Highlights
► AmiRNAs can be utilized to inhibit adenovirus replication in vitro. ► Adenoviral vectors are an effective expression vehicle for anti-adenoviral amiRNAs. ► A combination of amiRNA and cidofovir results in additive effects.
Keywords: Adenovirus, RNA interference, microRNA
Abstract
Human adenoviruses are rarely associated with life-threatening infections in healthy individuals. However, immunocompromised patients, and particularly allogeneic hematopoietic stem cell transplant recipients, are at high risk of developing disseminated and potentially fatal disease. The efficacy of commonly used drugs to treat adenovirus infections (i.e., cidofovir in most cases) is limited, and alternative treatment options are needed. Artificial microRNAs (amiRNAs) are a class of synthetic RNAs resembling cellular miRNAs, and, similar to their natural relatives, can mediate the knockdown of endogenous gene expression. This process, termed RNA interference, can be harnessed to target and potentially silence both cellular and viral genes. In this study, we designed amiRNAs directed against adenoviral E1A, DNA polymerase, and preterminal protein (pTP) mRNAs in order to inhibit adenoviral replication in vitro. For the expression of amiRNA-encoding sequences, we utilized replication-deficient adenoviral vectors. In cells transduced with the recombinant vectors and infected with the wild-type (wt) adenovirus, one particular amiRNA that was directed against the pTP mRNA was capable of decreasing the output of infectious wt virus progeny by 2.6 orders of magnitude. This inhibition rate could be achieved by concatemerizing amiRNA-encoding sequences to allow for high intracellular amiRNA concentrations. Because superinfecting wt virus induces the replication and amplification of the recombinant adenoviral vector, amiRNA concentrations were increased in cells infected with wt adenovirus. Furthermore, a combination of amiRNA expression and treatment of infected cells with cidofovir resulted in additive effects that manifested as a total reduction of infectious virus progeny by greater than 3 orders of magnitude.
1. Introduction
Human adenoviruses (Echavarria, 2008; Ison, 2006; Kojaoghlanian et al., 2003), belonging to the group of double-stranded (ds) DNA viruses, are a major cause of systemic infections with significant mortality rates in immunocompromised patients such as hematopoietic stem cell transplant recipients (Blanke et al., 1995; Hale et al., 1999; Howard et al., 1999; Lion et al., 2003; Munoz et al., 1998). Severe manifestations are mostly caused by adenoviruses belonging to species B and C (Kojaoghlanian et al., 2003), with a predominance of species C members reported in certain studies (Ebner et al., 2006; Lion et al., 2003, 2010). To date, the only drug with convincing anti-adenoviral activity in vivo is cidofovir (CDV).
However, regarding the treatment of adenoviral infections in immunocompromised patients, CDV is neither capable of fully preventing fatal outcomes in all instances (Lenaerts et al., 2008; Lindemans et al., 2010; Ljungman et al., 2003; Symeonidis et al., 2007; Yusuf et al., 2006), nor thought to be able to completely clear infections without the concomitant re-establishment of the immune system (Chakrabarti et al., 2002; Heemskerk et al., 2005; Lindemans et al., 2010). Moreover, it displays significant nephrotoxicity and limited bioavailability. Derivatives of CDV have been developed, but are still under investigation (Hartline et al., 2005; Paolino et al., 2011). Thus, there is a need for the development of alternative drugs or even alternative treatment strategies.
RNA interference (RNAi) is a post-transcriptional cellular process that results in gene silencing (Carthew and Sontheimer, 2009; Ghildiyal and Zamore, 2009; Huntzinger and Izaurralde, 2011; Hutvagner and Simard, 2008; Kawamata and Tomari, 2010). It is triggered by short (∼21–25 nt) dsRNAs displaying partial or complete complementarity to their target mRNAs (Fire et al., 1998). MicroRNAs (miRNAs) are members of this group of small RNAs. Their precursors, primary miRNAs (pri-miRNAs), are processed by Drosha/DGCR8 into 60–70 nt precursor miRNAs (pre-miRNAs) (Cullen, 2004), that are subsequently exported from the nucleus by Exportin-5 (Yi et al., 2003), and eventually processed into mature miRNAs by the ribonuclease-III enzyme Dicer (Cullen, 2004). The so-called guide strand is loaded into the RNA-induced silencing complex (RISC) (Sontheimer, 2005), where it mediates the cleavage or deadenylation of its target mRNA, or leads to translational repression (Huntzinger and Izaurralde, 2011).
RNAi has quickly been adopted as a tool to knock down the expression of disease-associated genes or to inhibit viral gene expression (Davidson and McCray, 2011). This is either mediated by synthetic short interfering RNAs (siRNAs) that are directly incorporated into RISC (Elbashir et al., 2001), short hairpin shRNAs that resemble pre-miRNAs (Burnett and Rossi, 2012), or artificial miRNAs (amiRNAs) that are analogs of pri-miRNAs (Zeng et al., 2002). RNAi-mediated inhibition of viral replication has been demonstrated for a wide range of viruses, both in vitro and in vivo (Arbuthnot, 2010; Haasnoot et al., 2007; Zhou and Rossi, 2011). We and others have recently demonstrated the successful in vitro inhibition of the replication of wild-type (wt) adenovirus (Ad) serotypes 1, 2, 5, and 6 (all belonging to species C and representing a main cause of severe adenovirus-related disease) (Kneidinger et al., 2012) and a mutated version of Ad5 lacking the E1B and E3 genes (Eckstein et al., 2010). The inhibition of an Ad 11 strain (2K2/507/KNIH; species B; isolated in Korea) has also been described (Chung et al., 2007). In all cases, inhibition of virus replication was mediated by exogenously added siRNAs, and mRNAs originating from viral genes directly or indirectly associated with viral DNA replication were identified as key targets (Kneidinger et al., 2012).
Although in vivo siRNA delivery has continuously been improved over the last years (Rettig and Behlke, 2012), it still represents a major challenge. In particular, targeted delivery into certain cell types or organs has proven tricky. In the past, viral vectors have frequently and successfully been employed for the delivery of protein-encoding DNA sequences into living organisms. Consequently, they have also been adopted for the delivery of shRNAs and amiRNAs (Liu and Berkhout, 2011; Mowa et al., 2010; Raoul et al., 2006). Depending on the type of target cell, organ, or delivery route, they may still outperform nonviral delivery systems in certain instances. Adenoviral vectors have been used for a long time to deliver DNA sequences into living organisms (Goncalves and de Vries, 2006). Since they display the same cell tropism as wt adenoviruses (when belonging to the same adenoviral species), they deliver transgenic DNA into exactly those cells that represent the main targets of their wt counterparts. Thus, adenoviral vectors may constitute a particularly attractive tool for the delivery of anti-adenoviral shRNAs or amiRNAs.
Consequently, in the present study, we generated a series of replication-deficient adenoviral amiRNA expression vectors for the silencing of selected Ad5 genes and investigated whether these amiRNAs are capable of efficiently inhibiting the replication of wt Ad5 upon transduction of a cell with the recombinant vector. The amiRNAs were designed to recognize those mRNAs that had been identified as candidate targets in our previous study (Kneidinger et al., 2012), i.e., mRNAs encoding the viral E1A protein, a key regulator of the infection cycle (Pelka et al., 2008), the preterminal protein (pTP), and the viral DNA polymerase, both essential for viral DNA replication (de Jong et al., 2003). Here, we present data demonstrating the efficient silencing of the wt Ad5 pTP gene upon transduction with amiRNA expression vectors. Moreover, we demonstrate an increase in the knockdown rate upon concatemerization of amiRNA-encoding sequences, and we show that amiRNA expression is strongly boosted in wt Ad5-infected cells, a prerequisite for the efficient targeting of high numbers of viral transcripts. Taken together, our data indicate that amiRNA-mediated knockdown of wt Ad5 gene expression significantly inhibits viral DNA replication and efficiently decreases the output of infectious virus progeny in vitro.
2. Materials and methods
2.1. Cell culture, virus production, and titer determination
HEK 293 (human embryonic kidney; ATCC CRL-1573), A549 (human epithelial lung carcinoma; ATCC CCL-185), HeLa (human epithelial carcinoma; ATCC CCL-2), SW480 (human colon carcinoma; ATCC CCL-228), RD-ES (Ewing’s sarcoma; ATCC HTB-166), and T-REx-293 cells (Life Technologies Austria, Vienna, Austria) were cultivated in Dulbecco’s Modified Eagles Medium (DMEM) with stabilized glutamine (PAA Laboratories, Pasching, Austria) and supplemented with 10% fetal bovine serum (FBS; PAA Laboratories) in a humidified 5% CO2 atmosphere at 37 °C. Recombinant adenoviral vectors expressing Ad5-directed amiRNAs were amplified in T-REx-293 cells. All other adenoviral vectors and wt Ad5 (ATCC VR-5) were amplified in HEK 293 cells. Titers of infectious adenoviruses expressing amiRNAs were determined on T-REx-293 cells by 50% tissue culture infective dose (TCID50) assays. Titers of wt Ad5 present in mixed virus suspensions containing both wt and recombinant virus as obtained in combined transduction/infection experiments were determined on A549 cells using the same method. All other TCID50 assays were performed with HEK 293 cells.
2.2. Vector constructions
The vectors employed in dual-luciferase assays for the screening of Ad5-directed amiRNAs have been described elsewhere (Kneidinger et al., 2012). The dual-luciferase target vector used for the determination of Renilla luciferase gene silencing in Ad5-infected cells was constructed as follows: a part of the modified coding region of the firefly (Photinus pyralis) luciferase open reading frame (ORF) representing the target sequence for the corresponding amiRNA was amplified by PCR with primers Fluc-f2 (5′-ATAAGGCTATCTCGAGATACGCCCTGGTTCC-3′) and Fluc-r2 (5′-AATGTCGTTCGCGGCCGCAACTGCAACTCCGAT-3′) from vector pGL3 (Promega, Mannheim, Germany). This fragment was restricted with XhoI and NotI and inserted into the corresponding sites located within the 3′UTR of the Renilla luciferase gene present on plasmid psiCHECK-2 (Promega, Mannheim, Germany). From the resulting vector (psiCHECK-FLuc2), a BglII-BamHI fragment comprising both the firefly and Renilla luciferase expression cassettes was transferred into pENTR4 (Life Technologies Austria, Vienna, Austria) that had been restricted with XmnI and EcoRV. From the resulting vector (pENTR-Luc), the entire expression cassette was eventually moved into the deleted E1 region of the adenoviral vector pAd/PL-DEST (Life Technologies Austria, Vienna, Austria) giving rise to vector Ad-Luc-as (Fig. 1). This final transfer was mediated by employing Life Technologies’ Gateway technology, i.e., by site-specific recombination between sequences flanking the expression cassette on pENTR-Luc and the corresponding sequences located on the adenoviral vector. The recombination reaction was performed according to the instructions of the manufacturer (Life Technologies Austria, Vienna, Austria). The adenoviral vector expressing the amiRNA directed against the target sequence present in the 3′UTR of the Renilla gene on Ad-Luc-as was constructed in a similar way by transferring the enhanced green fluorescence protein (EGFP)/amiRNA expression cassette of plasmid pcDNA6.2-GW/EmGFP-miR-luc (Life Technologies Austria, Vienna, Austria) into pAd/CMV/V5-DEST™ via site-specific recombination as before. A corresponding adenoviral vector carrying a negative control amiRNA cassette (originating from pcDNA6.2-GW/EmGFP-miR-neg; Life Technologies Austria, Vienna, Austria) was constructed analogously. The resulting adenoviral vectors were named Ad-Fluc-mi1 and Ad-mi-, respectively (Fig. 1).
