Abstract
Objective
To develop a juvenile mouse model to establish effects of in vivo hypothermia on expression of the inflammation-modulating cytokines tumor necrosis factor-α, interleukin-1β, interleukin-6, and interleukin-10. Although induced hypothermia is neuroprotective in some patients, the mechanisms of protection are not well understood and concerns remain over potential detrimental effects, particularly in the setting of infection. We previously showed that in vitro hypothermia increases production of tumor necrosis factor-α and interleukin-1β in lipopolysaccharide-treated monocytes.
Design
Laboratory investigation.
Setting
Research laboratory.
Subjects
Juvenile (4-wk) male C57BL/6 mice.
Interventions
Mice were given chlorpromazine to suspend thermoregulation and lipopolysaccharide to stimulate cytokine production. Core temperature was maintained at 32°C or 37°C for 6 hrs by adjusting environmental temperature. In separate experiments, lipopolysaccharide-treated mice were kept in a cooling chamber without chlorpromazine treatment.
Measurements and Main Results
Plasma and organs were collected for cytokine quantitation. Chlorpromazine-treated hypothermic mice had 2.3-fold and 1.8-fold higher plasma interleukin-6 and interleukin-10 levels at 6 hrs compared with identically treated normothermic mice (p < .05), whereas plasma tumor necrosis factor-α and interleukin-1β were not significantly different at 2 hrs or 6 hrs. Liver tumor necrosis factor-α and interleukin-6 were significantly higher in hypothermic vs. normothermic mice, but lung and brain cytokines were not different. Lipopolysaccharide-treated mice kept in a cooling chamber without chlorpromazine treatment developed varying degrees of hypothermia with associated increases in plasma interleukin-6 and interleukin-10. A nonspecific marker of stress (plasma corticosterone) was not affected by hypothermia in lipopolysaccharide-treated mice.
Conclusion
Further studies are necessary to determine the mechanism and physiologic consequences of augmented systemic interleukin-6 and interleukin-10 expression during induced hypothermia.
Keywords: hypothermia, cytokines, interleukin-10, interleukin-6, endotoxin
Numerous animal studies and an increasing number of clinical trials in humans have demonstrated neuro-protective properties of induced hypothermia (HT) in some forms of brain injury. In ischemia-reperfusion insults, such as cardiac arrest (1, 2) and perinatal hypoxic-ischemic encephalopathy (3–5), randomized trials have shown improved neurologic outcome in patients cooled to approximately 33°C for 12 to 72 hrs compared with normothermic controls. However, clinical trials of induced HT in adults with traumatic brain injury (TBI) have not consistently shown benefit (6 – 8) and a recent trial in pediatric patients with TBI showed no improvement in outcome with 24 hrs of HT therapy (6). Research is ongoing into other potential clinical applications of HT, including hepatic encephalopathy and acute lung injury (6–9).
The therapeutic potential of induced HT depends on matching mechanism of cytoprotection to mechanism of cellular injury. In ischemia-reperfusion injury, HT has been shown to decrease cerebral metabolic rate and preserve high-energy phosphates (10), reduce excitatory amino acid and free radical production (11), stabilize cell membranes, reduce cerebral edema (12), and inhibit apoptosis (13). The effects of HT on inflammation are less clear. Some animal models of traumatic brain injury and ex vivo studies of human cells have found that HT decreases or does not change production of inflammatory cytokines (14–17). Other studies, including animal models of hemorrhagic shock or heat shock, and in vitro studies of human leukocytes exposed to lipopolysaccharide (LPS), have shown increased levels of proinflammatory cytokines during HT (18–21). We have previously shown that, in LPS-stimulated human monocytes, HT causes a delayed release followed by an augmentation of tumor necrosis factor (TNF)-α and interleukin (IL)-1β production (22), mediated at least in part by prolonged activation of the transcription factor NF-κ B (22, 23). Although TNF-α and IL-1β are critical to mounting an adequate host response to infection, their overproduction has been associated with cell and tissue injury in the brain and other organs (24, 25). The current studies were designed to test the hypothesis that in vivo HT would augment production of both pro- and anti-inflammatory cytokines. Because HT is under investigation in pediatric patients whose cytokine regulation may differ from that of adults, we developed a juvenile mouse model of mild induced HT. LPS was used as a well-characterized stimulus for cytokine production which may be present in the circulation of patients with ischemia-reperfusion injury (4, 26). These studies focused on levels of the proinflammatory cytokines TNF-α and IL-1β as well as the counterregulatory cytokine IL-6 and the potent anti-inflammatory cytokine IL-10.
MATERIALS AND METHODS
Induction of HT and LPS Treatment
Male C57BL/6 mice (Charles River, Kronberg, Germany) were housed under standard conditions before experiments (temperature 22°C, 12-hr dark/light cycle, lights on at 7:00 AM). For preliminary experiments to determine effects of chlorpromazine treatment alone on cytokine expression, adult mice (8–10 wks old) were used. All other experiments utilized juvenile (4-wk old) mice. Preliminary studies in our laboratory with intraperitoneal temperature probes and continuous radiotelemetry established that juvenile C57BL/6 mice housed at 22°C had a usual temperature range of 36°C to 37.5°C, varying with time of day and level of activity. All experiments were begun at 9:00 AM to control for diurnal temperature variation as well as variability in hormones, such as endogenous glucocorticoids. Mice were housed individually for experiments and had free access to food and water. Animal protocols were approved by the Animal Care and Use Committee at the University of Virginia.
LPS (Escherichia coli 055:B5, Sigma-Aldrich, St. Louis, MO) 1 μg/g was given via intraperitoneal injection. Chlorpromazine (15 μg/g IP) was then given to suspend thermoregulation. In preliminary experiments, blood pressure and heart rate were measured in juvenile mice treated with LPS and chlorpromazine and maintained either hypothermic or normothermic (BP-2000, Visitech Systems, Apex, NC).
