Abstract
There are few clinical data on the combination abacavir/lamivudine plus raltegravir. We compared the outcomes of patients from the SPIRAL trial receiving either abacavir/lamivudine or tenofovir/emtricitabine at baseline who had taken at least one dose of either raltegravir or ritonavir-boosted protease inhibitors. For the purpose of this analysis, treatment failure was defined as virological failure (confirmed HIV-1 RNA ≥50 copies/ml) or discontinuation of abacavir/lamivudine or tenofovir/emtricitabine because of adverse events, consent withdrawal, or lost to follow-up. There were 143 (72.59%) patients with tenofovir/emtricitabine and 54 (27.41%) with abacavir/lamivudine. In the raltegravir group, there were three (11.11%) treatment failures with abacavir/lamivudine and eight (10.96%) with tenofovir/emtricitabine (estimated difference 0.15%; 95% CI −17.90 to 11.6). In the ritonavir-boosted protease inhibitor group, there were four (14.81%) treatment failures with abacavir/lamivudine and 12 (17.14%) with tenofovir/emtricitabine (estimated difference −2.33%; 95% CI −16.10 to 16.70). Triglycerides decreased and HDL cholesterol increased through the study more pronouncedly with abacavir/lamivudine than with tenofovir/emtricitabine and differences in the total-to-HDL cholesterol ratio between both combinations of nucleoside reverse transcriptase inhibitors (NRTIs) tended to be higher in the raltegravir group, although differences at 48 weeks were not significant. While no patient discontinued abacavir/lamivudine due to adverse events, four (2.80%) patients (all in the ritonavir-boosted protease inhibitor group) discontinued tenofovir/emtricitabine because of adverse events (p=0.2744). The results of this analysis do not suggest that outcomes of abacavir/lamivudine are worse than those of tenofovir/emtricitabine when combined with raltegravir in virologically suppressed HIV-infected adults.
Introduction
The efficacy of antiretroviral therapy has been improving over time allowing an increasing proportion of HIV-infected patients to achieve sustained suppression of viral replication in plasma. However, a considerable proportion of patients may still need to have their otherwise successful antiviral therapy changed because of comorbidities, drug–drug interactions, or other safety or convenience reasons.1,2 Regimens preferentially recommended in major guidelines at present include a combination of two nucleoside reverse transcriptase inhibitors (NRTIs) plus a third drug consisting of a nonnucleoside reverse transcriptase inhibitor (NNRTI), a ritonavir-boosted protease inhibitor (PI), or an integrase inhibitor.3–6 These recommendations are based on major randomized clinical trials in antiretroviral-naive patients, but not all currently used drugs have been similarly tested in settings other than antiretroviral-naive patients or when tested have not shown the same level of evidence. Evidence-based guidance for the use of alternative drugs when considering changing a virologically successful antiretroviral regimen is often scarce.
Abacavir/lamivudine is a currently used fixed-dose combination of NRTIs, but data available with new drugs such as raltegravir are more limited than that of tenofovir/emtricitabine and in both cases are limited to antiretroviral-naive patients. Because abacavir/lamivudine and tenofovir/emtricitabine may have a different impact on comorbitities, choosing between them could be helpful to customize the optimal therapy. Ritonavir-boosted PIs are recommended agents for both antiretroviral-naive and antiretroviral-experienced patients because of their potency and high barrier to resistance,3–6 but they may increase plasma lipids, have the potential for clinically meaningful drug–drug interactions, and have been associated with an increased risk for cardiovascular disease.7–10 In HIV-infected adults with sustained virological suppression on ritonavir-boosted PIs, the SPIRAL study demonstrated that switching from ritonavir-boosted PIs to raltegravir did not result in less efficacy and did result in a better lipid profile at 48 weeks than continuing the ritonavir-boosted PI component.11
Although abacavir/lamivudine could be used like tenofovir/emtricitabine in combination with raltegravir in virologically suppressed HIV-infected patients, there are no data comparing the two combinations of NRTIs in this setting. To gather further insight on the abacavir/lamivudine plus raltegravir regimen in virologically suppressed treatment-experienced patients, we compared the efficacy and safety of abacavir/lamivudine to that of tenofovir/emtricitabine when each was combined with either raltegravir or ritonavir-boosted PIs in the SPIRAL trial.
