Abstract
The effects of tuberculosis (TB) on the kinetics of CD4+ T cells among HIV-infected individuals with early combination antiretroviral therapy (cART) after TB therapy initiation are poorly characterized. We conducted a case-control study with 15 HIV-TB-coinfected patients who initiated TB treatment and early cART, and 30 controls without TB who had similar CD4+ T cell counts and viral loads at the time of starting cART. We compared the rate of CD4+ T cell increase for 5 years after cART. The time to CD4+ T cell increase >250 cells/mm3 was significantly slower in HIV-TB-coinfected patients (p=0.015, by log rank test). HIV-TB-coinfected patients had significantly lower median CD4+ T cell counts at 5 years after cART (p=0.048). The difference in CD4+ T cell increase was observed only during the first 6 months after cART initiation (p=0.002). These data suggest that TB slows the rate of CD4+ T cell recovery at an early period after cART. The effects of TB on the long-term immunity of HIV-infected patients should be further evaluated.
Introduction
Tuberculosis (TB) is one of the most common opportunistic diseases, and in developing countries, it is the most common cause of death in patients infected with human immunodeficiency virus (HIV).1 The number of patients coinfected with TB and HIV continues to grow rapidly.2 TB accelerates the progression of HIV infection, with an increased viral load, a fall in CD4+ T cell counts, and increased mortality.3–6 The immunological environment during active TB infection is characterized by cytokine and chemokine irregularities that are believed to increase T cell activation, enhance HIV replication, and result in a dysfunctional immune response.7–11
Some prospective studies have evaluated the optimal time for initiating combination antiretroviral therapy (cART) in HIV/TB-coinfected persons,12–14 and the studies demonstrated a mortality reduction among those starting cART during the early part of TB therapy compared to those who started later or after completion of TB therapy. The current WHO guideline recommends that cART should be initiated as soon as TB therapy is tolerated, ideally as early as 2 weeks and not later than 8 weeks, regardless of CD4+ T cell counts.15
The immunological effects of cART for HIV-infected patients, including reduction in viral load and restoration of CD4+ T cells, are well recognized.7,16,17 During CD4+ T cell recovery, the initial increase is rapid and usually lasts 3–6 months, followed by a phase of slower CD4+ T cell count recovery.18 However, in HIV/TB-coinfected patients, the initial increase inCD4+ T cell count may not be rapid under the influence of TB coinfection.
The effects of TB on the kinetics of CD4+ T cells among HIV-infected patients on early cART are poorly characterized. Especially, little is known about the study on the long-term effect of TB on CD4+ T cell recovery in HIV/TB-coinfected patients with early cART after TB therapy initiation. Therefore, we investigated the effects of TB on the kinetics of CD4+ T cell recovery among HIV/TB-coinfected patients with sustained viral suppression after early cART.
Materials and Methods
Study population
HIV-1-infected patients who continued cART for more than 5 years at a tertiary-care teaching hospital in Seoul, South Korea were included. Patients who were 18 years old or older at treatment initiation, and maintained a sustained suppressed viral load (HIV RNA <400 copies/ml) after 6 months on cART, were eligible for this study. Patients who had any treatment interruptions for more than 1 month were excluded. CD4+ T cell counts were evaluated at least every 6 months (within a window of±1 month). TB was defined as the isolation of Mycobacterium tuberculosis from a clinical specimen, the demonstration of acid-fast bacilli in a clinical specimen, or a histopathological lesion when a culture was not available in a person with signs or symptoms compatible with TB, or evidence of disease resolution when treatment with two or more anti-TB medications was prescribed and the patients were followed-up.
