Abstract
Development of an effective low-cost anti-acquired immunodeficiency syndrome (AIDS) drugs is needed for treatment of AIDS patients in developing countries. Host cell lipid raft microdomains, which are enriched with cholesterol, glycolipids, ceramide, and gangliosides, are important for human immunodeficiency virus type 1 (HIV-1) entry. Retinoid analogs have been shown to modulate ceramide levels in the cell membrane, while cholera toxin B subunit (CT-B) specifically binds to the ganglioside GM1. In this study, we found that the acyclic retinoid analogs geranylgeranoic acid (GGA) and NIK-333 as well as CT-B efficiently attenuate CXCR4-tropic, but not CCR5-tropic, HIV-1 vector infection. We also found that GGA and NIK-333 suppress CXCR4-tropic HIV-1 infection by attenuating CXCR4 expression. CT-B also attenuated CXCR4-tropic HIV-1 infection, but did not suppress CXCR4 expression. These results suggest a distinct role for lipid raft microdomains in CXCR4- and CCR5-tropic HIV-1 infections and illuminate novel agents for the development of AIDS therapy.
Introduction
Highly active antiretroviral therapy (HAART), which suppresses human immunodeficiency virus type 1 (HIV-1) reverse transcriptase, protease, and integrase, has been found to be an effective treatment against acquired immunodeficiency syndrome (AIDS). In fact, many patients infected with HIV-1 do not progress to AIDS in developed countries due to implementation of HAART. However, HIV-1/AIDS continues to be a serious problem, as many HIV-1-infected patients in developing countries do not have access to effective anti-HIV-1 drugs due to the prohibitive cost of the therapy, and thus, the numbers of HIV-1-infected patients are increasing worldwide. In addition, HIV-1 variants resistant to current drugs have appeared.1 To resolve these problems, novel, low-cost drugs that inhibit HIV-1 infection are critical.
Lipid raft microdomains of target cell membranes are required for HIV-1 infection.2–6 Lipid rafts are enriched with cholesterol, glycolipids, and ceramide.7 Extraction of cholesterol from cell membranes,4,6 binding of cholesterol with various factors,2,8 and inhibition of biosynthesis of cholesterol9,10 or glycolipids11–13 suppress HIV-1 infection, suggesting that cholesterol and glycolipids may be targets for novel anti-HIV-1 drugs. In this study, we examined the effects of lipid raft-associated factors, which were isolated from natural products, on HIV-1 vector infection.
Retinoic acid and its analogs modulate ceramide levels in cell membranes.14–20 Retinoid analogs may inhibit HIV-1 infection by altering ceramide levels of the target cell membrane. In fact, an all-trans retinoic acid21 and a weak nuclear retinoid receptor agonist, N-(4-hydroxyphenyl) retinamide (4-HPR),22 inhibit HIV-1 infection19, 23; however, because 4-HPR has severe toxicities, such as induction of vitamin A deficiency symptoms, clinical application of 4-HPR is restricted.24 Geranylgeranoic acid (GGA), which is a natural acyclic retinoid analog present in medicinal herbs,25 serves as a weak agonist for retinoid receptors, similar to 4-HPR.26, 27 NIK-333, which is an artificial acyclic retinoid analog with a structure similar to GGA (Fig. 1), prevents recurrence of hepatocellular carcinoma following oral administration without any obvious side effects in clinical studies of liver cancer patients.28,29 We analyzed the effects of the acyclic retinoid analogs GGA and NIK-333 on HIV-1 vector infection.
FIG. 1.
Chemical structures of 4-HPR, GGA, and NIK-333.
Cholesterol is enriched in lipid raft microdomains and requires their structural maintenance. Extraction of cholesterol from cell membranes by methyl-β-cyclodextrin (MβCD),4, 6 inhibition of cholesterol synthesis by statin,9,10 or binding of amphotericin B methyl ester to cholesterol8 suppresses HIV-1 infection. Plant sterols are cholesterol analogs that reduce serum cholesterol levels by replacing cholesterol.30 Therefore, plant sterols may function as anti-HIV-1 agents.
Because cholera toxin B subunit (CT-B) specifically binds to the ganglioside GM1, this subunit is frequently used as a lipid raft marker.4,6 The cytopathic determinant of cholera toxin is subunit A, which has the poly(ADP) ribosylation activity of G-proteins.31 In contrast, the B subunit has no cytopathic effect. GM1 is enriched in raft microdomains and has been reported to bind HIV-1 envelope (Env) glycoprotein.32 Additionally, CD4-positive lymphocytes that have elevated levels of another gangliosides, GM3, are highly susceptible to HIV-1 fusion and entry.11 Therefore, CT-B may inhibit HIV-1 infection without cytopathic effects.
In this study, we examined the effects of these raft-associated factors on HIV-1 vector infection. Our results showed that acyclic retinoid analogs and CT-B efficiently suppressed CXCR4-tropic HIV-1 vector infection, providing novel strategies for the development of CT-B or acyclic retinoid analog treatment for AIDS patients. In contrast, these factors did not affect CCR5-tropic HIV-1 vector infection, suggesting that raft microdomains are involved differently in CXCR4- and CCR5-tropic HIV-1 infections.
