Abstract
Purpose
Retinal ischemia-associated ocular disorders, such as retinal occlusive disorders, neovascular age-related macular degeneration, proliferative diabetic retinopathy, and glaucoma are vision-threatening. In this study, we examined whether and by what mechanisms resveratrol, a polyphenol found in red wine, is able to protect against retinal ischemia/reperfusion injury.
Methods
In vivo rat retinal ischemia was induced by high intraocular pressure (HIOP), namely, 120 mmHg for 60 min. The mechanism and management was evaluated by electroretinogram (ERG) b-wave amplitudes measurement, immunohistochemistry, and real-time polymerase chain reaction.
Results
The HIOP-induced retinal ischemic changes were characterized by a decrease in ERG b-wave amplitudes, a loss of choline acetyltransferase immunolabeling of amacrine cell bodies/neuronal processes, and increased vimentin immunoreactivity, which is a marker of Müller cells, together with upregulation of matrix metalloproteinase-9 (MMP-9), heme oxygenase-1 (HO-1), and inducible nitric oxide (iNOS), and downregulation of Thy-1, both at the mRNA level. The detrimental effects due to the ischemia were concentration-dependent (weaker effect at 0.05 nmole) and/or significantly (at 0.5 nmole) altered when resveratrol was applied 15 min before or after retina ischemia.
Conclusion
This study supports the hypothesis that resveratrol may be able to protect the retina against ischemia by downregulation of MMP-9 and iNOS, and upregulation of HO-1.
Introduction
Central/branch retinal artery/vein occlusion, diabetes, glaucoma and, possibly, age-related macular degeneration (AMD) are conditions associated with retinal ischemia.1–3 All these diseases may lead to severe sequelae. Therefore, the management of retinal ischemia is crucial.
Hollborn et al.4 have shown that oxidative stress in the human retinal pigment epitheliums results in upregulation of matrix metalloproteinase-9 (MMP-9), which is proteolytic and able to degrade the extracellular matrix.5 Additionally, ischemia has been proved to result in irreversible retinal ganglion cell (RGC) loss and has been shown to be accompanied by MMP-9 upregulation.6 In brain ischemia, MMP-9 expression is also known to be upregulated.7 In patients with AMD, an elevation in the plasma levels of MMP-2 and MMP-9 has also been noted.8 Thus, ischemia or oxidative stress would seem to result in the upregulation of MMP-9 levels.
There are three known isoforms of heme oxygenase (HO). HO-1 is an inducible isoform that is involved in the response to oxidative stress and hypoxia. HO-2 is a constitutive isoform that is expressed under homeostatic conditions. Both HO-1 and HO-2 are ubiquitously expressed and catalytically active. A third HO, HO-3, is not catalytically active, but is thought to be involved in oxygen sensing. HO-1 can exert a potent indirect antioxidative function by degrading heme to CO, iron, and biliverdin.9 Moreover, these byproducts have their own significance as part of an essential cellular metabolism and contribute to the suppression of oxidative stress. Furthermore, overexpression of HO-1 in photoreceptors protected them from subsequent cellular damage caused by intense light exposure.10 Oxidative stress might be one of the factors contributing to the development of AMD.11 Study of human eyes has disclosed that there may be an age-related decline in HO-1 expression in the retinal pigment epithelium (RPE).12 This explains why HO-1 is a representative mediator of antioxidants and cytoprotectants against ischemia or oxidative stresses. From a functional perspective, it is important to recognize that induction of the high-output iNOS usually occurs during the ischemic cascade, which involves oxidative stress. In the process, high levels of NO have the opportunity to react with superoxide leading to peroxynitrite formation and cell toxicity.1,2,13
Resveratrol appeared to exert retinal protective effects via modulation of NOS in oxygen-induced retinopathy.13 Oxidative stress damage plays a vital role in retinal ischemia/reperfusion (I/R) pathogenesis. Therefore, the possibility of an antioxidant therapy that is able to reduce I/R induced damage attracted our intense interest. Resveratrol is found in a number of dietary sources, such as grapes and red wine.14 Various studies have shown that resveratrol has strong antioxidant properties.14,15 It has also been confirmed that resveratrol has neuroprotective effects during cerebral I/R injury,15,16 during mice retina I/R17, and during in vitro experimental optic neuropathy.18 However, the effects of resveratrol on retinal neuron damage due to retinal ischemia are not completely understood. Therefore, we investigated whether and by what possible mechanisms resveratrol protects against retinal I/R.
Methods
Animals
All investigations involving the use of animals conformed to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmology and Vision Research and were approved by the Institutional Review Board of Cheng Hsin General Hospital (CHGH; Taipei, Taiwan). Six-week-old Wistar rats (BioLasco, Taipei, Taiwan) were kept in an animal house, where the humidity was 40%–60% and the temperature 19°C–23°C. They were kept on a 12-h light/dark cycle with 12–15 air changes/h. The animals were provided with food and water ad libitum.
