Abstract
Even in the setting of maximally suppressive antiretroviral therapy (ART), HIV persists indefinitely. Several mechanisms might contribute to this persistence, including chronic inflammation and immune dysfunction. In this study, we have explored a preclinical model for the evaluation of potential interventions that might serve to eradicate or to minimize the level of persistent virus. Given data that metabolic products of the inducible enzyme indoleamine 2,3-dioxygeanse (IDO) might foster inflammation and viral persistence, chronically simian immunodeficiency virus (SIV)-infected, ART-treated rhesus macaques were treated with the IDO inhibitor 1-methyl tryptophan (1mT). Orally administered 1mT achieved targeted plasma levels, but did not impact tryptophan metabolism or decrease viral RNA or DNA in plasma or in intestinal tissues beyond levels achieved by ART alone. Animals treated with 1mT showed no difference in the levels of T cell activation or differentiation, or in the kinetics or magnitude of viral rebound following cessation of ART. Notwithstanding these negative results, our observations suggest that the chronically SIV-infected rhesus macaque on suppressive ART can serve as a tractable model in which to test and to prioritize the selection of other potential interventions designed to eradicate HIV in vivo. In addition, this model might be used to optimize the route and dose by which such interventions are administered and the methods by which their effects are monitored.
Introduction
Despite the success of highly active antiretroviral therapy (HAART) in limiting circulating viral load, HIV continues to persist in treated patients, both as actively produced virus1,2 and as latent virus in long-lived cells.3 Such persistence is associated with chronically elevated inflammation, the cumulative impact of which is plausibly related to the observed increased incidence of non-AIDS morbidities, e.g., coronary artery disease, kidney disease, and neurocognitive decline,4 in optimally treated patients. Reciprocally, it is likely that inflammation contributes to virus persistence via a number of non-mutually exclusive mechanisms, including (1) expansion of the frequency and composition of target cells5; (2) favoring cell-to-cell contacts that can maintain or expand the viral reservoir (e.g., through inhibitory interactions, such as those with PD-16 or through T-DC viral transfer7); (3) generating proinflammatory cytokines such as tumor necrosis factor (TNF)-α that trigger increased viral replication8; and finally, (4) by resulting in T cell dysfunction9 and failure of host mechanisms to clear virus-producing cells.10,11
Another strategy for the management of HIV disease beyond simply suppressing HIV replication would be to eradicate all replication-competent virus, thereby allowing patients to live their lives free of antiretroviral medications. The feasibility of such eradication efforts has been validated by an apparent HIV cure in an individual who received a bone marrow transplant from a CCR5Δ32/Δ32 donor.12 Given this success, other candidate approaches have been advanced,13 including inhibition of histone deacetylases to free chromatin-bound integrated virus from transcriptional repression; activation of the T cell compartment to selectively deplete memory T cells that harbor the majority of virus; ex vivo exonuclease excision of CCR5 in autologous CD4+ T cells and/or in hematopoietic stem cells to create an immune system that is impervious to HIV infection; and inhibition of certain inflammatory mechanisms that might favor the persistence of viral reservoirs. To test the efficacy of these various approaches and to optimize them for clinical applications, a practical and relevant animal model is needed. We describe here one such model: the chronically SIVmac251-infected rhesus macaque treated with a highly suppressive antiretroviral regimen.
We started with the premise that the simian immunodeficiency virus (SIV)-infected macaque in the chronic phase of infection might represent an attractive model because (1) it is a well characterized and accepted model for chronic HIV disease, (2) chronically infected macaques might be less expensive to enroll into studies after their release from prior studies, and (3) viral replication might be more effectively suppressed in such animals with less intensive antiretroviral drug regimens. After placing animals on antiretroviral therapy, we tested the ability of a candidate intervention to diminish the reservoir of persistent virus by reducing the level of chronic inflammation that likely supports viral persistence.
We, and others, have shown that chronic inflammation in the context of lentiviral infection is likely sustained by stimulation of the innate immune system by persistent inflammatory signals (e.g., by virus and/or by translocated microbial products).14,15 These signals directly or indirectly induce interferon-α (IFN-α) and other proinflammatory molecules. Indeed, the degree of IFN-stimulated gene expression in the intestine is the strongest signal differentiating HIV controllers from progressors.16 In addition, a salient feature of HIV-associated immunodeficiency is the loss of intestinal barrier function, resulting in microbial translocation from the intestinal lumen to systemic circulation.15,17 The presence of these immunostimulatory products in tissue and circulation results in a systemic innate immune response characterized by markers of innate activation such as sCD14, interleukin (IL)-6, and CRP.18 Microbial translocation has also been linked to specific depletion of IL-17-producing CD4+ T cells (Th17) and enrichment of FoxP3+ regulatory T cells (Treg) in the intestine.14,19,20 In turn, the loss of IL-17 may augment continued microbial translocation and systemic inflammation in a feed-forward manner, as IL-17 is essential for intestinal epithelial cell homeostasis and maintenance of the epithelial barrier.21 In addition, the presence of Treg in the intestine might impair immune function,22 thereby facilitating the persistence of the virus. The balance of Th17 to Treg is thought to be controlled by tryptophan catabolites (e.g., 3-hydroxyanthranilic acid) produced by indoleamine-2,3-dioxygenase (IDO) activity.14 Since IDO expression and activity are significantly elevated in models of pathogenic SIV infection20,23,24 and during progressive HIV infection,14 we hypothesized that the induction of IDO, potentially by virus-induced inflammation, results in a vicious cycle of depletion of Th17, breakdown of the intestinal barrier, microbial translocation, systemic inflammation, and HIV (or SIV) persistence. We therefore undertook to inhibit IDO activity during antiretroviral-suppressed, chronic SIV infection to repair intestinal immunity and to break the self-sustaining virus/inflammation cycle to decrease or eliminate residual virus.
