Abstract
Although there is discordance between human immunodeficiency virus (HIV) blood plasma and seminal plasma viral loads (VL), little is known about the dynamics of VL rebound in these compartments upon discontinuation of highly active antiretroviral therapy (HAART). Therefore, we sought to examine the relationship between blood and semen VL rebound after discontinuation of HAART. Participants in this substudy were men enrolled from two centers of a multicenter, placebo-controlled randomized trial of HIV therapeutic vaccination using ALVAC with or without Remune. With at least 2 years of sustained virologic suppression and following a 20-week vaccination course, subjects underwent structured HAART interruption. Fourteen men provided semen samples. Seven to 12 weeks after HAART interruption, all 14 men had detectable blood VLs whereas 8 of 14 had detectable seminal VLs. There was a significant correlation between blood and seminal VLs (Spearman r=0.58, p=0.03) at the time of semen collection. An earlier time to detectable blood VL after HAART interruption was associated with higher seminal VL (Spearman r=−0.64, p=0.02). These findings support the compartmentalization of HIV and underscore the importance of understanding the genital tract as an HIV reservoir in the quest to minimize HIV transmission.
Sexual transmission accounts for approximately 80% of human immunodeficiency virus (HIV) infections.1 Although highly active antiretroviral therapy (HAART) confers a very significant reduction in the risk of HIV transmission,2 discontinuation of antiretrovirals (ARVs) results in rebound blood plasma viremia.3,4 However, it is currently unknown whether the kinetics of seminal viral rebound parallel that of viral rebound in the blood. Understanding the kinetics of seminal viral load (VL) rebound is critical as genital HIV RNA levels have been shown to predict HIV-1 transmission, independently of the peripheral blood VL.5 Therefore, we sought to explore the relationship between rebound plasma viremia in blood plasma and seminal plasma in HIV-infected men upon discontinuation of HAART.
Subjects were male volunteers from The Ottawa Hospital Immunodeficiency Clinic and The Maple Leaf Medical Clinic (MLMC) who were participating in the Canadian Institutes of Health Research (CIHR) Canadian HIV Trials Network (CTN) 173 Study. This was a multicenter, randomized, double-blind placebo-controlled trial of a combined therapeutic vaccine. The objective of this study was to determine if therapeutic vaccination would induce anti-HIV-specific immunity and delay viral rebound after discontinuation of HAART. The intervention included ALVAC, a vaccination that contains a canarypox virus vector vCP1452,6 designed to enhance CD8 T cell responses,7 and Remune, which contains chemically and physically inactivated gp120-depleted HIV-18 and induces helper T cell responses.9
Patients were randomized to receive either ALVAC plus Remune, ALVAC plus Remune placebo, or both placebos. Participants received Remune (or matched placebo) at weeks 0, 12, and 20 and ALVAC (or matched placebo) at weeks 8, 12, 16, and 20. At week 24, participants interrupted HAART providing their VL was maintained <50 copies/ml. Patients were monitored clinically and through laboratory parameters through week 48. Primary study results have been published previously.10
As a substudy, participants at The Ottawa Hospital Immunodeficiency Clinic and The MLMC had the option of donating a semen sample at baseline, week 24, and week 32. This latter time point was selected as it was expected that, by this time, plasma VL would have rebounded and VL set-point would have been established in the majority of subjects. Approval was obtained from the institutional ethics review boards from both sites.
As outlined by Angel et al.,10 individuals were eligible if they were >18 years of age with HIV infection and receiving a minimum of three ARV agents, with HIV RNA <50 copies/ml for >2 years. All subjects were on a protease inhibitor (PI) plus two nucleoside reverse transcriptase inhibitors (NRTIs) at the time of interrupting HAART. Additional eligibility requirements included absolute CD4 count >500 cells/μl, CD4 T cell nadir >250 cells/μl, and CD4 to CD8 ratio >0.5. Furthermore, individuals could not have any evidence of hepatitis B or C coinfection.
CD4 counts and blood plasma VLs (as measured by Chiron bDNA version 3.0 assay) at weeks −4, 0, 4, 8, 12, 16, 20, and 24 were determined. After week 24, plasma VLs were performed biweekly for 4 weeks, weekly for 8 weeks, and then monthly thereafter or until participants restarted therapy. CD4 counts continued to be measured every 4 weeks. Processing of semen was performed within 2 h of ejaculation. Ten milliliters of RPMI was added to each semen sample and the diluted semen samples were spun at 1500×g for 10 min. Eight to 10 ml of diluted seminal plasma was collected and stored at −150°C until analysis. VL in semen was determined on diluted seminal plasma samples by either bioMerieux Nuclisens HIV-1 QT assay with a limit of quantitation of 25 copies/ml (n=11) or Abbott RealTime HIV-1 assay with a limit of quantitation of 40 copies/ml (n=3). As HIV RNA levels were determined on diluted samples, a back-calculation was used to determine the quantity of HIV RNA within the seminal plasma.