Materials and Methods
Cells
COS7, 293T, NP2, TE671, and HeLa cells were cultured in Dulbecco's modified Eagle's medium (D-MEM) (Wako) supplemented with 8% fetal bovine serum (Biosource) at 37°C in 5% CO2. NP2 cells expressing CD4 and CXCR4 (NP2/CD4/X4) or CD4 and CCR5 (NP2/CD4/R5) were kindly provided by Dr. H. Hoshino.33 NP2 cells expressing CD4 and C-terminally HA-tagged CXCR4 (NP2/CD4/X4-HA) were constructed as previously reported.34 TE671, HeLa, and 293T cells expressing CD4 (TE671/CD4, HeLa/CD4, and 293T/CD4) were constructed with a CD4-encoding murine leukemia virus (MLV) vector as previously reported.35 MAGIC5 cells, which are derived from HeLa cells, express CD4 and CCR5 and contain the β-galactosidase (β-Gal) gene under control of the HIV-1 long terminal repeat.36
Expression plasmids
CXCR4-tropic HXB2 and CCR5-tropic JRFL HIV-1 Env expression plasmids were kindly provided by Dr. Y. Yokomaku (National Hospital Organization Nagoya Medical Center). A VSV-G expression plasmid and expression plasmids required for LacZ reporter gene-containing HIV-1 vector construction were obtained from Invitrogen. An expression plasmid encoding C-terminally HA-tagged CXCR4 was constructed as already reported.34
Transduction assay
To obtain HIV-1 vector particles, COS7 cells were transfected with the HIV-1 vector construction plasmids using Fugene transfection reagent (Roche). The transfected cells were washed with D-MEM medium 24 h after transfection and maintained in fresh medium for 24 h. Target cells were either left untreated or pretreated with the retinoid analogs 4-HPR (Sigma-Aldrich), GGA, or NIK-333 for 2 days or with CT-B (Sigma-Aldrich) or stigmasterol (Sigma-Aldrich) for 1 day. GGA and NIK-333 were synthesized by Kowa Company, Ltd. (Tokyo, Japan). The cells were inoculated with culture supernatants from the transfected COS7 cells and then stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (Nacalai) 2 days after inoculation. Blue cells were counted to estimate transduction titer. Approximately 104, 104, and 106 infected cells were detected among cells inoculated by the HXB2 Env-, JRFL Env-, and VSV-G-containing vectors, respectively. To normalize transduction titers, the VSV-G vector was diluted 100 times with medium.
Flow cytometry
To analyze cell surface CD4 expression, suspended cells were either left untreated or treated with an anti-CD4 antibody conjugated with FITC (Sigma-Aldrich). Cell surface expression of CXCR4 or CCR5 was analyzed in suspended cells treated with rat anti-CXCR4 (A80) or anti-CCR5 (T312) monoclonal antibody.37 As a control, cells were treated with a rat serum. The cells were then washed three times with phosphate-buffered saline (PBS) and treated with an FITC-conjugated anti-rat IgG antibody (Sigma-Aldrich). The stained cells were quantified using a flow cytometer (BD Biosciences).
Western immunoblotting
NP2/CD4/X4-HA cells were treated with the retinoid analogs, and cell lysates were prepared. The cell lysates were subjected to SDS polyacrylamide gel electrophoresis (Bio-Rad) and transferred onto a PVDF membrane (Millipore). The membrane was treated with a mouse anti-HA monoclonal antibody (Covance), and then with an HRP-conjugated anti-mouse IgG antibody (Bio-Rad).
Vector particle binding to target cells
Target cells were incubated with culture supernatants from the HIV-1 vector-producing cells for 1 h at 4°C. The cells were washed three times with PBS, and cell lysates were prepared. HIV-1 Gag p24 levels were measured with a p24 enzyme-linked immunosorbent assay (ELISA) (ZeptoMetrix) to estimate the numbers of HIV-1 vector particles bound to the target cells.
Cell fusion assay
The 293T cells were transfected with the HXB2 Env expression plasmid, which also encodes the Tat protein. As a control, 293T cells were transfected with a Tat expression plasmid. The transfected cells were cultured with MAGIC5 cells 24 h after transfection, and cell lysates were prepared from the cells 24 h after the mixed culture. Upon cell fusion, the Tat protein induced β-Gal expression. β-Gal activity in the cell lysates was measured to estimate cell fusion capability.
Statistical analysis
Differences between two groups were determined by the Student's t-test. The difference was considered statistically significant if the p-value was <0.05 for all tests.
Results
Acyclic retinoid analogs and CT-B inhibit CXCR4-tropic HIV-1 vector infection
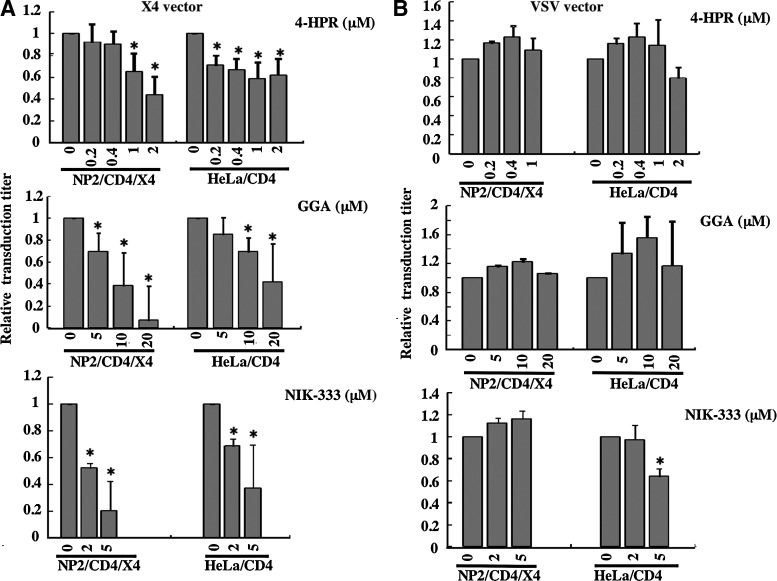
To assess whether retinoid analogs inhibit HIV-1 vector infection, target cells were pretreated with 4-HPR, GGA, or NIK-333 for 2 days. The chemical structures of the analogs are shown in Fig. 1. NP2 cells expressing CD4 and CXCR4 (NP2/CD4/X4), NP2 cells expressing CD4 and CCR5 (NP2/CD4/R5),33 and HeLa cells expressing CD4 (HeLa/CD4)35 were used as target cells. All of the retinoid analogs inhibited infection by a CXCR4-tropic HXB2 Env-carrying HIV-1 vector (Fig. 2A). Previous reports indicated that 4-HPR inhibits HIV-1 infection,23 and this result is consistent with our findings. In addition, cell viability was not affected by the analog treatment under these conditions. These results indicate that the acyclic retinoid analogs GGA and NIK-333 as well as 4-HPR inhibit CXCR4-tropic HIV-1 infection.
