress with stroke mortality and incident stroke over 6 years of follow-up.
Results
151 stroke deaths and 452 incident strokes were identified. Adjusting for age, race, and sex, the hazard ratio (HR) for each 1-SD increase in distress was 1.47 (95% confidence interval [CI]=1.28–1.70) for stroke mortality and 1.18 (95% CI, 1.07–1.30) for incident stroke. Associations were reduced following adjustment for stroke risk factors and remained significant for stroke mortality (HR=1.29; 95% CI=1.10–1.52) but not for incident stroke (HR=1.09; 95% CI=0.98–1.21). Secondary analyses of stroke subtypes showed that distress was strongly related to incident hemorrhagic strokes (HR=1.70; 95% CI=1.28–2.25) but not ischemic strokes (HR=1.02; 95% CI=0.91–1.15) in fully adjusted models.
Conclusions
Increasing levels of psychosocial distress are related to excess risk of both fatal and nonfatal stroke in older black and white adults. Additional research is needed to examine pathways linking psychosocial distress to cerebrovascular disease risk.
Keywords: epidemiology, psychosocial stress, risk factors, women & minorities
Stroke is a leading cause of death and disability in the United States.1 Diabetes, obesity, high blood pressure, cigarette smoking, and physical inactivity are widely recognized risk factors for stroke but they cannot account fully for the excess stroke risk observed in the population. Thus, research is needed to identify additional modifiable risk factors that may contribute to stroke risk. The literature on cardiovascular disease has clearly documented important associations between depression and cardiovascular outcomes, including stroke. Two recent meta-analyses concluded that depressive symptoms significantly increased stroke risk, particularly in women, but with most evidence from studies limited to whites.2,3 Recent research suggests, however, that cardiovascular risk may not be limited to specific negative mood states such as depression, but extends to psychosocial distress, a broader concept defined as the tendency to experience stress, negative events, and more generally a negative outlook on life.4–6 Less is known about the effect of psychosocial distress on risk for stroke morbidity and mortality, although available evidence is suggestive. Recent findings from the INTERSTROKE study, an international multi-center case-control study in 22 countries, indicate that both depression and psychosocial stress, defined in that study as experiencing at least several periods of general stress at work or home in the past year, may be significant risk factors for stroke.7 Little information was provided on the measure of psychosocial stress in INTERSTROKE and a single item was used to assess depression. These two measures may be tapping into the same larger construct of distress and their relationship to stroke risk may be better assessed as such. Given that psychosocial distress may be modifiable, more research is needed to understand how it contributes to stroke risk.
This study uses data from the Chicago Health and Aging Project (CHAP) to investigate the risk of stroke mortality and incident stroke in relation to psychosocial distress in a population-based cohort of older black and white adults. We hypothesized that the effects of distress on stroke risk would be independent of demographic characteristics, stroke risk factors and chronic conditions. Since stroke risk is known to vary by race, sex and age, we further examined whether the relation of distress to stroke risk varied by these demographic characteristics.
Methods
Study Design and Population
CHAP is an ongoing, longitudinal study investigating Alzheimer’s disease and other chronic illnesses of the elderly. Sampling for CHAP took place in three adjacent neighborhoods within the south side of Chicago, IL, whose residents are predominantly black and non-Hispanic white adults from a broad range of socioeconomic backgrounds.8 Eligibility for participation in CHAP was based solely on age. All residents of the three targeted neighborhoods age 65 years or older were invited to participate; 6,158 (78.7% of eligible) completed baseline interviews between 1993 and 1996.
Baseline interviews were conducted in participants’ homes and included questions on medical history, cognitive health, socioeconomic status, behavioral patterns, and psychosocial characteristics. Successive interviews were repeated in three-year cycles after the baseline interview. The 2nd cycle (1997–1999) of interviews assessed the broadest range of psychosocial characteristics, and serves as the baseline for this analysis. A total of 4,319 participants (87% of surviving cohort) completed cycle 2; of these, 4,120 had non-missing data on the psychosocial factors, demographic characteristics, and known vital status and thus were eligible for the current analyses. The Institutional Review Boards of Rush University Medical School and the University of Minnesota approved the study. All participants provided written, informed consent.