Chlorpromazine induced deep sedation within 15 mins and a temperature probe (2-mm diameter) was inserted 2 cm into the rectum. Mice were placed in incubators pre-warmed to 28°C or 32°C (to maintain rectal temperature 32°C or 37°C, respectively). Due to space restrictions in the incubators, each experiment was performed with three mice per temperature group and independent experiments were conducted to reach the “n” noted in results and in figure legends. Temperature was monitored continuously in one mouse in each temperature group and every 30 mins in the other two mice. Adjustments in incubator temperature were made to keep the mice at the target temperature. At 2 hrs or 6 hrs, mice were given ketamine/xylazine (60/6 mg/kg) immediately before blood collection and euthanasia. Control mice received vehicle or LPS alone and were housed at room temperature. Preliminary experiments showed that juvenile mice given LPS (1 μg/g) and kept at room temperature experienced approximately 1°C drop in core temperature with return to baseline within 90 mins.
In a subsequent series of experiments, cooling was attempted without sedation. Juvenile mice were given 1 μg/g of LPS and placed in a 4°C chamber for 6 hrs. To minimize stress in these nonsedated mice, temperature was measured noninvasively using an infrared thermometer (Thermoscan, Braun, Wilmington, MA) placed at the external auditory meatus and confirmed with a rectal temperature probe if ≤34°C (27).
Plasma and Organ Cytokine Assay
Blood was collected in heparinized tubes and plasma was separated and stored at −80°C. For chlorpromazine experiments, plasma TNF-α, IL-1β, IL-6, and IL-10 were measured using Luminex MAP technology (Bio-Rad, Hercules, CA). Diluted plasma samples and recombinant cytokine standards were incubated with fluorescent antibody-tagged microspheres, then with a biotin-labeled detection antibody followed by streptavidin-phycoerythrin. Microspheres were analyzed in a Bio-Plex 200 dual laser fluorometer (Bio-Rad) and cytokine concentrations calculated with Bio-Plex Manager 4.0 software. Samples and standards were analyzed in duplicate. Sensitivity was 12.5 pg/mL for TNF-α, 8 pg/mL for IL-1β, and 2 pg/mL for IL-6 and IL-10. For cooling chamber HT experiments, plasma IL-6 and IL-10 were measured by enzyme-linked immunosorbent assay, with a commercial kit (BioLegend, San Diego, CA) per the manufacturer’s recommendations. Standards and samples were assayed in duplicate, with lower limits of detection for IL-6 and IL-10 of 8 pg/mL and 16 pg/mL, respectively.
Liver (left lobe), lung (left), and brain (entire) were harvested and rinsed free from blood. Organs were placed in RIPA buffer with protease inhibitors in tubes containing Lysing Matrix D 1.4-mm ceramic spheres and lysed in a FastPrep FP120A instrument (MP Biomedicals, Solon, OH) with three cycles of 20 secs at 4°C. Organ lysates were cleared by centrifugation and protein quantitated, using a bicinchoninic acid assay (Pierce, Rockford, IL). Organ lysate cytokines were measured by the Luminex method and expressed as pg cytokine per mg tissue.
Corticosterone Assay
Plasma corticosterone was measured by competitive enzyme immunoassay, using a commercial kit (Cayman Chemical, Ann Arbor, MI). In wells precoated with mouse monoclonal anti-rabbit IgG, plasma samples and corticosterone standards were incubated with acetylcholinesterase-corticosterone tracer and rabbit anticorticosterone antibody. After washing, acetylcholinesterase was detected with Ellman’s reagent. The product of the enzymatic reaction is detected by spectrophotometric measurement at OD 412 nm. Samples and standards were analyzed in duplicate with a lower limit of detection of 16.4 pg/mL.
Statistics
Data are expressed as mean ± standard error, unless otherwise indicated. Due to the small sample size, nonparametric tests were employed exclusively. For environmental HT experiments, Lin’s concordance correlation coefficient was used to describe agreement between ear and rectal temperature readings. Similar to an intraclass correlation coefficient, a concordance correlation coefficient = 1 indicates perfect agreement (28). The Wilcoxon rank-sum test was used to compare plasma cytokine levels at 32°C vs. 37°C, with an exact p < .05 considered statistically significant. The Kruskal-Wallis test was used to compare corticosterone levels among the six groups. If the overall test was significant (p < .05), then the Wilcoxon rank-sum test (exact p value) was employed for the four pairwise comparisons of interest. A Bonferroni corrected significance level was used (α = 0.05/4 = 0.0125). The Spearman rank correlation coefficient was computed to assess the degree of linear association between temperature and IL-6 and IL-10. Analyses were performed utilizing SAS Version 9.1.
RESULTS
Chlorpromazine Induction of HT and Physiologic Effects
Chlorpromazine, a phenothiazine and potent sedative, is well described for inducing HT in mice (29, 30). Mice given 15 mg/kg IP chlorpromazine and 1 μg/g LPS were heavily sedated within 15 mins at which point their core temperature began to decrease. Mice were then placed in incubators prewarmed to 28°C or 32°C, temperatures which in preliminary studies were demonstrated to keep rectal temperature in mice approximately 32°C or 37°C, respectively. Rectal temperature was monitored continuously in one mouse per group of three and monitored every 30 mins in the others. Minor adjustments in incubator temperature were made, as needed, to keep temperatures in the target range. Incubator temperature was adjusted about every 15 mins for the first hour after chlorpromazine and then mouse and incubator temperatures were generally stable for the remaining time.
Preliminary experiments in adult mice showed that chlorpromazine alone did not significantly alter plasma cytokine levels, regardless of whether mice were maintained hypothermic or normothermic for 6 hrs. Plasma IL-6 levels were 94 ± 28 pg/mL, 128 ± 132 pg/mL, and 179 ± 197 pg/mL (mean ± SD) in sham, chlorpromazine hypothermic (Cpz32), and chlorpromazine normothermic (Cpz37) mice, respectively. IL-10 levels were 231 ± 52 pg/mL, 200 ± 126 pg/mL, and 173 ± 149 pg/mL in sham, Cpz32, and Cpz37 mice. TNF-α levels were 239 ± 156 pg/mL, 113 ± 102 pg/mL, and 58 ± 19 pg/mL and IL-1β levels were 52 ± 25 pg/mL, 50 ± 22 pg/mL, and 57 ± 41 pg/mL in sham, Cpz32, and Cpz37 mice, respectively (n = 3 mice in each group, p = NS for all cytokines).