FIG. 2.
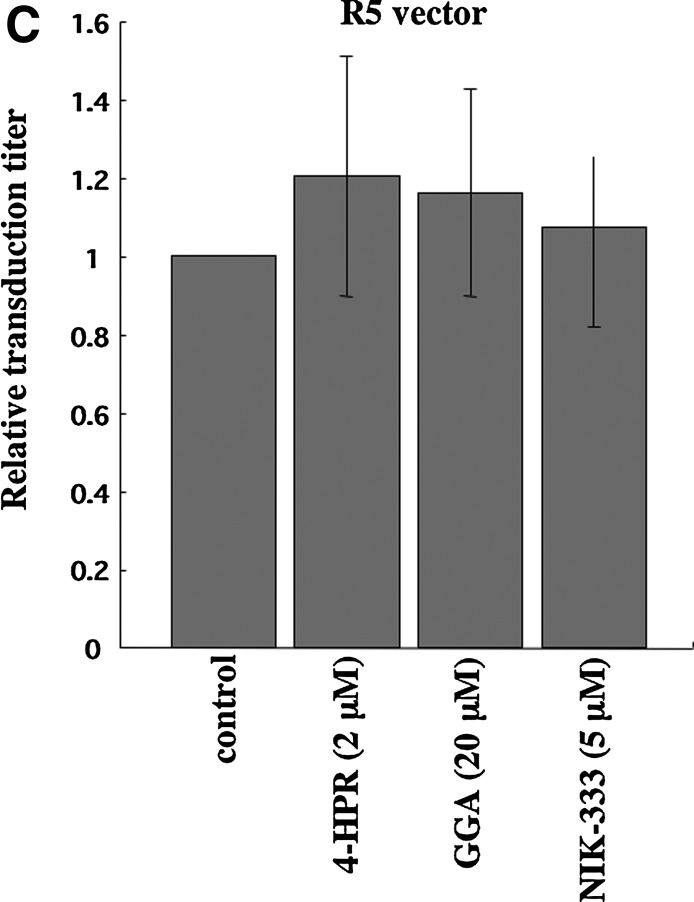
Retinoid analogs inhibit HIV-1 vector infection. Target cells (NP2/CD4/X4, TE671/CD4, and HeLa/CD4 cells) were either left untreated or pretreated with the retinoid analogs, 4-HPR, GGA, and NIK-333, for 2 days. The cells were then inoculated with the HXB2 Env- (A) or VSV-G- (B) pseudotyped HIV-1 vector. NP2/CD4/R5 cells were left untreated (control) or treated with the retinoid analogs for 2 days and then inoculated with the JRFL Env-pseudotyped HIV-1 vector (C). The transduction titers of untreated cells were set to 1. These experiments were repeated in triplicate, and results are shown as the mean+SD. Asterisks indicate statistically significant differences compared to untreated cells.
VSV-G-mediated infection is independent of lipid rafts,4,6 so we assessed whether VSV-G-pseudotyped HIV-1 vector infection is also attenuated by the retinoid analogs. VSV-G-pseudotyped HIV-1 vector infection was not significantly affected by the retinoid analogs (Fig. 2B). Similarly, infection by HIV-1 vector pseudotyped with the Env protein of the CCR5-tropic JRFL strain was not inhibited by the retinoid analogs (Fig. 2C). These results indicate that the retinoid analogs specifically suppress CXCR4-tropic HIV-1 Env-mediated infection but not VSV-G- and CCR5-tropic HIV-1 Env-mediated infection and that the retinoid analogs inhibit CXCR4-tropic HIV-1 infection by a mechanism other than a cytopathic effect.
We next assessed whether CT-B inhibits HIV-1 vector infection. CD4-expressing TE671 (TE671/CD4), HeLa/CD4, NP2/CD4/X4, and NP2/CD4/R5 cells were pretreated with CT-B for 24 h and then inoculated with HXB2 Env- or JRFL Env-bearing HIV-1 vector in the absence of CT-B. CT-B significantly attenuated CXCR4-tropic Env-mediated infection but not VSV-G-pseudotyped HIV-1 vector infection in TE671/CD4 (Fig. 3A), HeLa/CD4 (Fig. 3B), and NP2/CD4/X4 cells (Fig. 3C). However, CT-B did not inhibit CCR5-tropic Env-mediated infection in NP2/CD4/R5 cells (Fig. 3C). If CT-B inhibited cell growth, this toxin should also suppress VSV or CCR5-tropic vector infection; however, CT-B did not affect cell growth as analyzed by microscopic observation. These results indicate that CT-B specifically suppresses CXCR4-tropic HIV-1 infection by a mechanism other than cell growth inhibition.
FIG. 3.
Cholera toxin B (CT-B) inhibits HIV-1 vector infection. TE671/CD4 (A), HeLa/CD4 (B), and NP2/CD4/X4 and NP2/CD4/R5 (C) cells were either left untreated or treated with CT-B for 24 h. The TE671/CD4, HeLa/CD4, and NP2/CD4/X4 cells were inoculated with VSV-G- or HXB2 Env-pseudotyped vector. The NP2/CD4/R5 cells were inoculated with the JRFL Env-pseudotyped vector. The transduction titers in untreated cells were set to 1. These experiments were repeated in triplicate, and results are shown as the mean+SD. Asterisks indicate statistically significant differences compared to untreated cells.