Outcome Measures
Stroke Mortality
Information on vital status was obtained at each follow-up interview; reported deaths through 12/31/2007 were verified by linkages with the National Death Index (NDI). Primary, known causes of death were available for all NDI-verified deaths. Stroke deaths were defined by the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9) codes 430–438 for deaths prior to 1999, and Tenth Revision (ICD-10) codes I60-I69 beyond 1999. Stroke accounted for approximately 9% of deaths with known causes; 151 stroke deaths were identified over 6.7 (SD=3.5) years of follow-up.
Incident Stroke
Information on stroke hospitalizations within the CHAP cohort was ascertained via linkages with Medicare claims data from the Center for Medicare and Medicaid Services (CMS). CMS claims data were available through 12/31/2007; 88.2% of the CHAP cohort had matching claims data. Analyses of incident stroke excluded those who self-reported a history of stroke any time prior to the 2nd cycle of CHAP data collection (i.e., baseline for the analyses). Participants also were excluded from analysis for incident stroke any time during follow-up when they were actively involved in a health maintenance organization (HMO) since they would not simultaneously be enrolled in Medicare during those periods. Thus, for these analyses, the sample was reduced to 2,649 participants. For the participants with claims data, strokes were defined by ICD-9 codes 430–438 since CMS continues to use ICD-9 coding. Ischemic strokes were identified by ICD-9 codes 433.01, 433.1, 433.2, 433.21, 433.3, 433.31, 433.81, 434.01, 434.1, 434.11, 434.91, 435.2, 435.3, 435.8, 435.9, 436.0, 437.1, 437.7, 437.9, and 438.0. Hemorrhagic strokes were identified by ICD-9 codes 430, 431, 432.1, and 432.9. A total of 452 persons had at least one hospitalization for an incident stroke event (408 ischemic and 44 hemorrhagic) during follow-up; average follow-up time in these analyses was 6.0 (SD=3.4) years.
Measurement of Psychosocial Distress
Distress was measured by four psychosocial indicators at the 2nd cycle of CHAP data collection: depressive symptoms, perceived stress, neuroticism, and life satisfaction (reverse-coded). Prior analyses in CHAP showed that these four indicators loaded together on a single factor as a latent psychosocial construct that was theoretically and empirically justified and reliable across race and sex groups.9 Depressive symptoms were measured by the 10-item Center of Epidemiologic Studies-Depression (CES-D) scale, specifically developed for older populations;10 higher scores (range 0–10) indicate more depressive symptoms (Cronbach’s alpha, 0.76). Perceived stress was assessed by an abbreviated 6-item Perceived Stress Scale;11,12 higher scores indicate a higher level of perceived stress (Cronbach’s alpha, .82). Life satisfaction was measured by the 5-item Life Satisfaction Scale,13 reverse-coded such that higher scores (range, 0–15) reflected life dissatisfaction (Cronbach’s alpha=0.73). Neuroticism, a broad personality domain characterized by anxious, angry, and vulnerable traits, was measured by four items from the NEO Five-Factor Inventory (NEO-FFI).14 Consistent with prior work,15 the scale score was multiplied by three to achieve consistency with the full, 12-item neuroticism scale (range 0–45); higher scores reflect greater neuroticism. Shortening the scale resulted in a loss of internal consistency (Cronbach’s alpha=0.54); however, previous work shows that the abbreviated 4-item scale is significantly correlated with the full scale and equally predicts poor health outcomes.15,16
A Distress factor score was created by averaging the standardized values of the four psychosocial measures and dividing by its standard deviation. A higher factor score was indicative of higher levels of distress.