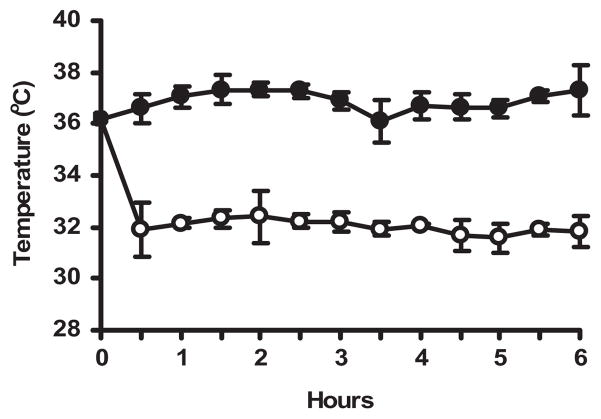
Juvenile mice were treated with 1 μg/g LPS IP followed immediately by chlorpromazine 15 μg/g IP. Using the temperature control strategy, rectal temperature for the 6-hr period of LPS treatment was 32.0 ± 0.46°C (mean ± SD) for the hypothermic group and 36.9 ± 0.48°C for the normothermic group (Fig. 1). There were no significant differences in heart rate or systolic blood pressure in a separate group of juvenile mice identically treated with chlorpromazine and LPS and maintained either hypothermic or normothermic. Mean ± SD values of heart rate and systolic blood pressure were 671 ± 59 beats/min and 114 ± 34 mm Hg, respectively, in hypothermic mice and 630 ± 57 beats/min and 130 ± 36 mm Hg in normothermic mice (n = 6; p > .30).
Figure 1.
Pharmacologic induction and maintenance of hypothermia. Juvenile (4 wk) C57BL/6 mice were given lipopolysaccharide 1 μg/g IP followed by chlorpromazine 15 μg/g IP, then placed in incubators preset to 28°C or 32°C air temperature to maintain rectal temperatures at approximately 32°C or 37°C. Rectal temperature was monitored continuously in one mouse in each group of three and checked every 30 mins in the other mice. Adjustments in incubator temperature were made to maintain rectal temperatures in the target range. Data points represent mean rectal temperature ± SD at 30-min intervals for 14 mice in each temperature group.
Chlorpromazine-Induced HT Increases LPS-Stimulated Plasma IL-6 and IL-10
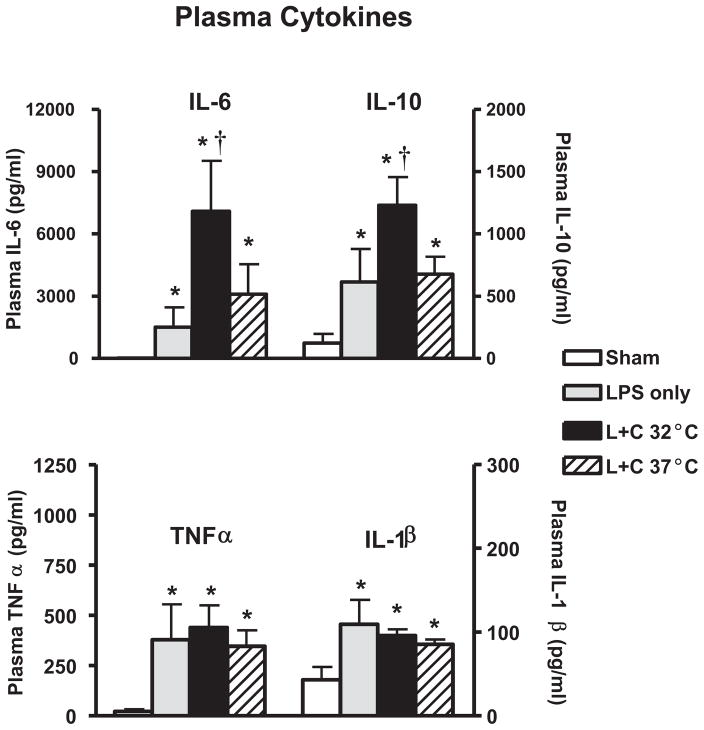
Compared with sham-treated controls, mice treated with 1 μg/g LPS had increased plasma levels of all four cytokines (Fig. 2). In mice treated with LPS and chlorpromazine and maintained hypothermic for 6 hrs, plasma levels of IL-6 were higher than those of identically treated mice maintained normothermic (7090 ± 2433 pg/mL at 32°C vs. 3091 ± 1455 pg/mL at 37°C, n = 14 per group, p = .02). IL-10 plasma levels were also higher in hypothermic than in normothermic LPS-treated mice (1229 ± 227 pg/mL at 32°C vs. 676 ± 139 pg/mL at 37°C, p = .02).
Figure 2.
Chlorpromazine-induced hypothermia increases plasma interleukin (IL)-6 and IL-10 but not tumor necrosis factor (TNF)-α or IL-1β in juvenile endotoxemic mice. Juvenile mice were treated with intraperitoneal lipopolysaccharide (LPS) followed immediately by chlorpromazine (L + C), and rectal temperature was regulated to approximately 32°C (n = 14) or 37°C (n = 14) in temperature-controlled incubators as shown in Figure 1. Other groups of mice received sham treatment (n = 6) or LPS alone (n = 6) (note IL-6 and TNF-α are below the level of visibility on the graph). Six hours after LPS administration, plasma was collected for analysis of IL-6, IL-10, TNF-α, and IL-1β. *p < .05 vs. sham-treated controls. †p < .05 in hypothermic vs. normothermic mice. The data represent five independent experiments.