Materials and Methods
The SPIRAL study was a 48-week, multicenter, open-label, randomized trial in which HIV-infected adults with <50 copies/ml of plasma HIV RNA for at least the previous 6 months on ritonavir-boosted PI-based therapy were randomized (1:1) to switch from the ritonavir-boosted PI to raltegravir or to continue on ritonavir-boosted PI-based therapy. The protocol was approved by the Ethics Committee at each center and by the Spanish Medicines Evaluation Agency. Written informed consent was obtained from all eligible patients before randomization. The SPIRAL trial is registered with ClinicalTrials.gov number NCT00528892. This analysis was planned after the parent study had been finished because abacavir/lamivudine and tenofovir/emtricitabine were the combinations of NRTIs most commonly used in the SPIRAL study and because of the paucity of data on the combination of abacavir/lamivudine plus raltegravir. For the purpose of this analysis, eligible patients were those who were receiving either abacavir/lamivudine or tenofovir/emtricitabine at baseline and who received at least one dose of either raltegravir or ritonavir-boosted PI. Patients were already taking the combinations of abacavir/lamivudine or tenofovir/emtricitabine when they were included in the SPIRAL study, and for this reason all of them were able to tolerate them. As the analysis did not ensure the homogeneity of the baseline characteristics between groups, comparisons in outcomes between groups were adjusted for differences in baseline characteristics.
Treatment failure was considered in all patients who had virological failure or discontinued abacavir/lamivudine or tenofovir/emtricitabine because of adverse events, consent withdrawal, or lost to follow-up. Virological failure was defined by a confirmed plasma HIV-1 RNA ≥50 copies/ml during treatment, whereas patients who withdrew consent, were lost, or switched or stopped abacavir/lamivudine or tenofovir/emtricitabine were censored. A sensitivity analysis for efficacy endpoints was done including all randomized patients. Fisher's exact test was used to compare proportions between treatment groups. The Mann–Whitney U-test was used for comparisons of continuous variables between groups. For testing overall differences of abacavir/lamivudine relative to tenofovir/emtricitabine and stratified by raltegravir and ritonavir-boosted PI use, 95% confidence intervals for the treatment difference were calculated by Newcombe's method.12 Simple comparisons were made with use of a two-sided alpha level of 0.05. Statistical analyses were performed with the use of SAS version 9.1.3 (SAS Institute, Cary, NC). The sponsors of the SPIRAL trial had no role in the study design, data collection, analysis, interpretation, or writing of this report. Esteban Martínez, Judit Pich, and Ignacio Perez had full access to all the data and had the final responsibility for the decision to submit this report for publication.
Results
Baseline characteristics
Of the 273 patients included in the SPIRAL study, 197 (72.16%) were included in this analysis. There were 143 (72.59%) patients treated with tenofovir/emtricitabine and 54 (27.41%) with abacavir/lamivudine. Tenofovir/emtricitabine and abacavir/lamivudine accounted for 76.56% of the combinations of NRTIs used in the SPIRAL study. In the overall population, patients taking abacavir/lamivudine were older, had a lower prevalence of previous virological failure, and had higher plasma levels of triglycerides, total and HDL cholesterol than patients taking tenofovir/emtricitabine (Table 1A). Baseline characteristics in the raltegravir and ritonavir-boosted PI groups are shown in Table 1B. In the population assigned to raltegravir (Table 1B), patients taking abacavir/lamivudine were significantly older and a higher proportion had suffered previous virological failure than those taking tenofovir/emtricitabine.
Table 1A.
Demographic and Baseline Characteristics (Abacavir/Lamivudine vs. Tenofovir/Emtricitabine)
| |
Abacavir/lamivudine |
Tenofovir/emtricitabine |
|
||
|---|---|---|---|---|---|
| (n=54) | (n=143) | p-value | |||
| Age, years [median (IQR)] | 46.5 | (42–56) | 44 | (40–48) | 0.0054 |