Fig. 1.
Schematic representation of adenoviral vectors. All vectors were based on the Ad5-derived vectors pAd/CMV/V5-DEST ™ or pAd/PL-DEST ™ (both from Life Technologies) and lack the E1 and E3 genes. Expression cassettes were inserted into the deleted E1 region in sense or antisense orientation with respect to the left inverted terminal repeat (ITR). Expression cassettes contained the indicated open reading frames (light grey) for firefly luciferase (F-Luc), Renilla luciferase (R-Luc), or enhanced green fluorescent protein (EGFP). The target sequence for amiRNA Luc-mi1 within the 3′UTR of the Renilla luciferase gene is indicated by a small, dark grey box. Gene expression was driven by the herpes simplex virus 1 thymidine kinase promoter (indicated as HSV), simian virus 40 early promoter (SV40), or cytomegalovirus promoter (CMV). Promoters are indicated as boxed grey arrows. The 2 copies of tetracycline repressor-binding sequences are indicated as 2xTetO2. Mmu-miR-155-derived sequences flanking the hairpins that give rise to mature amiRNAs are indicated as small, dark grey boxes. Polyadenylation sequences are indicated as p(A).
Construction of amiRNA expression vectors for the targeting of adenoviral mRNAs: amiRNAs were designed using Life Technologie’s BLOCK-iT™ RNAi Designer and target site accessibility, as calculated by RNAxs (http://rna.tbi.univie.ac.at/cgi-bin/RNAxs), was taken into account. The annealed, double-stranded (ds), oligonucleotides (Supplementary Table 1) supposed to give rise to pre-miRNA hairpins (Fig. 2) contained 4 nucleotide (nt), 5′ overhangs. Via these overhangs, the oligonucleotides were inserted into the pre-cut plasmid vector pcDNA6.2-GW/EmGFP-miR (Life Technologies Austria, Vienna, Austria) giving rise to amiRNA expression vectors for E1A silencing (pmiRE-E1A-mi1 to -mi4), Ad5 DNA polymerase silencing (pmiRE-Pol-mi1 to -mi7), and pTP silencing (pmiRE-pTP-mi1 to -mi5). In these vectors, the pri-miRNAs are located in the 3′UTR of an EGFP gene. Both the EGFP gene and the pri-mRNAs are co-expressed from a constitutive CMV promoter/enhancer. The analogous vector pcDNA6.2-GW/EmGFP-miR-neg (Life Technologies Austria, Vienna, Austria) harboring a universal, negative control amiRNA in the 3′UTR of the EGFP gene served as a negative control.
Fig. 2.
Predicted stem-loop structures giving rise to adenovirus-directed amiRNAs. The amiRNAs were designed to target E1A-13S and E1A-12S mRNAs (E1A-mi1 to E1A-mi4), DNA polymerase mRNA (Pol-mi1 to Pol-mi7), or pTP mRNA (pTP-mi1 to pTP-mi5). All pre-amiRNAs were based on the murine mmu-miR-155 pre-miRNA and contain 2-nt central mismatches. The sequences giving rise to the mature antisense strands are underlined.
Concatemerization of amiRNA-encoding sequences: the fragment supposed to be added to the existing copy of the amiRNA-encoding sequence was excised from the respective pcDNA6.2-GW/EmGFP-miR-based vector with SalI and BglII. The vector already harboring one copy was restricted with SalI and BamHI, and the second copy was inserted into those sites. Further fragments containing single copies or multiple copies were added analogously by excision/insertion using the same restriction enzymes. Concatermerization of pTP-mi5- and the negative amiRNA-encoding sequences gave rise to vectors pmiRE-pTP-mi5x2, pmiRE-pTP-mi5x3, pmiRE-pTP-mi5x6 and pmiREx2, pmiREx3, pmiREx6, respectively.
Construction of plasmid vectors for doxycycline-controlled EGFP/amiRNA expression: this series of vectors is based on pENTR4 (Life Technologies Austria, Vienna, Austria) and contains a fragment comprising a CMV promoter/enhancer followed by a 2xTetO2 tetracyclin repressor binding site, a multiple cloning site, and a BGH poly(A) site between the XmnI and XhoI sites of the pENTR4 backbone. This fragment was obtained by PCR from pcDNA4/TO (Life Technologies Austria, Vienna, Austria) using primers CMV-TO-f1 (5′-TTGCATTTCGAATCTGCTTAGGGTTAGG-3′) and BGHpA-r2 (5′-CCCAGCGAATTCTTTCCGCCTCAGAAG-3′). The BclI site located between the promoter/operator region and the BGH poly(A) site was subsequently used for the insertion of the individual EGFP/miRNA cassettes. These cassettes were amplified from the corresponding pcDNA6.2-GW/EmGFP-miR-based vectors lacking the tetracyclin repressor binding sites by PCR using primers pmiRE-f2 (5′-CAAAAATGATCACTTTAAAACCATGGTGAGC-3′) and pmiRE-r2 (5′- AAGCTGTGATCAGATATCTCGAGTGCGGC-3′). The resulting EGFP/miRNA expression vectors were termed pTO-mi- (carrying the negative control miRNA), pTO-E1A-mi3 (carrying amiRNA E1A-mi3), pTO-Pol-mi4 and pTO-Pol-mi7 (carrying the DNA polymerase-targeting amiRNAs Pol-mi4 and Pol-mi7, respectively), and pTO-pTP-mi5 (carrying the pTP-targeting amiRNA pTP-mi5). Versions of pTO-mi- carrying 2, 3, or 6 copies of the negative control miRNA-encoding sequence were generated in an analogous way and were named pTO-mi-x2, pTO-mi-x3, and pTO-mi-x6. Versions of pTO-pTP-mi5 carrying 2, 3, or 6 copies of the pTP-mi5-encoding sequence were termed pTO-pTP-mi5x2, pTO-pTP-mi5x3, and pTO-pTP-mi5x6.
Construction of adenoviral amiRNA expression vectors: eventually, the expression cassettes present in the pENTR4-based plasmid vectors were transferred into pAd/PL-DEST (Life Technologies Austria, Vienna, Austria) by site-specific recombination between sequences flanking the expression cassette and the corresponding respective sequences located on the adenoviral vector as described above. All resulting adenoviral vectors are depicted in Fig. 1. Restriction enzymes and DNA-modifying enzymes were purchased from Fermentas (St. Leon-Rot, Germany) or New England Biolabs (Frankfurt am Main, Germany). PCR reactions were performed with Pwo DNA polymerase obtained from Roche Diagnostics (Vienna, Austria) or PEQLAB (Erlangen, Germany).
2.3. Nucleic acid extraction
Circular plasmid DNA was extracted with an EasyPrep Pro Plasmid Miniprep Kit (Biozym, Oldendorf, Germany), or a HiSpeed Plasmid Midi Kit (QIAGEN, Hilden, Germany). PCR products were purified with a QIAquick PCR Purification Kit (QIAGEN, Hilden, Germany), and adenoviral DNA was isolated with a QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, Germany). Total RNA was extracted using a standard acid phenol/choloroform method.
2.4. Dual-luciferase assays
For amiRNA screens 1.2e + 05 HEK 293 or 1e + 05 HeLa cells were seeded into the wells of 96-well plates and reverse transfected with 100 ng of individual dual-luciferase reporter vectors and 200 ng of amiRNA expression vector using Lipofectamine 2000 (Life Technologies Austria, Vienna, Austria). For each well 0.5 μl Lipofectamine 2000 was diluted with 24.5 μL OptiMEM medium (Life Technologies Austria, Vienna, Austria), and after 5 min of incubation, 25 μL diluted Lipofectamine 2000 was mixed with 25 μL of plasmid DNA diluted in OptiMEM. After 20 min of incubation, the mixes were pipetted directly into the wells of a 96-well plate and freshly harvested cells were added. After 24 h of incubation, the medium was exchanged, and the cells were incubated for another 24 h. Firefly and Renilla luciferase activities were determined at 48 h post-transfection using the Dual-Glo luciferase assay (Promega, Mannheim, Germany), according to the manufacturer’s instructions. Luminescence was measured on a Wallac Victor 1420 Multilabel Counter (Perkin Elmer Austria, Brunn am Gebirge, Austria). Knockdown rates were calculated by normalizing Renilla luciferase activities to firefly luciferase activities, and comparing dual-luciferase ratios between the targeting amiRNAs and the non-targeting negative control amiRNA.
Knockdown experiments in which the firefly luciferase-specific amiRNA was employed were performed as follows: 1.5e + 05 HEK 293 cells or 2e + 04 A549 cells were seeded into the wells of a 96-well plate. Twenty-four hours thereafter, the cells were transduced with Ad-Luc-as at a multiplicity of infection (MOI) of 1 TCID50/cell and either Ad-FLuc-mi1 or Ad-mi-, each at an MOI of 10 TCID50/cell. In the case of A549 cells, the cells were additionally infected with wt Ad5 at an MOI of 100 TCID50/cell. Alternatively, 2e + 04 A549, 1.6e + 05 HEK 293, 1.6e + 05 SW480, or 1e + 04 RD-ES cells seeded into 96-well plates were infected with wt Ad5 at an MOI of 100 TCID50/cell, and 1 h after infection, cells were co-transfected with 100 ng of the target vector psiCHECK-FLuc2 and increasing amounts (25–200 ng) of the amiRNA expression vector pcDNA6.2-GW/EmGFP-miR-luc or its corresponding negative control vector pcDNA6.2-GW/EmGFP-miR-neg. Renilla luciferase activities in relation to firefly luciferase activities were determined 24 or 48 h post-infection as described above. Experiments in which the effect of chaining of amiRNA-encoding sequences present on plasmid vectors was investigated were carried out essentially in the same way except that 50 ng of amiRNA expression vector and 50 or 100 ng target vector was used for co-transfections. Analogous experiments with adenoviral vectors were carried out by first transfecting T-REx-293 cells with 100 ng of psiCHECK-pTP followed by transduction with adenoviral miRNA expression vectors at an MOI of 30 TCID50/cell and treatment of the cells with or without 1 μg/ml doxycycline. Luciferase activities were determined 24 h post-infection as before.
2.5. Determination of amiRNA and mRNA levels
Total RNA was isolated from cells using a standard acid phenol/chloroform extraction method and residual DNA was removed with TURBO™ DNase (Life Technologies Austria, Vienna, Austria). pTP-mi5 levels were determined with a custom-designed TaqMan small RNA assay (proprietary to Life Technologies Austria, Vienna, Austria) according to the instructions of the manufacturer. For the quantitation of mRNAs, total RNA was first revese transcribed using the High Capacity cDNA Reverse Transcription Kit (Life Technologies Austria, Vienna, Austria) and subsequently analyzed by real-time quantitative PCR (qPCR) using a LightCycler 480 Probes master mix (Roche Diagnostics, Vienna, Austria) and primer/probe sets specific for GAPDH (GAPDH-f1 5′-TGCACCACCAACTGCTTAGC-3′, GAPDH-r1 5′-GGCATGGACTGTGGTCATGAG-3′, GAPDH-p1 5′-CCTGGCCAAGGTCATCCATGACAACTT-3′), or Ad5 pTP (pTP-cDNA-f2 5′-AAACCAACGCTCGGTGCC-3′, pTP-cDNA-r2 5′-GGACGCGGTTCCAGATGTT-3′, pTP-cDNA-p2 5′-CGCGCGCAATCGTTGACGCT-3′). All qPCRs were performed in duplicates on a LightCycler 480 platform (Roche Applied Science, Vienna, Austria).