TNF-α and IL-1β plasma levels were not significantly different at 6 hrs in hypothermic compared with normothermic mice (Fig. 2). However, because these cytokines typically peak earlier than IL-6 and IL-10, additional experiments were performed to assess expression of TNF-α and IL-1β 2 hrs after LPS treatment in mice maintained hypothermic or normothermic with the chlorpromazine protocol. Plasma TNF-α had wide variability and was not significantly different at the 2-hr time point for the two body temperatures (388 ± 341 pg/mL [mean ± SD] in 32°C mice vs. 314 ± 166 pg/mL in mice maintained at 37°C, n = 8 per group). Likewise, plasma IL-1β did not show significant differences in the two temperature cohorts at the 2-hr time point (162 ± 114 pg/mL [mean ± SD] in 32°C mice vs. 122 ± 35 pg/mL in mice maintained at 37°C).
HT Increases Liver TNF-α and IL-6 But Does Not Affect Lung or Brain Cytokines
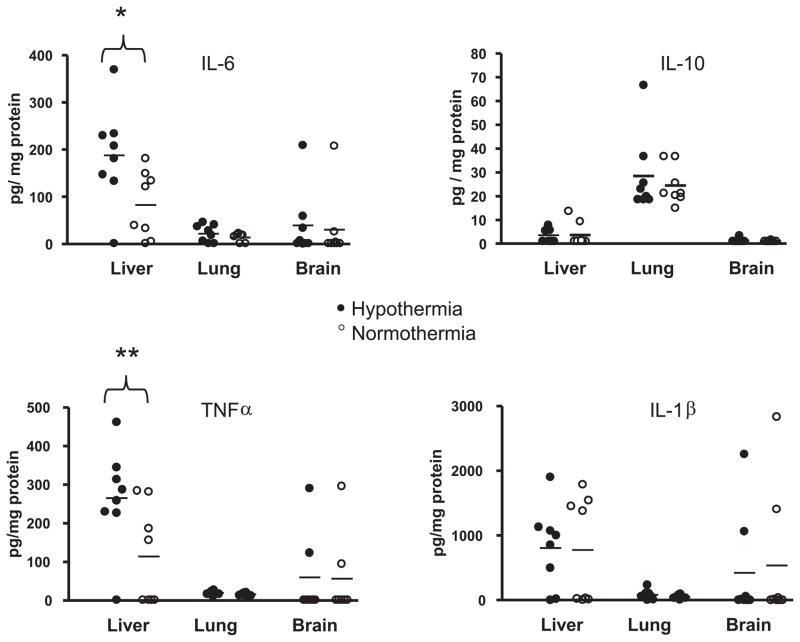
In light of our findings of increased plasma cytokines, we sought to determine whether 6 hrs of systemic HT affected cytokine levels in the organs of juvenile mice treated with 1 μg/g of LPS (n = 8 per temperature group). Brain levels of IL-6, TNF-α, and IL-10 were very low or undetectable in most mice 6 hrs after LPS treatment, whereas IL-1β was higher although not significantly altered by HT (Fig. 3). Lung levels of all four cytokines were low and not affected by temperature. Liver had very low IL-10 expression but higher levels of other cytokines compared with lung and brain. There was significantly more IL-6 and TNF-α in the liver of hypothermic compared with normothermic mice (IL-6: 188 ± 72 pg/mg [mean ± SD] hypothermic vs. 83 ± 63 pg/mg normothermic, n = 8, p = 0.05; TNF-α: 265 ± 86 pg/mg hypothermic vs. 114 ± 113 pg/mg normothermic, p = .03).
Figure 3.
Hypothermia increases liver tumor necrosis factor (TNF)-α and interleukin (IL)-6 but does not alter lung or brain cytokines. Juvenile mice were treated with lipopolysaccharide 1 μg/g and chlorpromazine 15 μg/g and rectal temperature targeted at 32°C (filled circles, n = 8) or 37°C (open circles, n = 8). At 6 hrs, liver, lung, and brain were homogenized and cytokines analyzed. *p = .05 hypothermia vs. normothermia; **p = .03 hypothermia vs. normothermia.
Environmental Cold Effects on Temperature and Plasma IL-6 and IL-10
To show that observed alterations in cytokine expression were not specific to chlorpromazine-induced HT, we attempted to induce HT using a nonpharmacologic system. To minimize stress from rectal temperature monitoring in these unsedated mice, we measured temperature at the external auditory meatus, using an infrared thermometer which registers as low as 34°C. If ear temperature was undetectable with this thermometer (<34°C), rectal temperature was measured. Preliminary experiments showed that the mean difference between ear and rectal temperature readings was 0.1°C (n = 13, 95% Confidence Interval: (−0.3, 0.4)). The concordance correlation coefficient for these 13 pairs was 0.87, indicating very good agreement between the two readings.
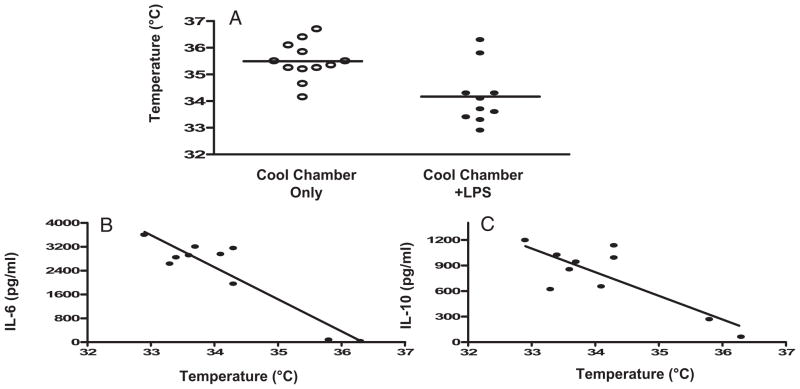
Mice which were sham treated and exposed to a 4°C environment for 6 hrs had a mean temperature of 35.5 ± 0.2°C (range = 34.2°C–36.7°C, n = 12) (Fig. 4A). Only two of 12 mice in this no-LPS group had a temperature <35°C. In contrast, juvenile mice treated with 1 μg/g LPS IP, then exposed to 4°C ambient temperature for 6 hrs, had a mean temperature of 34.2 ± 0.35°C (range = 32.9°C–36.3°C, n = 10). Eight of ten mice in this group experienced a drop in core temperature to <35°C.
Figure 4.