Previous studies of IDO inhibition during SIV infection have employed the tryptophan analog 1-methyl-d-tryptophan (45 mg/kg/day for 13 days) and demonstrated transient inhibition of in vivo enzymatic activity and significant impact on residual viral load during partially suppressive ART.25 We aimed to extend these earlier findings by providing a putatively more inhibitory regimen of both 1-methyl-d-tryptophan and 1-methyl-l-tryptophan (together referred to as 1mT), inhibiting both potentially active forms of isoforms of IDO (IDO1 and IDO2). 1mT was provided in dose escalation to chronically SIV-infected rhesus macaques per protocol to understand whether additional 1mT would prolong the transient inhibition observed previously.25 We expected to find that inhibition of IDO would lead to lower levels of inflammation and, hence, lower levels of persistent virus. Although these predictions were not borne out under the conditions of this experiment, we believe that the manipulations allowed by this animal model illustrate an approach that could be applied to other, potentially more effective, HIV eradication strategies in the future.
Materials and Methods
Animals
Animals were housed and handled in accordance with the Assessment and Accreditation of Laboratory Animal Care International, and these studies were in accordance with NIH guidelines and approved by the Institutional Animal Care and Use Committee at Advanced BioScience Laboratories, Bethesda, MD. To minimize the overall cost of the experiment, 12 animals with chronic SIV infection were enrolled after they had been engaged in a previous study of SIV vaccines (M. Vaccari, unpublished observations) in which the animals were infected 282 days previously with 470 TCID50 SIVmac251 intrarectally. Animals P013, P014, P016, P019, and M890 (5/6 animals) in the group receiving 1mT and animals P001, P008, P009, and M898 (4/6 animals) in the control group were vaccinated with a combination of DNA-SIVgpe,26 ALVAC-SIVgpe, and native SIVmac251 gp120 protein in alum adjuvant. The remaining macaques were given alum only. At the initiation of our study (day 0), the macaques were provided with a daily ART regimen consisting of 10 mg/kg 2,3-dideoxyinosine (ddI) per oral (po), 1.2 mg/kg 2-didehydro-3-deoxythymidine (d4T) po, and 20 mg/kg (R)-9-(2-phosphonylmethoxypropyl)adenine (PMPA) subcutaneously. At day 84, the animals were randomized to receive either saline or a racemic mixture of 1-methyl-d-tryptophan and 1-methyl-l-tryptophan (Sigma-Aldrich, St. Louis, MO); each was provided orally. The dose of 1mT was increased in 2-week dose levels of 45, 90, and 180 mg/kg/day, expressed as the sum of both isoforms of drug. Separate solutions of the d- and l- isomers of 1mT were prepared in distilled water with 0.09 M NaOH at 7.8 mg/ml and 6.4 mg/ml, respectively, and the pH of the solution was adjusted to 8.5 with 1 M HCl. Peripheral blood was obtained by venipuncture before and at varying times after the administration of ART. Rectal biopsies were taken with a rigid sigmoidoscope and biopsy forceps. Resected jejunal strip biopsies were obtained by abdominal surgery.
Virus quantitation
A real-time nucleic acid sequence-based amplification (NASBA) assay was used to quantitate SIV RNA in plasma.27 High-sensitivity assays for tissue-associated virus were performed as described by Hansen et al.28
Flow cytometry
Cells were prepared from peripheral blood by Ficoll density gradient centrifugation and from biopsy tissue by enzymatic and mechanical disruption. They were then stained for surface marker expression by standard methods, and permeabilized and stained intracellularly using the FoxP3 Fixation/Permeabilization Staining Set (eBiosciences, San Jose, CA). Acquisition was performed on a BD LSRII (BD Biosciences, San Jose, CA) and analyzed using FlowJo software, version 9 (Treestar, Portland, OR). The following antibodies [indicated as Target (Clone, Label, Dilution)] were used to discriminate phenotypic markers: Ki67 (B56, FITC, 1:100), CD4 (L200, PerCP-Cy5.5, 1:500), CD3 (SP34-2, Alexa700, 1:100), CD69 (L78, APC, 1:100), and HLA-DR (L243, PE-Cy7, 1:500) from BD Biosciences; CD8 (RPA-T8, APC-Cy7, 1:500) from Biolegend (San Diego, CA); and FoxP3 (PCH-101, PE, 1:100) from eBiosciences (San Diego, CA).