2.6. Virus replication experiments
3e + 04 A549 cells were seeded into the wells of a 96-well plate and transduced with the recombinant adenoviruses at an MOI of 100 TCID50/cell. Twenty-four hours later, cells were infected with wt Ad5 at an MOI of 0.01. If required, CDV was added to each well in concentrations ranging from 0 to 30 μM. The plates were incubated for 0, 2, 4, or 6 days without change of medium before freezing at −80 °C. Crude virus suspensions were obtained by freeze-thawing the plates thrice and removal of cell debris by centrifugation for 15 min at 2800 rpm. The replication rate of recombinant adenoviruses carrying different numbers of amiRNA-encoding sequences was assessed by infecting 1e + 05 T-REx-293 cells with the vectors at an MOI of 0.1 TCID50/cell. AmiRNA expression was induced by addition of 1 μg/ml doxycycline to the medium and cells were allowed to grow for an additional 48 h. Crude lysates were prepared as described above.
2.7. Determination of adenovirus genome copy numbers
Wt Ad5 DNA levels were determined by qPCR using the following TaqMan primer/probe set directed against the viral E1A gene (E1A-fwd 5′-GACGGCCCCCGAAGATC-3′, E1A-rev 5′-TCCTGCACCGCCAACATT-3′, and E1A-p 5′-CGAGGAGGCGGTTTCGCAGA-3′). Adenovirus genome copy numbers were calculated by using serial dilutions of an adenoviral reference DNA as a standard. DNA levels of amiRNA-expressing recombinant viruses were determined using a TaqMan primer/probe set specific for the adenoviral hexon gene (hexon-fwd 5′- CACTCATATTTCTTACATGCCCACTATT-3′, hexon-rev 5′- GGCCTGTTGGGCATAGATTG-3′, hexon-probe 5′- AGGAAGGTAACTCACGAGAACTAATGGGCCA -3′). Otherwise, qPCR conditions were as described above.
2.8. FACS analysis
EGFP expression rates were determined by FACS analysis. Cells transduced with EGFP-expressing adenoviruses were harvested by trypsinization, resuspended in normal cell culture medium, and pelleted by centrifugation at 1200 rpm for 5 min. Thereafter, cells were washed once with phosphate buffered saline (PBS) and fixed with 1% formaldehyde in PBS. Samples were analyzed with a FACS Calibur analyzer (Becton Dickinson, Heidelberg, Germany) and percentages of fluorescent cells and mean fluorescence intensities (MFIs) were calculated.
2.9. Statistical analysis
All the data are expressed as mean ± standard deviation (SD). To test for statistical significance, one-way ANOVA corrected with Bonferroni’s post hoc test was applied. A p value of <0.05 was considered statistically significant.
3. Results
3.1. Artificial miRNAs can mediate the knockdown of gene expression in adenovirus-infected cells
At late stages of infection, adenoviruses produce high amounts of the noncoding virus-associated RNAs (VA RNAs). These RNAs are at least partially processed into functional miRNAs (mivaRNAs), and their production has been reported to inhibit cellular RNAi (Andersson et al., 2005; Lu and Cullen, 2004). This inhibition is thought to be mediated by the saturation of the cellular RNAi machinery at different levels (i.e., cleavage of pri-miRNAs by Drosha, export of pre-miRNAs by Exportin-5, processing by Dicer, and loading into RISC). Such an inhibition of RNAi in adenovirus-infected cells may prevent successful knockdown of adenoviral gene expression by adenovirus-targeting amiRNAs. Thus, we first investigated whether expression of an amiRNA with proven activity at reasonably high levels could mediate efficient RNAi in adenovirus-infected cells. We made use of a plasmid vector (pcDNA6.2-GW/EmGFP-miR-luc) that produces an amiRNA from the 3′UTR of a transcript coding for EGFP. This amiRNA was directed against the mRNA of the luciferase gene of a humanized firefly variant, and the guide strand displayed 100% complementary to its target sequence, thus leading to the cleavage of its target RNA in an siRNA-like fashion. A corresponding vector (pcDNA6.2-GW/EmGFP-miR-neg) carrying a universal, non-targeting, negative control miRNA was employed as well. We inserted the corresponding luciferase miRNA target sequence into the 3′UTR of a Renilla luciferase gene located on a distinct plasmid vector which, for internal normalization, also harbored a firefly luciferase gene (with a sequence distinct from the one against which the amiRNA was directed). This vector was named psiCHECK-FLuc2. Since our goal was to deliver amiRNAs into target cells via adenoviral vectors, we moved the expression cassettes for the targeting and non-targeting amiRNAs into the deleted E1 region of a replication-deficient, Ad5-based vector, giving rise to the adenoviral miRNA expression vectors Ad-FLuc-mi1 and Ad-mi1-, respectively (Fig. 1). A corresponding adenoviral target vector (Ad-Luc-as; Fig. 1) carrying the dual-luciferase expression cassette, which included the amiRNA target site, was constructed in an analogous way.
When we co-transduced A549 cells with the adenoviral target vector and its corresponding amiRNA expression vector, we observed an efficient knockdown of Renilla luciferase gene expression (>90%) at 24 and 48 h after transduction when compared to the artificial negative control miRNA vector. This knockdown rate was not changed upon concomitant infection of the cells with high numbers of wt Ad5 (MOI = 100; Fig. 3A). This high amount of wt virus was chosen to assure high-level production of VA RNAs. We repeated the experiments with HEK 293 cells and observed similarly efficient knockdown rates of approximately 90% (Fig. 3B). In this experimental setting, infection with wt Ad5 was omitted because the presence of the E1 gene in the genome of HEK 293 cells promotes the replication of replication-deficient adenoviral vectors in this cell line, consequently enhancing the production of high amounts of VA RNAs in the absence of wt adenovirus. We also repeated the experiments in a slightly different way by expressing the amiRNA and its target gene from their respective nonviral plasmid vectors in wt Ad5-infected A549, HEK 293, SW480, and RD-ES cells and observed comparable knockdown rates (data not shown). There was, however, a slight tendency (albeit not statistically significant in all cases) toward a slightly less pronounced knockdown rate in A549 cells at 48 h post-infection in the presence of wt Ad5. This effect likely reflected the observations made by Andersson et al., (2005) and Lu and Cullen (2004). No such decrease in gene expression knockdown was detectable at 24 h post-infection.
Fig. 3.
Artificial miRNA-mediated RNAi in cells infected with wt Ad5 or packaging cells promoting the replication of the adenoviral miRNA expression vectors. A. A549 cells were co-transduced with the adenoviral target vector Ad-Luc-as carrying an miRNA target sequence in the 3′UTR of a Renilla luciferase gene at an MOI of 1 TCID50/cell and the adenoviral vector Ad-FLuc-mi1, which produces an amiRNA directed against the target sequence of the target vector at an MOI of 10 TCID50/cell. A corresponding adenoviral vector (Ad-mi-) producing an artificial, non-targeting, amiRNA was used as a control. Concomitantly, cells were infected with wt Ad5 at an MOI of 100 TCID50/cell or were mock-infected with wt Ad5. Renilla luciferase activities in relation to firefly luciferase activities were determined at 24 and 48 h post-infection. B. Co-infection of HEK 293 cells. Experimental set-up as in A, except that superinfection with wt Ad5 was omitted. Data represent relative light units (RLU; mean ± SD) in comparison to the non-targeting artificial control miRNA from representative triplicate infection experiments. ∗∗∗p < 0.001.
In any case, the data indicated that it is feasible to efficiently knock down the expression of a gene carried by a replicating adenovirus via an amiRNA provided by a second, co-infecting adenovirus with no decrease in the knockdown rate at least at 24 and 48 h post-infection. Considering that all amiRNAs we intended to design were supposed to target early viral processes and should thus be able to execute their functions, these results encouraged us to continue with the actual development of adenovirus-directed amiRNAs.
3.2. Prevention of amiRNA expression in packaging cells
Adenovirus-directed amiRNAs, when expressed from adenoviral vectors that carry the corresponding target sequence, would inevitably impair the amplification of these vectors in packaging cells, such as HEK 293 cells, consequently leading to poor virus titers. Thus, we needed to assure that amiRNA expression is abolished in these packaging cells. To this end, we generated an adenoviral expression system in which the expression of amiRNAs (encoded by sequences located in the 3′UTR of the EGFP gene, as above) is driven by a tetracycline (Tet) repressor-controlled CMV promoter containing binding sites for 2 Tet repressor homodimers downstream of its TATA box. Thus, this promoter was repressed in cells expressing the Tet repressor and active only in the presence of tetracycline or in cells lacking the repressor, such as the target cells into which the vectors would be delivered.
This expression cassette was moved into the adenoviral vector as before, and the adenoviral vectors were amplified and packaged in T-REx-293 cells, a derivative of HEK 293 cells harboring the Tet repressor. Since artificial pri-miRNAs are generated from longer transcripts encoding EGFP in their 5′ region, EGFP expression was used as a measure for the repression of pri-miRNA expression in the absence of doxycycline in T-REx-293 cells. FACS analysis of EGFP expression revealed that transcription from the CMV promoter is heavily reduced in the repressed state (i.e., in the absence of doxycycline), as exemplified for the adenoviral vector Ad-mi- in Fig. 4. These data demonstrated that the controllable system was also functional when incorporated into adenoviral vectors and importantly, upon replication of these vectors. EGFP expression from this viral vector-located expression cassette was high upon addition of doxycycline, comparable to the expression rate typically achievable with analogous vectors containing a constitutively active version of the CMV promoter (data not shown).
Fig. 4.
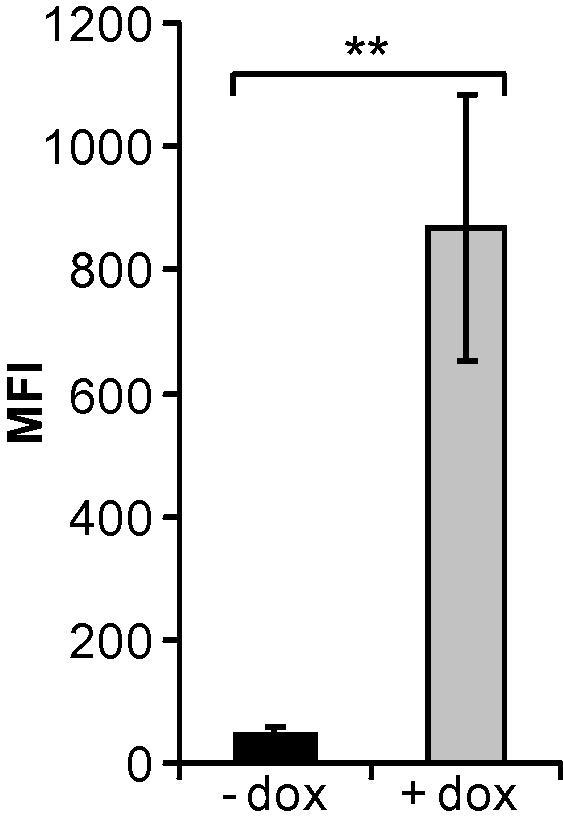
Expression of the EGFP/miRNA cassette from a tetracycline repressor-controlled CMV promoter is heavily decreased in adenovirus packaging cells in the absence of doxycycline. T-REx-293 cells were transduced with the adenoviral vector AdTO- and incubated with or without doxycycline. Twenty-four hours later, the cells were analyzed for EGFP expression by FACS analysis, and mean fluorescence intensities (MFIs) were calculated. Data represent the mean values of 3 independent experiments, each performed in triplicate (mean ± SD; n = 3). ∗∗p < 0.01.