| Female sex [n (%)] | 10 | (18.52) | 27 | (25.87) | 0.48 |
| Ritonavir-boosted protease inhibitors at entry [n (%)] | |||||
| Lopinavir/ritonavir | 25 | (46.3) | 68 | (47.55) | |
| Atazanavir/ritonavir | 20 | (37.04) | 47 | (32.87) | |
| Fosamprenavir/ritonavir | 9 | (16.67) | 14 | (9.79) | |
| Saquinavir/ritonavir | — | (0) | 2 | (1.4) | |
| Darunavir/ritonavir | — | (0) | 1 | (0.7) | |
| Tipranavir/ritonavir | — | (0) | 1 | (0.7) | |
| Patients on their first antiretroviral regimen [n (%)] | 9 | (16.67) | 18 | (12.59) | 0.48 |
| Exposure to antiretroviral therapy (years) [median (range)] | 9.17 | (4–11.77) | 10.13 | (3.7–12.76) | 0.56 |
| Exposure to protease inhibitor-based therapy (months) [median (range)] | 28 | (15.52–44.03) | 29.22 | (18.53–36.23) | 0.92 |
| Patients with previous suboptimal antiretroviral therapy [n (%)] | 15 | (27.78) | 55 | (38.46) | 0.18 |
| Patients with previous virological failure [n (%)] | 7 | (12.96) | 54 | (37.76) | <0.001 |
| Patients with previous suboptimal antiretroviral therapy or virological failure [n (%)] | 19 | (35.19) | 70 | (48.95) | 0.10 |
| Number of previous antiretroviral regimens [median (IQR)] | 4 | (2–7) | 5 | (2–7.5) | 0.71 |
| Number of previous suboptimal antiretroviral regimens [median (IQR)] | 2 | (1–2) | 2 | (1–3) | 0.41 |
| Number of previous virological failures [median (IQR)] | 2 | (1–2) | 1 | (1–3) | 0.86 |
| Patients with AIDS [n (%)] | 25 | (0.7) | 71 | 50 | 0.75 |
| CD4 cell count (cells/ml) [median (IQR)] | 514.5 | (375.15–779.7) | 486.5 | (355.5–720) | 0.52 |
| CD8 cell count (cells/ml) [median (IQR)] | 761.91 | (577.98–1085.76) | 799 | (575–1071.33) | 0.946 |
| Triglycerides (mg/dl) [median (IQR)] | 193 | (150.58–268) | 153.5 | (103–238) | 0.007 |
| Triglycerides >200 mg/dl [n (%)] | 23 | (42.59) | 48 | (33.57) | 0.24 |
| Total cholesterol (mg/dl) [median (IQR)] | 219.3 | (193–242) | 193.5 | (167.5–221) | 0.001 |
| Total cholesterol >240 mg/dl [n (%)] | 14 | (25.93) | 17 | (11.89) | 0.026 |
| LDL cholesterol (mg/dl) [median (IQR)] | 129.5 | (107.25–149.25) | 119 | (96–146) | 0.20 |
| LDL cholesterol >160 mg/dl [n (%)] | 16.67 | (54) | 11.19 | (143) | 0.33 |
| HDL cholesterol (mg/dl) [median (IQR)] | 49.62 | (38.85–57.31) | 42.31 | (34–52.7) | 0.008 |
| HDL cholesterol <40 mg/dl [n (%)] | 15 | (27.78) | 55 | (38.46) | 0.18 |
| Lipid-lowering therapy at entry [n (%)] | 14 | (25.93) | 19 | (13.29) | 0.052 |
Table 1B.
Demographic and Baseline Characteristics (Abacavir/Lamivudine vs. Tenofovir/Emtricitabine in Raltegravir and Ritonavir-Boosted Protease Inhibitor Groups)
| |
Raltegravir |
Ritonavir-boosted protease inhibitor |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Abacavir/lamivudine (n=27) | Tenofovir/emtricitabine (n=73) | p-value | Abacavir/lamivudine (n=27) | Tenofovir/emtricitabine (n=70) | p-value | |||||
| Age, years [median (IQR)] | 47 | (43–60) | 44 | (39–48) | 0.002 | 45 | (38–52) | 44 | (40–48) | 0.68 |
| Female sex [n (%)] | 3 | (11.11) | 17 | (23.29) | 0.26 | 7 | (25.93) | 20 | (28.57) | 1 |
| Ritonavir-boosted protease inhibitors at entry [n (%)] | ||||||||||
| Lopinavir/ritonavir | 11 | (40.74) | 35 | (47.95) | 14 | (51.85) | 33 | (47.14) | ||
| Atazanavir/ritonavir | 11 | (40.74) | 25 | (34.25) | 9 | (33.33) | 22 | (31.43) | ||
| Fosamprenavir/ritonavir | 5 | (18.52) | 7 | (9.59) | 4 | (14.81) | 7 | (10) | ||
| Saquinavir/ritonavir | — | (0) | 3 | (4.11) | — | (0) | 8 | (11.43) | ||
| Darunavir/ritonavir | — | (0) | 1 | (1.37) | ||||||
| Tipranavir/ritonavir | — | (0) | 1 | (1.37) | ||||||
| Patients on their first antiretroviral regimen [n (%)] | 4 | (14.81) | 10 | (13.7) | 1 | 5 | (18.52) | 8 | (11.43) | 0.50 |
| Exposure to antiretroviral therapy (years) [median (range)] | 10.65 | (5.02–12.73) | 9.22 | (2.7–12.33) | 0.39 | 8.17 | (3.81–10.89) | 10.73 | (4.44–13.15) | 0.05 |
| Exposure to protease inhibitor-based therapy (months) [median (range)] | 29.4 | (19.53–45.23) | 29.55 | (18.23–35.67) | 0.58 | 27.78 | (13–39.73) | 29.02 | (19–37.93) | 0.48 |
| Patients with previous suboptimal antiretroviral therapy [n (%)] | 11 | (40.74) | 26 | (35.62) | 0.64 | 4 | (14.81) | 29 | (41.43) | 0.01 |