Study design
A retrospective case-control study was conducted. The case group included 15 HIV-TB-coinfected patients. All subjects in the case group started early cART within 8 weeks after the initiation of TB treatment. The regimen of anti-TB treatment was 2 months of isoniazid+rifampicin/rifabutin+ethambutol+pyrazinamide, and then isoniazid+rifampicin/rifabutin+ethambutol. Thirty HIV-infected patients without TB who had similar CD4+ T cell counts (±5 cells/mm3) and viral loads (±0.1 log10 copies/ml) at the time of starting cART were selected as the control group. All enrolled patients were ART naive at the start of cART. We estimated the median time to reach a CD4+ T cell increase greater than 250 cells/mm3 after cART initiation, and the median times of the two groups were compared. The median times to reach a CD4+T cell recovery greater than 250 cells/mm3 after cART initiation in the case and control groups were 18 and 6 months, respectively. Based on these numbers, we could expect to have 0.91 power with enrolled HIV-1-infected patients. We also estimated the rate of CD4+ T cell recovery for every 6 months since the initiation of cART, and compared the rates of the two groups using a linear mixed model. This study was approved by the Institutional Review Board of the hospital (IRB #4-2011-0478).
Study variables
The following variables were assessed: age at the initiation of cART (years); gender; type of tuberculosis; reported route of infection; prior acquired immunodeficiency syndrome (AIDS) diagnosis at cART initiation; hepatitis B or C coinfection; cART regimens [two nucleoside analog reverse transcriptase inhibitors (NRTIs)+nonnucleoside reverse transcriptase inhibitor (NNRTI), two NRTIs+protease inhibitor (PI), or other combination] at cART initiation; CD4+ T cell count (cells/mm3); CD8+ T cell count (cells/mm3); and HIV viral load (log10 copies/ml) at cART initiation.
Statistical analysis
Continuous variables and categorical data were analyzed using independent sample t-tests and chi-square tests, respectively. Kaplan–Meier analysis was used to estimate the time to reach a CD4+ T cell recovery greater than 250 cells/mm3. The rate of CD4+ T cell increase per year was estimated using linear mixed model. All p-values were two-tailed, and a p-value<0.05 was considered statistically significant. All of the statistical analyses were performed using SAS ver. 9.2 (SAS Institute, Inc., Cary, NC). In addition, a post hoc power analysis was performed using PASS ver. 11 (NCSS, LLC, Kaysville, UT).
Results
Characteristics of the study population
There were no significant differences in demographic or clinical characteristics between the two groups (Table 1).
Table 1.
Clinical Characteristics of HIV-Infected Individuals With or Without Tuberculosis
| Variables | Case group (N=15) | Control group (N=30) | p-value |
|---|---|---|---|
| Male gender (%) | 15 (100) | 25 (83.3) | 0.153 |
| Age at starting cART, median years (range) | 43 (35–79) | 45 (35–75) | 0.554 |
| Type of TB | |||
| Pulmonary | 6 (40.0) | ||
| Extrapulmonary | 7 (46.7) | ||
| Mixture | 2 (13.3) | ||
| Mode of transmission (%) | |||
| Homosexual contacts | 5 (33.3) | 11 (36.7) | 1.000 |
| Heterosexual contacts | 3 (20.0) | 8 (26.7) | 0.756 |
| Bisexual contacts | 0 (0.0) | 1 (3.3) | 1.000 |
| Unknown | 6 (40.0) | 8 (26.7) | 0.356 |
| Pre-cART CD4+ T cell count, median (IQR), cells/mm3 | 114 (17–230) | 114 (17–231) | 1.000 |
| Pre-cART VL, median (IQR), log10 copies/ml | 5.36 (4.72–5.69) | 5.31 (3.88–6.30) | 0.921 |
| Initial cART (%) | |||
| PI-based | 5 (33.3) | 16 (53.3) | 0.342 |
| NNRTI-based | 10 (66.7) | 14 (46.7) | 0.342 |
| HBV coinfection at starting cART (%) | 0 (0.0) | 1 (3.3) | 1.000 |
TB, tuberculosis; HIV, human immunodeficiency virus; cART, combination antiretroviral therapy; IQR, interquartile range; VL, viral load; PI, protease inhibitor; NNRTI, nonnucleoside reverse-transcriptase inhibitor; HBV, hepatitis B virus.