Measurement of tryptophan, kynurenine, and 1mT concentrations in plasma
1-Methyl-d-tryptophan (1mT), tryptophan (Tryp), and kynurenine (Kyn) were measured by liquid chromatography-tandem mass spectrometry (LC/MS/MS). The plasma sample (100 μl) was mixed with 100 μl of internal standard consisting of kynurenine-d4 (Kyn-d4) and tryptophan-d8 (Tryp-d8) in water, and 20 μl of trifluoroacetic acid was added to precipitate the proteins. The sample was vortexed for 1 min and then centrifuged at 3000 rpm for 10 min. The supernatant was transferred to an autosampler vial and 5 μl was subjected to analysis by LC/MS/MS with one measurement per specimen. The mass detector was a Micromass Quattro Ultima using electrospray/positive ionization mode. The multiple reaction monitor was set at 219–160 m/z for 1mT, 205–88 m/z for Tryp, 209–192 m/z for Kyn, 213–196 m/z for Kyn-d4, and 213–195 m/z for Tryp-d8. The mobile phase (consisting of 2% acetonitrile, 5.4% methanol, and 0.1% formic acid) was pumped through a Synergi Polar RP (75×4.6 mm, 4 μm particle size) column at a flow rate of 1.0 ml/min. One quarter of the volume of the flow was split into the mass system. The standard curve was generated by adding 1mT, Tryp, and Kyn standard solutions to water, and treating these samples in the same manner as the plasma samples, with concentrations ranging from 31.25 to 2000 ng/ml for 1mT, 313 to 2000 ng/ml for Tryp, and 62.5 to 1000 ng/ml for Kyn. Assay variation for Tryp, Kyn, and 1mT were less than 15%.
Results
Study design
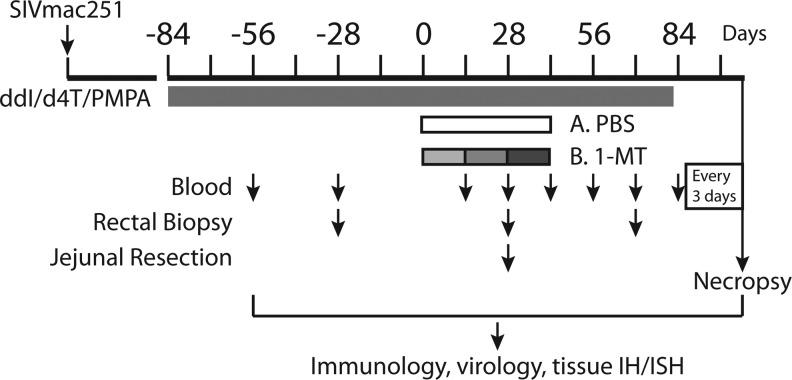
Twelve Indian-origin rhesus macaques (RM) that had been previously infected with SIVmac251 as part of a vaccine trial (9/12 animals in this study were in the vaccine arm, and distributed between the study groups here) were enrolled into the study shown schematically in Fig. 1. These animals had a normal course of SIV infection with peak viral loads ranging from 106 to 108 SIV RNA copies/ml plasma that decreased to set points between 103 to 106 SIV RNA copies/ml plasma at 250 days postinfection (Fig. 2A). A combination ART regimen (including ddI, d4T, and PMPA) was initiated at 282 days postinfection and continued for 84 days, at which time the animals were randomized to receive a racemic mixture of 1mT or saline by oral gavage. 1mT was given in escalating doses of 45 mg/kg/day for 14 days, followed by 90 mg/kg/day for 14 days, and then 180 mg/kg/day for a final 14 days, for a total treatment period of 42 days. Thereafter, ART was continued for an additional 28 days and then stopped. The kinetics and extent of viral rebound were then measured every 3–4 days over the next 21 days, at which point the animals were euthanized and several tissues were studied.
FIG. 1.
Schematic of study. Antiretroviral treatment (ART) is indicated by black bar, phosphate-buffered saline (PBS) or 1MT treatment is indicated by unshaded or shaded bars, respectively, and increasing doses of 1-methyl tryptophan (1mT) (see text) are indicated by varying shades of gray. The times of venipuncture, rectal biopsy, and jejunal resection are indicated by the corresponding arrows.