3.3. Construction of adenovirus-directed amiRNA expression vectors
All amiRNAs were designed to be first expressed as pri-miRNAs from the (nonviral) miRNA expression vector pcDNA6.2-GW/EmGFP-miR. In this vector context, amiRNA hairpins are embedded in the flanking sequences of the murine mmu-miR-155 miRNA. The pri-miRNA-encoding sequences are incorporated in the 3′UTR of a Renilla luciferase gene and are concomitantly expressed with the EGFP gene from a common, constitutive CMV promoter. AmiRNA-containing transcripts can then be generated and processed in the same way as naturally occurring pri-miRNAs/pre-miRNAs. However, the inserted sequences were designed to match their target sequences completely and were therefore expected to lead to the degradation of their target mRNAs.
Based on our results obtained with adenovirus-directed siRNAs, we designed amiRNAs directed against E1A, DNA polymerase, and pTP mRNAs of Ad5, which had previously been identified as promising targets (Kneidinger et al., 2012). For each target mRNA, at least 4 different amiRNAs were designed (Fig. 2), and the respective oligonucleotides containing the sequences of the pre-miRNA hairpins (Supplementary Table 1) were cloned into pcDNA 6.2-GW/EmGFP-miR giving rise to the plasmid expression vectors pmiRE-E1A-mi1 to -mi4, pmiRE-Pol-mi1 to -mi7, and pmiRE-pTP-mi1 to -mi5. A vector (pcDNA6.2-GW/EmGFP-miR-neg) encoding a universal, non-targeting amiRNA served as a reference for all other amiRNA expression vectors, thus allowing for comparison between groups of amiRNA expression vectors (i.e., amiRNA expression vectors for the targeting of distinct adenoviral transcripts).
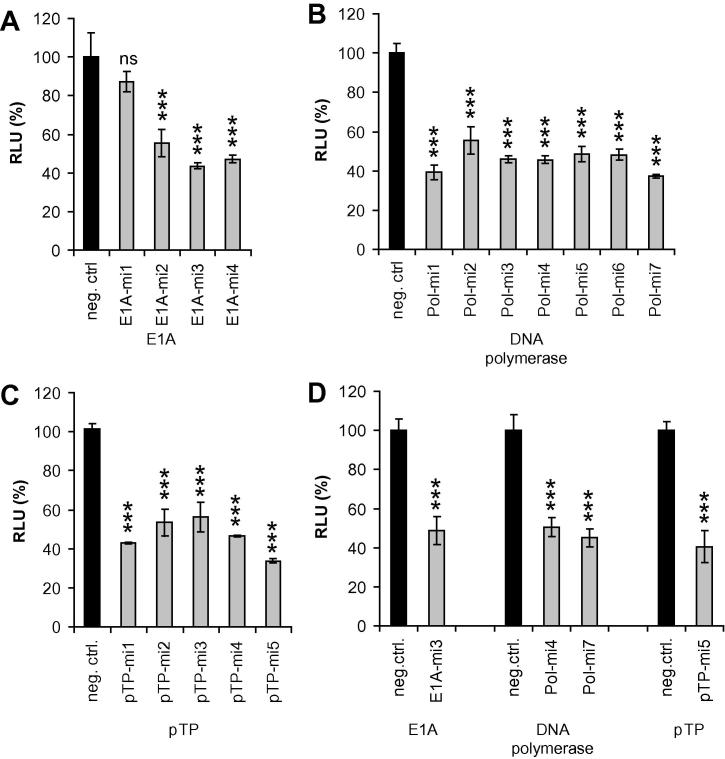
To select the most efficient amiRNAs, we employed the same dual-luciferase-based reporter system as described above. We first tested each group of amiRNAs (i.e., groups targeting either the E1A, DNA polymerase, or pTP mRNAs) individually in combination with reporter plasmid vectors harboring the respective target sequences in the 3′UTR of the Renilla luciferase mRNA (Fig. 5A–C). Finally, we compared amiRNAs selected from each group (i.e., E1A-mi3, Pol-mi4 and Pol-mi7, and pTP-mi5) side-by-side (Fig. 5D). The obtained knockdown rates were similar for all selected amiRNAs. Because the transfection rates were well below 100% in these experiments (but were identical for different vectors), as determined by parallel FACS experiments in which EGFP expression was measured (data not shown), the absolute knockdown rates were rather low. Thus, the knockdown rates observed in these experiments did not reflect the true capacities of the tested amiRNAs. For targeting of the DNA polymerase mRNA, we selected 2 distinct amiRNAs: Pol-mi7, which showed the highest knockdown rate, and Pol-mi4, which performed slightly worse, but contained the same seed sequence as Pol-si2, the most potent siRNA identified through our previous study (Kneidinger et al., 2012).
Fig. 5.
Screening for functional amiRNAs. Plasmid-based amiRNA expression vectors and their respective dual-luciferase target vectors were used to cotransfect HeLa cells (A, D) or HEK293 cells (B, C). Each group of artificial miRNAs, i.e., those directed against E1A (A), DNA polymerase (B), or pTP (C) target sequences, were first evaluated individually, and selected candidates were eventually evaluated side-by-side in a second round (D) by determining Renilla luciferase activities in relation to firefly luciferase activities. A plasmid vector expressing a universal non-targeting amiRNA served as a negative control (neg. ctrl.) in all experiments. Relative light units (RLU; mean ± SD, n = 3) in comparison to the non-targeting artificial control miRNA are shown. ∗∗∗p < 0.001; ns: not significant.
Next, we modified the expression system of the selected vectors by bringing the EGFP/amiRNA cassettes under the control of the tetracycline repressor-regulated CMV promoter and subsequently transferred these expression cassettes into the deleted E1 region of the Ad5-based replication-deficient adenoviral vector already employed for the experiments described in Section 3.1. The resulting adenoviral vectors AdTO-mi-, AdTO-E1A-mi3, AdTO-Pol-mi4, AdTO-Pol-mi7, and AdTO-pTP-mi5 are depicted in Fig. 1.
Since the dual-luciferase assay system represents an artificial set-up, the efficacy of amiRNAs must be properly evaluated in the biological context. To this end, we transduced T-REx-293 cells (which propagate the replication of otherwise replication-deficient adenoviral vectors lacking the E1 genes) with the individual adenoviral amiRNA expression vectors. The cells were cultivated in the presence of doxycycline to allow for amiRNA expression, which, in turn, was expected to lead to the attenuation of viral DNA replication in cases of highly efficient amiRNAs. Finally, we determined viral genome copy numbers for the time point 2 days post-infection by real-time qPCR using a primer/probe set directed against the adenoviral hexon gene. As shown in Fig. 6, expression of E1A-mi3, Pol-mi4, and Pol-mi7 did not cause a significant reduction in viral genome copy numbers. The only amiRNA that was able to decrease the amplification of its own vector significantly was pTP-mi5. In this case, the copy number of the vector was decreased to 26.9%. Thus, we selected the pTP-mi5 expression vector for further optimization.
Fig. 6.

Replication of adenoviral amiRNA expression vectors is inhibited in packaging cells upon amiRNA expression. T-REx-293 cells were transduced with the amiRNA expression vectors AdTO-mi- (indicated as neg. ctrl.), AdTO-E1A-mi3 (indicated as E1A-mi3), AdTO-Pol-mi4 (indicated as Pol-mi4), AdTO-Pol-mi7 (indicated as Pol-mi7), and AdTO-pTP-mi5 (indicated as pTP-mi5) at an MOI of 0.1 TCID50/cell, and amiRNA expression was induced by addition of doxycycline. Copy numbers of the vectors were determined for time points 0 and 2 days post-infection by qPCR using a TaqMan primer/probe set specific for the Ad5 hexon gene. Fold-increases of vector copy numbers were calculated for all vectors. Data represent the means ± SD of 3 independent experiments, each performed in triplicate. ∗p < 0.05.
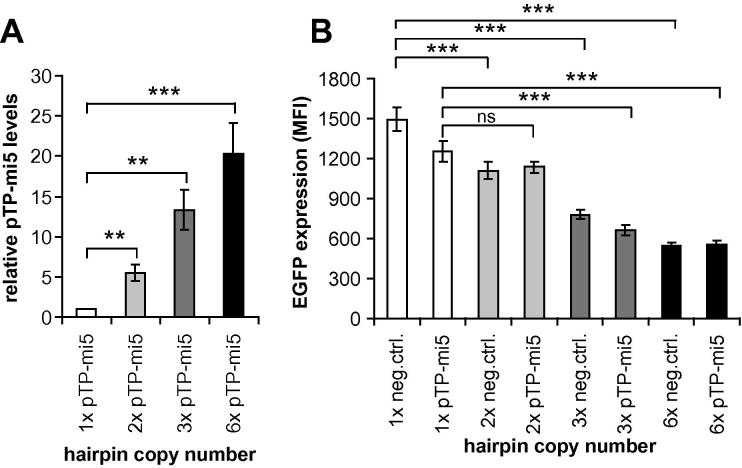
3.4. Concatemerization of pTP-mi5-encoding sequences increases pTP-mi5 levels
It has been reported that expression of shRNA or amiRNA hairpins as tandem copies can enhance knockdown efficacies (Chung et al., 2006; Wu et al., 2011). Consequently, we generated vectors in which the pTP-mi5 pre-mRNA hairpins were concatemerized. We first constructed additional pcDNA 6.2-GW/EmGFP-miR-based plasmid vectors containing 2, 3, or 6 copies of pTP-mi5-encoding sequences in the 3′UTR of the EGFP gene (vectors pmiRE-pTP-mi5x2, pmiRE-pTP-mi5x3, and pmiRE-pTP-mi5x6) and the respective negative control vectors carrying a corresponding number of negative control amiRNA hairpins (vectors pmiREx2, pmiREx3, and pmiREx6). Transfection of HEK 293 cells with pTP-mi5-encoding vectors revealed that the amount of mature pTP-mi5 increased with rising copy numbers in the constructs (Fig. 7A). The gain in the amount of pTP-mi5 present in the cells ranged from 6.8-fold (2 copies) to 20.3-fold (6 copies). Not surprisingly, there was an inverse correlation with EGFP expression: increased numbers of hairpins present in the 3′UTR of the EGFP gene led to decreased EGFP levels (Fig. 7B). This effect was not only evident for the pTP-mi5-encoding constructs but also for constructs encoding the negative control amiRNA. The observed decrease was likely due to enhanced processing of the primary transcripts by Drosha with increased amiRNA hairpin copy numbers, accelerated degradation of the processed forms due to lack of a 3′ poly(A) tail after Drosha cleavage, or decreased translation.