Assessment of covariates
Demographic characteristics obtained at the initial CHAP interview included self-reported age, race (non-Hispanic black or white), sex, and education (years of completed schooling). Stroke risk factors included as covariates were measured at the 2nd cycle of data collection. Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared and modeled categorically based on clinically established BMI categories.17 Systolic blood pressure (mmHg) was measured as the average of two consecutive mercury sphygmomanometer readings, taken in the seated position.18 Self-reported cigarette smoking was reported as never, former (ever), and current. Physical activity was measured by the sum of nine dichotomous response items inquiring about activities (e.g., walking, gardening, dancing) over the last two weeks.19 History of chronic health conditions was based on affirmative responses to inquiries about four physician-diagnosed conditions: cardiovascular diseases, hip fracture, diabetes, and cancer;20 each chronic condition was modeled separately as a dichotomous variable. History of stroke was indicated separately as a dichotomous variable based on self-reported, physician-diagnosed stroke. Chronic conditions and history of stroke took into account participants’ reports from both the first and second cycles of data collection in CHAP. Self-reported current usage of anti-hypertensives, lipid-lowering medications, and anti-depressants, confirmed by medication review, were modeled as three separate dichotomous variables.
Statistical Analysis
Descriptive statistics were calculated for baseline sociodemographic characteristics, distress, and health-related variables for all participants and by categorical distress scores. Cox proportional hazard models examined the association between distress at baseline and subsequent stroke mortality and incident stroke. Subequent analyses examined incident ischemic and incident hemorrhagic strokes separately. The unit of time in all Cox models was one year; proportional hazards assumptions were checked analytically and graphically and all assumptions were met. Analyses for all outcomes were conducted in a similar manner, as follows. The initial model included adjustments for age, race, and sex. We also tested for age by distress, race by distress, and sex by distress interactions in a minimally adjusted model. The second model added covariates adjusting for education, BMI, systolic blood pressure, smoking status, physical activity, chronic conditions, and medication usage. Adjusted analyses for stroke mortality also included history of stroke. Due to missing data on some covariates, sample sizes for risk factor-adjusted models were 3,916 for stroke mortality (142 events) and 2,539 for incident stroke (426 events). Distress was modeled continuously and categorically (based on quartiles) in separate analyses. SAS software version 9.2 was used for all analyses.
Results
Population Characteristics
Baseline characteristics are shown in Table 1 for all participants and by distress quartiles. The 4,120 eligible participants were predominantly black and female, average age was 77 years old, most had a high school education, and an average of one chronic condition; 13.1% reported a history of stroke. Nearly all characteristics varied by distress level; those in the most distressed group (Q4) were older, more likely to be female and black, less educated, less physically active, and reported a higher prevalence of most chronic health conditions and use of anti-depressants.
Table 1.
Participant Characteristics at Baseline, 1997–1999