The time to reach a CD4+ T cell recovery greater than 250 cells/mm3 between HIV-T -coinfected patients and HIV-monoinfected patients receiving cART therapy
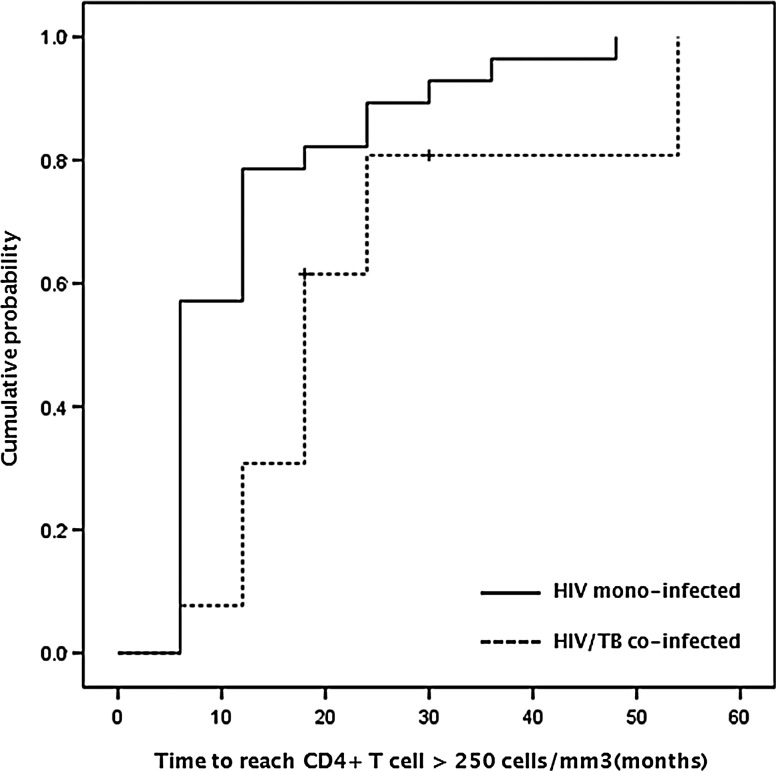
We estimated the median time to reach a CD4+ T cell recovery greater than 250 cells/mm3 after cART initiation, and the median times of the two groups were compared. The case group had a longer median time than the control group (18 and 6 months, respectively), and the Kaplan–Meier curve summarizing the time to a CD4+ T cell increase greater than 250 cells/mm3 indicated that the rate of CD4+ T cell recovery was slower for HIV-TB-coinfected than monoinfected patients (p=0.015, by log rank test) (Fig. 1).
FIG. 1.
Cumulative probability of CD4+ T cell increase >250 cells/mm3 from baseline.
Changes in median CD4+ T cell counts between HIV-TB-coinfected patients and HIV-monoinfected patients receiving cART therapy
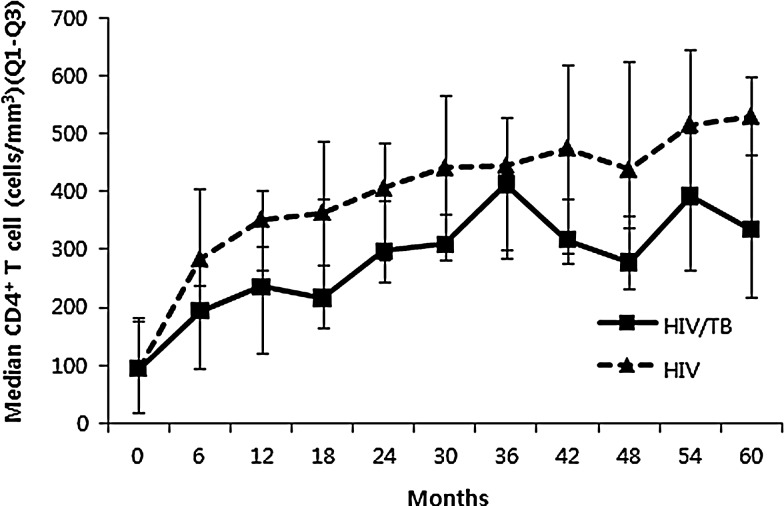
To better evaluate whether the effect of TB on the rate of increasing CD4+ T cell counts was dependent on time after cART, we estimated the rate of CD4+ T cell recovery every 6 months after initiating cART, and compared the median CD4+ T cell counts of the two groups using a linear mixed model. HIV-TB-coinfected patients had significantly lower median CD4+ T cell counts at 5 years after cART initiation (p=0.048). However, the difference in CD4+ T cell recovery was observed only during the first 6 months after cART initiation (p=0.002), and not from 6 months to 5 years (Fig. 2).