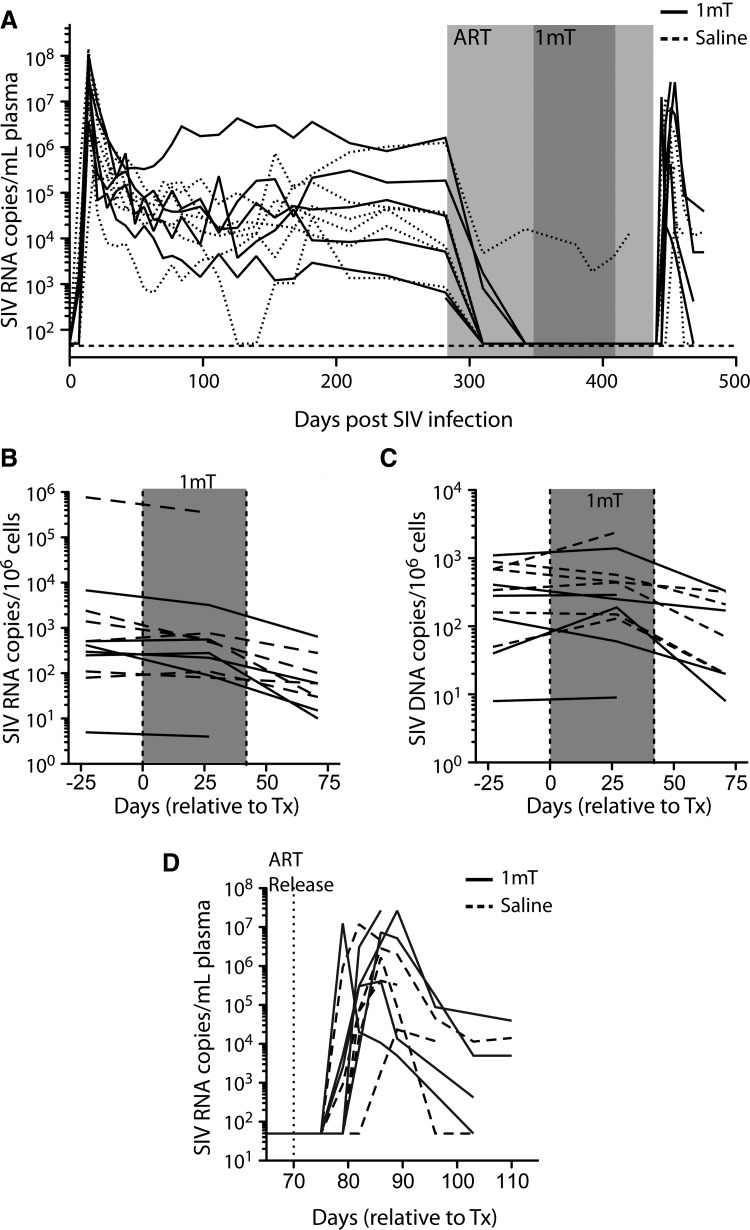
FIG. 2.
Plasma and tissue viral loads during ART and 1mT administration. ART was provided from day 282 to 436 relative to infection (light gray box). During this time (from days 380 to 408; dark gray box), either 1mT (at varying doses) or saline was provided as well. Plasma viral loads were measured by nucleic acid sequence-based amplification (NASBA) (A) before and after treatment with 1mT or saline. Tissue viral RNA (B) and DNA (C) levels were measured by PCR-based methods, and normalized to copies per 106 cells by copies of genomic DNA. (D) Viral loads were measured in plasma by NASBA after cessation of ART.
Viral load is effectively suppressed by ART but not impacted by 1mT
By example of our previous work,25,29–31 including a previous study combining 1mT and ART in rhesus macaques that demonstrated a significant decrease in residual viral replication with the addition of 1mT,25 an ART regimen including ddI, d4T, and PMPA was chosen for use in this experiment. In the chronically SIV-infected animals enrolled into our study, this regimen rapidly suppressed the plasma viral load to levels below the limit of detection (50 copy/ml) in 11/12 animals (Fig. 2A). These results confirm that effective suppression of viremia can be achieved using this three-drug regimen in chronically SIV-infected macaques25,29–31 and that the kinetics of viral suppression over this time period roughly approximate those seen in humans.32 It is important to note that six of 12 animals developed diabetes during treatment, with the remaining six animals having no evidence of metabolic abnormalities. The affected animals were evenly distributed among the 1mT and phosphate-buffered saline (PBS) arms, and no discernible differences arose with respect to viral loads, drug kinetics, or immunological features of the study (data not shown). This phenomenon is likely associated with the use of high-dose ddI during this study, indicating that ddI use might be avoided in the future.
At day 84 after the initiation of ART, six animals received increasing doses of 1mT (see Materials and Methods) and six received saline. Over the course of the next 42 days, plasma viral remained undetectable in 11/12 animals (Fig. 2A) while viral RNA and DNA levels declined in rectal biopsy tissue (Fig. 2B and C, respectively), with no apparent difference between the 1mT and saline groups. After 27 days of treatment with 1mT, viral RNA and DNA levels were also measured in resected jejunal tissue and again the administration of 1mT was found to have no effect (data not shown).
To more definitively understand the impact of 1mT on viral reservoir, ART was interrupted after a total of 154 days of treatment so that the kinetics and extent of viral rebound could be observed (Fig. 2D). Within 4 days of interruption, circulating virus was detectable in 5/10 animals (3/5 remaining in the 1mT group and 2/5 in the control group); by day 10, all animals were viremic, with no difference in the absolute virus load between animals treated or not treated with 1mT. The time to postinterruption peak viremia and the magnitude of the peak were also similar between animals treated or not treated with 1mT. In aggregate, these data indicate that 1mT has no effect on ongoing virus production or on the viral reservoir, at least as administered and as measured in this study.