| Patients with previous virological failure [n (%)] | 4 | (14.81) | 28 | (38.36) | 0.033 | 3 | (11.11) | 26 | (37.14) | 0.01 |
| Patients with previous suboptimal antiretroviral therapy or virological failure [n (%)] | 14 | (51.85) | 35 | (47.95) | 0.82 | 5 | (18.52) | 35 | (50) | <0.001 |
| Number of previous antiretroviral regimens [median (IQR)] | 5.5 | (2–9) | 4 | (2–6) | 0.22 | 4 | (2–4) | 5 | (3–8) | 0.04 |
| Number of previous suboptimal antiretroviral regimens [median (IQR)] | 2 | (1–3) | 2 | (1–3) | 0.76 | 1.5 | (1–2) | 2 | (1–3) | 0.31 |
| Number of previous virological failures [median (IQR)] | 1.5 | (1–2) | 1 | (1–3) | 0.73 | 2 | (1–4) | 1 | (1–2) | 0.48 |
| Patients with AIDS [n (%)] | 11 | (40.74) | 37 | (50.68) | 0.05 | 14 | (51.84) | 34 | (48.57) | 0.82 |
| CD4 cell count (cells/ml) [median (IQR)] | 565.5 | (416–806) | 477.4 | (348.14–731.4) | 0.23 | 467.04 | (350.4–735.05) | 501 | (384–685.1) | 0.76 |
| CD8 cell count (cells/ml) [median (IQR)] | 845.34 | (577.98–1275) | 832.8 | (680.95–1071.9) | 0.78 | 756 | (577.5–974.4) | 718.1 | (504–1071.33) | 0.558781 |
| Triglycerides (mg/dl) [median (IQR)] | 168.29 | (149.9–268) | 151.29 | (100.5–242.5) | 0.11 | 198 | (150.58–282) | 157 | (104–231.15) | 0.02 |
| Triglycerides >200 mg/dl [n (%)] | 10 | (37.04) | 25 | (34.25) | 0.81 | 13 | (48.15) | 23 | (32.86) | 0.17 |
| Total cholesterol (mg/dl) [median (IQR)] | 217.69 | (193–244) | 198 | (168.5–226.5) | 0.04 | 221 | (187–242) | 190.5 | (167.5–217.5) | <0.001 |
| Total cholesterol >240 mg/dl [n (%)] | 7 | (25.93) | 9 | (12.33) | 0.12 | 7 | (25.93) | 8 | (11.43) | 0.11 |
| LDL cholesterol (mg/dl) [median (IQR)] | 128.5 | (108.5–139) | 124 | (95.8–147) | 0.83 | 138.46 | (100–163) | 113 | (96.9–144) | 0.12 |
| LDL cholesterol >160 mg/dl [n (%)] | 9 | (33.33) | 28 | (38.36) | 0.81 | 6 | (22.22) | 27 | (38.57) | 0.15 |
| HDL cholesterol (mg/dl) [median (IQR)] | 45 | (38.85–58.85) | 44.7 | (33–54) | 0.19 | 49.81 | (41–56) | 41.62 | (34.25–49.35) | 0.01 |
| HDL cholesterol <40 mg/dl [n (%)] | 2 | (7.41) | 12 | (16.44) | 0.34 | 7 | (25.93) | 4 | (5.71) | <0.001 |
| Lipid-lowering therapy at entry [n (%)] | 5 | (18.52) | 8 | (10.96) | 0.32 | 9 | (33.33) | 11 | (15.71) | 0.09 |
Efficacy
There were no deaths or new AIDS-defining events. In the overall population, there were 7/54 (12.96%) treatment failures in the abacavir/lamivudine group (three virological failures and four abacavir/lamivudine discontinuations) and 20/143 (14%) in the tenofovir/emtricitabine group (seven virological failures and 13 tenofovir/emtricitabine discontinuations) (estimated difference −1.02%; 95% confidence interval −10.30 to 11.40). In the raltegravir group, there were 3/27 (11.11%) treatment failures in the abacavir/lamivudine group and 8/73 (10.96%) in the tenofovir/emtricitabine group (estimated difference 0.15%; 95% confidence interval −17.90 to 11.6). In the ritonavir-boosted PI group, there were 4/27 (14.81%) treatment failures in the abacavir/lamivudine group and 12/70 (17.14%) in the tenofovir/emtricitabine group (estimated difference −2.33%; 95% confidence interval −16.10 to 16.70). Additional efficacy analyses according to prior virological failure or suboptimal therapy and a sensitivity analysis including all randomized patients did not significantly affect the overall results (data not shown).
In the overall population, there were 3/54 (5.56%) virological failures in the abacavir/lamivudine group and 7/143 (4.90%) in the tenofovir/emtricitabine group (estimated difference 0.66%; 95% confidence interval −10.50 to 5.40). In the raltegravir group, there were 1/27 (3.70%) virological failures in the abacavir/lamivudine group and 3/73 (4.11%) in the tenofovir/emtricitabine group (estimated difference −0.41%; 95% confidence interval −8.30 to 14.4). In the ritonavir-boosted PI group, there were 2/27 (7.41%) virological failures in the abacavir/lamivudine group and 4/70 (5.71%) in the tenofovir/emtricitabine group (estimated difference 1.69%; 95% confidence interval −18.00 to 8.00). Again, additional efficacy analyses according to prior virological failure or suboptimal therapy and a sensitivity analysis including all randomized patients did not significantly affect the overall results (data not shown).