In the overall study, 52 men from five centers were randomized between May 2004 and May 2006. Nineteen were randomized to ALVAC plus Remune, 18 to ALVAC plus Remune placebo, and 15 to both placebos. In this substudy, 14 subjects (median age 48, IQR 43, 59) provided semen samples (Table 1). Eleven men provided semen samples at all three time points. For three men, semen samples were not available at week 24. Final seminal plasma VL measurements for all 14 subjects were performed between 46 and 87 days (7–12 weeks) after HAART discontinuation with matching blood plasma VL available for all subjects.
Table 1.
Immunization Treatment Allocation and Highly Active Antiretroviral Therapy Regimen
| Subject | Immunization treatment allocation | HAART regimen |
|---|---|---|
| 1 | Placebo | Lopinavir/r, 3TC, ABC |
| 2 | Remune + ALVAC | Lopinavir/r, 3TC, ABC |
| 3 | Placebo | Lopinavir/r, 3TC, d4T |
| 4 | Remune + ALVAC | Lopinavir/r, 3TC, AZT |
| 5 | Remune + ALVAC | Lopinavir/r, 3TC, ABC |
| 6 | ALVAC | Lopinavir/r, 3TC, AZT |
| 7 | Remune + ALVAC | Saquinavir/r, 3TC, AZT |
| 8 | Placebo | Lopinavir/r, 3TC, ABC |
| 9 | Remune + ALVAC | Nelfinavir, 3TC, AZT, ABC |
| 10 | Placebo | Lopinavir/r, 3TC, TDF |
| 11 | Remune + ALVAC | Lopinavir/r, TDF, FTC |
| 12 | ALVAC | Lopinavir/r, 3TC, d4T |
| 13 | Placebo | Lopinavir/r, 3TC, d4T |
| 14 | Remune + ALVAC | Indinavir, AZT, 3TC |
HAART, highly active antiretroviral therapy; r, ritonavir boosting dose; 3TC, lamivudine; ABC, abacavir; d4T, stavudine; AZT, zidovudine; TDF, tenofovir; FTC, emtricitabine.
As vaccination group allocation did not have any impact on semen VL rebound (Fisher t test, p=1.0) results for all individuals were analyzed together. At week 0 all men had blood and seminal plasma VL below the level of quantitation (BLQ). At week 24, the time of HAART discontinuation, median CD4 count was 866 cells/μl (IQR 791, 1,410) and median CD4% was 37.8% (IQR 33.3%, 49.5%). Plasma VL was BLQ in all 14 subjects and all 11 men who provided semen samples at week 24 had seminal plasma VLs BLQ. At the time of semen sample collection, 7–12 weeks after discontinuation of HAART, virus was detectable in the semen of 8 out of 14 men and in the plasma of all 14 subjects.