Fig. 7.
Concatemerization of amiRNA-encoding sequences increases amiRNA levels and decreases EGFP expression. HEK293 cells were transfected with identical amounts of plasmid expression vectors carrying 1–6 copies of pTP-mi5 (i.e., vectors pmiRE-pTP-mi5, pmiRE-pTP-mi5x2, pmiRE-pTP-mi5x3, and pmiRE-pTP-mi5x6) or the corresponding negative control amiRNA (i.e., vectors pcDNA6.2-GW/EmGFP-miR-neg, pmiREx2, pmiREx3, and pmiREx6) in the 3′UTR of the EGFP gene. Forty-eight hours after transfection, pTP-mi5 levels were measured by RT-qPCR (A), and EGFP expression was determined by FACS analysis (B). Means of relative pTP-mi5 levels and mean fluorescent intensities (MFIs) from 3 independent transfections (mean ± SD, n = 3) are shown. ∗p < 0.05, ∗∗p < 0.01, ns: not significant.
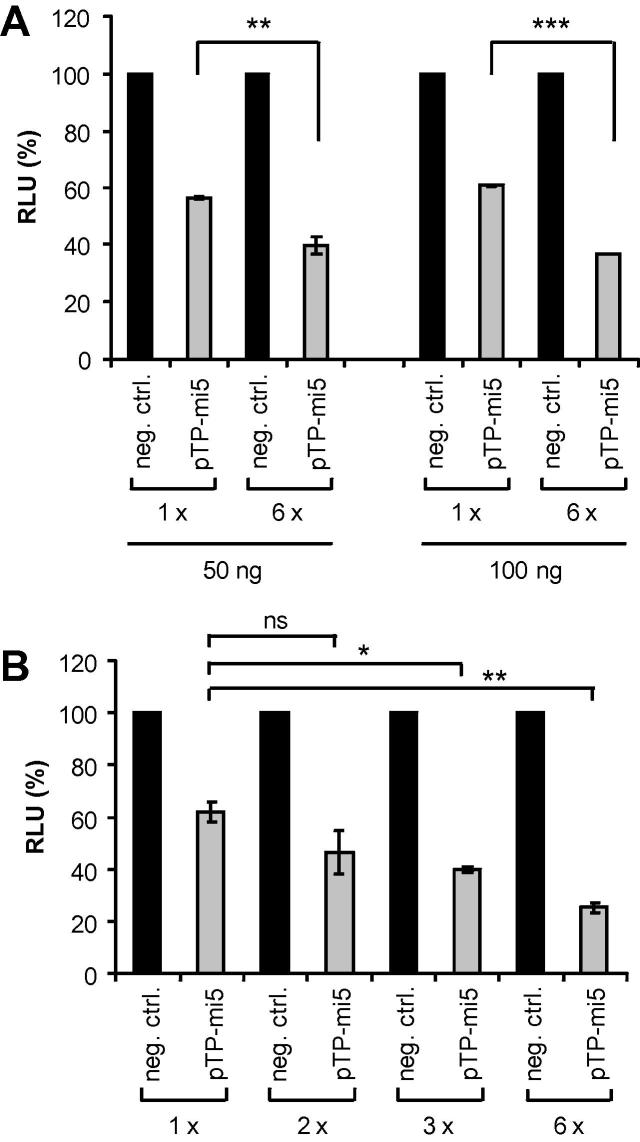
3.5. Concatemerization of pTP-mi5-encoding sequences increases the knockdown rate
To determine whether elevated levels of pTP-mi5 produced by pmiRE-pTP-mi5x6 in comparison to pmiRE-pTP-mi5 had a positive effect on the knockdown rate, we performed dual-luciferase-based knockdown experiments as before. Knockdown of Renilla luciferase expression in HEK 293 cells transfected with the target vector psiCHECK-pTP was significantly increased upon raising the copy number of pTP-mi5 from 1 to 6 (Fig. 8A), revealing the expected positive correlation between amiRNA levels and knockdown capacities.
Fig. 8.
Concatemerization of pTP-mi5 hairpins increases the knockdown rate. A. HEK 293 cells were transfected with 50 ng of the plasmid vectors pmiRE-pTP-mi5 and pmiRE-pTP-mi5x6 carrying 1 or 6 copies of the sequence encoding pTP-mi5, respectively, and either 50 or 100 ng of the dual-luciferase reporter vector psiCHECK-pTP harboring the target sequence of pTP-mi5. The vectors pmiRE and pmiREx6, carrying 1 or 6 copies of a sequence encoding the non-targeting amiRNA, served as negative controls (neg. ctrl.). Numbers of hairpins for amiRNAs in the individual vectors are indicated as 1 x and 6 x, respectively. Renilla luciferase activities in relation to firefly luciferase activities were determined 24 h post-transfection. Relative light units (RLU) obtained with the vectors carrying pTP-mi5 in relation to their respective negative control vectors are shown. Data represent the means ± SD of 2 independent experiments, each performed in triplicate. B. HEK 293 cells were transfected with 100 ng of psiCHECK-pTP. Twenty-four hours after transfection, the cells were transduced with the adenoviral vectors AdTO-pTP-mi5, AdTO-pTP-mi5x2, AdTO-pTP-mi5x3, or AdTO-pTP-mi5x6 expressing pTP-mi5 or AdTO-mi-, AdTO-mi-x2, AdTO-mi-x3, or AdTO-mi-x6 expressing the negative control amiRNA at an MOI of 30 TCID50/cell. The copy number of the amiRNA hairpins is indicated beneath the figure. Renilla luciferase activities in relation to firefly luciferase activities were determined 24 h post-infection. Relative light units (RLU) obtained with the vectors carrying pTP-mi5 in relation to their respective negative control vectors are shown. Data represent the means ± SD of 2 independent experiments, each performed in triplicate. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ns: not significant.
Next, we modified these plasmid vectors by replacing the constitutive CMV promoter with the tetracycline-regulated CMV promoter and subsequently converted those intermediate vectors into adenoviral vectors as before. The final set of adenoviral vectors (Fig. 1) contained 1, 2, 3, or 6 copies of the pTP-mi5-encoding sequence (vectors AdTO-pTP-mi5, AdTO-pTP-mi5x2, AdTO-pTP-mi5x3, and AdTO-pTP-mi5x6), or a corresponding number of copies of the sequence encoding the negative control amiRNA (vectors AdTO-mi-, AdTO-mi-x2, AdTO-mi-x3, and AdTO-mi-x6). We evaluated this set of vectors by again performing dual-luciferase assays; briefly, we transfected T-REx-293 cells with the pTP-mi5 target vector psiCHECK-pTP and subsequently transduced those cells with the adenoviral vectors at an MOI of 30 TCID50/cell. The cells were cultivated in the presence of doxycycline for an additional 24 h to allow for the expression of amiRNA before determining luciferase activities. As shown in Fig. 8B, Renilla luciferase expression showed a steady decrease with increasing copy numbers of pTP-mi5-encoding sequences present on the vectors. This indicated that the amiRNA expression cassette giving rise to highest number of pTP-mi5 hairpins was the most effective when incorporated into the adenoviral vector backbone.
The positive effect of incorporating 6 copies of pTP-mi5 hairpins was also reflected by the increased inhibition of viral vector amplification in T-REx-293 cells when the cells were cultivated in the presence of doxycycline, i.e., upon derepression of EGFP and pTP-mi5 expression (Fig. 9). No such effect was observed for vectors encoding the negative control amiRNA, indicating that the decrease in vector copy number was specifically related to pTP-mi5 expression and not to the treatment of the cells with doxycycline. Viral DNA synthesis was decreased by 0.9 orders of magnitude (86.2%) for the vector containing 1 copy of the pTP-mi5 hairpin. There was no significant difference in the inhibition rate when the copy number was raised to 2 or 3. However, doubling the copy number further from 3 to 6 generated a markedly increased inhibitory effect on vector amplification. Here, viral DNA synthesis was decreased by 1.6 orders of magnitude (97.6%) compared to the negative control vector. We also monitored the amplification kinetics of the vector containing 6 copies of the pTP-mi5-encoding sequence over a 6-day period and found a pronounced decrease in vector copy numbers also at later time points in the presence of doxycycline (Supplementary Fig. 1).
Fig. 9.
Concatemerization of pTP-mi5 hairpins increases the inhibition of adenoviral pTP-mi5 expression vectors in packaging cells. T-REx-293 cells were transduced with the adenoviral pTP-mi5 expression vectors AdTO-pTP-mi5, AdTO-pTP-mi5x2, AdTO-pTP-mi5x3, or AdTO-pTP-mi5x6 or their respective negative control vectors AdTO-mi-, AdTO-mi-x2, AdTO-mi-x3, or AdTO-mi-x6 at an MOI of 0.1 TCID50/cell. The vectors carried amiRNA-encoding sequences with copy numbers increasing from 1 (A, B) to 2 (C, D), 3 (E, F), or 6 (G, H). The transduced cells were cultivated in the absence (A, C, E, G) or presence (B, D, F, H) of doxycycline. Copy numbers of the vectors were determined for time points 0 and 2 days post-infection by qPCR using a TaqMan primer/probe set specific for the Ad5 hexon gene. Fold-increases in vector copy numbers were calculated for all vectors. Data represent the means ± SD of 3 independent experiments, each performed in triplicate. ∗p < 0.05, ns: not significant.
Because all experiments clearly suggested that chaining of pTP-mi5 hairpins was a prerequisite for the successful inhibition of adenovirus replication, all subsequent experiments were performed with the vector carrying 6 copies of pTP-mi5.
3.6. Levels of amiRNAs, when expressed from a replication-deficient adenoviral vector, are increased in wt adenovirus-infected cells
Replication-deficient adenoviral vectors were chosen for the expression of amiRNAs based on the assumption that net levels of amiRNA should increase upon exposure of the recombinant vector to wt adenovirus in infected cells. Provided the amiRNA was not capable of completely blocking viral DNA replication, amiRNA gene copy numbers should increase upon onset of replication of the recombinant vector, which should be induced by E1A generated by the co-infecting wt adenovirus. Indeed, we found pTP-mi5 levels increased by ∼6-fold in A549 cells infected with wt Ad5 (Fig. 10A).
Fig. 10.