At 48 weeks, CD4 cells (mean±SD) increased 55.74 (±227.70) per mm3 in the abacavir/lamivudine group and 50.14 (±162.47) per mm3 in the tenofovir/emtricitabine group (p=0.5992). In the raltegravir group, CD4 cells (mean±SD) increased 33.34 (187.86) per mm3 in the abacavir/lamivudine group and 27.89 (±170.14) per mm3 in the tenofovir/emtricitabine group (p=0.6794) at 48 weeks. In the ritonavir-boosted PI group, CD4 cells (mean±SD) increased 79.92 (±266.02) per mm3 in the abacavir/lamivudine group and 74.48 (±151.23) per mm3 in the tenofovir/emtricitabine group (p=0.7329) at 48 weeks.
Safety
At 48 weeks, changes in triglycerides (mean percent change, −17.65% vs. −18.12%, p=0.4224), total cholesterol (mean percent change, −7.38% vs. −5.00%, p=0.4874), LDL cholesterol (mean percent change, −12.22% vs. −5.33%, p=0.5291), HDL cholesterol (mean percent change, +0.72% vs. −2.56%, p=5661), and total-to-HDL cholesterol ratio (mean percent change, −0.49 vs. −0.17, p=0.1747) were not significantly different between the abacavir/lamivudine and tenofovir/emtricitabine groups, respectively. At 48 weeks, the proportion of patients showing triglycerides >200 mg/dl (n=12, 22.64% vs. n=29, 21.01%, p=0.8449), total cholesterol >240 mg/dl (n=8, 15.09% vs. n=8, 5.80%, p=0.0753), or LDL cholesterol >160 mg/dl (n=1, 1.89% vs. n=4, 2.90%, p=1) was not different between the abacavir/lamivudine or tenofovir/emtricitabine groups, respectively.
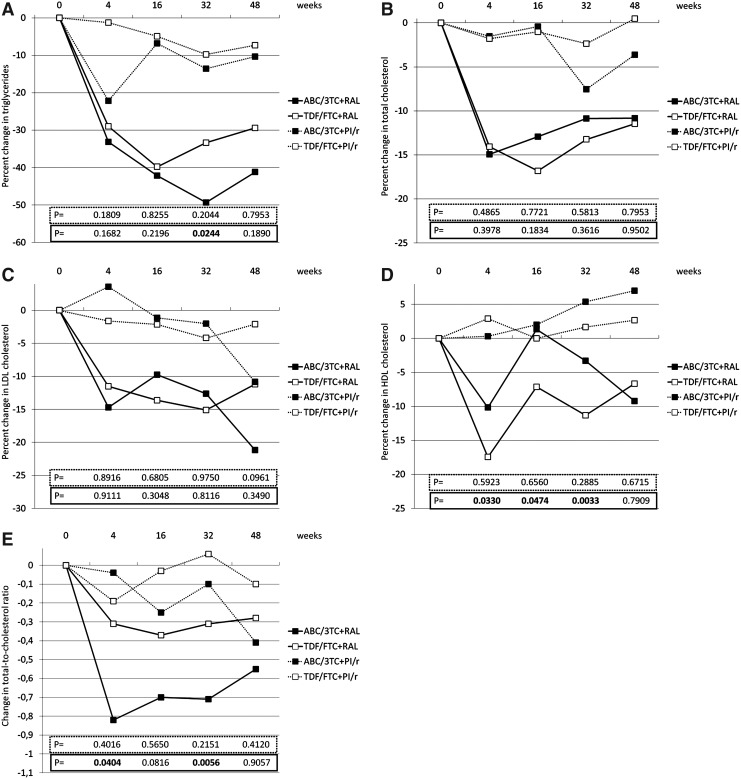
However, the proportion of patients showing HDL cholesterol <40 mg/dl at 48 weeks was significantly lower with abacavir/lamivudine (n=11, 20.75%) relative to that with tenofovir/emtricitabine (n=63, 45.65%) (p=0.0016). Median changes in plasma lipids in patients receiving either abacavir/lamivudine or tenofovir/emtricitabine in combination with raltegravir or ritonavir-boosted PI are shown in Fig. 1. As expected, decreases in plasma lipids were higher in patients switching from ritonavir-boosted PIs to raltegravir than in those continuing on ritonavir-boosted PIs. Interestingly, decreases in triglycerides and increases in HDL cholesterol through the study tended to be more pronounced in patients receiving abacavir/lamivudine than in those receiving tenofovir/emtricitabine, and differences in the total-to-HDL cholesterol ratio between both combinations of NRTIs tended to be higher in the raltegravir group, although differences at 48 weeks were not significant in any case (Fig. 1). Four (7.41%) patients treated with abacavir/lamivudine and seven (4.90%) patients treated with tenofovir/emtricitabine discontinued lipid-lowering therapies during the study (p=0.4972).
FIG. 1.