| All Participants (N=4,120) | Quartiles of Distress | |||||
|---|---|---|---|---|---|---|
| Q1 (N=1032) | Q2 (N=1028) | Q3 (N=1031) | Q4 (N=1029) | P-value | ||
|
| ||||||
| Age, mean (SD), years | 77.1 (6.3) | 75.7 (5.5) | 76.7 (6.1) | 77.5 (6.4) | 78.5 (6.9) | <0.0001 |
| Women, N (%) | 2545 (61.8) | 573 (55.5) | 614 (59.7) | 648 (62.9) | 710 (69.0) | <0.0001 |
| Black, N (%) | 2531 (61.4) | 587 (56.9) | 566 (55.1) | 642 (62.3) | 736 (71.5) | <0.0001 |
| Education, mean (SD), years | 12.0 (3.7) | 13.0 (3.6) | 12.6 (3.5) | 11.7 (3.6) | 10.9 (3.6) | <0.0001 |
| Systolic blood pressure, mmHg, mean (SD) | 135.0 (18.8) | 136.3 (17.8) | 135.0 (18.1) | 134.9 (19.5) | 133.6 (19.6) | 0.019 |
| BMI, kg/m2, mean (SD) | 26.7 (5.7) | 27.1 (5.0) | 26.9 (5.5) | 26.7 (6.1) | 26.3 (6.2) | <0.0001 |
| BMI<18.5, N (%) | 179 (4.4) | 24 (2.4) | 33 (3.2) | 53 (5.2) | 69 (7.0) | <0.0001 |
| 18.5≤BMI<25 | 1518 (37.5) | 339 (33.0) | 378 (37.1) | 402 (39.5) | 399 (40.3) | |
| 25≤BMI<30 | 1381 (34.1) | 421 (41.2) | 362 (35.6) | 318 (31.2) | 280 (28.3) | |
| BMI≥30 | 971 (24.0) | 239 (23.4) | 245 (24.1) | 245 (24.1) | 242 (24.4) | |
| Smoking Status, N (%) | 0.043 | |||||
| Never smoker | 1902 (46.2) | 502 (48.7) | 475 (46.2) | 445 (43.2) | 480 (46.7) | |
| Ever smoker | 1719 (41.8) | 420 (40.7) | 439 (42.7) | 433 (42.0) | 427 (41.6) | |
| Current smoker | 495 (12.0) | 109 (10.6) | 114 (11.1) | 152 (14.8) | 120 (11.7) | |
| Physical activity sum, mean (SD) | 2.9 (4.8) | 4.1 (5.4) | 3.4 (5.3) | 2.5 (4.6) | 1.4 (1.1) | <0.0001 |
| Use of anti-hypertensives, N (%) | 2,564 (62.2) | 587 (56.9) | 636 (61.9) | 678 (65.8) | 663 (64.4) | 0.0002 |
| Use of lipid-lowering medication, N (%) | 443 (10.8) | 134 (13.0) | 122 (11.9) | 104 (10.1) | 83 (8.1) | 0.002 |
| Use of anti-depressants, N (%) | 152 (3.7) | 12 (1.2) | 31 (3.0) | 38 (3.7) | 71 (6.9) | <0.0001 |
| Chronic health conditions, mean (SD) | 1.2 (0.98) | 0.97 (0.86) | 1.2 (0.93) | 1.3 (0.98) | 1.4 (1.08) | <0.0001 |
| History of CVD, N (%) | 675 (16.4) | 122 (11.8) | 143 (13.9) | 184 (17.9) | 226 (22.0) | <0.0001 |
| History of diabetes, N (%) | 367 (8.9) | 60 (5.8) | 84 (8.2) | 100 (9.7) | 123 (11.9) | <0.0001 |
| History of cancer, N (%) | 941 (22.8) | 204 (19.8) | 252 (24.5) | 241 (23.4) | 244 (23.7) | 0.051 |
| History of hip fracture, N (%) | 178 (4.3) | 24 (2.3) | 44 (4.3) | 43 (4.2) | 67 (6.5) | <0.0001 |
| History of stroke, N (%) | 538 (13.1) | 83 (8.0) | 117 (11.4) | 128 (12.4) | 210 (20.4) | <0.0001 |
Note: The study sample size was 4,120, but due to missing data, the N for covariates ranged from 3,988 to 4,120. Q1–Q4 indicate quartiles of the distress measure based on four approximate divisions of the overall distress factor score. Number of chronic health conditions is the count of a participant’s history of cardiovascular diseases, hip fracture, diabetes, and cancer. Demographic statistics were similar for the sample populations included in the stroke mortality (N=4,120) and incident stroke (N=2,649) analyses. P-values are from Chi-square tests or analysis of variance, as appropriate.
Distress and Stroke Mortality
Adjusting for age, race, and sex, each 1-SD higher distress score was significantly related to a 47% greater risk of dying from stroke (HR=1.47; 95% CI=1.28–1.70; p<.0001). Further adjustments for education, stroke risk factors, chronic conditions, medication usage, and history of stroke reduced the hazard ratio (HR) but the association remained significant (HR=1.29; 95% CI=1.10–1.52; p=.0018). There were no age, race, or sex interactions with distress (all p>.20; results not shown).