Additionally, we assessed whether a plant sterol, stigmasterol, inhibits HIV-1 vector infection. The target cells were pretreated with stigmasterol (80 μg/ml) for 24 h. The transduction efficiency of the HIV-1 vector was not affected by the treatment (data not shown).
Retinoid analogs inhibit CXCR4 cell surface expression
As the acyclic retinoid analogs inhibited CXCR4-tropic HIV-1 vector infection, we next assessed whether these retinoid analogs suppressed cell surface expression of the HIV-1 infection receptors, CD4, CXCR4, and CCR5. 4-HPR did not affect CD4 cell surface expression in HeLa/CD4 cells (Fig. 4A). GGA and NIK-333 treatment elevated CD4 expression, though the acyclic retinoid analogs inhibited CXCR4-tropic HIV-1 vector infection. In contrast, all of these retinoid analogs reduced cell surface CXCR4 expression. Similar results were observed in NP2/CD4/X4 cells, in which CXCR4 is artificially expressed (data not shown). Furthermore, these retinoid analogs did not affect CCR5 expression (Fig. 4B). These results suggest that the retinoid analogs inhibit CXCR4-tropic HIV-1 infection by suppressing CXCR4 cell surface expression.
FIG. 4.
Retinoid analogs inhibit cell surface expression of CXCR4. Cell surface expression of CD4 and CXCR4 in retinoid analog-treated HeLa/CD4 cells (A), CCR5 in retinoid analog- or CT-B-treated NP2/CD4/R5 cells (B), and CD4 and CXCR4 in CT-B-treated TE671/CD4 cells (D) were analyzed by flow cytometry. Closed and open areas indicate untreated cells stained with control serum or with anti-CD4, -CXCR4, or -CCR5 antibody, respectively. Representative results are shown. Expression of C-terminally HA-tagged CXCR4 was analyzed by Western immunoblotting using an anti-HA antibody (C). As a control, actin expression was also analyzed.
When NP2 cells expressing C-terminally HA-tagged CXCR4 were treated with the retinoid analogs, expression levels of the HA-tagged CXCR4 were not altered, analyzed by Western immunoblotting using an anti-HA antibody (Fig. 4C). This result suggests that the retinoid analogs inhibit the trafficking of CXCR4 to the cell surface, but do not inhibit CXCR4 expression.
CT-B also inhibited CXCR4-tropic HIV-1 vector infection but not CCR5-tropic HIV-1 vector infection; however, CT-B did not affect cell surface expression of CCR5 (Fig. 4B), CXCR4, or CD4 (Fig. 4C). These results indicate that CT-B inhibits CXCR4-tropic infection by a mechanism other than suppression of CXCR4 expression.
Retinoid analogs and CT-B do not affect HIV-1 particle binding to host cells
We analyzed the effects of the retinoid analogs and CT-B on CXCR4-tropic HIV-1 vector particle binding to the target cells by p24 ELISA. The amount of p24 protein bound to CD4-expressing HeLa cells was higher than that bound to CD4-negative HeLa cells, indicating that vector particle binding is CD4-dependent (Fig. 5A). None of the retinoid analogs (Fig. 5B) or CT-B (Fig. 5C) affected HIV-1 vector particle binding to the CD4-expressing target cells. These results show that the retinoid analogs and CT-B inhibit CXCR4-tropic HIV-1 infection by a mechanism other than suppression of CD4-dependent virion binding to target cells.
FIG. 5.
Retinoid analogs and CT-B do not affect HIV-1 vector particle binding to target cells. HeLa/CD4 and HeLa cells were incubated with the HXB2 Env-containing HIV-1 vector particles at 4°C for 1 h and then washed with phosphate buffered saline (PBS) (A). HIV-1 particles bound to the target cells were measured by p24 ELISA. The p24 levels in untreated HeLa/CD4 cells were set to 1. HIV-1 vector particles bound to the retinoid analog-treated NP2/CD4/X4 or HeLa/CD4 cells (B) or to CT-B-treated TE671/CD4 cells (C) were measured. The p24 levels in untreated cells were set to 1. These experiments were repeated in triplicate, and results are shown as the mean+SD.
Retinoid analogs and CT-B inhibit membrane fusion activity of HIV-1 Env protein
To assess whether the retinoid analogs or CT-B inhibit HIV-1 Env-mediated membrane fusion activity, we analyzed the effects of these agents on HIV-1 Env-induced syncytium formation. HEK293T cells transfected with the plasmid encoding the HIV-1 HXB2 Env and Tat proteins were cocultured with MAGIC5 cells for 24 h, and β-galactosidase activity was measured in the cell lysates. The retinoid analogs (Fig. 6A) and CT-B (Fig. 6B) suppressed syncytium formation. Direct inhibition of the HIV-1 Env-mediated membrane fusion reaction by these factors would suppress both CXCR4- and CCR5-tropic HIV-1 infections; however, the factors did not affect CCR5-tropic HIV-1 infection (Fig. 2C). Taken together, these results support the hypothesis that retinoid analogs inhibit CXCR4-tropic HIV-1 infection by attenuating CXCR4 expression, although CT-B may affect the HIV-1 entry process between vector particle binding to target cells and membrane fusion.
FIG. 6.
Retinoid analogs and CT-B inhibit CXCR4-tropic HIV-1 Env-induced syncytium formation. Cell fusion activity of the HXB2 HIV-1 Env protein was measured in untreated and retinoid analog- (A) or CT-B-treated (B) cells (see Materials and Methods). The β-Gal activities in untreated cells were set to 1. These experiments were repeated in triplicate, and results are shown as the mean+SD. Asterisks indicate statistically significant differences compared to untreated cells.
Discussion
HAART has dramatically reduced the mortality and morbidity of HIV-1-infected patients in developed countries. However, due to the high cost of HAART, this therapy is limited in developing countries. In addition, HIV-1 variants that are resistant to HAART have emerged. Therefore, development of novel low-cost drugs that inhibit HIV-1 replication is essential.
