Abstract
Intracellular Ca2+ stores play a central role in the regulation of cellular [Ca2+]i and the generation of complex [Ca2+] signals such as oscillations and waves. Ca2+ signalling is of particular significance in sperm cells, where it is a central regulator in many key activities (including capacitation, hyperactivation, chemotaxis and acrosome reaction) yet mature sperm lack endoplasmic reticulum and several other organelles which serve as Ca2+ stores in somatic cells. Here we review (i) the evidence for the expression in sperm of the molecular components (pumps and channels) which are functionally significant in the activity of Ca2+ stores of somatic cells and (ii) the evidence for the existence of functional Ca2+ stores in sperm. This evidence supports the existence of at least two storage organelles in mammalian sperm, one in the acrosomal region and another in the region of the sperm neck and midpiece. We then go on to discuss the likely identity of these organelles and their discrete functions: regulation by the acrosome of its own secretion and regulation by membranous organelles at the sperm neck (and possibly by the mitochondria) of flagellar activity and hyperactivation. Finally we consider the ability of the sperm discretely to control mobilisation of these stores and the functional interaction of stored Ca2+ at the sperm neck/midpiece with CatSper channels in the principal piece in regulation of the activities of mammalian sperm.
1. [Ca2+]i - a central regulator in sperm function
Regulation of cellular activity, in response to signals from other cells or from the extracellular environment, can occur at a number of levels. Long term regulation is achieved by control of gene expression. This process can occur through control of translation and/or transcription and also by more subtle regulation of mRNA transcripts and turnover of the protein product. Effects of this type are typically exerted over time periods measured in hours rather than minutes or seconds. Regulation of cellular activity over shorter time periods is achieved by rapid, ‘post-translational’ modification of the function of proteins already present. Various pathways have been characterised, by which the actions of extracellular signals such as hormones, growth factors and transmitters are transduced, leading to appropriate modification of protein function. One such mechanism is through changes in the intracellular Ca2+ concentration ([Ca2+]i).
In sperm, which lack endoplasmic reticulum and have a highly condensed nucleus, regulation of function by translation/transcription (if it occurs at all) will be very limited. Post-translational mechanisms must, therefore, control all activities of the cell. Regulation of protein function through Ca2+ signalling is central to a range of activities that are pivotal to sperm function, including hyperactivation, chemotaxis and acrosome reaction (Publicover et al., 2007). Impairment of Ca2+ signalling in sperm is associated with male sub-fertility (Krausz et al, 1995; Baldi et al, 1999; Espino et al, 2009)
Signalling though [Ca2+]i is achieved by permitting Ca2+ to enter the cytoplasm (where concentration is maintained very low) from the extracellular space and/or from intracellular organelles, where the Ca2+ concentration is up to four orders of magnitude higher. Signal initiation requires merely that Ca2+ permeable membrane channels are opened, allowing the ions to flow down their electrochemical gradient. The presence of Ca2+ channels in the plasma membrane of sperm cells is well established, as is their significance in the key activities of sperm. A number of thorough reviews on the various types and distribution of these channels are available (Darszon et al. 1999, 2007; Jimenez-Gonzalez et al, 2006; Felix et al, 2005; Navarro et al, 2008). Here we will review evidence for the existence, identity and role(s) of Ca2+ storage organelles in sperm.
2. Ca2+ stores in somatic cells and their associated Ca2+ transporters
Somatic cells contain a number of membrane-bound organelles that undertake various biochemical reactions vital to the maintenance of cellular homeostasis and viability (Berridge et al., 1998). Many of these organelles also act as Ca2+ reservoirs or Ca2+ stores, which contribute to the regulation of Ca2+-dependent processes (Michelangeli et al. 2005). In order to be classified as a bona fide Ca2+ store, an organelle must have at least two types of Ca2+ transporters, enabling both loading of the store and release of stored Ca2+ in a controlled fashion.