Anesthesia and euthanasia of animals
The rats were anesthetized with an intraperitoneal (i.p.) injection of ketamine (100 mg/kg) and xylazine (5 mg/kg). The Wistar rats were killed using a humane method (Scientific Procedures Acts 1986), namely, an overdose (at least 140 mg/kg) of sodium pentobarbitone injected into the i.p. cavity.
Induction of retinal ischemia
The rats (200–250 g) were anesthetized and placed in a stereotaxic frame. The anterior chamber of one eye of a rat was cannulated with a 30-gauge needle connected to an elevated 0.9% saline reservoir; this caused a high intraocular pressure (HIOP) of 120 mmHg for 60 min. Eye fundus whitening confirmed the induction of an ischemic insult.1,2 A sham procedure was carried out on the controls in which the saline bottle was not elevated.
Drug administration
Drug administration involved either preischemic administration (pretreatment: 15 min before 60-min HIOP-induced retinal ischemia) or postischemic administration (post-treatment: 15 min after 60-min HIOP-induced retinal ischemia) of resveratrol (0.05, 0.5 nmole; Sigma-Aldrich, St Louis, MO) or vehicle (ethanol; control). The ischemic eye of each test rat was treated with a single intravitreous injection of 5 μL of the test compound. The paired normal eye was untreated.
Recording of flash electroretinogram
Flash electroretinogram (ERG) recordings were performed on all the rats before retinal ischemia (day 0) as well as at 1 day after ischemia and treatment with the defined chemicals. The animals were dark adapted for at least 8 h, were anesthetized during the ERG recordings, and the pupils were dilated with 1% tropicamide and 2.5% phenylephrine.1,2 A strobe was placed 2 cm in front of the animal to provide a stimulus of 0.5 Hz. Fifteen consecutive responses were recorded at 2 s intervals and at 10 kHz; the responses were amplified and averaged using an amplifier P511/regulated power supply RPS 107/stimulator PS22 (Grass-Telefactor; Astro-Med Inc., West Warwick, RI). For comparative purposes, the b-wave ratio, namely, the b-wave amplitude of the treated ischemic/sham operation eye when compared with that of the untreated contralateral normal eye, was calculated.1,2
Immunofluorescence analysis
The rats were sacrificed and intracardially perfused with 0.9% normal saline (w/v); then the rat retinas were removed, fixed with 4% (w/v) paraformaldehyde for 45 min, and transferred to 30% sucrose for cryosectioning.1,2 Sampling was carried out 1 and 7 days after induction of retinal ischemia and pretreatment with resveratrol (0.5 nmole) or vehicle, or the sham operation. The retinal sections from the eyes were incubated overnight with primary antibodies, namely, either the goat anti-ChAT polyclonal antibody (1:100, AB144p; Chemicon, Temecula, CA) or the mouse anti-vimentin monoclonal antibody (1:100, V6630; Sigma-Aldrich). Next, the retinal sections were incubated with appropriate secondary antibodies, rhodamine-conjugated rabbit anti-goat antibody (1:500, AP106R; Chemicon); fluorescein isothiocyanate-conjugated goat anti-mouse IgG (1:500, AP124F; Milipore, Temecula, SA). At the same time, the nuclei of the cells were labeled by 4,6-diamidine-2-phenylindole dihydrochloride (DAPI; 30 nM; Molecular Probes, Eugene, OR). The retinal sections were examined using a fluorescence microscope (Olympus BX61, Center Valley, PA).
Assessment of the levels of various retinal mRNAs by real-time polymerase chain reaction
The mRNA levels of Thy-1, MMP-9, HO-1, and iNOS present in the retinas were determined using real-time polymerase chain reaction (PCR).1 Twenty four hours after retinal ischemia and treatment with the defined chemicals or a sham-operation, the rats were sacrificed and the retinas were removed. Each retina was then sonicated in TriReagent (Sigma Chemical, St Louis, MO). Total retinal RNA was isolated and first-strand complementary DNA (cDNA) synthesis was performed on 2 μg deoxyribonuclease (DNase)-treated RNA using High-Capacity RNA-to-cDNA Master Mix. The first-strand cDNA then underwent real-time PCR using Fast SYBR Green Master Mix. The PCR was initiated by incubation at 95°C for 20 s; then 40 cycles of 95°C for 3 s and 60°C for 30 s were performed. Cycling was carried out on a StepOnePlus™ Real-Time PCR System. Relative quantification (a comparative method) was performed using the house keeping gene β-actin as the internal standard. This process allows the normalized quantification of the mRNA target (Ct) and takes into account the differences in the amount of total RNA added to each reaction (ΔCt). The relative Thy-1/MMP-9/HO-1/iNOS expression changes were calculated as fold changes relative to the control with respect to the calibrator (ΔΔ Ct), which was represented by the control retina. Relative quantification of gene expression was calculated according the method of 2−ΔΔCt, as described in the manufacturer's instructions, and was carried out by the accompanying software (RQ, ver. 1.3). The PCR reagents, software, and machine were purchased from AB Applied Biosystems (Foster City, CA). The data obtained for each treatment were pooled, and a total percentage change relative to the control was calculated. The PCR oligonucleotide primers were obtained from MISSION BIOTECH (Taipei, Taiwan) and were as follows:
β-actin forward primer 5′-GAACCGCTCATTGCCGATAGTG-3′
reverse primer 5′-TTGTCCCTGTATGCCTCTGGTCG-3′
Thy-1 forward primer 5′-ACCAAGGATGAGGGCGA CTA-3′
reverse primer 5′-CAGGCTTATGCCACCACACTT-3′
MMP-9 forward primer 5′-TGCGCTGGGCTTAGATC ATT -3′
reverse primer 5′- TGGATGCCTTTTATGTCGTCTTC-3′
HO-1 forward primer 5′-CAGGTGTCCAGAGAAGGC TTT-3′
reverse primer 5′-TCTTCCAGGGCCGTGTAGAT-3′
iNOS forward primer 5′-AGGCTTGGGTCTTGTTAGCC TAGT-3′
reverse primer 5′-ATTCTGTGCAGTCCCAGTGAGGAA-3
Statistical analysis
One-way analysis of variance (ANOVA) was performed to compare three or more independent groups. Following the one-way ANOVA, the Tukey multiple-comparison test1 was utilized to compare the control (such as vehicle-treated ischemic retinas) with all other groups (such as resveratrol-treated ischemic retinas). A value of P<0.05 was considered significant.