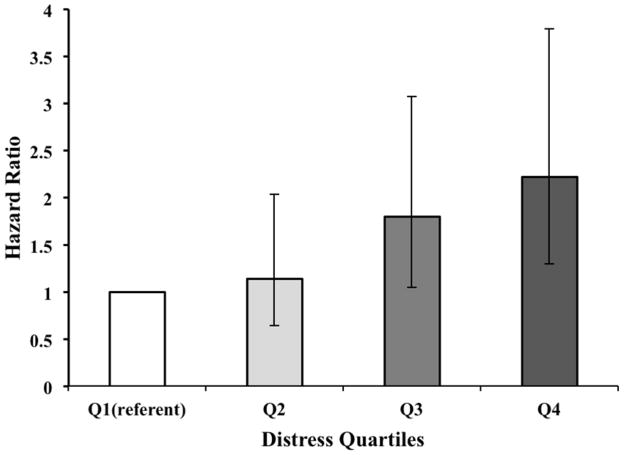
With distress modeled categorically and adjusting for age, race, and sex, participants in the highest quartile had nearly three times (HR=2.97; 95% CI=1.81–4.88; p<.0001) and those in the third quartile had nearly two times (HR=1.98; 95% CI=1.19–3.30; p=.0091) greater risk of dying from stroke relative to those with the lowest distress scores. The second quartile was not at increased risk (HR=1.24; 95% CI=0.71–2.15). Results from the risk factor-adjusted model are shown in Figure 1. The dose-response pattern of stroke mortality risk by level of distress is clearly evident; associations remained significant for the top two quartiles of distress.
Figure 1.
Hazard Ratios & 95% Confidence Intervals For Stroke Mortality By Categories of Distress. Q1–Q4 indicate quartiles of the distress measure from low to high based on four approximate divisions of the overall distress factor score (N=3,916, with 142 stroke deaths). Error bars indicate 95% confidence intervals. The data in the graph are based on the risk factor-adjusted model, controlling for age, race, sex, education, systolic blood pressure, body mass index, physical activities, smoking status, chronic health conditions, medication usage, and history of stroke.
Distress and Incident Stroke
Each 1-SD higher distress score predicted 18% greater risk of incident stroke during follow-up, adjusting for age, race, and sex (HR=1.18; 95% CI=1.07–1.30; p=.001). Further adjustment for education, stroke risk factors, chronic conditions, and medication usage attenuated these results (HR=1.09; 95% CI=0.98–1.21; p=.109). There were no interactions between distress and age, race, or sex (all p>.12; data not shown).
With distress modeled categorically, a dose-response pattern of risk for incident stroke was observed. Relative to the least distressed quartile, the HRs for the second, third, and fourth quartiles were 1.27 (95% CI=0.98–1.65; p=.067), 1.44 (95% CI=1.10–1.87; p=.0068) and 1.54 (95% CI=1.16–2.04; p=.0025), respectively, in a model adjusted for age, race and sex. Associations were reduced after adding adjustments for stroke risk factors (Q2: HR=1.21; 95% CI=0.93–1.58; p=.165); Q3: HR=1.22; 95% CI=0.92–1.61; p=.16; Q4: HR=1.31; 95% CI=0.97–1.76; p=.08).
We further evaluated type of stroke in relation to distress, categorizing strokes into ischemic and hemorrhagic strokes as described. Distress was related to incident ischemic stroke in the minimally adjusted model (HR=1.12; 95% CI=1.01–1.24; p=.031) but this relation was attenuated in the risk factor-adjusted model (HR=1.02; 95% CI=0.91–1.15; p=.69). In contrast, a robust association of distress with hemorrhagic stroke was observed; each 1-SD increase in distress was associated with a 72% (HR=1.72; 95% CI=1.32–2.24; p<.0001) increased risk of hemorrhagic stroke adjusting for age, sex, and race, which was little changed following risk factor adjustment (HR=1.70; 95% CI=1.28–2.25; p=.0003).
Individual Psychosocial Measures and Stroke Outcomes
Finally, we examined the four individual psychosocial measures used to create the Distress factor score in relation to stroke mortality and incident stroke. Each measure was modeled as a z-score in separate analyses and covariates were the same as in our main analyses. For stroke mortality, HR were 1.34, 1.29, 1.36, and 1.47 for each 1-SD increase in depressive symptoms, neuroticism, life satisfaction (reverse-coded), and perceived stress, respectively (all p≤.001), adjusted for age, race and sex; with risk factor adjustments, the HR ranged from 1.19 to 1.27 (all p≤.05). For incident stroke, the HR ranged from 1.11 to 1.13 (all p<.04) in the minimally adjusted models and 1.05 to 1.08 and non-significant with risk factor adjustment. For incident hemorrhagic stroke, however, the adjusted associations were robust; HR were 1.63 (p=.0003), 1.30 (p<.10), 1.52 (p<.01), and 1.74 (p<.001) for depressive symptoms, neuroticism, life satisfaction and perceived stress, respectively.