1mT is orally bioavailable but does not durably impact systemic IDO activity
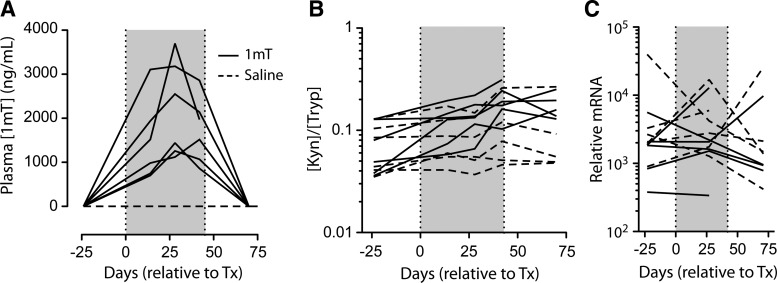
To determine whether the lack of an apparent 1mT effect might be due to inadequate absorption after oral administration, drug levels were measured in plasma by LC/MS/MS and found to be in the range of 704–3700 ng/ml (3.2–16.9 μM), levels comparable to those observed previously25 (Fig. 3A). Of note, maximum drug levels were reached at the end of the second dosing stage (of 90 mg/kg/day) and were lower after the third dosing stage (of 180 mg/kg/day), suggesting the induction of drug clearance mechanisms.
FIG. 3.
Plasma levels of 1mT, kynurenine, and tryptophan. Levels of 1mT (A) and the kynurenine to tryptophan ratio (B) in circulation were measured by liquid chromatography-tandem mass spectrometry (LC/MS/MS). (C) Relative expression of 2,3-dioxygeanse (IDO) mRNA in rectal biopsy tissue. The shaded box indicates the period of 1mT treatment.
To understand the impact of 1mT administration on IDO activity, the plasma concentrations of tryptophan (Tryp) and its IDO-catalyzed metabolite kynurenine (Kyn) were measured by LC/MS/MS. Effective inhibition of IDO activity should increase the concentration of Tryp and decrease the concentration of Kyn, thereby decreasing the Kyn:Tryp ratio. Instead, after 14 days of 1mT and throughout the treatment phase, Tryp levels fell while Kyn levels rose (data not shown), increasing the Kyn:Tryp ratio in the plasma of 1mT-treated animals (p=0.0071 for treatment in two-way ANOVA; Fig. 3B). By contrast, there was no apparent change in this ratio in the control animals. While we did not measure a long-term, systemic impact on IDO activity, similar regimens have demonstrated a shorter-term effect on IDO activity, lasting 2–5 days.25 This intriguing finding potentially indicates that feedback mechanisms were induced over the course of the 48-day treatment period that cleared excess Tryp and/or that induced additional IDO activity. Importantly, we did not detect induction of IDO mRNA in colon biopsies, making induction of the enzyme itself a less likely explanation for this paradoxical finding (Fig. 3C). This observation is at odds with studies showing that 1mT can inhibit IDO activity over the short (2–5 day) term25 and suggests, as has been previously described,33 that provision of 1mT over a longer term may actually induce IDO activity.
1mT does not impact innate immune activation or T cell activation
Anticipating from previous studies14,20,24 that 1mT administration might result in changes in various immune parameters, CD4+ T cells (Fig. 4A), Ki67+CD8+ T cells (Fig. 4B), and FoxP3+CD4+ Treg (Fig. 4C) were measured in the peripheral blood and in rectal biopsy tissue during 1mT treatment. As in the case of viral loads (above), the frequency of each of these cell populations was unchanged by 1mT treatment. To further explore changes that might have occurred in the balance of Treg and Th17 cells, FoxP3 and IL-17 transcripts were quantitated in mRNA extracted from rectal biopsy tissue; again, no consistent changes were found in the relative expression values or in the ratio of these transcripts (Fig. 4D). To determine any measurable impact that 1mT might have on Type 1 interferon responses, we measured mRNA levels of myxovirus resistance protein 1 (MX-1), an interferon–stimulated gene previously associated with intestinal inflammation during HIV infection.20,24,34 As in the case of IL-17 and FoxP3 transcripts, MX-1 levels did not change upon treatment with 1mT (Fig. 4D). Lastly, 1mT had no apparent impact on the levels of T cell activation, measured as the percentage of cells expressing Ki67 (Fig. 4B), HLA-DR, and/or CD69 (data not shown) in peripheral blood or in rectal biopsy tissue. Thus, at least under the conditions of this experiment, 1mT had no discernible effect on parameters of systemic or tissue-based immune activation.
FIG. 4.
Immunologic analysis of peripheral blood mononuclear cell (PBMC) and rectal biopsy (GALT) tissue. The fractions of CD4+ cells among CD3+ T cells (A), of Ki67+ cells among CD8+ T cells (B), and of FoxP3+ cells among CD4+ T cells (C) were measured in PBMCs (left) and in rectal biopsy tissue (right). (D) The relative expression of IL-17, FoxP3, and MX1 mRNA was measured in rectal biopsy tissue. The 1mT group is denoted by solid lines and the saline group is denoted by dashed lines.