In this study, we found that the acyclic retinoid analogs, GGA and NIK-333, suppress CXCR4-tropic HIV-1 vector infection similarly to 4-HPR.23 Additionally, retinoids repress expression of the HIV-1 promoter,38–40 suggesting that retinoid analogs are possible candidates for a novel anti-HIV-1 therapy. Many reports indicate that vitamin A (retinol) supplementation reduces the mortality of HIV-1-infected patients.41–44 NIK333, a synthetic acyclic retinoid, is orally effective against liver cancer without severe side effects.28 GGA also suppresses HIV-1 vector infection and is present in medicinal herbs. These results suggest that oral intake of a natural acyclic retinoid analog may be novel low-cost therapy against AIDS.
We also found that CT-B efficiently suppresses CXCR4-tropic HIV-1 vector infection. Gauthier and Tremblay have shown that CT-B does not inhibit HIV-1 infection, although the concentration of CT-B (10 ng/ml) used in their study was too low to inhibit HIV-1 infection.31 Similar to our results with CT-B, pertussis toxin B subunit also inhibits HIV-1 infection.45–47 Although the receptor for pertussis toxin B oligomer has not yet been identified, the receptor appears to belong to a class of sialylated glycoproteins, with likely candidates being a 43-kDa protein48 and CD11b/CD18 integrin.49 Because the CT-B receptor GM1 is not the pertussis toxin B subunit receptor, the mechanisms by which these bacterial toxin B subunits inhibit HIV-1 infection appear to be different.
One route of HIV-1 transmission is through anal sex. As such, if gut bacteria that secrete nontoxic CT-B are present in the rectum, HIV-1 transmission through this route may be suppressed. Gut bacteria genetically engineered to express CT-B may be a useful novel low-cost strategy to prevent HIV-1 transmission through anal sex.
Use of these factors in vivo, however, should be approached cautiously. First, our study suggests that the acyclic retinoid analogs modulate cell surface expression of CD4 and CXCR4. Second, CT-B is used as an adjuvant for vaccination.50,51 Therefore, these agents may induce unexpected effects in vivo via activation or perturbation of the human immune system. Further study is required to address this issue.
Other retinoid analogs have been reported to reduce cell surface expression of CXCR4,52,53 similar to the retinoid analogs used in this study. This down-regulation of CXCR4 expression is one of the mechanisms by which retinoid analogs inhibit CXCR4-tropic HIV-1 infection. HIV-1 infection is suppressed and influenza virus infection is elevated by 4-HPR through activation of endocytosis23 without suppression of CXCR4 expression. In this study, VSV-G-mediated infection, which occurs via the endosomes, was not affected by the retinoid analogs. Further study is needed to understand the mechanism of HIV-1 infection inhibition by the retinoid analogs.
The retinoid analogs inhibited CXCR4 expression, while CT-B did not, suggesting that the mechanism of HIV-1 infection inhibition by CT-B differs from that by the retinoid analogs. Interestingly, CT-B inhibited CXCR4-tropic HIV-1 infection but not CCR5-tropic infection. Thus, CT-B may inhibit CXCR4-tropic HIV-1 entry at some point between virion binding to host cells and membrane fusion. CD4 and CCR5, but not CXCR4,4–6 localize to lipid raft microdomains and constitutively interact.54,55 It has been reported that CCR5-tropic HIV-1 infection is not dependent upon raft localization of CD4 and CCR5.56 These results, together with our findings, suggest that CT-B inhibits the HIV-1 Env-induced interaction of CD4 and CXCR4 in lipid rafts and that raft microdomains are differentially involved in CXCR4- and CCR5-tropic HIV-1 infections. CT-B may have no effect on CCR5-tropic HIV-1 infection, because CD4 and CCR5 constitutively interact without binding HIV-1 Env. Recruitment of CXCR4 to CD4-containing raft microdomains by HIV-1 Env, however, has been observed in studies using CT-B as the raft marker.5,6 Further study is required to understand the mechanism by which CT-B inhibits HIV-1 infection.
The plant sterol stigmasterol did not suppress HIV-1 vector infection. Our group previously reported that MβCD inhibits HIV-1 vector infection and that the addition of cholesterol to the MβCD-treated cells at 50 μg/ml for 30 min recovers infection, suggesting that cholesterol is incorporated into the cell membrane by the addition of cholesterol.4 Therefore, treatment of cells with stigmasterol at 80 μg/ml for 24 h likely induces uptake of the plant sterol to the cell membrane. These results indicate that this plant sterol does not affect HIV-1 infection. Similar to mammalian cells, plant cells also have lipid raft microdomains in their membranes,57 and these raft domains are enriched with plant sterols. Therefore, even upon replacement of cholesterol with stigmasterol in mammalian cells, the lipid raft structure is maintained, and HIV-1 infection remains unaffected.
In summary, the acyclic retinoid analogs, GGA and NIK-333, as well as CT-B, efficiently suppress HIV-1 vector infection. Another retinoid analog, 4-HPR, inhibits HIV-1 infection23 but induces a vitamin A-deficiency syndrome. In contrast, NIK-333 induces no clinical side effects in patients with liver cancer.28,29 This study suggests that NIK-333 can be used as a novel anti-HIV-1 agent without severe side effects. Additionally, CT-B inhibits CXCR4-tropic, but not CCR5-tropic.
HIV-1 infection, suggesting that host cell lipid raft microdomains are differentially involved in CXCR4- and CCR5-tropic HIV-1 infections.