Nonpharmacologic hypothermia increases plasma interleukin (IL)-6 and IL-10. Juvenile mice were sham treated (n = 12) or given lipopolysaccharide (LPS) 1 μg/g IP (n = 10). All mice were then placed in a cool chamber with an ambient temperature of 4°C for 6 hrs. Temperature was monitored every 2 hrs and plasma was collected at 6 hrs for measurements of IL-6 and IL-10. A) Mean temperature with sham or LPS treatment in cool chamber. B, C) Correlation of plasma IL-6 and IL-10 to body temperature in LPS-treated mice (Spearman IL-6 r = −.58 (p = .0765) and IL-10 r = −.49 (p = .1482).
Plasma IL-6 and IL-10 were measured in all cold-exposed mice (n = 22). In sham-treated cold-exposed mice (n = 12), plasma cytokines were undetectable. In contrast, all ten mice which received LPS 1 μg/g IP before cold exposure had detectable plasma IL-6 and IL-10 (Fig. 4B, C). The eight mice which became hypothermic (temperature = <35°C) with LPS plus cool chamber exposure had significantly higher plasma levels of IL-6 and IL-10 compared with the two mice identically treated which did not develop HT (IL-6: 2905 ± 170 pg/mL vs. 53 ± 26 pg/mL; IL-10: 927 ± 74 pg/mL vs. 165 ± 103 pg/mL, p < .001). In this group of LPS plus cool chamber-exposed mice, there was a trend toward a negative correlation between body temperature and plasma IL-6 and IL-10 levels (Fig. 4B, C) (Spearman r = −.58 [p =.0765] and −.49 [p = .1482], respectively).
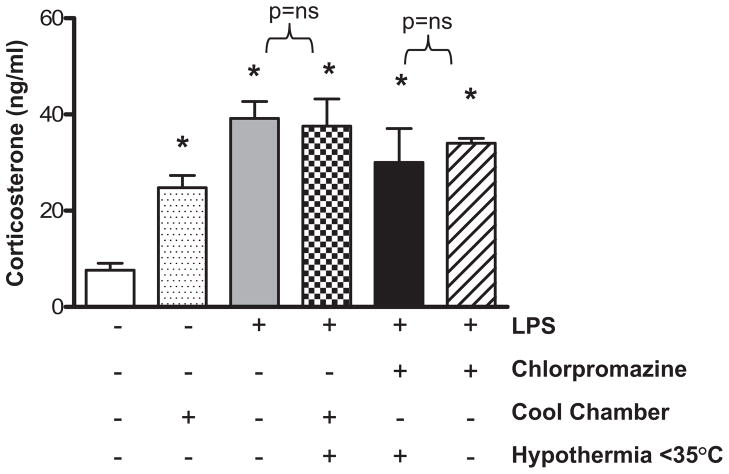
HT Does Not Increase Corticosterone in LPS-Treated Mice
Endogenous glucocorticoids are released in response to physiologic stress and influence production of pro- and anti-inflammatory cytokines (31). We therefore sought to establish whether HT modified corticosterone production in our system. Compared with sham-treated controls (n = 8), mice kept in a cool chamber for 6 hrs had significantly higher plasma corticosterone levels (n = 12) (Fig. 5). LPS treatment alone (n = 6) also significantly increased corticosterone levels over baseline. Mice with combined LPS and cool chamber exposure (mean rectal temperature = 34.2 ± 0.35°C, n = 7) had comparable corticosterone levels to mice with LPS treatment alone which were normothermic. Similarly, mice treated with combination chlorpromazine and LPS and maintained hypothermic (32.0 ± 0.46°C) had comparable corticosterone levels with mice identically treated but maintained normothermic (n = 3 per group). These data suggest that, in our system, HT-induced changes in cytokine expression are not related to a nonspecific stress response.
Figure 5.
In lipopolysaccharide (LPS)-treated mice, pharmacologic or environmental hypothermia do not alter plasma corticosterone levels. Plasma corticosterone levels were measured in mice that were sham-treated or treated for 6 hrs with intraperitoneal LPS, chlorpromazine, cool chamber exposure, or combination treatment (n = 3–12 per group, *p < .05 compared with control).
DISCUSSION
Maintaining an appropriate balance of pro- and anti-inflammatory cytokines is critically important to host defense in the face of illness or injury. Given our previous findings that LPS-stimulated human monocytes overproduce TNF-α and IL-1β during exposure to hypothermic temperatures (32°C), we developed the current model to determine whether in vivo HT increases proinflammatory cytokines. On the contrary, we found that TNF-α and IL-1β were not significantly increased in the plasma of mice exposed to endotoxin and induced HT for 2 hrs or 6 hrs compared with identically treated mice maintained normothermic. However, the anti-inflammatory cytokine IL-10 and the counterregulatory cytokine IL-6 were significantly increased by in vivo HT.
LPS was used in these studies to stimulate a systemic inflammatory response. In pediatric patients undergoing HT therapy, including children with cardiac arrest and neonates with hypoxic-ischemic encephalopathy (HIE), infection may be present as either a coexisting or a contributing factor to the asphyxial event. In a large randomized, controlled trial of HT therapy for neonatal HIE, maternal fever was present in 10% of cases and 5% of enrolled neonates had culture-proven sepsis (4). Most patients undergoing HT therapy will be treated with antibiotics, but bacterial toxins may nonetheless be present and contribute to a systemic inflammatory response. Even in the absence of infection, patients with ischemia/reperfusion injury, such as cardiac arrest, have been shown to have detectable plasma endotoxin and high levels of circulating cytokines (26). In our mouse model, we gave a relatively low dose of LPS, which induces cytokine production yet does not cause significant hemodynamic compromise, signs of illness, or histopathologic changes; thus, we were unable to determine whether tissue injury or inflammation was impacted by HT. We did, however, find that liver expression of TNF-α and IL-6 was higher in hypothermic compared with normothermic mice, suggesting that Kupffer cells or hepatocytes may be a source of excess cytokine production.