2.1 Ca2+ uptake and release mechanisms
Ca2+ accumulation into stores normally occurs against the electrochemical gradient for the ion and therefore requires expenditure of energy. Typically this is achieved by ATPase ‘pumps’ such as the sarcoplasmic-endoplasmic reticulum Ca2+ ATPase (SERCA) or secretory pathway Ca2+ ATPases (SPCA), though Ca2+ exchangers (co-transporters) may also be used. In contrast, controlled release of Ca2+ can be achieved by gating of Ca2+-permeable ion channels in the membrane of the organelle. These are usually regulated by second messengers (or putative second messengers) such as inositol 1,4,5-trisphosphate (InsP3), cyclic adenine nucleotide phosphate ribose (cADP-ribose), nicotinic acid adenine dinucleotide phosphate (NAADP) and even by Ca2+ itself, via a Ca2+-induced Ca2+ release mechanism (CICR) (Bootman et al, 2001). The difference in mechanisms for uptake and release of stored Ca2+ has significant effects upon the rates at which Ca2+ translocation occurs. SERCAs must undergo multiple binding and conformational states during translocation of Ca2+ and transport only a few ions per ATPase molecule per second, whereas a single release channel can transport 100,000’s of Ca2+ in the same period (Taylor, 1995).
2.2 Ca2+ storage organelles
2.2.1 Endoplasmic reticulum
In the early 1980s it was first demonstrated that the endoplasmic reticulum (ER) acted as a Ca2+ store that could release its Ca2+ in the presence of the agonist-generated second messenger InsP3 (Berridge, 2002). This release was later shown to occur via activation of Ins3P receptors (Ins3PRs), InsP3-activated Ca2+ channels located on the ER membranes (Michelangeli et al, 1995). From analogous studies on striated muscle sarcoplasmic reticulum (SR), it was shown that ER membranes also contain SERCA pumps for Ca2+ accumulation and ryanodine receptor (RyR) type Ca2+ channels, named for their sensitivity to the drug ryanodine, but activated in vivo by Ca2+ itself (CICR) and possibly by cADP-ribose (Michelangeli et al., 2005). Though there seems little doubt that the ER is the primary store of Ca2+ that is used in intracellular Ca2+ signalling, other organelles may also play a role (Michelangeli et al., 2005).
2.2.2 Nuclear, golgi and lysosomal Ca2+ storage
Immunohistochemical and biochemical studies have shown that the nuclear envelope, the outer membrane of which is continuous with the ER, also contains both SERCA Ca2+ pumps and InsP3 receptor Ca2+ channels (Lanini et al 1992, Humbert et al., 1996). RyR type Ca2+ channels have also been identified on the nuclear membrane (Gerasimenko et al., 2003). In some cells the nuclear membrane forms a complex tubular network which penetrates deep into the nucleus and which is particularly enriched in InsP3 receptors (Echevarria et al, 2003). This has lead to the suggestion that Ca2+ mobilisation, leading to localised increases in [Ca2+] within distinct regions of the nucleus, may affect gene transcription.
The Golgi apparatus, involved in both post-translational protein modification and protein trafficking, has also been shown to contain InsP3 receptors and SERCA Ca2+ pumps (Surra & Wolff, 2000). These transporters are localised to the cis Golgi region, while membranes of the trans Golgi region contain the SPCA pump (Missiaen et al., 2004; Wootton et al., 2005), which has different transport properties compared to SERCA.
A role for lysosomes in Ca2+ signalling is suggested by the observation that they release Ca2+ when treated with the NAD metabolite and putative second messenger NAADP, which activates the NAADP-sensitive Ca2+ channel (Churchill et al., 2002; Kinnear et al., 2004) of the two-pore (TPC) family Calcraft et al, 2009). These organelles are believed to be filled by a H+/Ca2+ exchanger utilising the proton gradient across the membrane maintained by the vacuolar H+ ATPase (Churchill et al., 2002).