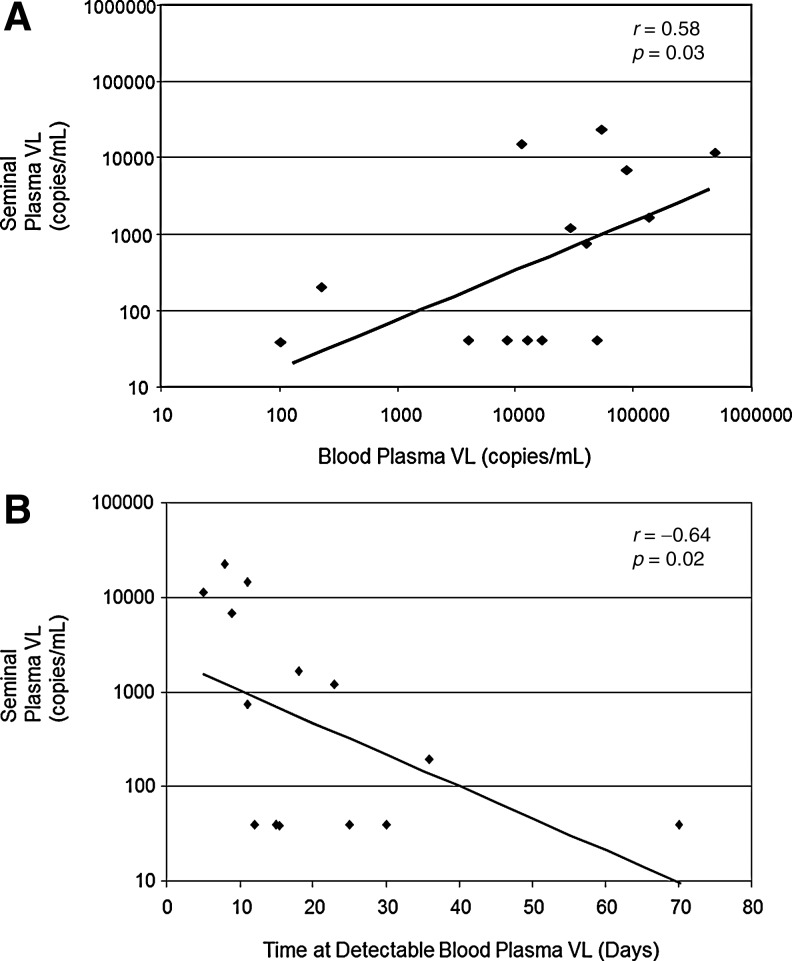
Blood plasma VL was greater than seminal plasma VL in 13 out of 14 men. There was also a significant correlation between blood and seminal plasma VL at the time seminal and plasma VL was evaluated (Spearman r=0.58, p=0.03) (Fig. 1A). The primary outcome of the main study was related to the time to detectable blood plasma VL after HAART interruption. The frequent measurements of blood plasma VL allowed for the ability to determine if the rapidity of VL rebound in the blood correlated with the magnitude of VL in semen 8 weeks after HAART interruption. An earlier time to detectable blood VL after HAART discontinuation was found to be associated with higher seminal VL (Spearman r=−0.64, p=0.02) (Fig. 1B). Mean time to VL rebound in the blood tended to be shorter in subjects with detectable virus in the semen 7–12 weeks after HAART interruption (15.1±10.2 days) compared to those with seminal VL BLQ (27.8±21.7 days) although this did not reach statistical significance (p=0.169). With six out of 14 men having seminal plasma VL BLQ and none with blood plasma BLQ at week 32, there was a statistically significant difference in the likelihood of an undetectable VL in blood versus seminal plasma after HAART interruption (McNemar's X2=–4.16, p=0.041), consistent with differing kinetics of viral replication and further indicating the compartmentalization of HIV.
FIG. 1.
(A) Seminal plasma and blood plasma HIV viral load (VL) 7–12 weeks after discontinuation of highly active antiretroviral therapy (HAART). (B) Seminal plasma HIV VL 7–12 weeks after discontinuation of HAART versus time to detectable blood plasma viremia.
Since vaccination group allocation did not have any impact on semen VL rebound, we sought to examine the kinetics of VL rebound in seminal plasma compared to blood plasma after discontinuation of HAART. Our findings demonstrated a correlation between time to detectable blood plasma VL and magnitude of semen VL. This result is noteworthy given that we were able to detect a correlation despite our small sample size. Moreover, given that blood plasma VLs are used to monitor control of HIV replication in clinical practice, this finding has implications for patient counseling. Specifically, it suggests that men whose blood plasma VLs become detectable rapidly upon HAART discontinuation are more likely to harbor higher levels of HIV within their seminal plasma. Therefore, these men may be more likely to transmit HIV infection to their partners compared to men whose blood plasma VLs take longer to rebound and extra vigilance is thus required to minimize the risk of HIV transmission. Furthermore, 6 of 14 individuals had seminal VLs BLQ while none had blood VLs BLQ 7–12 weeks upon HAART discontinuation. The finding that approximately half of the subjects had seminal VLs BLQ, and that blood VLs tended to be greater than seminal VLs, supports the concept of differential viral replication and compartmentalization.11–14 However, as phylogenetic studies were not performed, there remains the possibility that a degree of spillover of virus from peripheral blood, in addition to localized viral production, has occurred.