It is not surprising to find differences between in vivo and in vitro cytokine responses to HT because interactions between different cell types impact cytokine production in a whole animal model. Increased plasma IL-6 and IL-10 during in vivo HT may, for example, lead to dampening of TNF-α and IL-1β production by circulating leukocytes. In vitro studies also do not mimic physiologic and metabolic changes occurring in the hypothermic animal, which may affect cytokine production, such as release of stress hormones or catecholamines. In our model, LPS treatment or exposure to a cold environment without sedation each caused a significant increase in plasma corticosterone levels. However, in LPS-treated mice, addition of cold exposure or chlorpromazine-induced HT did not result in a further increase in corticosterone levels, suggesting that changes in cytokines in this model are not due to an exaggerated glucocorticoid stress response. We did not measure plasma catecholamines, which may be elevated during induced HT (32) and which have been shown to increase IL-10 production (33).
The mechanism and consequences of increased systemic IL-10 expression during induced HT remain speculative. Although in vitro HT has been reported to decrease IL-10 production by human monocytes (34) and rat microglia (35), a study in rats showed that induction of HT increased ex vivo IL-10 production by T lymphocytes (36). A number of other studies in various animal injury models have reported increased systemic IL-10 expression during induced HT (19, 37). This is not a universal finding, as a recent study in a rat model of cardiac arrest found decreased plasma IL-10 in animals maintained hypothermic without pharmacologic intervention (38). These apparently discrepant results could be explained by differences in species, injury model, depth and mode of inducing HT, or timing of measurements. It is important to elucidate effects of induced HT on IL-10 expression because IL-10 has been postulated to have a protective role in acute brain injury, possibly by dampening production of cytokines, such as TNF-α and IL-1β, which may be directly toxic to brain cells and can alter cerebral temperature and blood flow (39). IL-10 may also be neuroprotective by decreasing glial activation and inhibiting macrophage infiltration into the brain (40, 41). On the other hand, prolonged overexpression of IL-10 has been linked to immunosuppression and adverse outcomes in sepsis (42). It is possible that HT could be a double-edged sword, conferring neuroprotection to some patients but predisposing to comorbidities, such as nosocomial sepsis.
IL-6 levels have previously been reported to be decreased (16, 43–45), increased (19, 46 –48), or unchanged (18, 38, 49, 50) in hypothermic vs. normothermic rodents or humans with a variety of insults. In a report of pediatric patients with traumatic brain injury, patients randomized to induced HT had similar cerebrospinal fluid cytokines including IL-6 compared with normothermic TBI patients, but serum cytokine levels were not reported (50). High IL-6 expression has been associated with adverse outcome in some conditions, such as sepsis (48), yet increasing evidence suggests that IL-6 may confer protection in acute brain injury. Neuroprotective properties attributed to IL-6 include stimulating production of neurotrophic factors, activating immune cells involved in neuroregeneration, and protecting against apoptosis (51). We did not find that HT increased IL-6 expression in the brain but our model was not designed to induce brain injury or inflammation.
Two important considerations in interpreting results in our model include the nature of the injury and the method and duration of induced HT. We chose an endotoxin model to parallel our previous in vitro studies. Although some patients undergoing HT have circulating endotoxin, this model does not mimic an ischemia-reperfusion insult in which multiple other mechanisms of increased cytokine production and tissue injury are at play. Our results do, however, suggest that HT-induced changes in inflammatory cytokines and tissue inflammation should be considered in studies of hypothermic protection in various injury models. As for the technical aspects of inducing HT, the small size and high metabolic rate of mice makes monitoring and lowering their core temperature particularly challenging. Pharmacologic agents are used in both animals and humans undergoing HT to block thermogenic processes and reduce stress. Chlorpromazine, although not commonly used for lowering core temperature in humans, induces essentially a poikilothermic state in mice (29, 30). This allowed us to treat mice identically with chlorpromazine and LPS and maintain them either hypothermic or normothermic in a warmed environment so that the only experimental variable was body temperature. The duration of action of a single dose of chlorpromazine allowed us to study a relatively short period of HT. Important future challenges in animal studies of therapeutic HT include extending the duration of cooling toward that used in human applications (at least 12–24 hrs) and developing methods for lowering core temperature that mimic those used in the intensive care unit.
In conclusion, we developed a novel juvenile mouse HT system and showed that a 5°C drop in core temperature significantly alters expression of critical inflammation-modulating cytokines. IL-6 and IL-10 have potential cytoprotective properties, and their increased expression could play a role in the beneficial effects of HT in some patients. On the other hand, in certain clinical situations, such as sepsis, prolonged overexpression of IL-10 could be detrimental to host defense. Elucidating the mechanisms by which temperature alters these and other cytokines and chemokines may lead to a better understanding of how therapeutic HT will affect acutely ill or injured patients.
Acknowledgments
This work was supported, in part, by a University of Virginia Children’s Hospital Fellow Grant (CRS) and Grant 0365547U from the American Heart Association, Jeffress Memorial Research Trust, and Grant 5K08HD051609-02 (KDF) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
The authors have not disclosed any potential conflicts of interest.