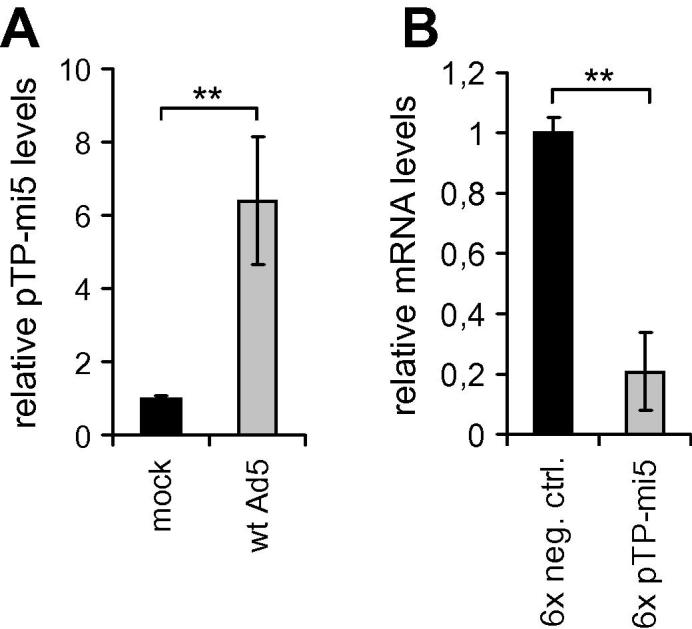
Adenoviral vector-mediated expression of pTP-mi5 increased upon superinfection with wt Ad5 and led to knockdown of pTP expression. A549 cells were transduced with the adenoviral pTP-mi5 expression vector AdTO-mi-x6 or its respective negative control vector AdTO-mi-x6 at an MOI of 100 TCID50/cell. Twenty-four hours later, cells were infected with wt Ad5 at an MOI of 100 TCID50/cell or were mock-infected. Another 24 h later, cells were analyzed for relative pTP-mi5 levels (A) and relative pTP mRNA levels (B) by RT-qPCR. Data represent the means of 3 independent infections (mean ± SD; n = 3). ∗∗p < 0.01.
To determine whether and to what extent pTP-mi5 inhibited the expression of pTP during virus replication, we transduced A549 cells with the adenoviral pTP-mi5 expression vector AdTO-pTP-mi5x6 or its corresponding negative control amiRNA expression vector AdTO-mi-x6. Subsequently, we infected the cells with wt Ad5 and determined pTP mRNA levels at 24 h post-infection with wt Ad5 by RT-qPCR. As shown in Fig. 10B, pTP-mi5 expression decreased pTP mRNA levels by nearly 80% compared to the negative control amiRNA.
3.7. Adenoviral vector-based expression of pTP-mi5 inhibits the replication of wt Ad5
To finally investigate whether pTP-mi5 was capable of inhibiting the replication of wt Ad5, we transduced A549 cells with AdTO-pTP-mi5x6 or the negative control vector AdTO-mi-x6 and infected them with wt Ad5. To assure that all cells were transduced with the recombinant vectors, we used rather high MOIs of 100 TCID50/cell and transduced the cells with the recombinant vectors 24 h prior to infection with wt Ad5. Wt Ad5 genome copy numbers were determined at 0, 2, 4, and 6 days post-infection by real-time qPCR using a primer/probe set directed against a part of the E1A gene. As shown in Fig. 11A, wt Ad5 DNA levels were decreased by 1.24, 1.21, and 1.77 orders of magnitude (94.2%, 93.8%, and 98.4%) on days 2, 4, and 6, respectively, in cells expressing pTP-mi5, as compared to cells expressing the negative control amiRNA. The negative control amiRNA itself did not significantly inhibit wt Ad5 replication.
Fig. 11.
Adenoviral vector-mediated expression of pTP-mi5 decreases the replication of wt Ad5. A549 cells were transduced with the adenoviral pTP-mi5 expression vector AdTO-pTP-mi5x6 (indicated as 6x pTP-mi5) or its respective negative control vector AdTO-mi-x6 (indicated as neg. ctrl.) at an MOI of 100 TCID50/cell or were mock-transduced (indicated as no vector). Twenty-four hours after infection, cells were infected with wt Ad5 at an MOI of 0.01 TCID50/cell. Cells were then cultivated for an additional 6 days. A. Genome copy numbers of wt Ad5 were determined for time points 0, 2, 4, and 6 days post-infection with wt Ad5 by qPCR using a primer/probe set specific for the E1A gene present only on wt Ad5. Data represent the means ± SD of 3 independent experiments, each performed in triplicate. B. Output of infectious virus progeny for time point 2 days post-infection was determined by TCID50 assays. Data represent the means ± SD of 3 independent experiments, each performed in triplicate.
As a consequence of the inhibition of viral DNA synthesis, the generation of infectious wt Ad5 progeny was also heavily inhibited. The number of infectious wt Ad5 virions as determined by TCID50 analysis using A549 cells as indicator cells (which permitted the specific detection of wt Ad5 replication) was decreased by 2.6 orders of magnitude (99.8%) in cultures transduced with the pTP-mi5-expressing vector compared to control cultures expressing the negative control amiRNA (Fig. 11B).
The amiRNA-mediated inhibitory effect on wt Ad5 DNA replication was also revealed when the cells were infected with wt Ad5 at higher MOIs of up to 100 TCID50/cell (Supplementary Fig. 2). No differences were observed for MOIs ranging between 0.01 and 1 (Supplementary Fig. 2A–C). At higher MOIs of 10 and 100 (Supplementary Fig. 2D and E) there was a tendency toward a decrease in the difference between wt Ad5 genome copy numbers of cells transduced with the pTP-mi5 amiRNA expession vector and its negative control vector. However, this decrease was not due to a diminished inhibition of wt Ad5 DNA replication by the amiRNA, because the slope from day 0 to day 2 was comparable for pTP-mi5-expressing cells regardsless of which MOI was used for wt Ad5 infection. The observed effect was rather due to a decrease in the gain of wt Ad5 DNA from day 0 to day 2 when cells were infected with wt Ad5 at high MOIs (compare slopes for cells not transduced with any recombinant vector or with the amiRNA control vector at different MOIs).
The inhibitory effect described above was revealed with cells that had been transduced with the recombinant amiRNA expression vector 24 h prior to infection with wt Ad5. However, an inhibitory effect on wt Ad5 replication was also observed when cells were transduced with the pTP-mi5 expression vector only 6 h prior to, concomitant with, or 6 h after infection with wt Ad5 (Supplementary Fig. 3). Wt Ad5 replication was inhibited at all MOIs. However, we observed a tendency toward a slightly decreased inhibition rate when cells were infected with wt Ad5 prior to transduction with the recombinant vectors and when low MOIs were used for wt Ad5 infection (compare slopes for cells transduced with the pTP-mi5 expression vector in panels A, B, and C at wt Ad5 MOIs of 0.01–1).
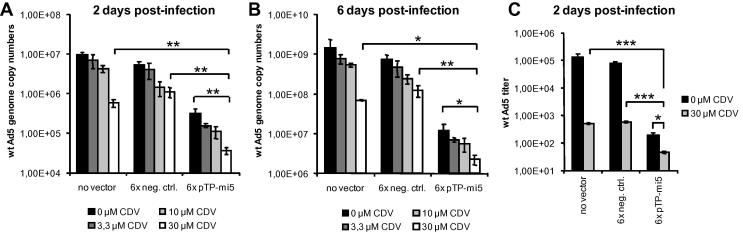
3.8. AmiRNA expression and concomitant treatment with CDV results in an additive inhibitory effect
The inhibitory effect of pTP-mi5 when expressed from and delivered with a replication-deficient adenoviral vector was very pronounced, but not complete. Thus, we investigated whether knockdown of pTP expression by pTP-mi5 and concomitant treatment of infected cells with CDV may result in additive inhibitory effects. To this end, we transduced and infected A549 cells as before and treated them with therapeutically relevant concentrations of CDV. The highest dose of CDV (30 μM) corresponded to in vivo peak serum concentrations typically measured after intravenous administration (Cundy, 1999). We assessed the inhibition of wt Ad5 replication by determining wt Ad5 genome copy numbers at time points 2 and 6 days post-infection (Fig. 12A and B). In our experimental setting, adenoviral vector-mediated expression of pTP-mi5 was generally more effective in inhibiting wt Ad5 replication than was treatment with CDV. However, the inhibitory effect of pTP-mi5 could clearly be further increased by concomitant treatment of the cells with CDV. pTP-mi5 expression alone decreased wt Ad5 genome copy numbers by 1.2 orders of magnitude (94.2%) at day 2 post-infection and by 1.8 orders of magnitude (98.4%) at day 6 post-infection when compared to the negative control amiRNA. However, concomitant treatment of the cells with 30 μM CDV decreased wt Ad5 genome copy numbers by 2.2 orders of magnitude (99.3%) at day 2 and by 2.5 orders of magnitude (99.7%) at day 6. This clear additive effect also manifested as a further drop in the output of infectious virus progeny (Fig. 12C); concomitant treatment with 30 μM CDV decreased the titer of wt Ad5 by another 0.6 orders of magnitude and resulted in a total reduction rate of 3.2 orders of magnitude (99.9%).
Fig. 12.
Adenoviral vector-mediated expression of pTP-mi5 and concomitant treatment with CDV has an additive effect on the inhibition of wt Ad5 replication. A549 cells transduced with AdTO-pTP-mi5x6 at an MOI of 100 TCID50/cell were infected with wt Ad5 at an MOI of 0.01 TCID50/cell and were concomitantly treated with 0, 3.3, 10, or 30 μM CDV. Two days (A) and 6 days after infection with wt Ad5 (B), genome copy numbers of wt Ad5 were determined by qPCR using a primer/probe set specific for the E1A gene present only on wt Ad5. Data represent the means ± SD of 3 independent experiments, each performed in triplicate. C. Output of infectious virus progeny for time point 2 days post-infection was determined by TCID50 assays. Data represent the means ± SD of 3 independent experiments, each performed in triplicate. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
4. Discussion
In contrast to anti-adenoviral siRNAs such as the ones used in our previous study (Kneidinger et al., 2012), the generation of anti-adenoviral amiRNAs is dependent on intracellular processing steps which may be disturbed in adenovirus-infected cells due to the saturation of several components of the RNAi pathway by mivaRNAs (Andersson et al., 2005; Lu and Cullen, 2004). We estimated the performance of amiRNAs during the first 48 h of adenovirus infection as being especially critical, because viral DNA replication – the viral process which we intended to target – largely takes place within this time frame. However, we found that amiRNA function was not affected during these stages of adenovirus infection when the amiRNA was delivered via an adenoviral vector (Fig. 3). This is likely due to the fact that mivaRNAs reach high levels only at very late stages of infection, and pTP mRNA-targeting amiRNAs prevent the otherwise steady increase in VA-RNA gene copy numbers after the onset of viral DNA replication.
The design of amiRNAs follows slightly different rules compared to those required for the design of 25-nt-long, blunt-ended siRNAs. Although we designed certain amiRNAs (i.e., pTP-mi5 and Pol-mi4) to contain the same seed sequences as their successful siRNA relatives used in our previous study (Kneidinger et al., 2012), these amiRNAs did not necessarily represent the most efficient amiRNAs (see Pol-mi4), indicating that it was not always feasible to automatically convert an effective siRNA into a potent amiRNA. This may be due to the different lengths of amiRNAs and siRNAs, their different types of ends (i.e., blunt ends in the case of siRNAs and 2-nt 3′ overhangs in the case of amiRNAs), and the lack of any chemical modifications within amiRNAs.
Concatemerization of identical amiRNA-encoding sequences has been shown to increase knockdown rates (Chung et al., 2006; Wu et al., 2011). Consequently, we concatemerized pTP-mi5-encoding sequences to increase the inhibition of adenoviral replication. While inhibition of the replication of the vector carrying the pTP-mi5 expression cassette was limited to 0.9 orders of magnitude (86.2%) when only one copy was present, increasing the copy number from 1 to 6 resulted in a decrease of viral genome copy number by 1.6 orders of magnitude (97.6%; Fig. 9). This effect correlated with an increase in pTP-mi5 levels (Fig. 7A). However, the increase in the amount of mature amiRNA was disproportionally higher compared to the increase in the number of hairpins present on primary transcripts. This effect may be related to an observation made byothers when placing a pre-amiRNA hairpin onto a miRNA polycistron: when combined with other amiRNA hairpins, the silencing capacity of the individual amiRNA was increased (Liu et al., 2008).