Discussion
Tremendous obstacles confront the goal of providing life-long ART to all of those infected with HIV. Even if this goal can be reached, it is not clear that all would achieve a normal state of health: patients on HAART harbor persistent virus, are more susceptible to a number of life-threatening illnesses, and tend to die at a younger age than their uninfected, age-matched counterparts.4 Consequently, there is an urgent need to find interventions that can either completely eradicate virus or, alternatively, diminish it to levels that can be contained absent treatment. A number of such interventions have been proposed to achieve this goal and most if not all might be best evaluated in a preclinical model prior to use in humans, for the following reasons: (1) many have not been used in humans before and may have unknown adverse effects; (2) head-to-head comparison of potential interventions might serve to prioritize those most likely to succeed; and (3) methods for the definition of “success” might be established, prior to movement into the clinic.
In this study, we examined the impact of one eradication strategy in the context of chronically SIVmac251-infected rhesus macaques. Given prior data that activation of IDO might contribute to viral replication and persistence in vivo,14,20 and that IDO inhibition with 1-methly-d-tryptophan (45 mg/kg/day×13 days) during ART resulted in decreased viral load,25 we chose to administer a racemic mixture of 1-methyl-d- and 1-methyl-l-tryptophan at higher doses (including 45 mg/kg/day, 90 mg/kg/day, and 180 mg/kg/day, each for 14 days). Administration of an ART regimen of ddI, d4T, and PMPA (as used in Boasso et al.25) was found to effectively suppress viremia in most animals, with 11/12 suppressed below detection (Fig. 2A). Viral suppression in this study was more effective than in other reports employing the same ART regimen25,29–31 as well as other two-to-three drug, single class regimens,35,36 and more advanced multiclass regimens.37,38 When, however, oral 1mT was administered in increasing doses of 45, 90, and 180 mg/kg daily, inhibition of IDO-catalyzed conversion of Tryp to Kyn was not observed and the predicted changes that might have occurred in T cell activation, proliferation, and Treg differentiation were also not seen. These findings indicate that 1mT delivered in this fashion does not affect systemic IDO activity or interfere with the putative inflammatory feedback loop that was described in a previous study.14 They do not, however, rule out a role for IDO in the context of persistent inflammation and viral persistence nor do they prove that IDO inhibition will provide no benefit. Certainly, it is still conceivable that IDO inhibition might contribute to diminished levels of persistent virus during ART, though it is also clear that such inhibition must be mediated by more potent IDO inhibitors provided in a more effective manner.
Notwithstanding these otherwise negative results, we present these observations because we believe that the strategy used in this study (testing potential interventions in the chronically SIV-infected rhesus macaque on ART) is applicable to the evaluation of other eradication strategies in the future. Important considerations contributing to the development of a model system for eradication of HIV include model animal species, protective allele expression, viral isolate, duration of chronic infection, inclusion or exclusion of animals with nonnormal disease course, ART regimen and duration, and method of reservoir measurement. The model system described here, based on chronic SIVmac251 infection of rhesus macaques, offers a number of significant advantages. First, in the rhesus macaque (as opposed to other nonhuman primates), there is not only a substantial body of prior knowledge on which to base measurements and experimental design, there also exist robust virologic and immunologic tools to evaluate interventions. Second, the SIVmac251-infected rhesus macaque is a well-validated model of pathogenesis and of viral dynamics that appears to closely approximate the course of HIV disease in humans and that does not use engineered chimeric viruses of unknown in vivo relevance. Finally, the model that we describe here is a practical one for use: chronically infected macaques available after completion of participation in other studies might be enrolled in a study at considerable cost savings relative to those infected de novo.
Use of chronically infected macaques does, however, raise a number of potentially confounding variables: (1) the duration of prior infection and the starting stage of disease are likely to impact the effectiveness of a given intervention; (2) there may exist unintended or unmeasured effects of earlier interventions; (3) the enrolled animals may have a varied and incompletely defined pretreatment history; and (4) variation in set point viral loads, such as that seen when comparing this study to a previous study of IDO inhibition,25 might have a significant impact of the efficacy of subsequent ART and putative eradication regimens. It is worth noting, however, that these considerations also prevail in the clinical setting, wherein such variables are often present.
Three observations made during the course of this study warrant further consideration in the development of related eradication protocols in the future. First, we found that virus levels were still falling in tissue, even after 84 days of ART, indicating that a longer period of treatment may be required to decrease viral load to a level that can be impacted by eradication strategies. The optimal duration of therapy for nonhuman primate eradication studies needs to be defined. Carefully performed studies in humans suggest it may take 3–4 years to achieve a true steady-state level of viremia32 and it will be logistically and financially impossible to treat large cohorts of monkeys for this duration. Second, six of the animals in the study developed ART-related diabetes independent of 1mT treatment, likely due to the high dose of ddI in the ART regimen. This potentially inflammatory outcome might have affected our primary metabolic pathways of interest and may have obscured the impact of IDO inhibition. This should be taken into account in the design of future ART regimens in macaques, potentially avoiding the use of ddI. Finally, we chose to directly assess the unbiased whole body viral reservoir and the potential for viral rebound by interrupting ART and measuring viral rebound. This strategy prevents in-depth measurement of tissue-based virus after intervention, except in small surgical biopsies, but presents an overall picture of the state of virus. These animals showed a remarkable diversity of rebound kinetics (in terms of time to rebound, time to peak, and magnitude of rebound) that was unrelated to acute peak viremia or plasma viremia pretherapy (data not shown). Possibly, this diversity is reflective of the state of the reservoir after treatment and not of other preexisting virus–host relationships. It will be important in the future to understand the tissue-level correlates of viral rebound to develop monitoring strategies for eventual human trials and treatments and future studies might incorporate both modes of reservoir analysis in smaller subgroups of animals. As has been observed in other recent nonhuman primate studies,39 changes in more readily sampled reservoirs may be different from those that can be observed with more intensive postmortem tissue analyses.