Acknowledgments
We thank Dr. Y. Yokomaku for the HXB2 and JRFL Env expression plasmids and Dr. H. Hoshino for the NP2/CD4/X4 and NP2/CD4/R5 cells. We also thank Ms. Y. Kobayashi and Ms. F. Tsujita for assistance with laboratory work. This study was supported by the Japan Society for the Promotion of Science (JSPS) (No. 09J07637), a Health Science Research Grant from the Ministry of Health, Labor, and Welfare of Japan, and Kowa Company, Ltd. H. Kamiyama is a special research fellow of JSPS.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Kolber MA. Development of drug resistance mutations in patients on highly active antiretroviral therapy: Does competitive advantage drive evolution. AIDS Rev. 2007;9:68–74. [PubMed] [Google Scholar]
- 2.Carter GC. Bernstone L. Sangani D. Bee JW. Harder T. James W. HIV entry in macrophages is dependent on intact lipid rafts. Virology. 2009;386:192–202. doi: 10.1016/j.virol.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Real G. Jimenez-Baranda S. Lacalle RA, et al. Blocking of HIV-1 infection by targeting CD4 to nonraft membrane domains. J Exp Med. 2002;196:293–301. doi: 10.1084/jem.20020308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamiyama H. Yoshii H. Tanaka Y. Sato H. Yamamoto N. Kubo Y. Raft localization of CXCR4 is primarily required for X4-tropic human immunodeficiency virus type 1 infection. Virology. 2009;386:23–31. doi: 10.1016/j.virol.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 5.Manes S. del Real G. Lacalle RA, et al. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep. 2000;1:190–196. doi: 10.1093/embo-reports/kvd025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Popik W. Alce TM. Au WC. Human immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for productive entry into CD4(+) T cells. J Virol. 2002;76:4709–4722. doi: 10.1128/JVI.76.10.4709-4722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michel V. Bakovic M. Lipid rafts in health and disease. Biol Cell. 2007;99:129–140. doi: 10.1042/BC20060051. [DOI] [PubMed] [Google Scholar]
- 8.Waheed AA. Ablan SD. Mankowski MK, et al. Inhibition of HIV-1 replication by amphotericin B methyl ester: Selection for resistant variants. J Biol Chem. 2006;281:28699–28711. doi: 10.1074/jbc.M603609200. [DOI] [PubMed] [Google Scholar]
- 9.Giguere JF. Tremblay MJ. Statin compounds reduce human immunodeficiency virus type 1 replication by preventing the interaction between virion-associated host intercellular adhesion molecule 1 and its natural cell surface ligand LFA-1. J Virol. 2004;78:12062–12065. doi: 10.1128/JVI.78.21.12062-12065.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.del Real G. Jimenez-Baranda S. Mira E, et al. Statins inhibit HIV-1 infection by down-regulating Rho activity. J Exp Med. 2004;200:541–547. doi: 10.1084/jem.20040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puri A. Rawat SS. Lin HM, et al. An inhibitor of glycosphingolipid metabolism blocks HIV-1 infection of primary T-cells. AIDS. 2004;18:849–858. doi: 10.1097/00002030-200404090-00002. [DOI] [PubMed] [Google Scholar]
- 12.Mizrachi Y. Lev M. Harish Z. Sundaram SK. Rubinstein A. L-Cycloserine, an inhibitor of sphingolipid biosynthesis, inhibits HIV-1 cytopathic effects, replication, and infectivity. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;11:137–141. doi: 10.1097/00042560-199602010-00004. [DOI] [PubMed] [Google Scholar]
- 13.Hug P. Lin HM. Korte T, et al. Glycosphingolipids promote entry of a broad range of human immunodeficiency virus type 1 isolates into cell lines expressing CD4, CXCR4, and/or CCR5. J Virol. 2000;74:6377–6385. doi: 10.1128/jvi.74.14.6377-6385.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erdreich-Epstein A. Tran LB. Bowman NN, et al. Ceramide signaling in fenretinide-induced endothelial cell apoptosis. J Biol Chem. 2002;277:49531–49537. doi: 10.1074/jbc.M209962200. [DOI] [PubMed] [Google Scholar]
- 15.Maurer BJ. Metelitsa LS. Seeger RC. Cabot MC. Reynolds CP. Increase of ceramide and induction of mixed apoptosis/necrosis by N-(4-hydroxyphenyl)-retinamide in neuroblastoma cell lines. J Natl Cancer Inst. 1999;91:1138–1146. doi: 10.1093/jnci/91.13.1138. [DOI] [PubMed] [Google Scholar]
- 16.O'Donnell PH. Guo WX. Reynolds CP. Maurer BJ. N-(4-Hydroxyphenyl)retinamide increases ceramide and is cytotoxic to acute lymphoblastic leukemia cell lines, but not to non-malignant lymphocytes. Leukemia. 2002;16:902–910. doi: 10.1038/sj.leu.2402485. [DOI] [PubMed] [Google Scholar]
- 17.Wang H. Maurer BJ. Reynolds CP. Cabot MC. N-(4-Hydroxyphenyl)retinamide elevates ceramide in neuroblastoma cell lines by coordinate activation of serine palmitoyltransferase and ceramide synthase. Cancer Res. 2001;61:5102–5105. [PubMed] [Google Scholar]
- 18.Wiegandt H. Helland R. Radsak K. Retinoic acid alters the metabolic 3H-labelling of glycosphingolipids. Biochem Biophys Res Commun. 1987;143:525–531. doi: 10.1016/0006-291x(87)91385-4. [DOI] [PubMed] [Google Scholar]