References
- 1.Group THaCAS. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 2.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 3.Eicher DJ, Wagner CL, Katikaneni LP, et al. Moderate hypothermia in neonatal encephalopathy: Efficacy outcomes. Pediatr Neurol. 2005;32:11–17. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 5.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: Multicentre randomised trial. Lancet. 2005;365:663– 670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 6.Hutchison JS, Ward RE, Lacroix J, et al. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358:2447–2456. doi: 10.1056/NEJMoa0706930. [DOI] [PubMed] [Google Scholar]
- 7.Chu SJ, Perng WC, Hung CM, et al. Effects of various body temperatures after lipopolysaccharide-induced lung injury in rats. Chest. 2005;128:327–336. doi: 10.1378/chest.128.1.327. [DOI] [PubMed] [Google Scholar]
- 8.Hong SB, Koh Y, Lee IC, et al. Induced hypothermia as a new approach to lung rest for the acutely injured lung. Crit Care Med. 2005;33:2049–2055. doi: 10.1097/01.ccm.0000178186.37167.53. [DOI] [PubMed] [Google Scholar]
- 9.Stravitz RT, Lee WM, Kramer AH, et al. Therapeutic hypothermia for acute liver failure: Toward a randomized, controlled trial in patients with advanced hepatic encephalopathy. Neurocrit Care. 2008;9:90–96. doi: 10.1007/s12028-008-9090-y. [DOI] [PubMed] [Google Scholar]
- 10.Laptook AR, Corbett RJ, Sterett R, et al. Quantitative relationship between brain temperature and energy utilization rate measured in vivo using 31P and 1H magnetic resonance spectroscopy. Pediatr Res. 1995;38:919–925. doi: 10.1203/00006450-199512000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Globus MY, Alonso O, Dietrich WD, et al. Glutamate release and free radical production following brain injury: Effects of post-traumatic hypothermia. J Neurochem. 1995;65:1704–1711. doi: 10.1046/j.1471-4159.1995.65041704.x. [DOI] [PubMed] [Google Scholar]
- 12.Gunn AJ, Gunn TR, de Haan HH, et al. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest. 1997;99:248–256. doi: 10.1172/JCI119153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ginsberg MD, Belayev L. BIological and Molecular Mechanisms of Hypothermic Neuroprotection. In: Mayer SA, Sessler DI, editors. Therapeutic Hypothermia. New York: Marcel Decker; 2005. pp. 85–140. [Google Scholar]
- 14.Beilin B, Shavit Y, Razumovsky J, et al. Effects of mild perioperative hypothermia on cellular immune responses. Anesthesiology. 1998;89:1133–1140. doi: 10.1097/00000542-199811000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Kinoshita K, Chatzipanteli K, Vitarbo E, et al. Interleukin-1beta messenger ribonucleic acid and protein levels after fluid-percussion brain injury in rats: importance of injury severity and brain temperature. Neurosurgery. 2002;51:195–203. doi: 10.1097/00006123-200207000-00027. discussion 203. [DOI] [PubMed] [Google Scholar]
- 16.Aibiki M, Maekawa S, Ogura S, et al. Effect of moderate hypothermia on systemic and internal jugular plasma IL-6 levels after traumatic brain injury in humans. J Neurotrauma. 1999;16:225–232. doi: 10.1089/neu.1999.16.225. [DOI] [PubMed] [Google Scholar]
- 17.Sutcliffe IT, Smith HA, Stanimirovic D, et al. Effects of moderate hypothermia on IL-1 beta-induced leukocyte rolling and adhesion in pial microcirculation of mice and on proinflammatory gene expression in human cerebral endothelial cells. J Cereb Blood Flow Metab. 2001;21:1310–1319. doi: 10.1097/00004647-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Wu X, Stezoski J, Safar P, et al. Mild hypothermia during hemorrhagic shock in rats improves survival without significant effects on inflammatory responses. Crit Care Med. 2003;31:195–202. doi: 10.1097/00003246-200301000-00030. [DOI] [PubMed] [Google Scholar]
- 19.Leon LR, Blaha MD, DuBose DA. Time course of cytokine, corticosterone, and tissue injury responses in mice during heat strain recovery. J Appl Physiol. 2006;100:1400–1409. doi: 10.1152/japplphysiol.01040.2005. [DOI] [PubMed] [Google Scholar]
- 20.Kentner R, Rollwagen FM, Prueckner S, et al. Effects of mild hypothermia on survival and serum cytokines in uncontrolled hemorrhagic shock in rats. Shock. 2002;17:521–526. doi: 10.1097/00024382-200206000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Luhm J, Schromm AB, Seydel U, et al. Hypothermia enhances the biological activity of lipopolysaccharide by altering its fluidity state. Eur J Biochem. 1998;256:325–333. doi: 10.1046/j.1432-1327.1998.2560325.x. [DOI] [PubMed] [Google Scholar]
- 22.Fairchild KD, Singh IS, Patel S, et al. Hypothermia prolongs activation of NF-kappaB and augments generation of inflammatory cytokines. Am J Physiol Cell Physiol. 2004;287:C422–C431. doi: 10.1152/ajpcell.00507.2003. [DOI] [PubMed] [Google Scholar]
- 23.Fairchild KD, Singh IS, Carter HC, et al. Hypothermia enhances phosphorylation of I{kappa}B kinase and prolongs nuclear localization of NF-{kappa}B in lipopolysaccharide-activated macrophages. Am J Physiol Cell Physiol. 2005;289:C1114–C1121. doi: 10.1152/ajpcell.00152.2005. [DOI] [PubMed] [Google Scholar]
- 24.Aly H, Khashaba MT, El-Ayouty M, et al. IL-1beta, IL-6 and TNF-alpha and outcomes of neonatal hypoxic ischemic encephalopathy. Brain Dev. 2006;28:178–182. doi: 10.1016/j.braindev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Stoll G, Jander S, Schroeter M. Detrimental and beneficial effects of injury-induced inflammation and cytokine expression in the nervous system. Adv Exp Med Biol. 2002;513:87–113. doi: 10.1007/978-1-4615-0123-7_3. [DOI] [PubMed] [Google Scholar]
- 26.Adrie C, Adib-Conquy M, Laurent I, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106:562–568. doi: 10.1161/01.cir.0000023891.80661.ad. [DOI] [PubMed] [Google Scholar]
- 27.Saegusa Y, Tabata H. Usefulness of infrared thermometry in determining body temperature in mice. J Vet Med Sci. 2003;65:1365–1367. doi: 10.1292/jvms.65.1365. [DOI] [PubMed] [Google Scholar]