In summary, we were not able to find a treatment regimen with 1mT that could durably inhibit IDO and/or have a measurable effect on viremia in the chronically SIVmac251-infected macaque on effective ART. We do believe, however, that this model may prove useful in the future analysis of alternative interventions aimed at the eradication of HIV.
Acknowledgments
The authors would like to acknowledge technical support from Melvin N. Doster and flow cytometry support from Katherine McKinnon and the Vaccine Branch FACS Core Facility at the National Cancer Institute. In addition, the authors acknowledge the outstanding support from Deborah Weiss and Jim Treece at Advanced BioScience Laboratories, Inc.
This work was supported in part by the Foundation for AIDS Research (amfAR 106710-40-RGRL), the UCSF/Gladstone Center for AIDS Research (P30 AI27763), the Martin Delaney AIDS Research Enterprise (DARE) Collaboratory (U19 AI096109), the UCSF Clinical and Translational Science Institute (UL1 RR024131-01), the National Institutes of Health (K24 AI069994), federal funds from the National Cancer Institute, under contract no. HHSN261200800001E, and the Harvey V. Berneking Living Trust. Richard M. Dunham is supported by a postdoctoral fellowship from NIAID (F32 AI91534). Joseph M. McCune is a recipient of the NIH Director's Pioneer Award Program, part of the NIH Roadmap for Medical Research, through grant DPI OD00329.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Maldarelli F. Palmer S. King MS, et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog. 2007;3(4):e46. doi: 10.1371/journal.ppat.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yukl SA. Shergill AK. McQuaid K, et al. Effect of raltegravir-containing intensification on HIV burden and T-cell activation in multiple gut sites of HIV-positive adults on suppressive antiretroviral therapy. AIDS. 2010;24(16):2451–2460. doi: 10.1097/QAD.0b013e32833ef7bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chun T-W. Nickle DC. Justement JS, et al. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J Infect Dis. 2008;197(5):714–720. doi: 10.1086/527324. [DOI] [PubMed] [Google Scholar]
- 4.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoddart CA. Keir ME. McCune JM. IFN-alpha-induced upregulation of CCR5 leads to expanded HIV tropism in vivo. PLoS Pathog. 2010;6(2):e1000766. doi: 10.1371/journal.ppat.1000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Said EA. Dupuy FP. Trautmann L, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med. 2010;16(4):452–459. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lekkerkerker AN. van Kooyk Y. Geijtenbeek TBH. Viral piracy: HIV-1 targets dendritic cells for transmission. Curr HIV Res. 2006;4(2):169–176. doi: 10.2174/157016206776055020. [DOI] [PubMed] [Google Scholar]
- 8.Williams SA. Kwon H. Chen L-F. Greene WC. Sustained induction of NF-kappa B is required for efficient expression of latent human immunodeficiency virus type 1. J Virol. 2007;81(11):6043–6056. doi: 10.1128/JVI.02074-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terawaki S. Chikuma S. Shibayama S, et al. IFN-alpha directly promotes programmed cell death-1 transcription and limits the duration of T cell-mediated immunity. J Immunol. 2011;186(5):2772–2779. doi: 10.4049/jimmunol.1003208. [DOI] [PubMed] [Google Scholar]
- 10.Trautmann L. Janbazian L. Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nature Med. 2006;12(10):1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 11.Day CL. Kaufmann DE. Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 12.Allers K. Hütter G. Hofmann J, et al. Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. Blood 3. 2011;117(10):2791–2799. doi: 10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- 13.Smith MZ. Wightman F. Lewin SR. HIV reservoirs and strategies for eradication. Curr HIV/AIDS Rep. 2012;9(1):5–15. doi: 10.1007/s11904-011-0108-2. [DOI] [PubMed] [Google Scholar]
- 14.Favre D. Mold J. Hunt PW, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Translation Med. 2010;2(32):32ra36. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenchley JM. Price DA. Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 16.Loke P. Favre D. Hunt PW, et al. Correlating cellular and molecular signatures of mucosal immunity that distinguish HIV controllers from noncontrollers. Blood. 2010;115(15):e20–32. doi: 10.1182/blood-2009-12-257451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Estes JD. Harris LD. Klatt NR, et al. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog. 2010;6(8) doi: 10.1371/journal.ppat.1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nixon DE. Landay AL. Biomarkers of immune dysfunction in HIV. Curr Opin HIV AIDS. 2010;5(6):498–503. doi: 10.1097/COH.0b013e32833ed6f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenchley JM. Paiardini M. Knox KS, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112(7):2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Favre D. Lederer S. Kanwar B, et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5(2):e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marks BR. Craft J. Barrier immunity and IL-17. Semin Immunol. 2009;21(3):164–171. doi: 10.1016/j.smim.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Estes JD. Wietgrefe S. Schacker T, et al. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J Infect Dis. 2007;195(4):551–561. doi: 10.1086/510852. [DOI] [PubMed] [Google Scholar]