Discussion
This study identified a robust relationship between psychosocial distress and increased risk of stroke mortality and incident stroke in over six years of follow-up in a population-based cohort of older black and white adults. Moreover, a clear dose-response pattern of associations was evident, with the most distressed participants (top quartile of the distribution) experiencing more than a two-fold increased risk of stroke mortality and a 31% increased risk of incident stroke, compared to their least distressed peers, after adjusting for potential confounders and stroke risk factors. Analyses of stroke subtypes revealed that distress was significantly related to incident hemorrhagic strokes, but not to ischemic strokes after adjustment for covariates. Our findings clearly document important adverse effects of psychosocial distress on cerebrovascular disease risk in the elderly.
Results from the present study are largely consistent with three other studies of stroke outcomes have included measures labeled as “psychological distress” although the measures used in those studies mostly captured depressive symptoms. One study reported that high scores (≥5) on the 30-item General Health Questionnaire (GHQ) were associated with fatal but not nonfatal ischemic stroke or transient ischemic attacks in middle-aged men in South Wales.21 The GHQ was described as a measure of psychological distress and high scorers were considered to possibly have a “minor psychiatric disorder” though the GHQ was discussed largely as a measure of depressive symptoms. A more recent study used a 12-item version of the GHQ and reported that high scorers had elevated risk of death from cerebrovascular disease and ischemic heart disease over 8 years of follow-up among more than 68,000 British adults; incident stroke was not reported.22 Another large prospective cohort study of initially stroke-free middle-aged and older adults examined whether the association of mood status with stroke risk was related to symptoms of depression, measured by the 5-item Mental Health Inventory and labeled as distress, or to major depressive disorder and showed that only the symptom measure predicted increased stroke risk.2 Our study found that a composite measure of distress, assessed by four indicators of psychosocial functioning, contributes to excess stroke risk. Each of the individual psychosocial measures related to our outcomes as well, at approximately similar levels of risk. This study extends the prior work by including a racially diverse cohort of older men and women.
The pathways by which distress may increase stroke risk are not fully understood. Interestingly, our analyses of incident stroke events show that the critical biologic pathways may not be ischemic in origin. Several plausible pathways were not considered due to lack of data in the present study, but could help explain our observations. For example, chronic stress and negative emotional states have known neuroendocrine and inflammatory effects that could affect stroke risk.4 An extensive literature documents stress-induced dysregulation in various physiologic systems – variously termed ‘allostatic load’ or ‘cumulative biologic risk’ that could contribute to accelerated aging.9,23,24 Recent literature suggests that HPA dysregulation related to stress and depression may cause an increase in circulating catecholamines, endothelial dysfunction, and platelet activation, culminating in a “hypercoaguable state” and thereby increasing stroke risk.25,26 These pathways likely are more important for ischemic than hemorrhagic stroke, however, and we observed much stronger findings for hemorrhagic stroke, so additional pathways need to be considered. It is possible that the burden of biologic risk that occurs with chronic exposure to stress and distress leads to alterations in physiologic regulation across multiple systems, which together could lead to the accumulation or exacerbation of cerebrovascular pathology and excess stroke risk of any subtype. Although no studies to date have tested this hypothesis, future analyses evaluating the relationship between multiple clinical neuroimaging markers of cerebrovascular disease (e.g., infarct size, number and location) to distress and incident stroke would be useful.
Psychological and behavioral factors also may be important. Persons who experience a high level of distress may be less likely or less able to adhere to treatment recommendations, e.g., compliance with medications or maintaining a healthy lifestyle. Our most distressed participants were less physically active, and had a higher prevalence of CVD and diabetes, suggesting potentially greater disease burden in this group, which could make lifestyle management more challenging. Nonetheless, controlling for these factors had little effect on the relation between distress and either stroke mortality or hemorrhagic strokes.