FIG. 2.
Changes in median CD4+ T cell counts in HIV-TB-coinfected and HIV-monoinfected patients receiving combination antiretroviral therapy.
Discussion
We showed that HIV-TB-coinfected patients with sustained viral suppression after early cART took a longer time to reach a CD4+ T cell count increase >250 cells/mm3 and had significantly lower median CD4+ T cell counts at 5 years after early cART initiation compared to HIV monoinfected patients. Moreover, coinfected patients had a slower rate of CD4+ T cell recovery, but the difference was observed only during the early period after cART initiation.
Importantly, we showed long-term effects of TB on immune restoration during sustained viral suppression after early cART. Although the current WHO guideline recommends that cART should be initiated as soon as TB therapy is tolerated, ideally as early as 2 weeks and not later than 8 weeks with data favoring early cART,15 there have been few studies of the long-term (>5 years) effects of TB on the kinetics of CD4+ T cell recovery among HIV-infected patients with sustained viral suppression after early cART. A recent study by Hermans and colleagues showed that patients with incident TB within 12 months after cART had significantly lower median CD4+ T cell counts at 2 years compared to patients who were TB free throughout the follow-up period.19 However, it was not clear whether cART initiation was later than 8 weeks after TB therapy.
We also showed a lower rate of CD4+ T cell recovery for HIV-TB-coinfected patients only during the initial period after early cART. Although the underlying mechanisms of immunological nonresponse in HIV-TB coinfection are not understood, one study has suggested that a complex model of immune activation, T cell turnover, and homeostatic regulation is responsible for CD4+ T cell loss.20 In particular, excessive T cell destruction due to T cell activation in HIV-TB-coinfected patients plays an important role, and has been shown to persist even after virological suppression occurs.21–23
Our data suggest that TB in HIV-infected patients influences the redistribution of lymphocytes during the early phase of immune restoration, and the effects of TB on immune restoration are not overt after the treatment of TB. Generally, in HIV-infected patients, CD4+ T cell recovery in response to cART is thought to be biphasic, with an initial rapid increase during the first 3–6 months after cART initiation, due to the redistribution of cells located within the lymph reticular system. This is followed by a slower recovery, thought to be due to the generation of naive CD4+ T cells through cell division or from the thymus.18,24,25 Further study of the effects of TB on the peripheral redistribution of CD4+ T cells and generation of naive CD4+ T cells through cell division or from the thymus after cART initiation is needed.
In our study, NNRTI-based regimens were used more frequently than PI-based regimens as an initial cART for HIV/TB-coinfected patients. This was due to drug interactions between protease inhibitors and rifamycin.26 Thus, the rate of CD4+ T cell recovery might have been influenced by this difference in initial cART regimens. However, several studies have reported that initial treatment with either an NNRTI-based regimen resulted in similar immunological outcomes in long-term antiretroviral management in treatment-naive patients.27–29 Additionally, the mean duration of TB treatment was 11±3.3 (mean±standard deviation) months in this study. TB treatment might influence the slow CD4+ T cell recovery phase.
Our study has several limitations. First, the sample of HIV-TB-coinfected patients was collected from a single center. Second, the sample size was small. Third, as in all retrospective studies, there was the potential for bias and inaccurate data collection. Further prospective studies with larger patient populations involving multiple centers are necessary to ascertain more accurately the relevant influencing factors.
In conclusion, HIV/TB-coinfected patients with sustained viral suppression after early cART took a longer time to reach a CD4+ T cell count >250 cells/mm3 and seem to have a slower rate of CD4+ T cell increase during the early period after cART, compared to HIV-monoinfected patients. However, the slower CD4+ T cell increase was observed only during the first 6 months after cART initiation. As it is uncertain whether this long-lasting immunosuppressive effect of TB coinfection has a real influence on immunological function in HIV-infected individuals, further study is needed.