- 23.Bosinger SE. Li Q. Gordon SN, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119(12):3556–3572. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lederer S. Favre D. Walters K-A, et al. Transcriptional profiling in pathogenic and non-pathogenic SIV infections reveals significant distinctions in kinetics and tissue compartmentalization. PLoS Pathog. 2009;5(2):e1000296. doi: 10.1371/journal.ppat.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boasso A. Vaccari M. Fuchs D, et al. Combined effect of antiretroviral therapy and blockade of IDO in SIV-infected rhesus macaques. J Immunol. 2009;182(7):4313–4320. doi: 10.4049/jimmunol.0803314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosati M. Bergamaschi C. Valentin A, et al. DNA vaccination in rhesus macaques induces potent immune responses and decreases acute and chronic viremia after SIVmac251 challenge. Proc Natl Acad Sci USA. 2009;106(37):15831–15836. doi: 10.1073/pnas.0902628106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romano JW. Williams KG. Shurtliff RN. Ginocchio C. Kaplan M. NASBA technology: Isothermal RNA amplification in qualitative and quantitative diagnostics. Immunol Invest. 1997;26(1–2):15–28. doi: 10.3109/08820139709048912. [DOI] [PubMed] [Google Scholar]
- 28.Hansen SG. Ford JC. Lewis MS, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473(7348):523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hryniewicz A. Boasso A. Edghill-Smith Y, et al. CTLA-4 blockade decreases TGF-beta, IDO, and viral RNA expression in tissues of SIVmac251-infected macaques. Blood. 2006;108(12):3834–3842. doi: 10.1182/blood-2006-04-010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaccari M. Boasso A. Fenizia C, et al. Fatal pancreatitis in simian immunodeficiency virus SIV(mac251)-infected macaques treated with 2',3'-dideoxyinosine and stavudine following cytotoxic-T-lymphocyte-associated antigen 4 and indoleamine 2,3-dioxygenase blockade. J Virol. 2012;86(1):108–113. doi: 10.1128/JVI.05609-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cecchinato V. Tryniszewska E. Ma ZM, et al. Immune activation driven by CTLA-4 blockade augments viral replication at mucosal sites in simian immunodeficiency virus infection. J Immunol. 2008;180(8):5439–5447. doi: 10.4049/jimmunol.180.8.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmer S. Maldarelli F. Wiegand A, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci USA. 2008;105(10):3879–3884. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Opitz CA. Litzenburger UM. Opitz U, et al. The indoleamine-2,3-dioxygenase (IDO) inhibitor 1-methyl-D-tryptophan upregulates IDO1 in human cancer cells. PLoS One. 2011;6(5):e19823. doi: 10.1371/journal.pone.0019823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guadalupe M. Sankaran S. George MD, et al. Viral suppression and immune restoration in the gastrointestinal mucosa of human immunodeficiency virus type 1-infected patients initiating therapy during primary or chronic infection. J Virol. 2006;80(16):8236–8247. doi: 10.1128/JVI.00120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verhoeven D. Sankaran S. Dandekar S. Simian immunodeficiency virus infection induces severe loss of intestinal central memory T cells which impairs CD4+ T-cell restoration during antiretroviral therapy. J Med Primatol. 2007;36(4–5):219–227. doi: 10.1111/j.1600-0684.2007.00239.x. [DOI] [PubMed] [Google Scholar]
- 36.Boyer JD. Kumar S. Robinson T, et al. Initiation of antiretroviral therapy during chronic SIV infection leads to rapid reduction in viral loads and the level of T-cell immune response. J Med Primatol. 2006;35(4–5):202–209. doi: 10.1111/j.1600-0684.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 37.Horiike M. Iwami S. Kodama M, et al. Lymph nodes harbor viral reservoirs that cause rebound of plasma viremia in SIV-infected macaques upon cessation of combined antiretroviral therapy. Virology. 2012;423(2):107–118. doi: 10.1016/j.virol.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 38.Fuller DH. Rajakumar P. Che JW, et al. Therapeutic DNA vaccine induces broad T cell responses in the gut and sustained protection from viral rebound and AIDS in SIV-infected rhesus macaques. PLoS One. 2012;7(3):e33715. doi: 10.1371/journal.pone.0033715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.North TW. Higgins J. Deere JD, et al. Viral sanctuaries during highly active antiretroviral therapy in a nonhuman primate model for AIDS. J Virol. 2010;84(6):2913–2922. doi: 10.1128/JVI.02356-09. [DOI] [PMC free article] [PubMed] [Google Scholar]