Results
The effect of preischemic or postischemic resveratrol on the b-wave
As shown in Figure 1, in the sham procedure eye (sham; a/c), the ERG b-wave amplitude was measured 0.35 (a)/0.23 mV (c) as a control. When the IOP was raised to induce retinal ischemia followed by reperfusion for 1 day, the b-wave amplitude was drastically reduced. This reduction was not affected by preischemic or postischemic vehicle [I/R+Preischemic Vehicle or I/R+Postischemic Vehicle: 0.16 (a) or 0.10 mV (c)]. However, preischemic or postischemic resveratrol [I/R+Preischemic Res 0.05 vs. 0.5 nmole (a) or I/R+Postischemic Res 0.05 vs. 0.5 nmole (c)] dose dependently attenuated the ischemia-induced reduction in b-wave amplitude and raised the value to 0.20 versus 0.29 mV(a) or 0.22 versus 0.27 mV(c) 1 day after I/R.
FIG. 1.
Electroretinogram (ERG) (a, c). In comparison with the sham procedure (Sham) eye, the b-wave amplitude was drastically decreased after 1-day I/R and preischemic (pretreatment) or postischemic administration (posttreatment) of vehicle in one rat from the I/R+Preischemic Vehicle group or I/R+Postischemic Vehicle group. This decrease was dramatically attenuated by pretreatment or posttreatment with resveratrol (0.05 or 0.5 nmole) in one rat from the I/R+Preischemic Res 0.05 nmole group (a), I/R+Postischemic Res 0.05 nmole group (c), I/R+Preischemic Res 0.5 nmole group (a) or I/R+Postischemic Res 0.5 nmole group (c). (b, d) As compared to the sham procedure group, there was a significant reduction (*P<0.05) in the vehicle-pretreated or -posttreated ischemic retina at 1 day after ischemia/reperfusion. This significant reduction was dose dependently (with a weaker effect at 0.05 nmole) and significantly (at 0.5 nmole; †P<0.05) alleviated by pretreatment or posttreatment with resveratrol. The results are the mean±SEM [pretreatment (n=5; b) or post-treatment (n=3; d)]. ERG, electroretinogram; I/R, ischemia/reperfusion.
In Figure 1b (n=5) or d (n=3), when compared to the preischemia b-wave ratio (baseline: day 0, 107.53%±6.84% or 93.70%±6.00%), 1 day after I/R and pretreated or post-treated vehicle (I/R+Preischemic Vehicle or I/R+Postischemic Vehicle), there is a significant (P<0.05) b-wave ratio reduction (42.78%±6.12% or 39.91%±2.00%). When pretreated or post-treated with resveratrol (I/R+Preischemic Res or I/R+Postischemic Res), there was a concentration-dependent (0.05 vs. 0.5 nmole) and significant (P<0.05; at 0.5 nmole) improvement in the ischemia-reduced b-wave ratio on day 1 (51.77%±2.72% vs. 88.90%±4.30% or 57.19%±3.00% vs. 78.80%±0.26%) after I/R. In this context, the preischemia b-wave ratio (day 0) was found to be as follows: (99.65%±6.11% vs. 101.84%±5.98% or 103.50%±9.00% vs. 93.20%±4.00%). Furthermore, there was a significant (P<0.05) difference between the attenuating effect of 0.5 nmole resveratrol and that of 0.05 nmole resveratrol, via either preischemic (b) or postischemic administration (d). When the ERG b-wave ratios of the sham procedure eye versus the fellow normal eye were compared as controls [presham (day 0): 103.23%±4.95% (n=5, b), 99.45%±6.00% (n=3, d); postsham (day 1): 97.96%±4.44% (n=5, b), 100.96%±3.00% (n=3, d)], there was no significant difference between the ERG b-wave ratio before the sham procedure (day 0) and on day 1 after the sham procedure group.