- 28.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- 29.Lundgren-Eriksson L, Carlsson A, Ottosson-Lönn S, et al. Chlorpromazine-induced hypothermia: Effect of tumour progression and survival in mice. Anticancer Res. 1996;16:333–335. [PubMed] [Google Scholar]
- 30.Boschi G, Launay N, Rips R. Neuroleptic-induced hypothermia in mice: Lack of evidence for a central mechanism. Br J Pharmacol. 1987;90:745–751. doi: 10.1111/j.1476-5381.1987.tb11228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Druker J, Liberman AC, Acuna M, et al. Molecular understanding of cytokine-steroid hormone dialogue: Implications for human diseases. Ann N Y Acad Sci. 2006;1088:297–306. doi: 10.1196/annals.1366.007. [DOI] [PubMed] [Google Scholar]
- 32.Chernow B, Lake CR, Zaritsky A, et al. Sympathetic nervous system “switch off” with severe hypothermia. Crit Care Med. 1983;11:677– 680. doi: 10.1097/00003246-198309000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Elenkov IJ, Papanicolaou DA, Wilder RL, et al. Modulatory effects of glucocorticoids and catecholamines on human interleukin-12 and interleukin-10 production: clinical implications. Proc Assoc Am Physicians. 1996;108:374–381. [PubMed] [Google Scholar]
- 34.Matsui T, Ishikawa T, Takeuchi H, et al. Mild hypothermia inhibits IL-10 production in peripheral blood mononuclear cells. Acta Anaesthesiol Scand. 2004;48:205–210. doi: 10.1111/j.0001-5172.2004.00293.x. [DOI] [PubMed] [Google Scholar]
- 35.Matsui T, Kakeda T. IL-10 production is reduced by hypothermia but augmented by hyperthermia in rat microglia. J Neurotrauma. 2008;25:709–715. doi: 10.1089/neu.2007.0482. [DOI] [PubMed] [Google Scholar]
- 36.Lee SL, Battistella FD, Go K. Hypothermia induces T-cell production of immunosuppressive cytokines. J Surg Res. 2001;100:150–153. doi: 10.1006/jsre.2001.6230. [DOI] [PubMed] [Google Scholar]
- 37.Scumpia PO, Sarcia PJ, Kelly KM, et al. Hypothermia induces anti-inflammatory cytokines and inhibits nitric oxide and myeloperoxidase-mediated damage in the hearts of endotoxemic rats. Chest. 2004;125:1483–1491. doi: 10.1378/chest.125.4.1483. [DOI] [PubMed] [Google Scholar]
- 38.Callaway CW, Rittenberger JC, Logue ES, et al. Hypothermia after cardiac arrest does not alter serum inflammatory markers. Crit Care Med. 2008;36:2607–2612. doi: 10.1097/CCM.0b013e318184443b. [DOI] [PubMed] [Google Scholar]
- 39.Monroy M, Kuluz JW, He D, et al. Role of nitric oxide in the cerebrovascular and thermoregulatory response to interleukin-1 beta. Am J Physiol Heart Circ Physiol. 2001;280:H1448–H1453. doi: 10.1152/ajpheart.2001.280.4.H1448. [DOI] [PubMed] [Google Scholar]
- 40.Dietrich WD, Busto R, Bethea JR. Postischemic hypothermia and IL-10 treatment provide long-lasting neuroprotection of CA1 hippocampus following transient global ischemia in rats. Exp Neurol. 1999;158:444– 450. doi: 10.1006/exnr.1999.7115. [DOI] [PubMed] [Google Scholar]
- 41.Pang Y, Rodts-Palenik S, Cai Z, et al. Suppression of glial activation is involved in the protection of IL-10 on maternal E.coli induced neonatal white matter injury. Brain Res Dev Brain Res. 2005;157:141–149. doi: 10.1016/j.devbrainres.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 42.Albaiceta GM, Pedreira PR, Garcia-Prieto E, et al. Therapeutic implications of immuno-paralysis in critically ill patients. Inflamm Allergy Drug Targets. 2007;6:191–196. doi: 10.2174/187152807783334337. [DOI] [PubMed] [Google Scholar]
- 43.Torossian A, Ruehlmann S, Middeke M, et al. Mild preseptic hypothermia is detrimental in rats. Crit Care Med. 2004;32:1899–1903. doi: 10.1097/01.ccm.0000139608.34486.fd. [DOI] [PubMed] [Google Scholar]
- 44.Yanagawa Y, Kawakami M, Okada Y. Moderate hypothermia alters interleukin-6 and interleukin-1alpha reactions in ischemic brain in mice. Resuscitation. 2002;53:93–99. doi: 10.1016/s0300-9572(01)00499-3. [DOI] [PubMed] [Google Scholar]
- 45.Gundersen Y, Vaagenes P, Pharo A, et al. Moderate hypothermia blunts the inflammatory response and reduces organ injury after acute haemorrhage. Acta Anaesthesiol Scand. 2001;45:994–1001. doi: 10.1034/j.1399-6576.2001.450812.x. [DOI] [PubMed] [Google Scholar]
- 46.Grunenfelder J, Zund G, Schoeberlein A, et al. Expression of adhesion molecules and cytokines after coronary artery bypass grafting during normothermic and hypothermic cardiac arrest. Eur J Cardiothorac Surg. 2000;17:723–728. doi: 10.1016/s1010-7940(00)00401-2. [DOI] [PubMed] [Google Scholar]
- 47.Horan M, Ichiba S, Firmin RK, et al. A pilot investigation of mild hypothermia in neonates receiving extracorporeal membrane oxygenation (ECMO) J Pediatr. 2004;144:301–308. doi: 10.1016/j.jpeds.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 48.Remick DG, Bolgos G, Copeland S, et al. Role of interleukin-6 in mortality from and physiologic response to sepsis. Infect Immun. 2005;73:2751–2757. doi: 10.1128/IAI.73.5.2751-2757.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Truettner JS, Suzuki T, Dietrich WD. The effect of therapeutic hypothermia on the expression of inflammatory response genes following moderate traumatic brain injury in the rat. Brain Res Mol Brain Res. 2005;138:124–134. doi: 10.1016/j.molbrainres.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 50.Buttram SD, Wisniewski SR, Jackson EK, et al. Multiplex assessment of cytokine and chemokine levels in cerebrospinal fluid following severe pediatric traumatic brain injury: Effects of moderate hypothermia. J Neurotrauma. 2007;24:1707–1718. doi: 10.1089/neu.2007.0349. [DOI] [PubMed] [Google Scholar]
- 51.Carlson NG, Wieggel WA, Chen J, et al. Inflammatory cytokines IL-1 alpha, IL-1 beta, IL-6, and TNF-alpha impart neuroprotection to an excitotoxin through distinct pathways. J Immunol. 1999;163:3963–3968. [PubMed] [Google Scholar]