- 19.Finnegan CM. Rawat SS. Puri A. Wang JM. Ruscetti FW. Blumenthal R. Ceramide, a target for antiretroviral therapy. Proc Natl Acad Sci USA. 2004;101:15452–15457. doi: 10.1073/pnas.0402874101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke CJ. Mediwala K. Jenkins RW. Sutton CA. Tholanikunnel BG. Hannun YA. Neutral sphingomyelinase-2 mediates growth arrest by retinoic acid through modulation of ribosomal S6 kinase. J Biol Chem. 2011;286:21565–21576. doi: 10.1074/jbc.M110.193375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakashima H. Harada S. Yamamoto N. Effect of retinoic acid on the replication of human immunodeficiency virus in HTLV-I-positive MT-4 cells. Med Microbiol Immunol. 1987;176:189–198. doi: 10.1007/BF00196686. [DOI] [PubMed] [Google Scholar]
- 22.Anding AL. Chapman JS. Barnett DW. Curley RW., Jr Clagett-Dame M. The unhydrolyzable fenretinide analogue 4-hydroxybenzylretinone induces the proapoptotic genes GADD153 (CHOP) and Bcl-2-binding component 3 (PUMA) and apoptosis that is caspase- dependent and independent of the retinoic acid receptor. Cancer Res. 2007;67:6270–6277. doi: 10.1158/0008-5472.CAN-07-0727. [DOI] [PubMed] [Google Scholar]
- 23.Finnegan CM. Blumenthal R. Fenretinide inhibits HIV infection by promoting viral endocytosis. Antiviral Res. 2006;69:116–123. doi: 10.1016/j.antiviral.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Sani BP. Meeks RG. Subacute toxicity of all-trans- and 13-cis-isomers of N-ethyl retinamide, N-2-hydroxyethyl retinamide, and N-4-hydroxyphenyl retinamide. Toxicol Appl Pharmacol. 1983;70:228–235. doi: 10.1016/0041-008x(83)90098-4. [DOI] [PubMed] [Google Scholar]
- 25.Shidoji Y. Ogawa H. Natural occurrence of cancer-preventive geranylgeranoic acid in medicinal herbs. J Lipid Res. 2004;45:1092–1103. doi: 10.1194/jlr.M300502-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Araki H. Shidoji Y. Yamada Y. Moriwaki H. Muto Y. Retinoid agonist activities of synthetic geranyl geranoic acid derivatives. Biochem Biophys Res Commun. 1995;209:66–72. doi: 10.1006/bbrc.1995.1471. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto K. Sakimoto Y. Imai K. Senoo H. Shidoji Y. Induction of an incomplete autophagic response by cancer-preventive geranylgeranoic acid (GGA) in a human hepatoma-derived cell line. Biochem J. 2011;440:63–71. doi: 10.1042/BJ20110610. [DOI] [PubMed] [Google Scholar]
- 28.Muto Y. Moriwaki H. Ninomiya M, et al. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. Hepatoma Prevention Study Group. N Engl J Med. 1996;334:1561–1567. doi: 10.1056/NEJM199606133342402. [DOI] [PubMed] [Google Scholar]
- 29.Okusaka T. Ueno H. Ikeda M. Morizane C. Phase I and pharmacokinetic clinical trial of oral administration of the acyclic retinoid NIK-333. Hepatol Res. 2011;41:542–552. doi: 10.1111/j.1872-034X.2011.00800.x. [DOI] [PubMed] [Google Scholar]
- 30.Baumgartner S. Mensink RP. Plat J. Plant sterols and stanols in the treatment of dyslipidemia: New insights into targets and mechanisms related to cardiovascular risk. Curr Pharm Des. 2011;17:922–932. doi: 10.2174/138161211795428795. [DOI] [PubMed] [Google Scholar]
- 31.Gauthier S. Tremblay MJ. Cholera toxin inhibits HIV-1 replication in human colorectal epithelial HT-29 cells through adenylate cyclase activation. Antiviral Res. 2011;88:207–216. doi: 10.1016/j.antiviral.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 32.Hammache D. Pieroni G. Yahi N, et al. Specific interaction of HIV-1 and HIV-2 surface envelope glycoproteins with monolayers of galactosylceramide and ganglioside GM3. J Biol Chem. 1998;273:7967–7971. doi: 10.1074/jbc.273.14.7967. [DOI] [PubMed] [Google Scholar]
- 33.Soda Y. Shimizu N. Jinno A, et al. Establishment of a new system for determination of coreceptor usages of HIV based on the human glioma NP-2 cell line. Biochem Biophys Res Commun. 1999;258:313–321. doi: 10.1006/bbrc.1999.0633. [DOI] [PubMed] [Google Scholar]
- 34.Kubo Y. Yokoyama M. Yoshii H, et al. Inhibitory role of CXCR4 glycan in CD4-independent X4-tropic human immunodeficiency virus type 1 infection and its abrogation in CD4-dependent infection. J Gen Virol. 2007;88:3139–3144. doi: 10.1099/vir.0.83202-0. [DOI] [PubMed] [Google Scholar]
- 35.Kubo Y. Yoshii H. Kamiyama H, et al. Ezrin, Radixin, and Moesin (ERM) proteins function as pleiotropic regulators of human immunodeficiency virus type 1 infection. Virology. 2008;375:130–140. doi: 10.1016/j.virol.2008.01.047. [DOI] [PubMed] [Google Scholar]
- 36.Tobiume M. Takahoko M. Tatsumi M. Matsuda M. Establishment of a MAGI-derived indicator cell line that detects the Nef enhancement of HIV-1 infectivity with high sensitivity. J Virol Methods. 2001;97:151–158. doi: 10.1016/s0166-0934(01)00349-4. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka R. Yoshida A. Murakami T, et al. Unique monoclonal antibody recognizing the third extracellular loop of CXCR4 induces lymphocyte agglutination and enhances human immunodeficiency virus type 1-mediated syncytium formation and productive infection. J Virol. 2001;75:11534–11543. doi: 10.1128/JVI.75.23.11534-11543.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maciaszek JW. Coniglio SJ. Talmage DA. Viglianti GA. Retinoid-induced repression of human immunodeficiency virus type 1 core promoter activity inhibits virus replication. J Virol. 1998;72:5862–5869. doi: 10.1128/jvi.72.7.5862-5869.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanley TM. Kiefer HL. Schnitzler AC. Marcello JE. Viglianti GA. Retinoid-dependent restriction of human immunodeficiency virus type 1 replication in monocytes/macrophages. J Virol. 2004;78:2819–2830. doi: 10.1128/JVI.78.6.2819-2830.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiefer HL. Hanley TM. Marcello JE. Karthik AG. Viglianti GA. Retinoic acid inhibition of chromatin remodeling at the human immunodeficiency virus type 1 promoter. Uncoupling of histone acetylation and chromatin remodeling. J Biol Chem. 2004;279:43604–43613. doi: 10.1074/jbc.M408069200. [DOI] [PubMed] [Google Scholar]