The effect of resveratrol on ChAT immunoreactivity
As shown in Figure 2, at 1 and 7 days after the sham procedure, when the sham retina (control: b and f) were examined, ChAT immunoreactivity (red) was found to be associated with the amacrine cell bodies (short arrows; Sham 1d, b: 39.00±3.00; Sham 7d, f: 34.50±1.38/field) that are found in the inner nuclear layer (INL) and the ganglion cell layer (GCL); furthermore, the neuronal processes can be seen to have a two-band pattern in the inner plexiform layer (IPL; long arrows). In the ischemic retina pretreated with vehicle, the immunolabeling of amacrine cell bodies (I/R 1d+Veh, c: 12.00±3.00; I/R 7d+Veh, g: 7.50±1.04/field) had almost disappeared at 1 and 7 days after I/R; furthermore, labeling in the IPL was hardly visible. Importantly, the effect of I/R was obviously counteracted by pretreatment with 0.5 nmole resveratrol (I/R 1d+Res, d: 25.00±3.00; I/R 7d+Res, h: 22.67±1.32) and the results after treatment with resveratrol show a retina that is quite similar to the control retina.
FIG. 2.
ChAT immunoreactivity. In the sham procedure retina (Sham 1d and 7d; b and f), ChAT immunoreactivity (red) is associated with amacrine perikarya (short arrows) in the INL and the GCL as well as their neuronal processes in the IPL, which are seen as two clearly defined strata of immunoreactivity (long arrows). I/R caused an almost complete obliteration of the ChAT immunoreactivity in the IPL and the number of ChAT immunopositive amacrine cell bodies was drastically reduced; the ischemia-induced alterations were not affected by pretreatment with vehicle (I/R 1d+Veh, c; I/R 7d+Veh, g). However, the effect of ischemia/reperfusion was obviously nullified by pretreatment of 0.5 nmole resveratrol (I/R 1d+Res, d; I/R 7d+Res, h). DAPI (blue) was used to counterstain the nuclei in the sham procedure retina (a and e). The merge images of ChAT and DAPI labeling are shown in pictures b–d and f–h. ONL: outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; DAPI, 4,6-diamidine-2-phenylindole dihydrochloride; ChAT, choline acetyltransferase. Scale bar=50 μm.
The effect of resveratrol on vimentin immunoreactivity
As shown in Figure 3, in the sham retina 1 and 7 days after the sham procedure (control; Sham 1d and 7d; b and f), the vimentin immunolabeled Müller cell processes can be seen to extend from the end foot region to the IPL as well as into the INL and into the outer nuclear layer (ONL). Immunoreactivity was increased after I/R and pretreatment with vehicle (I/R 1d+Veh, c; I/R 7d+Veh, g). However, importantly, the ischemic effect was attenuated by pretreatment with 0.5 nmole resveratrol (I/R 1d+Res, d; I/R 7d+Res, h) and producing results after treatment with resveratrol that are quite similar to the control retina.
FIG. 3.
Vimentin immunoreactivity. At 1 and 7 days after the sham procedure (Sham 1d, b; Sham 7d, f), antivimentin (green) immunolabeled Müller cell end foot (arrow heads) as well as processes were visible in the IPL (arrows), INL, and ONL. As compared to the sham-operation retina (b and f), antivimentin immunolabeling was increased after ischemia/reperfusion and pretreatment with vehicle (I/R 1d+Veh, c; I/R 7d+Veh, g). This increase was counteracted 1 and 7 days after I/R by pretreatment with 0.5 nmol resveratrol (I/R 1d+Res, d; I/R 7d+Res, h). DAPI (blue) was used to counterstain the nuclei in the sham procedure retina (a and e). Scale bar=50 μm.
The effect of resveratrol on the mRNA levels of Thy-1, MMP-9, HO-1, or iNOS in the retina
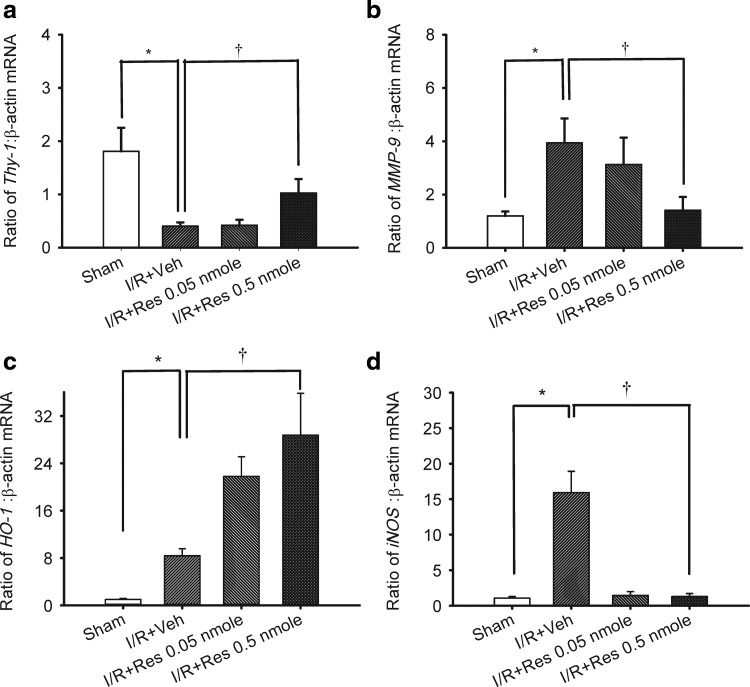
In Fig. 4 (n=5), as compared to the control retina [Thy-1 (1.81±0.44; a), MMP-9 (1.20±0.16; b), HO-1 (1.00±0.41; c), and iNOS (1.07±0.22; d)], the ratios for Thy-1 (0.40±0.07; a), MMP-9 (3.95±0.90; b), HO-1 (10.50±2.58; c), and iNOS (15.92±3.00; d) in the vehicle-pretreated ischemic retina were significantly (P<0.05) changed after I/R.