The only other published study examining rebound seminal viremia in the context of HAART discontinuation was that performed by Liuzzi et al.15 They examined VL rebound in blood and seminal plasma in 12 HIV-infected males who discontinued HAART after at least 1 year of therapy. VL rebound in plasma and seminal VL was detected in all patients. While the magnitude of rebound was generally greater in blood plasma, two patients had higher VLs in seminal plasma. The major difference between this study and ours was the fact that one-third of their subjects did not have suppressed blood plasma VLs at the time of HAART interruption. Ongoing viral replication at the time of HAART interruption undoubtedly has an impact on seminal VL kinetics. Another important difference between the Liuzzi et al. study and our study is that treatment interruptions in the former were not structured but were often precipitated by adverse effects of ARVs. Furthermore, in the study by Liuzzi et al. VL rebound was examined at variable lengths of time after treatment interruption (range 1–8 months after HAART interruption) and following unknown adherence to HAART.
In addition to the small sample size, another limitation of our study is that specimens were collected at only a single point in time after HAART discontinuation and may not be reflective of the relationship between blood and seminal plasma VL rebound at later time points. We also did not account for the possibility of sexually transmitted infections (STIs), which can facilitate the transmission of HIV by inducing a local inflammatory response.16 Furthermore, semen itself contains many substances that both enhance and inhibit HIV infectivity.17–20 For example, Munch et al. have described a semen-derived enhancer of viral infectivity (SEV1). This substance forms aggregates of amyloid fibrils that “trap” HIV virions, culminating in a potential 5-fold increase in HIV infectious titer.19 As we did not examine seminal samples for the presence of inhibitors, we cannot entirely exclude the possibility that these substances played a role in influencing our results.
In the first study to explore the relationship between plasma genital HIV RNA level and risk of heterosexual HIV transmission, Beaton et al. prospectively examined 2,521 HIV serodiscordant African couples.5 Specimens included cervical samples from 1805 women and seminal samples from 716 men. The investigators found that each 1.0 log10 increase in HIV genital HIV-1 RNA was associated with a 2.20-fold and 1.79-fold increased risk of HIV transmission for endocervical and seminal specimens, respectively. Importantly, a higher genital HIV RNA level was associated with increased risk of heterosexual transmission, independently of blood plasma VL. As genital HIV RNA was measured at a single time point, another important observation from this study was that a single genital HIV RNA measurement appeared to be sufficient to estimate the risk of HIV transmissibility.5
It is becoming increasingly clear that understanding the genital tract as an HIV reservoir is important in the quest to minimize HIV transmission. As alluded to by Beaton et al., it is plausible that VL in the genital tract may be a surrogate marker of HIV infectivity and that the impact of new interventions aimed at reducing HIV-1 transmission can be estimated by determining the genital HIV RNA level.5 Although our sample size was small, our findings suggest that time to VL rebound within blood plasma upon HAART discontinuation may be another surrogate marker that can be used in clinical practice to estimate the propensity for HIV transmission. Further studies are required to estimate the impact of seminal VL and time to blood plasma VL rebound on transmissibility in the context of chronic, as well as acute, HIV infection. Similarly, elucidating the mechanisms by which different factors mediate the transmission process is also essential in the quest to minimize the spread of HIV infection.
Acknowledgments
The authors would like to thank Paul Sandstrom, Richard Pilon, and John Kim from the National HIV and Retrovirology Laboratories, Public Health Agency of Canada for analysis of seminal plasma viral loads. The authors would also like to thank Tim Ramsay and Elham El-Sabri from the Ottawa Hospital Research Institute Methods Center for statistical assistance.
This study was supported by operating grants to J.B.A. from the Canadian Foundation for AIDS Research (CanFAR) (grant 017024), the Canadian Institutes of Health Research (CIHR) (grant 44179), and the Ontario HIV Treatment Network (OHTN) (grant ROGA103). Support was also provided by the CIHR Canadian HIV Trials Network (CTN). Sanofi Pasteur provided financial support. J.B.A. is supported by a Career Scientist Award from the OHTN. R.P.S. is supported by a Canada Research Chair. J.P.R. is a clinician-scientist receiving support from Fonds de la recherche en santé du Québec (FRSQ). C.T.C. is a CTN Fellowship recipient.
Author Disclosure Statement
J.B.A. and R.P.S. have conducted contract research for Sanofi Pasteur. S.G. is an employee of Sanofi Pasteur.