2.2.3 Mitochondria
It has been known for some time that mitochondria can accumulate Ca2+ into the matrix space, primarily through the mitochondrial Ca2+ uniporter (MCU) located on the inner mitochondrial membrane. Ca2+ uptake is driven by the negative membrane potential of the mitochondrial matrix. Recent studies have shown the MCU to be a Ca2+ channel of relatively low conductance, with a complex gating mechanism (Kirichok et al., 2004). Controlled release of mitochondrial Ca2+ can occur through a Na+/Ca2+ exchanger (Bernardi, 1999). Under conditions where ‘resting’ [Ca2+]i is elevated, Ca2+ uptake by mitochondria both activates a number of key tricarboxylic acid cycle dehydrogenases and also acts as a Ca2+ sink in order to buffer cytosolic Ca2+ levels (Gunter et al., 2004). If excessive mitochondrial Ca2+ accumulation occurs this can lead to activation of the permeability transition pore (PTP), which permits release from the mitochondrial matrix of factors that initiate cell death (Dong et al, 2007; Orrenius et al., 2003; Jeong & Seol, 2008). However, under physiological conditions mitochondria also play an important role in Ca2+ buffering and signalling, shaping (and often extending) the kinetics of Ca2+ signals (Bianchi et al, 2004; Rimessi et al, 2008).
Over the last few years researchers have begun to investigate potential interactions between different Ca2+ stores, some recent evidence indicating that such interactions may contribute to the complexity of spatio-temporal intracellular [Ca2+] profiles (Michelangeli et al., 2005). Current research is now focussing on identifying these interactions and assessing their roles in controlling complex physiological processes.
3. Do sperm have Ca2+ stores?
In somatic cells the ER is the primary Ca2+ storage organelle. A mature sperm has no recognisable ER but does have a nuclear membrane, an acrosome (a single capshaped vesicle that surrounds the anterior nucleus), mitochondria (which are concentrated in the midpiece) and some poorly-defined, irregular membranous structures in the region of the sperm neck from where the cytoplasmic droplet has been shed (fig 1a). Since organelles other than the ER can participate in storage and release of Ca2+ in somatic cells (see section 2.2 above), any or several of the membranous structures of sperm may act as releasable Ca2+ stores.
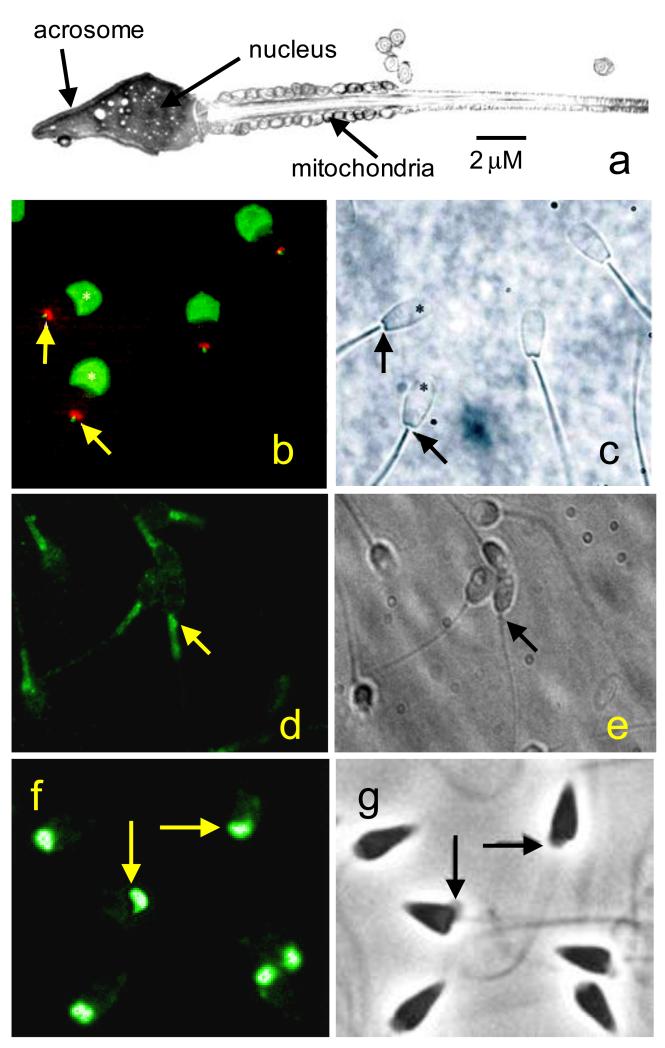
Figure 1.