FIG. 4.
Real-time polymerase chain reaction analysis of the expression of Thy-1 (a), MMP-9 (b), HO-1 (c), iNOS (d), and β-actin. Twenty four hours after ischemia plus reperfusion (I/R), whole retinal extracts were isolated from the sham procedure eyes (control) or the ischemic eyes (subjected to 60-min high intraocular pressure) preadministered (15-min before ischemia) with intravitreous vehicle (ethanol) or resveratrol (0.05 nmole; 0.5 nmole). The effect of either treatment on the ratio of Thy-1/MMP-9/HO-1/iNOS: β-actin mRNA was determined compared to the control, which is β-actin and is a housekeeping gene. */† represent significant differences (P<0.05) between the vehicle-pretreated ischemic group and the sham procedure group and significant differences (P<0.05) between the vehicle-pretreated ischemic group and the resveratrol-pretreated ischemic group, respectively. MMP-9, matrix metalloproteinase-9; HO-1, heme oxygenase-1; iNOS, inducible nitric oxide synthase; Res, resveratrol. The results are the mean±SEM (n=5).
When compared with the vehicle-pretreated ischemic retina, the ischemic retina pretreated with resveratrol (0.05 or 0.5 nmole) showed a dose-dependent and/or significant (P<0.05) counteraction of the upregulation of MMP-9 (I/R+resveratrol 0.05 vs. 0.5 nmole: 3.13±1.01 vs. 1.42±0.50; b) and iNOS (I/R+resveratrol 0.05 vs. 0.5 nmole: 1.43±0.56 vs. 1.30±0.42; d), together with a significant (P<0.05) counteraction of downregulation of Thy-1 (I/R+resveratrol 0.05 vs. 0.5 nmole: 0.42±0.10 vs. 1.03±0.26; a). This was complemented by a further significant (P=0.021) upregulation of HO-1 (I/R+resveratrol 0.05 vs. 0.5 nmole: 21.80±3.32 vs. 28.75±7.05; c).
Discussion
In a previous study,19 resveratrol from grape has been reported to be a sirutins (SIRT1) activator that has potential clinical important effects on various disorders, including type 2 diabetes, inflammation, neurodegeneration, and heart disease. Marambaud et al.20 also have demonstrated that resveratrol has therapeutic potential in the treatment of Alzheimer's disease by markedly lowering the levels of amyloid-β peptides. Moreover, Dasgupta et al.21 have suggested that neuronal activation of AMP-activated kinase (AMPK) by resveratrol may affect neuronal energy homeostasis and contribute to the neuroprotective effects of resveratrol. In addition, it has been indicated that the nucleotide-binding and oligomerization domain-like receptor (NLR) protein (NLRP3) inflammasome plays a role in a variety of inflammatory diseases, including cellular stress.22 Reactive oxygen species (ROS) have been proved to be a major endogenous activator of NLRP3 inflammasome assembly, which activates caspase-1, IL-1β, and IL-18 causing an inflammatory response and apoptosis.23 In addition to the above, the polyphenol antioxidant resveratrol24 has been reported to exert its neuroprotective effect by decreasing the expression of caspase-3, increasing the amounts of an antioxidant enzyme superoxide dismutase, and upregulating expression of transcription factor Nrf2 during brain ischemia.25 Therefore, it is possible that resveratrol might inhibit inflammasome assembly through an inhibition of upstream ROS formation. Sakata et al.26 and Ren et al.25 have also reported that resveratrol selectively induces HO-1 in a dose-dependent and time-dependent manner in in vitro mouse or in vivo rat cortical neurons and provided neuroprotection against free-radical or excitotoxicity damage. Moreover, resveratrol is known to reduce the elevated level of MMP-9 that is induced by cerebral I/R in mice.7 Furthermore, resveratrol has been shown to inhibit the iNOS pathway in macrophages.27 A number of evidences support the present novel results that the preischemic or postischemic administration of resveratrol both protected against retinal ischemia and possibly reversed the ischemia-induced upregulation of MMP-9, HO-1, and iNOS (an indicator of oxidative stress) as well as downregulation of Thy-1 (a marker of the neuron RGC) at the mRNA level.