References
- 1.Donnell D. Baeten JM. Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: A prospective cohort analysis. Lancet. 2010;375:2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.HIV Prevention Trials Network: Initiation of antiretroviral treatment protects uninfected sexual partners from HIV infection (HPTN Study 052) May 12, 2011. http://www.hptn.org/web%20documents/PressReleases/HPTN052PressReleaseFINAL5_12_118am.pdf http://www.hptn.org/web%20documents/PressReleases/HPTN052PressReleaseFINAL5_12_118am.pdf
- 3.Davey RT. Bhat N. Yoder C, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci USA. 1999;96:15109–15114. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chun TW. Justement JS. Murray D, et al. Rebound plasma viremia following cessation of antiretroviral therapy despite profoundly low levels of HIV reservoir: Implications for eradication. AIDS. 2010;24:2803–2808. doi: 10.1097/QAD.0b013e328340a239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaton JM. Kahle E. Lingappa JR, et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med. 2011;3:77ra29. doi: 10.1126/scitranslmed.3001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasteur A, editor. ALVAC-HIV (vCP1452) Adventis Pasteur. 2003.
- 7.Jin X. Ramanathan M. Barsoum S, et al. Safety and immunogenicity of ALVAC vCP1452 and recombinant gp160 in newly human immunodeficiency virus type 1-infected patients treated with prolonged highly active antiretroviral therapy. J Virol. 2002;76:2206–2216. doi: 10.1128/jvi.76.5.2206-2216.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Remune® (HIV-1 Immunogen): In Corporation TIR (Ed.) VI. Carlsbad, CA: 2004. [Google Scholar]
- 9.Robbins GK. Addo MM. Troung H, et al. Augmentation of HIV-1 specific T helper cell responses in chronic HIV-1 infection by therapeutic immunization. AIDS. 2003;17:1121–1126. doi: 10.1097/00002030-200305230-00002. [DOI] [PubMed] [Google Scholar]
- 10.Angel JB. Routy JP. Tremblay C, et al. A randomized controlled trial of HIV therapeutic vaccination using ALVAC with or without Remune. AIDS. 2010;25:731–739. doi: 10.1097/QAD.0b013e328344cea5. [DOI] [PubMed] [Google Scholar]
- 11.Sheth PM. Kovacs C. Kemal KS, et al. Persistent HIV RNA shedding in semen despite effective antiretroviral therapy. AIDS. 2009;24:2050–2054. doi: 10.1097/QAD.0b013e3283303e04. [DOI] [PubMed] [Google Scholar]
- 12.Lorello G. la Porte C. Pilon R. Zhang G. Karnauchow T. MacPherson P. Discordance in HIV-1 viral loads and antiretroviral drug concentrations comparing semen and blood plasma. HIV Med. 2009;10:548–554. doi: 10.1111/j.1468-1293.2009.00725.x. [DOI] [PubMed] [Google Scholar]
- 13.Vernazza PL. Troiani L. Flepp MJ, et al. Potent antiretroviral treatment in HIV-1 infection results in suppression of seminal shedding of HIV. The Swiss HIV Cohort. AIDS. 2000;14:117–121. doi: 10.1097/00002030-200001280-00006. [DOI] [PubMed] [Google Scholar]
- 14.Curran R. Ball JK. Concordance between semen-derived HIV- 1 proviral DNA and viral RNA hypervariable region 3 (V3) envelope sequences in cases where semen populations are distinct from those present in blood. J Med Virol. 2002;67:9–19. doi: 10.1002/jmv.2186. [DOI] [PubMed] [Google Scholar]
- 15.Liuzzi G. Chirianni A. Bagnarelli P. Valenza A. Tullio Cataldo P. Piazza M. A combination of nucleoside analogues and a protease inhibitor reduces HIV-1 RNA levels in semen: Implications for sexual transmission of HIV infection. Antivir Ther. 1999;4:95–99. [PubMed] [Google Scholar]
- 16.Cohen MS. Hoffman IF. Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: Implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868–1873. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 17.Roan NR. Munch J. Arhel N, et al. The cationic properties of SEVI underlie its ability to enhance human immunodeficiency virus infection. J Virol. 2009;83:73–80. doi: 10.1128/JVI.01366-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doncel GF. Joseph T. Thurman AR. Role of semen in HIV-1 transmission: Inhibitor or facilitator? Am J Reprod Immunol. 2011;65:292–301. doi: 10.1111/j.1600-0897.2010.00931.x. [DOI] [PubMed] [Google Scholar]
- 19.Munch J. Rucker E. Standker L, et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell. 2007;131:1059–1071. doi: 10.1016/j.cell.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Kim KA. Yolamanova M. Zirafi O, et al. Semen-mediated enhancement of HIV infection is donor-dependent and correlates with the levels of SEVI. Retrovirology. 2010;7:55. doi: 10.1186/1742-4690-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]