Median percent change in triglycerides (A), total cholesterol (B), LDL cholesterol (C), and HDL cholesterol (D), and median change in total-to-HDL cholesterol ratio (E) at different time points during the study. Black squares denote abacavir/lamivudine, and white squares denote tenofovir/emtricitabine. Continuous lines denote raltegravir, and dashed lines denote ritonavir-boosted protease inhibitors. p-values within the dashed lines refer to comparisons between ritonavir-boosted protease inhibitor (PI/r)-containing regimens and p-values within the continuous lines refer to comparisons between raltegravir (RAL)-containing regimens at different time points.
The overall incidence of adverse effects was similar in the abacavir/lamivudine (n=33, 61.11%) and the tenofovir/emtricitabine (n=82, 57.34%) groups (p=0.6335). Although no patient discontinued abacavir/lamivudine due to adverse events, four (2.80%) patients (all in the ritonavir-boosted PI group) discontinued tenofovir/emtricitabine because of kidney (progressive decrease in glomerular filtration rate, n=3) or bone (progressive decrease in bone mineral density, n=1) events (p=0.2744).
Discussion
This analysis of the SPIRAL trial suggests that abacavir/lamivudine may display similar efficacy and be as well tolerated as tenofovir/emtricitabine when combined with raltegravir in virologically suppressed HIV-infected adults. Prior comparisons between both fixed-dose NRTI combinations in virologically suppressed HIV-infected adults have also shown similar results in the BICOMBO and STEAL trials,13,14 although in the BICOMBO trial there were more discontinuations with abacavir/lamivudine due to hypersensitivity because patients had not been previously tested for HLA-B5701. In the SPIRAL study, patients taking abacavir/lamivudine or tenofovir/emtricitabine had been taking these combinations for months or years and therefore they were already able to tolerate them.
Approximately 40% of treatment failures in the abacavir/lamivudine and tenofovir/emtricitabine groups in the SPIRAL trial were due to virological failure. There are no available data on the use of raltegravir combined with tenofovir/emtricitabine or abacavir/lamivudine in other than antiretroviral-naive patients. In combination with tenofovir/emtricitabine, raltegravir (n=281) demonstrated no inferiority when compared with efavirenz (n=282) in the STARTMRK study (53% of the patients had HIV-1 RNA >100,000 copies/ml). The proportion of patients treated with tenofovir/emtricitabine plus raltegravir showing HIV-1 RNA <50 copies/ml was 86% at 48 weeks, 81% at 96 weeks, and 75% at 156 weeks.15–17 Data with abacavir/lamivudine plus raltegravir are more limited. The SHIELD trial (34% of the patients had HIV-1 RNA >100,000 copies/ml) was a prospective, observational study enrolling 35 antiretroviral-naive patients who initiated abacavir/lamivudine plus raltegravir. The proportion of patients showing HIV-1 RNA <50 copies/ml was 91% at 48 weeks and 77% at 96 weeks.18,19
In accordance with other studies comparing abacavir/lamivudine and tenofovir/emtricitabine, patients taking abacavir/lamivudine showed higher plasma lipids at baseline as compared to patients taking tenofovir/emtricitabine.13,14,20–22 Although differences were not significant at 48 weeks, it may be of interest that through the study decreases in triglycerides, decreases in total-to-HDL cholesterol, and increases in HDL cholesterol tended to be higher when raltegravir (instead of ritonavir-boosted PIs) was combined with abacavir/lamivudine than with tenofovir/emtricitabine. This was also consistent with a significantly lower proportion of patients showing HDL cholesterol <40 mg/dl at 48 weeks when treated with abacavir/lamivudine compared to tenofovir/emtricitabine, and a nonsignificant higher decrease in the total-to-HDL cholesterol ratio at 48 weeks in patients treated with abacavir/lamivudine plus raltegravir relative to patients treated with tenofovir/emtricitabine plus raltegravir.
These data suggest that the improvement in plasma lipids expected when PIs are replaced by raltegravir in virologically suppressed HIV-infected patients should not be worse when the combination of NRTIs used is abacavir/lamivudine than when it is tenofovir/lamivudine. This finding was unexpected and the reason is not clear. Potential explanations might be that baseline lipids with abacavir/lamivudine were already higher that those with tenofovir/emtricitabine when combined with ritonavir-boosted protease inhibitors and/or that the lipid-lowering effect of discontinuing ritonavir-boosted protease inhibitors may be greater with abacavir/lamivudine than with tenofovir/emtricitabine. However, these results should be taken with caution because of the small sample size and the lack of significance at 48 weeks in most lipid changes. Nevertheless, because of the paucity of data concerning the combination of abacavir/lamivudine plus raltegravir and because the design of the SPIRAL study included only antiretroviral-experienced, virologically suppressed HIV-infected patients, it would make sense to accurately investigate the lipid profile in future studies assessing the effects of this antiretroviral combination in other patient settings.