Acknowledgments
This study was supported by a faculty research grant from Yonsei University College of Medicine 2011 (6-2011-0166), a National Research Foundation of Korea grant funded by the Korean government (NRF-2011-220-E00015), and a grant from the Chronic Infectious Disease Cohort (4800-4859-304-260) from the Korea Centers for Disease Control and Prevention.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Reid A. Scano F. Getahun H, et al. Towards universal access to HIV prevention, treatment, care, and support: The role of tuberculosis/HIV collaboration. Lancet Infect Dis. 2006;6(8):483–495. doi: 10.1016/S1473-3099(06)70549-7. [DOI] [PubMed] [Google Scholar]
- 2.Karim SS. Durban 2000 to Toronto 2006: The evolving challenges in implementing AIDS treatment in Africa. AIDS. 2006;20(15):N7–9. doi: 10.1097/01.aids.0000247110.51338.73. [DOI] [PubMed] [Google Scholar]
- 3.Aliyu MH. Salihu HM. Tuberculosis and HIV disease: Two decades of a dual epidemic. Wien Klin Wochenschr. 2003;115(19–20):685–697. doi: 10.1007/BF03040884. [DOI] [PubMed] [Google Scholar]
- 4.Kizza HM. Rodriguez B. Quinones-Mateu M, et al. Persistent replication of human immunodeficiency virus type 1 despite treatment of pulmonary tuberculosis in dually infected subjects. Clin Diagn Lab Immunol. 2005;12(11):1298–1304. doi: 10.1128/CDLI.12.11.1298-1304.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whalen CC. Nsubuga P. Okwera A, et al. Impact of pulmonary tuberculosis on survival of HIV-infected adults: A prospective epidemiologic study in Uganda. AIDS. 2000;14(9):1219–1228. doi: 10.1097/00002030-200006160-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Gatell H. Cole SR. Margolick JB, et al. Effect of tuberculosis on the survival of HIV-infected men in a country with low tuberculosis incidence. AIDS. 2008;22(14):1869–1873. doi: 10.1097/QAD.0b013e32830e010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lancioni CL. Mahan CS. Johnson DF, et al. Effects of antiretroviral therapy on immune function of HIV-infected adults with pulmonary tuberculosis and CD4+ >350 cells/mm3. J Infect Dis. 2011;203(7):992–1001. doi: 10.1093/infdis/jiq141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toossi Z. Johnson JL. Kanost RA, et al. Increased replication of HIV-1 at sites of Mycobacterium tuberculosis infection: Potential mechanisms of viral activation. J Acquir Immune Defic Syndr. 2001;28(1):1–8. doi: 10.1097/00042560-200109010-00001. [DOI] [PubMed] [Google Scholar]
- 9.Goletti D. Weissman D. Jackson RW, et al. Effect of Mycobacterium tuberculosis on HIV replication. Role of immune activation. J Immunol. 1996;157(3):1271–1278. [PubMed] [Google Scholar]
- 10.Hertoghe T. Wajja A. Ntambi L, et al. T cell activation, apoptosis and cytokine dysregulation in the (co)pathogenesis of HIV and pulmonary tuberculosis (TB) Clin Exp Immunol. 2000;122(3):350–357. doi: 10.1046/j.1365-2249.2000.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang M. Gong J. Iyer DV. Jones BE. Modlin RL. Barnes PF. T cell cytokine responses in persons with tuberculosis and human immunodeficiency virus infection. J Clin Invest. 1994;94(6):2435–2442. doi: 10.1172/JCI117611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdool Karim SS. Naidoo K. Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362(8):697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Havlir DV. Kendall MA. Ive P, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365(16):1482–1491. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdool Karim SS. Naidoo K. Grobler A, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011;365(16):1492–1501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO. Antiretroviral therapy for HIV infection in adults adolescents: Recommendations for a public health approach 2010 version. 2010. [PubMed]