Localisation of Ca2+ store components in sperm. a; Electron microscope image of a human sperm showing the localisation of the acrosome, nucleus and mitochondria. b: immunolocalisation of InsP3Rs (green) and nuclear pore complex proteins (red) in bovine sperm. InsP3 receptors are localised over the acrosome (asterisks) and also at the sperm neck (arrows). Nuclear pore complex proteins indicate site of redundant nuclear envelope. c: phase image of the cells in panel b. d: immunolocalisation of secretory pathway Ca2+ ATPase (SPCA) (green) in human sperm. SPCA is restricted to the neck and midpiece. In many cells there is a particular concentration at the sperm neck (arrows). e: phase image of the cells in panel d. f: immunolocalisation of secretory pathway Ca2+ ATPase (SPCA) (green) in sperm of the sea urchin Strongylocentrotus purpuratus. SPCA localises to the giant mitochondrion in each cell (arrows). g: phase image of the cells in panel f. Panels b and c from Ho and Suarez (2003) with permission. Panels f and g from Gunaratne & Vacquier (2006) with permission.
3.1. Components of Ca2+ storage organelles expressed in sperm
3.1.1 Inositol trisphosphate receptors
The first clear evidence that intracellular organelles in mature mammalian sperm might act as Ca2+ stores was the finding of Walensky & Snyder (1995) that components of the phosphoinositide signalling system are present in mammalian sperm. Both the G protein Gαq/11 and the β1 isoform of phospholipase C, which generates the Ca2+-mobilising intracellular ligand InsP3, were identified in the acrosomal (anterior head) region. InsP3Rs were also present, primarily in the anterior head, though a second, smaller concentration of receptors was detected in the anterior midpiece. InsP3Rs were enriched in acrosomal fractions and were lost from the sperm into the medium upon acrosome reaction, consistent with localisation to the outer acrosomal membrane. Tomes et al (1996) identified PLCγ in the head of mouse sperm. The enzyme was transferred to the particulate fraction during capacitation. Stimulation of the sperm with solubilised zona pellucida increased tyrphostin-sensitive PLC activity, suggesting that the γ isoform of PLC was activated during induction of acrosome reaction. Since these initial reports, the presence of InsP3Rs in the sperm of a number of mammals has been confirmed (Dragileva et al 1999; Kuroda et al 1999; Naaby-Hansen et al, 2001; Ho & Suarez, 2001; 2003). Though the exact pattern of staining that was reported varied somewhat between species and between studies, two concentrations of Ins3PRs were generally reported, one over the acrosome and the other at the sperm neck or anterior midpiece (fig. 1b). In sea urchin sperm an InsP3-binding protein has also been identified. An antibody against the type I InsP3R recognised a protein present in the sperm plasma membrane (Zapata et al, 1997)
3.1.2 Ryanodine receptors
The evidence regarding expression of ryanodine receptors (RyRs) in mammalian sperm is less clear. We have observed staining of mature human sperm in the region of the sperm neck both with BODIPY FL-X ryanodine (a fluorescently-tagged ryanodine derivative) and with antibodies against RyRs 1 and 2 (Harper et al, 2004; Lefievre et al, 2007). In contrast, others have reported no staining with BODIPY FL-X ryanodine in bovine sperm (Ho & Suarez, 2001) and staining only for RyR3 in mature rodent sperm (Trevino et al, 1998). The conductance of RyRs (>100 pS; Zalk et al, 2007) is particularly high for Ca2+-permeable channels so it is possible that RyRs, if present in sperm, are expressed at extremely low levels. Only one or two channels may be present in each cell, Ca2+ flux being regulated by the proportion of time for which the channel is open as it flickers between open and closed states.