There were no discontinuations of any combination of NRTIs due to adverse events when combined with raltegravir. In patients taking ritonavir-boosted PIs, four patients discontinued tenofovir/emtricitabine due to adverse events as compared to no patient discontinuing abacavir/lamivudine. Although this difference was not significant, the results are not unexpected due to the negative impact of protease inhibitors on tenofovir-related kidney or bone toxicity.23,24
The small sample size of the abacavir-lamivudine group is a limitation of the study. However, the only existing data on the combination abacavir/lamivudine plus raltegravir included 35 antiretroviral-naive patients, and thus the number of patients with this combination of interest was almost double in our study. The NRTI backbone was not the randomized component, although comparisons in outcomes were adjusted for baseline characteristics showing differences between groups. In addition, the analyses of efficacy outcomes may have been affected by the reduced power given to the relatively low frequency of the outcome measures. However, the study has also strengths as there are few data on the combination abacavir/lamivudine plus raltegravir and the data available are restricted to antiretroviral-naive patients.
In summary, this analysis of the SPIRAL trial does not suggest that outcomes of abacavir/lamivudine are worse than those of tenofovir/emtricitabine when combined with raltegravir in virologically suppressed HIV-infected adults.
Acknowledgments
We thank all the patients who participated in the SPIRAL study. We gratefully acknowledge the coordinators and monitors who ensured adequate collection of data and recognize the important contributions of the SPIRAL study investigators who enrolled their patients.
The SPIRAL trial was supported in part by research grants from Merck Sharp & Dohme, and from Red Temática Cooperativa de Investigación en SIDA (RIS G03/173), Ministerio de Sanidad, Política Social e Igualdad, Spain.
E. Martínez and J.M. Gatell designed the study, helped with analyses of data, and drafted the manuscript. P.M. d'Albuquerque and J. Pich helped design the study, interpreted results, and drafted and revised the manuscript. I. Pérez performed statistical analyses and led the interpretation of the results. All authors critically reviewed and subsequently approved the final version.
Author Disclosure Statement
The following authors have received research funding, consultancy fees, or lecture sponsorships, or served on advisory boards: Esteban Martínez: Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb, Ferrer Group, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Theratechnologies, Tibotec, and ViiV Healthcare. José M. Gatell: Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb, Ferrer Group, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Theratechnologies, Tibotec, and ViiV Healthcare.
References
- 1.Vo TT. Ledergerber B. Keiser O. Hirschel B. Furrer H. Battegay M, et al. Durability and outcome of initial antiretroviral treatments received during 2000–2005 by patients in the Swiss HIV Cohort Study. J Infect Dis. 2008;197:1685–1694. doi: 10.1086/588141. [DOI] [PubMed] [Google Scholar]
- 2.Gazzola L. Tincati C. d'Arminio Monforte A. Noninfectious HIV-related comorbidities and HAART toxicities: Choosing alternative antiretroviral strategies. HIV Ther. 2010;5:553–565. [Google Scholar]
- 3.Thompson MA. Aberg JA. Cahn P. Montaner JS. Rizzardini G. Telenti A, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA. 2010;304:321–333. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 4.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults, adolescents. Department of Health and Human Services. Oct 14, 2011. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [May 25;2012 ]. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf
- 5.European AIDS Clinical Society Guidelines: Version 6–October 2011. http://www.europeanaidsclinicalsociety.org/images/stories/EACS-Pdf/eacsguidelines-v6_english.pdf. [May 25;2012 ]. http://www.europeanaidsclinicalsociety.org/images/stories/EACS-Pdf/eacsguidelines-v6_english.pdf
- 6.Panel de expertos de GESIDA y Plan Nacional sobre el Sida: National consensus document by GESIDA/National AIDS Plan on antiretroviral treatment in adults infected by the human immunodeficiency virus (January 2011 update) Enferm Infecc Microbiol Clin. 2011;29 doi: 10.1016/j.eimc.2010.12.004. 209.e1-103. [DOI] [PubMed] [Google Scholar]
- 7.Moyle GJ. Back D. Principles and practice of HIV-protease inhibitor pharmacoenhancement. HIV Med. 2001;2:105–113. doi: 10.1046/j.1468-1293.2001.00063.x. [DOI] [PubMed] [Google Scholar]