- 16.Bisset LR. Cone RW. Huber W, et al. Highly active antiretroviral therapy during early HIV infection reverses T-cell activation and maturation abnormalities. Swiss HIV Cohort Study. AIDS. 1998;12(16):2115–2123. doi: 10.1097/00002030-199816000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Plana M. Garcia F. Gallart T, et al. Immunological benefits of antiretroviral therapy in very early stages of asymptomatic chronic HIV-1 infection. AIDS. 2000;14(13):1921–1933. doi: 10.1097/00002030-200009080-00007. [DOI] [PubMed] [Google Scholar]
- 18.Pakker NG. Notermans DW. de Boer RJ, et al. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: A composite of redistribution and proliferation. Nat Med. 1998;4(2):208–214. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 19.Hermans SM. Kiragga AN. Schaefer P. Kambugu A. Hoepelman AI. Manabe YC. Incident tuberculosis during antiretroviral therapy contributes to suboptimal immune reconstitution in a large urban HIV clinic in sub-Saharan Africa. PLoS One. 2010;5(5):e10527. doi: 10.1371/journal.pone.0010527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gazzola L. Tincati C. Bellistri GM. Monforte A. Marchetti G. The absence of CD4+ T cell count recovery despite receipt of virologically suppressive highly active antiretroviral therapy: Clinical risk, immunological gaps, and therapeutic options. Clin Infect Dis. 2009;48(3):328–337. doi: 10.1086/595851. [DOI] [PubMed] [Google Scholar]
- 21.Hunt PW. Martin JN. Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187(10):1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 22.Marchetti G. Gori A. Casabianca A, et al. Comparative analysis of T-cell turnover and homeostatic parameters in HIV-infected patients with discordant immune-virological responses to HAART. AIDS. 2006;20(13):1727–1736. doi: 10.1097/01.aids.0000242819.72839.db. [DOI] [PubMed] [Google Scholar]
- 23.Hansjee N. Kaufmann GR. Strub C. Weber R. Battegay M. Erb P. Persistent apoptosis in HIV-1-infected individuals receiving potent antiretroviral therapy is associated with poor recovery of CD4 T lymphocytes. J Acquir Immune Defic Syndr. 2004;36(2):671–677. doi: 10.1097/00126334-200406010-00003. [DOI] [PubMed] [Google Scholar]
- 24.Smith CJ. Sabin CA. Lampe FC, et al. The potential for CD4 cell increases in HIV-positive individuals who control viraemia with highly active antiretroviral therapy. AIDS. 2003;17(7):963–969. doi: 10.1097/00002030-200305020-00004. [DOI] [PubMed] [Google Scholar]
- 25.Staszewski S. Miller V. Sabin C, et al. Determinants of sustainable CD4 lymphocyte count increases in response to antiretroviral therapy. AIDS. 1999;13(8):951–956. doi: 10.1097/00002030-199905280-00011. [DOI] [PubMed] [Google Scholar]
- 26.Boulanger C. Hollender E. Farrell K, et al. Pharmacokinetic evaluation of rifabutin in combination with lopinavir-ritonavir in patients with HIV infection and active tuberculosis. Clin Infect Dis. 2009;49(9):1305–1311. doi: 10.1086/606056. [DOI] [PubMed] [Google Scholar]
- 27.Chou R. Fu R. Huffman LH. Korthuis PT. Initial highly-active antiretroviral therapy with a protease inhibitor versus a non-nucleoside reverse transcriptase inhibitor: Discrepancies between direct and indirect meta-analyses. Lancet. 2006;368(9546):1503–1515. doi: 10.1016/S0140-6736(06)69638-4. [DOI] [PubMed] [Google Scholar]
- 28.MacArthur RD. Novak RM. Peng G, et al. A comparison of three highly active antiretroviral treatment strategies consisting of non-nucleoside reverse transcriptase inhibitors, protease inhibitors, or both in the presence of nucleoside reverse transcriptase inhibitors as initial therapy (CPCRA 058 FIRST Study): A long-term randomised trial. Lancet. 2006;368(9553):2125–2135. doi: 10.1016/S0140-6736(06)69861-9. [DOI] [PubMed] [Google Scholar]
- 29.Springer SA. Friedland GH. Doros G. Pesanti E. Altice FL. Antiretroviral treatment regimen outcomes among HIV-infected prisoners. HIV Clin Trials. 2007;8(4):205–212. doi: 10.1310/hct0804-205. [DOI] [PMC free article] [PubMed] [Google Scholar]