- 41.Chatterjee A. Bosch RJ. Hunter DJ. Manji K. Msamanga GI. Fawzi WW. Vitamin A and vitamin B-12 concentrations in relation to mortality and morbidity among children born to HIV-infected women. J Trop Pediatr. 2011;56:27–35. doi: 10.1093/tropej/fmp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Humphrey JH. Iliff PJ. Marinda ET, et al. Effects of a single large dose of vitamin A, given during the postpartum period to HIV-positive women and their infants, on child HIV infection, HIV-free survival, and mortality. J Infect Dis. 2006;193:860–871. doi: 10.1086/500366. [DOI] [PubMed] [Google Scholar]
- 43.Mehta S. Fawzi W. Effects of vitamins, including vitamin A, on HIV/AIDS patients. Vitam Horm. 2007;75:355–383. doi: 10.1016/S0083-6729(06)75013-0. [DOI] [PubMed] [Google Scholar]
- 44.Semba RD. Ndugwa C. Perry RT, et al. Effect of periodic vitamin A supplementation on mortality and morbidity of human immunodeficiency virus-infected children in Uganda: A controlled clinical trial. Nutrition. 2005;21:25–31. doi: 10.1016/j.nut.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Iordanskiy S. Iordanskaya T. Quivy V. Van Lint C. Bukrinsky M. B-oligomer of pertussis toxin inhibits HIV-1 LTR-driven transcription through suppression of NF-kappaB p65 subunit activity. Virology. 2002;302:195–206. doi: 10.1006/viro.2002.1618. [DOI] [PubMed] [Google Scholar]
- 46.Alfano M. Schmidtmayerova H. Amella CA. Pushkarsky T. Bukrinsky M. The B-oligomer of pertussis toxin deactivates CC chemokine receptor 5 and blocks entry of M-tropic HIV-1 strains. J Exp Med. 1999;190:597–605. doi: 10.1084/jem.190.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alfano M. Pushkarsky T. Poli G. Bukrinsky M. The B-oligomer of pertussis toxin inhibits human immunodeficiency virus type 1 replication at multiple stages. J Virol. 2000;74:8767–8770. doi: 10.1128/jvi.74.18.8767-8770.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogers TS. Corey SJ. Rosoff PM. Identification of a 43-kilodalton human T lymphocyte membrane protein as a receptor for pertussis toxin. J Immunol. 1990;145:678–683. [PubMed] [Google Scholar]
- 49.Wong WS. Simon DI. Rosoff PM. Rao NK. Chapman HA. Mechanisms of pertussis toxin-induced myelomonocytic cell adhesion: Role of Mac-1(CD11b/CD18) and urokinase receptor (CD87) Immunology. 1996;88:90–97. doi: 10.1046/j.1365-2567.1996.d01-646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang SM. Yao Q. Guo L. Compans RW. Mucosal immunization with virus-like particles of simian immunodeficiency virus conjugated with cholera toxin subunit B. J Virol. 2003;77:9823–9830. doi: 10.1128/JVI.77.18.9823-9830.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun JB. Czerkinsky C. Holmgren J. Mucosally induced immunological tolerance, regulatory T cells and the adjuvant effect by cholera toxin B subunit. Scand J Immunol. 2011;71:1–11. doi: 10.1111/j.1365-3083.2009.02321.x. [DOI] [PubMed] [Google Scholar]
- 52.Villablanca EJ. Zhou D. Valentinis B, et al. Selected natural and synthetic retinoids impair CCR7- and CXCR4-dependent cell migration in vitro and in vivo. J Leukoc Biol. 2008;84:871–879. doi: 10.1189/jlb.0108047. [DOI] [PubMed] [Google Scholar]
- 53.Matsumoto T. Jimi S. Hara S. Takamatsu Y. Suzumiya J. Tamura K. Am80 inhibits stromal cell-derived factor-1-induced chemotaxis in T-cell acute lymphoblastic leukemia cells. Leuk Lymphoma. 2011;51:507–514. doi: 10.3109/10428190903560180. [DOI] [PubMed] [Google Scholar]
- 54.Baker AM. Sauliere A. Gaibelet G, et al. CD4 interacts constitutively with multiple CCR5 at the plasma membrane of living cells. A fluorescence recovery after photobleaching at variable radii approach. J Biol Chem. 2007;282:35163–35168. doi: 10.1074/jbc.M705617200. [DOI] [PubMed] [Google Scholar]
- 55.Gaibelet G. Planchenault T. Mazeres S, et al. CD4 and CCR5 constitutively interact at the plasma membrane of living cells: A confocal fluorescence resonance energy transfer-based approach. J Biol Chem. 2006;281:37921–37929. doi: 10.1074/jbc.M607103200. [DOI] [PubMed] [Google Scholar]
- 56.Percherancier Y. Lagane B. Planchenault T, et al. HIV-1 entry into T-cells is not dependent on CD4 and CCR5 localization to sphingolipid-enriched, detergent-resistant, raft membrane domains. J Biol Chem. 2003;278:3153–3161. doi: 10.1074/jbc.M207371200. [DOI] [PubMed] [Google Scholar]
- 57.Lefebvre B. Furt F. Hartmann MA, et al. Characterization of lipid rafts from Medicago truncatula root plasma membranes: a proteomic study reveals the presence of a raft-associated redox system. Plant Physiol. 2007;144:402–418. doi: 10.1104/pp.106.094102. [DOI] [PMC free article] [PubMed] [Google Scholar]