Expression of pTP-mi5 from the adenoviral vector inhibited the replication of superinfecting wt Ad5 by approximately 1.2 orders of magnitude (94%) at 2 days post-infection with wt Ad5. This inhibitory effect was also evident by the suppression of infectious wt Ad5 progeny output by 2.6 orders of magnitude (99.8%). Although we used a low MOI of 0.01 TCID50/cell for wt Ad5 in most experiments to allow for monitoring of virus spreading within the cultures, the high burst size of adenovirus quickly led to infection of the entire culture. Consequently, the exponential increase in virus multiplication at later time points was disproportionately prevented in cultures in which replication was not attenuated by amiRNAs. Thus, regardless of the readout system, the pTP-mi5-mediated inhibition rate at late time points (4 or 6 days post-infection) is probably underestimated.
Both CDV and pTP-mi5 target the same viral process, namely viral DNA replication. However, while pTP-mi5 decreases the number of functional protein complexes that have to be formed for efficient initiation of viral DNA synthesis, CDV, as a nucleoside analog, acts downstream of this step by preventing DNA polymerization (Cundy, 1999). Thus, it was conceivable that a combination of both mechanisms may result in additive inhibitory effects; while pTP-mi5 would in a first step limit the number of available DNA replication complexes, CDV would in a second step inhibit residual DNA synthesis that could not be prevented by the amiRNA. Indeed, a combination of pTP-mi5 expression and treatment with CDV resulted in a further decrease of wt Ad5 genome copy numbers and infectious virus progeny by an additional 1 and 0.6 orders of magnitude, respectively, at 2 days post-infection with wt Ad5 (Fig. 12A and C).
The delivery of amiRNAs, shRNAs, or siRNAs into living organisms is a challenging task. Based on the development of a plethora of different delivery vehicles, nonviral delivery methods have constantly been improved but are still far from perfect (Rettig and Behlke, 2012). In this regard, the delivery of anti-adenoviral amiRNAs, via a replication-deficient adenoviral vector, may have several unique advantages. For example, it may allow for the amplification of amiRNA expression cassette copy numbers upon exposure of the recombinant virus to the wt virus as demonstrated in our in vitro experiments (Fig. 10) and theoretically ensure a constant supply of recombinant vector as long as wt adenovirus is present. Moreover, based on the shared organ tropism of the adenoviral vector and its wt counterpart, this type of delivery may also permit the directing of amiRNAs predominantly to those cells that are also the preferred targets of the wt virus.
It may be argued that treating a virus infection with a vector derived from the very same virus may generally be dangerous. For example, recombination events between the wt virus and the recombinant virus are conceivable, which may result in the generation of a replication competent virus. This potential problem could generally be circumvented by the generation of “gutted” versions of the vectors presented here. Such gutted adenoviral vectors lack all parts of the viral genome except for the 5′ and 3′ inverted terminal repeats and the packaging signal (Ψ) required for replication and DNA packaging, respectively (Alba et al., 2005). In general, due to the presence of the inhibitory amiRNA sequences on the vector, a virus emerging from a recombination between the recombinant virus and the wt virus would be attenuated in its replication. At the same time, such a recombination event would likely render the “donor” wt virus replication-deficient. Thus, the generation of a virus that is more dangerous than the parent wt virus seems unlikely. In any case, this issue would have to be addressed in animal studies.
Such animal studies are also needed to eventually clarify, which of the 2 RNAi-based approaches, i.e., silencing of adenoviral gene expression by siRNAs, such as the ones presented in our previous study (Kneidinger et al., 2012), or by amiRNAs expressed from and delivered by adenoviral vectors (this study), provide a greater probability to permit efficient inhibition of adenovirus multiplication in vivo.
5. Conclusion
Taken together, our data indicate that (i) adenoviral vector-based delivery and expression of amiRNAs can mediate significant gene expression knockdown in cells infected with wt adenovirus; (ii) targeting of adenoviral pTP mRNA by amiRNA can inhibit the replication of wt adenovirus in vitro; (iii) efficient inhibition requires a sufficiently high intracellular concentration of amiRNA, which can be achieved by concatemerization of amiRNA hairpins in primary transcripts; (iv) the intracellular amiRNA concentration can be further increased upon the encounter of the recombinant vector with its co-infecting wt counterpart; and (v) amiRNA expression in cells infected by wt virus and their concomitant treatment with CDV can result in additive inhibitory effects.
Acknowledgments
This work was supported by the Austrian Science Fund through grant L665-B13.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.antiviral.2012.10.008.
Appendix A. Supplementary data
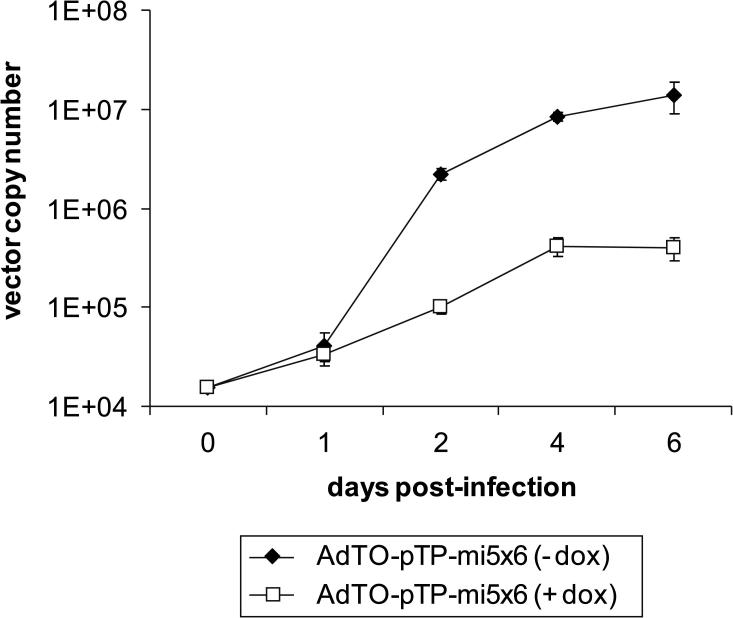
Supplementary Figure 1.
The replication rate of the adenoviral pTP-mi5 expression vector is decreased in packaging cells when pTP-mi5 expression is permitted. T-REx-293 cells were transduced with the adenoviral pTP-mi5 expression vector AdTO-pTP-mi5x6 at an MOI of 0.1 TCID50/cell. The transduced cells were cultivated in the absence (- dox) or presence of doxycycline (+ dox). Amplification of the vector was monitored by determining vector copy numbers at time points 0, 2, 4, and 6 days post-infection (mean ± SD, n = 3) by qPCR using a TaqMan primer/probe set specific for the Ad5 hexon gene.
Supplementary Figure 2.
Adenoviral vector-mediated expression of pTP-mi5 decreases the replication of wt Ad5 also at higher MOIs. A549 cells were transduced with the adenoviral pTP-mi5 expression vector AdTO-pTP-mi5x6 (indicated as 6x pTP-mi5) or its respective negative control vector AdTO-mi-x6 (indicated as 6x neg. ctrl.) at an MOI of 100 TCID50/cell or were mock-transduced (indicated as no vector). Twenty-four hours after infection, cells were infected with wt Ad5 at MOIs of 0.01 (A), 0.1 (B), 1 (C), 10 (D), or 100 TCID50/cell (E). Cells were then cultivated for an additional 2 days. Genome copy numbers of wt Ad5 were determined for time points 0 and 2 days post-infection with wt Ad5 by qPCR using a primer/probe set specific for the E1A gene present only on wt Ad5. Data represent the means ± SD of 3 independent experiments, each performed in triplicate. ∗∗∗p < 0.001.
Supplementary Figure 3.
Infection of cells with the adenoviral pTP-mi5 expression vector concomitant with or after infection with wt Ad5 decreases the replication of wt Ad5. A549 cells were transduced with the adenoviral pTP-mi5 expression vector AdTO-pTP-mi5x6 (indicated as 6x pTP-mi5) or its respective negative control vector AdTO-mi-x6 (indicated as 6x neg. ctrl.) at an MOI of 100 TCID50/cell or were mock-transduced (indicated as no vector). The same cells were also infected with wt Ad5 at MOIs ranging from 0.01 to 100 at time points 6 h after infection with the recombinant vectors (A), concomitant with (B), or 6 h prior to infection with the recombinant vectors. Cells were then cultivated for an additional 2 days. Genome copy numbers of wt Ad5 were determined for time points 0 and 2 days post-infection with wt Ad5 by qPCR using a primer/probe set specific for the E1A gene present only on wt Ad5. Data represent the means ± SD of 3 independent infections. ∗∗∗p < 0.001.