In contrast, Fig. 3b of Li et al.,17 seems to indicate that resveratrol, respectively, reduces or prevents ischemia-induced loss of cells (Brn3a labeling RGCs on flat-mounts or GCL cells, such as displaced amacrines and RGCs by standard histology) in a dose-dependent, but not significant manner. Whereas, the current study shows significant and obvious protection by means of extensive electrophysiological measurements (b-wave reflecting the function of the inner retina, such as INL and GCL), immunohistochemistry (ChAT indexing cholinergic amacrine cells), and biochemistry (Thy-1 mRNA indexing RGCs). Specifically, there are various differences between the present study and that of Li et al.,17 namely, the species used (Wistar rats vs. C57BL/6 mice), the markers studied (MMP-9/HO-1/iNOS mRNAs vs. ER stress markers: eIF2α, IRE1α, Xbp1 splicing, Bip), the measurement timing (1 vs. 4 days after retinal I/R), the drug administration (different doses; time points and routes: 15-min preischemia or postischemia via intravitreous injection vs. 2-day preischemia by oral ingestion). Therefore, for the first time, the present study reveals the protective effects of resveratrol on the rat retinal I/R (vs. on the mice retinal I/R),17 together with an analysis of presumed underlying biochemical mechanisms that are involved, namely, changes in expression of HO-1, MMP-9, and iNOS.
However, whether these mRNA changes are a direct effect of resveratrol needs further confirmation. Additional experiments are needed, including observing these changes at the protein level, exploring the differences between treatment with 0.5 nmole resveratrol and with 0.05 nmole resveratrol in terms of the mRNA level and investigating the effects of pharmacological inhibitors of HO-1, MMP-9, and iNOS.
In the present study, resveratrol was intravitreously administered 15 min before or after I/R. I/R in the rat causes severe retinal alterations that especially affects the inner retina and are characterized by a decline in the retinal physiological function (Fig. 1), by a decrease in the number of RGCs indexed by Thy-1 mRNA (Fig. 4a), by a reduction in the number/distribution of cell bodies/neuronal processes of cholinergic amacrine cells (Fig 2c, g), and by an increase in vimentin immunoreactivity, a marker for Müller cells (Fig. 3c, g), as well as by overall increases in the mRNA levels of MMP-9, HO-1, and iNOS (Fig. 4b–d).
Recently, vimentin-immunoreactivity has been shown to be increased in Müller cells after I/R28 and this was confirmed in the present study (Fig. 3c, g) after I/R and pretreatment with vehicle. The ischemia-injured Müller cells reduced the b-wave;1,2 this was confirmed in the present study (Fig. 1b). When the ischemic retinas were pretreated with vehicle, the increase in vimentin-immunoreactivity (Fig. 3c, g) parallels the reduction in b-wave (Fig. 1b). Of clinical importance, we found that all of these ischemia-induced alterations were ameliorated by pretreatment with 0.5 nmole resveratrol (Fig. 1b and Fig. 3d, h).
It is widely acknowledged that RGC death plays a major role in retinal ischemia or experimental glaucoma.1,2 Since the inner retina is mainly injured during retinal ischemia, both RGCs and neighboring cells, namely, amacrine cells, are directly or indirectly damaged.1,2 Amacrine cells are neurons that use various neurotransmitters and make lateral synaptic contacts onto other amacrine cells as well as with ganglion cells and bipolar cells. Cholinergic amacrine cells use acetylcholine as a neurotransmitter and these are localized in the INL and the GCL. In the present study, we have found that the number of cholinergic amacrine cells was reduced by retinal ischemia and pretreatment with vehicle (Fig. 2c, g). The same observations in relation to the ChAT immunoreactivity reduction have been described previously in the ischemic rat retina.1,2 It has been previously reported that functionality of the RGCs is compromised when there is a loss of cholinergic signaling in the laser ablated retina.29 In this study of retinal ischemia, we have also clearly shown that both RGCs and amacrine cells are affected and these findings are based on the Thy-1 mRNA and ChAT immunoreactivity results. Consistent with the results of Dong et al.,30 it would seem that highly selectively synaptic connections between starburst amacrine cells and direction-selective ganglion cells are necessary for functionality. Importantly, our results also show that the ischemia-induced decrease in ChAT immunoreactivity and Thy-1 mRNA was obviously or significantly attenuated by pretreatment with resveratrol, respectively (Fig. 2d, h and Fig. 4a).