- 8.The DAD Study Group: Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 9.Hsu A. Granneman GR. Witt G. Locke C. Denissen J. Molla A, et al. Multiple-dose pharmacokinetics of ritonavir in human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother. 1997;41:898–905. doi: 10.1128/aac.41.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shafran SD. Mashiter LD. Roberts SE. The effect of low-dose ritonavir monotherapy on fasting serum lipid concentrations. HIV Med. 2005;6:421–425. doi: 10.1111/j.1468-1293.2005.00328.x. [DOI] [PubMed] [Google Scholar]
- 11.Martinez E. Larrousse M. Llibre JM. Gutierrez F. Saumoy M. Antela A, et al. Substitution of raltegravir for ritonavir-boosted protease inhibitors in HIV-infected patients: The SPIRAL study. AIDS. 2010;24:1697–1707. doi: 10.1097/QAD.0b013e32833a608a. [DOI] [PubMed] [Google Scholar]
- 12.Newcombe RG. Interval estimation for the difference between independent proportions: Comparison of eleven methods. StatMed. 1998;17:873–890. doi: 10.1002/(sici)1097-0258(19980430)17:8<873::aid-sim779>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 13.Martinez E. Arranz JA. Podzamczer D. Loncá M. Sanz J. Barragán P, et al. A simplification trial switching from nucleoside reverse transcriptase inhibitors to once-daily fixed-dose abacavir/lamivudine or tenofovir/emtricitabine in HIV-1-infected patients with virological suppression. J Acquir Immune Defic Syndr. 2009;51:290–297. doi: 10.1097/QAI.0b013e3181aa12d5. [DOI] [PubMed] [Google Scholar]
- 14.Martin A. Bloch M. Amin J. Baker D. Cooper DA. Emery S, et al. Simplification of antiretroviral therapy with tenofovir-emtricitabine or abacavir-lamivudine: A randomized, 96-week trial. Clin Infect Dis. 2009;49:1591–1601. doi: 10.1086/644769. [DOI] [PubMed] [Google Scholar]
- 15.Lennox JL. DeJesus E. Lazzarin A. Pollard RB. Madruga JV. Berger DS, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: A multicentre, double-blind randomised controlled trial. Lancet. 2009;374:796–806. doi: 10.1016/S0140-6736(09)60918-1. [DOI] [PubMed] [Google Scholar]
- 16.Lennox JL. Dejesus E. Berger DS. Lazzarin A. Pollard RB. Ramalho Madruga JV, et al. Raltegravir versus efavirenz regimens in treatment-naive HIV-1-infected patients: 96-week efficacy, durability, subgroup, safety, and metabolic analyses. J Acquir Immune Defic Syndr. 2010;55:39–48. doi: 10.1097/QAI.0b013e3181da1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rockstroh JK. Lennox JL. Dejesus E. Saag MS. Lazzarin A. Wan H, et al. Long-term treatment with raltegravir or efavirenz combined with tenofovir/emtricitabine for treatment-naive human immunodeficiency virus-1-infected patients: 156-week results from STARTMRK. Clin Infect Dis. 2011;53:807–816. doi: 10.1093/cid/cir510. [DOI] [PubMed] [Google Scholar]
- 18.Young B. Vanig T. Dejesus E. Hawkins T. St Clair M. Yau L, et al. A pilot study of abacavir/lamivudine and raltegravir in antiretroviral-naïve HIV-1-infected patients: 48-week results of the SHIELD trial. HIV Clin Trials. 2010;11:260–269. doi: 10.1310/hct1105-260. [DOI] [PubMed] [Google Scholar]
- 19.Young B. Vanig T. Dejesus E. Hawkins T. St Clair M. Stancil B, et al. 96-week results of a pilot study of abacavir/lamivudine and raltegravir in antiretroviral-naïve HIV-1-infected patients: The SHIELD trial. HIV Clin Trials. 2010;12:228–233. doi: 10.1310/hct1204-228. [DOI] [PubMed] [Google Scholar]
- 20.Smith KY. Patel P. Fine D. Bellos N. Sloan L. Lackey P, et al. Randomized, double-blind, placebo-matched, multicenter trial of abacavir/lamivudine or tenofovir/emtricitabine with lopinavir/ritonavir for initial HIV treatment. AIDS. 2009;23:1547–1556. doi: 10.1097/QAD.0b013e32832cbcc2. [DOI] [PubMed] [Google Scholar]
- 21.Sax PE. Tierney C. Collier AC. Fischl MA. Mollan K. Peeples L, et al. Abacavir-lamivudine versus tenofovir-emtricitabine for initial HIV-1 therapy. N Engl J Med. 2009;361:2230–2240. doi: 10.1056/NEJMoa0906768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Post FA. Moyle GJ. Stellbrink HJ. Domingo P. Podzamczer D. Fisher M, et al. Randomized comparison of renal effects, efficacy, and safety with once-daily abacavir/lamivudine versus tenofovir/emtricitabine, administered with efavirenz, in antiretroviral-naive, HIV-1-infected adults: 48-week results from the ASSERT study. J Acquir Immune Defic Syndr. 2010;55:49–57. doi: 10.1097/QAI.0b013e3181dd911e. [DOI] [PubMed] [Google Scholar]
- 23.Mocroft A. Kirk O. Reiss P. De Wit S. Sedlacek D. Beniowski M, et al. Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. AIDS. 2010;24:1667–1678. doi: 10.1097/QAD.0b013e328339fe53. [DOI] [PubMed] [Google Scholar]
- 24.Bedimo R. Maalouf NM. Zhang S. Drechsler H. Tebas P. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. AIDS. 2012;26:825–831. doi: 10.1097/QAD.0b013e32835192ae. [DOI] [PubMed] [Google Scholar]