References
- Alba R., Bosch A., Chillon M. Gutless adenovirus: last-generation adenovirus for gene therapy. Gene Ther. 2005;12(Suppl. 1):S18–S27. doi: 10.1038/sj.gt.3302612. [DOI] [PubMed] [Google Scholar]
- Andersson M.G., Haasnoot P.C., Xu N., Berenjian S., Berkhout B., Akusjarvi G. Suppression of RNA interference by adenovirus virus-associated RNA. J. Virol. 2005;79:9556–9565. doi: 10.1128/JVI.79.15.9556-9565.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuthnot P. Harnessing RNA interference for the treatment of viral infections. Drug News Perspect. 2010;23:341–350. doi: 10.1358/dnp.2010.23.6.1437713. [DOI] [PubMed] [Google Scholar]
- Blanke C., Clark C., Broun E.R., Tricot G., Cunningham I., Cornetta K., Hedderman A., Hromas R. Evolving pathogens in allogeneic bone marrow transplantation: increased fatal adenoviral infections. Am. J. Med. 1995;99:326–328. doi: 10.1016/s0002-9343(99)80169-7. [DOI] [PubMed] [Google Scholar]
- Burnett J.C., Rossi J.J. RNA-based therapeutics: current progress and future prospects. Chem. Biol. 2012;19:60–71. doi: 10.1016/j.chembiol.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew R.W., Sontheimer E.J. Origins and mechanisms of miRNAs and siRNAs. Nat. Rev. Mol. Cell Biol. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Mautner V., Osman H., Collingham K.E., Fegan C.D., Klapper P.E., Moss P.A., Milligan D.W. Adenovirus infections following allogeneic stem cell transplantation: incidence and outcome in relation to graft manipulation, immunosuppression, and immune recovery. Blood. 2002;100:1619–1627. doi: 10.1182/blood-2002-02-0377. [DOI] [PubMed] [Google Scholar]
- Chung K.H., Hart C.C., Al-Bassam S., Avery A., Taylor J., Patel P.D., Vojtek A.B., Turner D.L. Polycistronic RNA polymerase II expression vectors for RNA interference based on BIC/miR-155. Nucleic Acids Res. 2006;34:e53. doi: 10.1093/nar/gkl143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y.S., Kim M.K., Lee W.J., Kang C. Silencing E1A mRNA by RNA interference inhibits adenovirus replication. Arch. Virol. 2007;152:1305–1314. doi: 10.1007/s00705-007-0951-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B.R. Transcription and processing of human microRNA precursors. Nat. Rev. Mol. Cell Biol. 2004;16:861–865. doi: 10.1016/j.molcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Cundy K.C. Clinical pharmacokinetics of the antiviral nucleotide analogues cidofovir and adefovir. Clin. Pharmacokinetics. 1999;36:127–143. doi: 10.2165/00003088-199936020-00004. [DOI] [PubMed] [Google Scholar]
- Davidson B.L., McCray P.B., Jr. Current prospects for RNA interference-based therapies. Nat. Rev. Genet. 2011;12:329–340. doi: 10.1038/nrg2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong R.N., van der Vliet P.C., Brenkman A.B. Adenovirus DNA replication: protein priming, jumping back and the role of the DNA binding protein DBP. Curr. Topics Microbiol. Immunol. 2003;272:187–211. doi: 10.1007/978-3-662-05597-7_7. [DOI] [PubMed] [Google Scholar]
- Ebner K., Rauch M., Preuner S., Lion T. Typing of human adenoviruses in specimens from immunosuppressed patients by PCR-fragment length analysis and real-time quantitative PCR. J. Clin. Microbiol. 2006;44:2808–2815. doi: 10.1128/JCM.00048-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echavarria M. Adenoviruses in immunocompromised hosts. Clin. Microbiol. Rev. 2008;21:704–715. doi: 10.1128/CMR.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein A., Grossl T., Geisler A., Wang X., Pinkert S., Pozzuto T., Schwer C., Kurreck J., Weger S., Vetter R., Poller W., Fechner H. Inhibition of adenovirus infections by siRNA-mediated silencing of early and late adenoviral gene functions. Antiviral Res. 2010;88:86–94. doi: 10.1016/j.antiviral.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Elbashir S.M., Harborth J., Lendeckel W., Yalcin A., Weber K., Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Ghildiyal M., Zamore P.D. Small silencing RNAs: an expanding universe. Nat. Rev. Genet. 2009;10:94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves M.A., de Vries A.A. Adenovirus: from foe to friend. Rev. Med. Virol. 2006;16:167–186. doi: 10.1002/rmv.494. [DOI] [PubMed] [Google Scholar]
- Haasnoot J., Westerhout E.M., Berkhout B. RNA interference against viruses: strike and counterstrike. Nat. Biotechnol. 2007;25:1435–1443. doi: 10.1038/nbt1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale G.A., Heslop H.E., Krance R.A., Brenner M.A., Jayawardene D., Srivastava D.K., Patrick C.C. Adenovirus infection after pediatric bone marrow transplantation. Bone Marrow Transplant. 1999;23:277–282. doi: 10.1038/sj.bmt.1701563. [DOI] [PubMed] [Google Scholar]
- Hartline C.B., Gustin K.M., Wan W.B., Ciesla S.L., Beadle J.R., Hostetler K.Y., Kern E.R. Ether lipid-ester prodrugs of acyclic nucleoside phosphonates: activity against adenovirus replication in vitro. J. Infect. Dis. 2005;191:396–399. doi: 10.1086/426831. [DOI] [PubMed] [Google Scholar]
- Heemskerk B., Lankester A.C., van Vreeswijk T., Beersma M.F., Claas E.C., Veltrop-Duits L.A., Kroes A.C., Vossen J.M., Schilham M.W., van Tol M.J. Immune reconstitution and clearance of human adenovirus viremia in pediatric stem-cell recipients. J. Infect. Dis. 2005;191:520–530. doi: 10.1086/427513. [DOI] [PubMed] [Google Scholar]
- Howard D.S., Phillips I.G., Reece D.E., Munn R.K., Henslee-Downey J., Pittard M., Barker M., Pomeroy C. Adenovirus infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 1999;29:1494–1501. doi: 10.1086/313514. [DOI] [PubMed] [Google Scholar]
- Huntzinger E., Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat. Rev. Genet. 2011;12:99–110. doi: 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- Hutvagner G., Simard M.J. Argonaute proteins: key players in RNA silencing. Nat. Rev. Mol. Cell Biol. 2008;9:22–32. doi: 10.1038/nrm2321. [DOI] [PubMed] [Google Scholar]
- Ison M.G. Adenovirus infections in transplant recipients. Clin. Infect. Dis. 2006;43:331–339. doi: 10.1086/505498. [DOI] [PubMed] [Google Scholar]
- Kawamata T., Tomari Y. Making RISC. Trends Biochem. Sci. 2010;35:368–376. doi: 10.1016/j.tibs.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Kneidinger D., Ibrisimovic M., Lion T., Klein R. Inhibition of adenovirus multiplication by short interfering RNAs directly or indirectly targeting the viral DNA replication machinery. Antiviral Res. 2012;94:195–207. doi: 10.1016/j.antiviral.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojaoghlanian T., Flomenberg P., Horwitz M.S. The impact of adenovirus infection on the immunocompromised host. Rev. Med. Virol. 2003;13:155–171. doi: 10.1002/rmv.386. [DOI] [PubMed] [Google Scholar]
- Lenaerts L., De Clercq E., Naesens L. Clinical features and treatment of adenovirus infections. Rev. Med. Virol. 2008;18:357–374. doi: 10.1002/rmv.589. [DOI] [PubMed] [Google Scholar]
- Lindemans C.A., Leen A.M., Boelens J.J. How i treat adenovirus in hematopoietic stem cell transplant recipients. Blood. 2010;116:5476–5485. doi: 10.1182/blood-2010-04-259291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lion T., Baumgartinger R., Watzinger F., Matthes-Martin S., Suda M., Preuner S., Futterknecht B., Lawitschka A., Peters C., Potschger U., Gadner H. Molecular monitoring of adenovirus in peripheral blood after allogeneic bone marrow transplantation permits early diagnosis of disseminated disease. Blood. 2003;102:1114–1120. doi: 10.1182/blood-2002-07-2152. [DOI] [PubMed] [Google Scholar]
- Lion T., Kosulin K., Landlinger C., Rauch M., Preuner S., Jugovic D., Potschger U., Lawitschka A., Peters C., Fritsch G., Matthes-Martin S. Monitoring of adenovirus load in stool by real-time PCR permits early detection of impending invasive infection in patients after allogeneic stem cell transplantation. Leukemia. 2010;24:706–714. doi: 10.1038/leu.2010.4. [DOI] [PubMed] [Google Scholar]
- Liu Y.P., Berkhout B. MiRNA cassettes in viral vectors: problems and solutions. Biochim. Biophys. Acta. 2011;1809:732–745. doi: 10.1016/j.bbagrm.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Liu Y.P., Haasnoot J., ter Brake O., Berkhout B., Konstantinova P. Inhibition of HIV-1 by multiple siRNAs expressed from a single microRNA polycistron. Nucleic Acids Res. 2008;36:2811–2824. doi: 10.1093/nar/gkn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungman P., Ribaud P., Eyrich M., Matthes-Martin S., Einsele H., Bleakley M., Machaczka M., Bierings M., Bosi A., Gratecos N., Cordonnier C. Cidofovir for adenovirus infections after allogeneic hematopoietic stem cell transplantation: a survey by the infectious diseases working party of the european group for blood and marrow transplantation. Bone Marrow Transplant. 2003;31:481–486. doi: 10.1038/sj.bmt.1703798. [DOI] [PubMed] [Google Scholar]
- Lu S., Cullen B.R. Adenovirus VA1 noncoding RNA can inhibit small interfering RNA and microRNA biogenesis. J. Virol. 2004;78:12868–12876. doi: 10.1128/JVI.78.23.12868-12876.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowa M.B., Crowther C., Arbuthnot P. Therapeutic potential of adenoviral vectors for delivery of expressed RNAi activators. Expert Opin. Drug Deliv. 2010;7:1373–1385. doi: 10.1517/17425247.2010.533655. [DOI] [PubMed] [Google Scholar]
- Munoz F.M., Piedra P.A., Demmler G.J. Disseminated adenovirus disease in immunocompromised and immunocompetent children. Clin. Infect. Dis. 1998;27:1194–1200. doi: 10.1086/514978. [DOI] [PubMed] [Google Scholar]
- Paolino K., Sande J., Perez E., Loechelt B., Jantausch B., Painter W., Anderson M., Tippin T., Lanier E.R., Fry T., DeBiasi R.L. Eradication of disseminated adenovirus infection in a pediatric hematopoietic stem cell transplantation recipient using the novel antiviral agent CMX001. J. Clin. Virol. 2011;50:167–170. doi: 10.1016/j.jcv.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Pelka P., Ablack J.N., Fonseca G.J., Yousef A.F., Mymryk J.S. Intrinsic structural disorder in adenovirus E1A: a viral molecular hub linking multiple diverse processes. J. Virol. 2008;82:7252–7263. doi: 10.1128/JVI.00104-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoul C., Barker S.D., Aebischer P. Viral-based modelling and correction of neurodegenerative diseases by RNA interference. Gene Ther. 2006;13:487–495. doi: 10.1038/sj.gt.3302690. [DOI] [PubMed] [Google Scholar]
- Rettig G.R., Behlke M.A. Progress toward in vivo use of siRNAs-II. Mol. Ther. 2012;20:483–512. doi: 10.1038/mt.2011.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer E.J. Assembly and function of RNA silencing complexes. Nat. Rev. Mol. Cell Biol. 2005;6:127–138. doi: 10.1038/nrm1568. [DOI] [PubMed] [Google Scholar]
- Symeonidis N., Jakubowski A., Pierre-Louis S., Jaffe D., Pamer E., Sepkowitz K., O’Reilly R.J., Papanicolaou G.A. Invasive adenoviral infections in T-cell-depleted allogeneic hematopoietic stem cell transplantation: high mortality in the era of cidofovir. Transpl. Infect. Dis. 2007;9:108–113. doi: 10.1111/j.1399-3062.2006.00184.x. [DOI] [PubMed] [Google Scholar]
- Wu Z., Xue Y., Wang B., Du J., Jin Q. Broad-spectrum antiviral activity of RNA interference against four genotypes of Japanese encephalitis virus based on single microRNA polycistrons. PLoS ONE. 2011;6:e26304. doi: 10.1371/journal.pone.0026304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi R., Qin Y., Macara I.G., Cullen B.R. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf U., Hale G.A., Carr J., Gu Z., Benaim E., Woodard P., Kasow K.A., Horwitz E.M., Leung W., Srivastava D.K., Handgretinger R., Hayden R.T. Cidofovir for the treatment of adenoviral infection in pediatric hematopoietic stem cell transplant patients. Transplantation. 2006;81:1398–1404. doi: 10.1097/01.tp.0000209195.95115.8e. [DOI] [PubMed] [Google Scholar]
- Zeng Y., Wagner E.J., Cullen B.R. Both natural and designed microRNAs can inhibit the expression of cognate mRNAs when expressed in human cells. Nat. Rev. Mol. Cell Biol. 2002;9:1327–1333. doi: 10.1016/s1097-2765(02)00541-5. [DOI] [PubMed] [Google Scholar]
- Zhou J., Rossi J.J. Progress in RNAi-based antiviral therapeutics. Methods Mol. Biol. 2011;721:67–75. doi: 10.1007/978-1-61779-037-9_4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.