The present study has notable strengths and weaknesses. We included a sizable population-based sample of older blacks and non-Hispanic whites from a large, urban Midwestern city, making our findings generalizable to other similar populations. However, findings may not hold in other minority or age groups. Moreover, due to differences in health outcomes across regions within the US, our findings may not equally translate to other geographic regions. In particular, differences may occur in high-risk regions such as the southeastern region often regarded as the “Stroke Belt.” CHAP includes extensive data on sociodemographic characteristics and stroke risk factors, enabling us to conduct analyses with important multi-variable adjustments. Yet, we lacked data on important inflammatory and neuroendocrine biomarkers, therefore limiting our ability to test additional important pathways that may link psychosocial distress to stroke risk. CHAP does not have concurrent imaging data on participants, which could provide important information and clinical details about the types of strokes experienced and potentially further our understanding of the observed relationships. This study does not have clinically adjudicated outcome data, although such data are rare in most large-scale epidemiologic studies such as CHAP. Utilizing the CMS Medicare Claims data to ascertain incident stroke events (hospitalizations) in a study of persons 65 and older has a distinct advantage, however, because there is little to no bias in outcome data due to differential health care access, which can be a problem in younger populations. Finally, with psychosocial assessments at just one point in time, we are unable to determine whether changes in distress levels occurred or if such changes influence stroke risk.
In sum, this study provides important evidence linking psychosocial distress to risk of both fatal and nonfatal stroke outcomes in elderly blacks and whites. The biologic mechanisms underlying these associations remain to be determined, although our data suggest that pathways related to non-ischemic disease mechanisms may be critical. Better understanding of the psychosocial risk pathways for cerebrovascular disease may lead to future interventions that could reduce the risk of stroke in the elderly.
Acknowledgments
The authors thank all participants in the Chicago Health and Aging Project, as well as all administrative and field staff for community development, oversight of project coordination, study coordination and data collection.
Sources of Funding
This work was supported by NIH grants, including grant HL084209 from the National Heart, Lung and Blood Institute (NHLBI), grant AG11101 from the National Institutes on Aging (NIA), and grant ES10902 from the National Institute on Environmental Health Sciences (NIEHS), grant K01HL092591 (Dr. Lewis) from NHLBI, grant 1KL2RR033182-01 (Dr. Clark) from the National Center For Research Resources (NCRR), grant 8UL1TR000114-02 from the National Center for Advancing Translational Sciences (NCATS), and by grant UL1RR033183 from NCRR to the University of Minnesota Clinical and Translational Science Institute (CTSI). Dr. Everson-Rose also supported in part by grant 1P60MD003422 from the National Institute on Minority Health and Health Disparities (NIMHD). Contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, NHLBI, NIA, NIEHS, NIMHD, NCATS, NCRR, or CTSI. The University of Minnesota CTSI is part of a national Clinical and Translational Science Award consortium created to accelerate laboratory discoveries into treatments for patients. Additional support provided by the Program in Health Disparities Research and the Applied Clinical Research Program (Dr. Clark; Dr. Everson-Rose; Mrs. Henderson) at the University of Minnesota.