As described in the Introduction, HO-1 can be induced in response to oxidative stress and ischemia. Ischemia/hypoxia has been proved to induce an overexpression of MMP-9.1,3,6,8 Induction of the high-output iNOS usually occurs in an ischemia-associated oxidative environment as has been mentioned previously.1,2,13 Oxidative stress damage, thus, plays a vital role in retinal I/R pathogenesis. In the present study, MMP-9, HO-1, and iNOS mRNA levels (Fig. 4b–d) were significantly upregulated in the retinal cells after pressure-induced ischemic insult. Furthermore, the significant upregulation of MMP-9, iNOS, and HO-1 was each found to be significantly affected by pretreatment with 0.5 nmole resveratrol; such changes did not occur when the eye was treated with vehicle. Oxidative stress-related retinal ischemia and neovascular AMD (nvAMD) are associated with an upregulation of MMP-9 and iNOS as well as downregulation of HO-1, as mentioned extensively elsewhere.1,3,6,8,9,12,13 Thus, resveratrol seems to have the potential to manage problematic retinal ischemia and nvAMD. This thought is supported by a previous report of Sheu et al., “Resveratrol might ameliorate oxidative stress-induced or age-related RPE degeneration as occurred in AMD.”31 A recent result has also indicated that resveratrol significantly inhibited Bcl-2 or vascular endothelial growth factor expression in the neonatal rat retina after oxygen-induced retinopathy of prematurity (ROP) and might protect against ROP.32
In conclusion, characteristic ischemic changes can be observed using electrophysiology (b-wave), by immunohistochemistry (ChAT-immunoreactivity or vimentin-immunoreactivity), and by biochemical analysis (Thy-1, HO-1, MMP-9, or iNOS expression). Clinically, it is important that all of these changes were found to be significantly attenuated or enhanced, as appropriate, by resveratrol treatment before or after retinal I/R. This study supports the hypothesis that resveratrol may be able to protect the retina against ischemia by acting to suppress the upregulation of MMP-9 and iNOS, while also acting to further increase the HO-1 levels.
Acknowledgments
The authors thank Cheng Hsin General Hospital for providing grants (CHGH98-77) and the relevant facilities at the Core Laboratory to carry out this study. The authors are also indebted to Ms. Shu-Hua Wu for her efficient administrative and technical assistance. They also express their sincere gratitude to Prof. Ralph Kirby at the National Yang-Ming University for professional revision.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Chen Y.Q. Pan W.H.T. Liu J.H, et al. The effects and underlying mechanisms of S-Allyl L-Cysteine treatment of the retina after ischemia/reperfusion. J. Ocul. Pharmacol. Ther. 2012;28:110–117. doi: 10.1089/jop.2011.0099. [DOI] [PubMed] [Google Scholar]
- 2.Osborne N.N. Casson R.J. Wood J.P.M. et al. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog. Retin. Eye Res. 2004;23:91–147. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Zarbin M.A. Current concepts in the pathogenesis of age related macular degeneration. Arch. Ophthalmol. 2004;122:598–614. doi: 10.1001/archopht.122.4.598. [DOI] [PubMed] [Google Scholar]
- 4.Hollborn M. Stathopoulos C. Steffen A., et al. Positive feedback regulation between MMP-9 and VEGF in human RPE cells. Invest. Ophthalmol. Vis. Sci. 2007;48:4360–4367. doi: 10.1167/iovs.06-1234. [DOI] [PubMed] [Google Scholar]
- 5.Stetler-Stevenson W.G. Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin. Invest. 1999;103:1237–1241. doi: 10.1172/JCI6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo L. Moss S.E. Alexander R.A. et al. Retinal ganglion cell apoptosis in glaucoma is related to intraocular pressure and IOP-induced effects on extracellular matrix. Invest. Ophthalmol. Vis. Sci. 2005;46:175–182. doi: 10.1167/iovs.04-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao D. Zhang X. Jiang X. et al. Resveratrol reduces the elevated level of MMP-9 induced by cerebral ischemia-reperfusion in mice. Life Sci. 2006;78:2564–2570. doi: 10.1016/j.lfs.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 8.Chau K.Y. Sivaprasad S. Patel N. et al. Plasma levels of matrix metalloproteinase-2 and−9 (MMP-2 and MMP-9) in age-related macular degeneration. Eye (Lond) 2007;21:1511–1515. doi: 10.1038/sj.eye.6702722. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J. Tan S. Liu F., et al. Heme oxygenase and ocular disease: a review of the literature. Curr. Eye Res. 2012;37:955–960. doi: 10.3109/02713683.2012.700753. [DOI] [PubMed] [Google Scholar]
- 10.Sun M.H. Pang J.H. Chen S.L., et al. Photoreceptor protection against light damage by AAV-mediated overexpression of heme oxygenase-1. Invest. Ophthalmol. Vis. Sci. 2008;49:893. doi: 10.1167/iovs.07-0340. [DOI] [PubMed] [Google Scholar]