Footnotes
Disclosures: None.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2012 update: A report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB. Depression and risk of stroke morbidity and mortality: A meta-analysis and systematic review. JAMA. 2011;306:1241–1249. doi: 10.1001/jama.2011.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong JY, Zhang YH, Tong J, Qin LQ. Depression and risk of stroke: A meta-analysis of prospective studies. Stroke. 2011;4:188–196. [Google Scholar]
- 4.Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev Public Health. 2005;26:469–500. doi: 10.1146/annurev.publhealth.26.021304.144542. [DOI] [PubMed] [Google Scholar]
- 5.Brotman DJ, Golden SH, Wittstein IS. The cardiovascular toll of stress. Lancet. 2007;370:1089–1100. doi: 10.1016/S0140-6736(07)61305-1. [DOI] [PubMed] [Google Scholar]
- 6.Hamer M, Molloy GJ, Stamatakis E. Psychological distress as a risk factor for cardiovascular events: Pathophysiological and behavioral mechanisms. Am Coll Cardiol. 2008;52:2156–2162. doi: 10.1016/j.jacc.2008.08.057. [DOI] [PubMed] [Google Scholar]
- 7.O’Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): A case-control study. Lancet. 2010;376:112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 8.Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA. Design of the Chicago Health And Aging Project (CHAP) J Alzheimers Dis. 2003;5:349–355. doi: 10.3233/jad-2003-5501. [DOI] [PubMed] [Google Scholar]
- 9.Clark CJ, Henderson KM, Mendes de Leon CF, Guo H, Lunos S, Evans DA, et al. Latent constructs in psychosocial factors associated with cardiovascular disease: An examination by race and sex. Frontiers in Psychiatry. 2012;3:1–9. doi: 10.3389/fpsyt.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 11.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 12.Cohen S, Williamson G. The Social Psychology of Health: Claremont Symposium on Applied Social Psychology. Newbury Park, CA: Sage; 1988. Perceived stress in a probability sample of the United States. [Google Scholar]
- 13.Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. J Pers Assess. 1985;49:71–75. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- 14.Costa PT, McCrae RR. Revised NEO personality inventory (NEO-PI-R) and NEO five-factor inventory (NEO-FFI) professional manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- 15.Wilson RS, Krueger KR, Gu L, Bienias JL, Mendes de Leon CF, Evans DA. Neuroticism, extraversion, and mortality in a defined population of older persons. Psychosom Med. 2005;67:841–845. doi: 10.1097/01.psy.0000190615.20656.83. [DOI] [PubMed] [Google Scholar]
- 16.Wilson RS, Barnes LL, Bennett DA, Li Y, Bienias JL, Mendes de Leon CF, et al. Proneness to psychological distress and risk of Alzheimer Disease in a biracial community. Neurology. 2005;64:380–382. doi: 10.1212/01.WNL.0000149525.53525.E7. [DOI] [PubMed] [Google Scholar]
- 17.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults--the evidence report. National Institutes of Health. Obes Res. 1998;6 (Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 18.Variability of blood pressure and the results of screening in the hypertension detection and follow-up program. J Chronic Dis. 1978;31:651–667. doi: 10.1016/0021-9681(78)90069-3. [DOI] [PubMed] [Google Scholar]
- 19.McPhillips JB, Pellettera KM, Barrett-Connor E, Wingard DL, Criqui MH. Exercise patterns in a population of older adults. Am J Prev Med. 1989;5:65–72. [PubMed] [Google Scholar]
- 20.Cornoni-Huntley J, Brock D, Ostfeld A, Taylor J, Wallace R. Established Populations For Epidemiological Studies Of The Elderly Resouce Data Book. Washington, DC: US Department of Health and Human Services; 1986. [Google Scholar]
- 21.May M, McCarron P, Stansfeld S, Ben-Shlomo Y, Gallacher J, Yarnell J, et al. Does psychological distress predict the risk of ischemic stroke and transient ischemic attack? The Caerphilly Study. Stroke. 2002;33:7–12. doi: 10.1161/hs0102.100529. [DOI] [PubMed] [Google Scholar]
- 22.Hamer M, Kivimaki M, Stamatakis E, Batty GD. Psychological distress as a risk factor for death from cerebrovascular disease. CMAJ. 2012;184:1461–1466. doi: 10.1503/cmaj.111719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 24.King KE, Morenoff JD, House JS. Neighborhood context and social disparities in cumulative biological risk factors. Psychosom Med. 2011;73:572–579. doi: 10.1097/PSY.0b013e318227b062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emsley HC, Hopkins SJ. Acute ischaemic stroke and infection: Recent and emerging concepts. Lancet Neurol. 2008;7:341–353. doi: 10.1016/S1474-4422(08)70061-9. [DOI] [PubMed] [Google Scholar]
- 26.Elkind MS. Why now? Moving from stroke risk factors to stroke triggers. Curr Opin Neurol. 2007;20:51–57. doi: 10.1097/WCO.0b013e328012da75. [DOI] [PubMed] [Google Scholar]