- 11.Beatty S. Koh H. Phil M., et al. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000;45:115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 12.Miyamura N. Ogawa T. Boylan S., et al. Topographic and age-dependent expression of heme oxygenase-1 and catalase in the human retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2004;45:1562–1565. doi: 10.1167/iovs.02-0761. [DOI] [PubMed] [Google Scholar]
- 13.Kim W.T. Suh E.S. Retinal protective effects of resveratrol via modulation of nitric oxide synthase on oxygen-induced retinopathy. Korean J. Ophthalmol. 2010;24:108–118. doi: 10.3341/kjo.2010.24.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De la Lastra C.A. Villegas I. Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and clinical implications. Biochem. Soc. Trans. 2007;35:1156–1160. doi: 10.1042/BST0351156. [DOI] [PubMed] [Google Scholar]
- 15.Ban J.Y. Cho S.O. Choi S.H., et al. Neuroprotective effect of Smilacis chinae rhizome on NMDA-induced neurotoxicity in vitro and focal cerebral ischemia in vivo. J. Pharmacol. Sci. 2008;106:68–77. doi: 10.1254/jphs.fp0071206. [DOI] [PubMed] [Google Scholar]
- 16.Shin J.A. Lee H. Lim Y.K., et al. Therapeutic effects of resveratrol during acute periods following experimental ischemic stroke. J. Neuroimmunol. 2010;227:93–100. doi: 10.1016/j.jneuroim.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Li C. Wang L. Huang K., et al. Endoplasmic reticulum stress in retinal vascular degeneration: protective role of resveratrol. Invest. Ophthalmol. Vis. Sci. 2012;53:3241–3249. doi: 10.1167/iovs.11-8406. [DOI] [PubMed] [Google Scholar]
- 18.Liu Q. Ju W.K. Crowston J.G., et al. Oxidative stress is an early event in hydrostatic pressure induced retinal ganglion cell damage. Invest. Ophthalmol. Vis. Sci. 2007;48:4580–4589. doi: 10.1167/iovs.07-0170. [DOI] [PubMed] [Google Scholar]
- 19.Lavu S. Boss O. Elliott P.J., et al. Sirtuins-novel therapeutic targets to treat age-associated diseases. Nat. Rev./Drug Dis. 2008;7:841–853. doi: 10.1038/nrd2665. [DOI] [PubMed] [Google Scholar]
- 20.Marambaud P. Zhao H. Davies P. Reseveratrol promotes clearance of Alzheimer's disease amyloid-β peptides. J. Biol. Chem. 2005;280:37377–37382. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- 21.Dasgupta B. Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc. Natl. Acad. Sci. U.S.A. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang C.S. Shin D.M. Jo E.K. The role of NLR-related protein 3 inflammasome in host defense and inflammatory diseases. Int. Neurourol. J. 2012;16:2–12. doi: 10.5213/inj.2012.16.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schroder K. Tschoop J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 24.Frankel E.N. Waterhouse A.L. Kinsella J.E. Inhibition of human LDL oxidation by resveratrol. Lancet. 1993;341:1103–1104. doi: 10.1016/0140-6736(93)92472-6. [DOI] [PubMed] [Google Scholar]
- 25.Ren J. Fan C. Chen N., et al. Resveratrol pretreatment attenuates cerebral ischemic injury by upregulating expression of transcription factor Nrf2 and HO-1 in rats. Neurochem. Res. 2011;36:2352–2362. doi: 10.1007/s11064-011-0561-8. [DOI] [PubMed] [Google Scholar]
- 26.Sakata Y. Zhuang H. Kwansa H., et al. Resveratrol protects against experimental stroke: putative neuroprotective role of heme oxygenase 1. Exp. Neurol. 2010;224:325–329. doi: 10.1016/j.expneurol.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan M.M. Mattiacci J.A. Hwang H.S., et al. Synergy between ethanol and grape polyphenols, quercetin, and resveratrol, in the inhibition of the inducible nitric oxide synthase pathway. Biochem. Pharmacol. 2000;60:1539–1548. doi: 10.1016/s0006-2952(00)00471-8. [DOI] [PubMed] [Google Scholar]
- 28.Wurm A. Iandiev I. Uhlmann S., et al. Effects of ischemia-reperfusion on physiological properties of Müller glial cells in the porcine retina. Invest. Ophthalmol. Vis. Sci. 2011;52:3360–3367. doi: 10.1167/iovs.10-6901. [DOI] [PubMed] [Google Scholar]
- 29.He S. Masland R.H. Retinal direction selectivity after targeted laser ablation of starburst amacrine cell. Nature. 1997;389:378–382. doi: 10.1038/38723. [DOI] [PubMed] [Google Scholar]
- 30.Dong W. Sun W. Zhang Y., et al. Dendritic relationship between starburst amacrine cells and direction-selective ganglion cells in the rabbit retina. J. Physiol. 2004;556((Pt 1)):11–17. doi: 10.1113/jphysiol.2004.060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheu S.J. Liu N.C. Chen J.L. Resveratrol protects human retinal pigment epithelial cells from acrolein-induced damage. J. Ocul. Pharmacol. Ther. 2010;26:231–236. doi: 10.1089/jop.2009.0137. [DOI] [PubMed] [Google Scholar]
- 32.Li W. Jiang D. Effect of resveratrol on bcl-2 and VEGF expression in oxygen-induced retinopathy of prematurity. J Pediatr. Ophthalmol. Strabismus. 2012;49:230–235. doi: 10.3928/01913913-20111129-01. [DOI] [PubMed] [Google Scholar]