3.1.3 Ca2+ store pumps and Ca2+ chelating proteins
Rossato et al (2001) used the BODIPY derivative of thapsigargin, a highly potent and specific blocker of SERCAs (Treiman et al, 1998), to probe for the presence of SERCAs in human sperm. Similarly to staining patterns for InsP3Rs, localisation of BODIPY thapsigargin was observed over the acrosome and also the midpiece. More recently Lawson et al (2007), using antibodies to SERCA type 2, obtained a similar pattern of staining in human, bovine and mouse sperm. SERCA 2 was also detected by Western blot. Subcellular fractionation showed that binding occurred primarily in the membrane fraction of the cells and also suggested that different splice variants of SERCA2 were present in different sub-cellular locations. In contrast, using immunolocalisation and western blotting we were unable to detect SERCAs in human sperm using a pan-SERCA antibody, but did detect secretory pathway Ca2+ ATPase 1 (SPCA1), another intracellular Ca2+ pump. SPCA1 immuno-staining was observed only at the sperm neck/midpiece (Harper et al, 2005; fig. 1d, e). Similar results were obtained with sperm of the sea urchin (Gunaratne & Vacquier, 2006) (fig. 1f,g). If SERCAs are present in sperm their role is far from clear since mobilisation of stored Ca2+ in sperm by exposure to thapsigargin requires high (non-specific) doses of the drug (Harper et al, 2005; see section 3.2 below).
A characteristic of Ca2+ stores in somatic cells is the protein calreticulin, which acts as a chelator of Ca2+ within the storage organelle. This protein is present in the acrosome of developing rat sperm (Nakamura et al. 1992; 1993) and is present in both the acrosomal and neck regions of human and bovine sperm (Naaby-Hansen et al, 2001; Ho & Suarez, 2003)
3.2. Evidence for functional calcium storage in sperm
Direct assessment of uptake and release of Ca2+ by sperm organelles or organelle membranes has been attempted in only a few studies. Walensky & Snyder (1995) measured accumulation and release of 45Ca2+ in digitonin-permeabilised rat sperm and demonstrated an ATP-dependent accumulation of Ca2+ into an intracellular site that was sensitive to thapsigargin (10 μM). Accumulated Ca2+ was released (partially) by 10 μM InsP3. Spungin & Breitbart (1996) reported that purified acrosomal membranes from bovine sperm possessed a thapsigargin-sensitive Ca2+ uptake pump and a cAMP-activated Ca2+-release channel. These authors suggested that generation of cAMP (and consequent mobilisation of stored Ca2+) could occur upon interaction with the zona pellucida (Breitbart & Spungin, 1997; Breitbart, 2002).
An alternative approach has been indirectly to assess Ca2+ movements attributable to store uptake and release in intact sperm by using fluorescent Ca2+ indicators to monitor cytoplasmic [Ca2+]. Blackmore (1993) showed that treatment of human sperm with the SERCA inhibitor thapsigargin, to release Ca2+ from intracellular stores, caused a sustained increase in [Ca2+]i due to opening of channels at the plasma membrane. No elevation of [Ca2+]i was seen when extracellular [Ca2+] was buffered with EGTA but upon subsequent addition of Ca2+ to the extracellular medium there was a sustained rise in [Ca2+]i. Rossato et al (2001) and Williams & Ford (2003) reported similar observations but in these studies a transient (and much smaller) increase in [Ca2+]i was also observed when the drug was applied to cells bathed in Ca2+-free saline, confirming that mobilisation of stored Ca2+ was indeed occurring. Similar types of response have been observed in sperm of rams (Dragileva et al 1999), mice (O’Toole et al, 2000) and sea urchins (Gonzalez-Martinez et al, 2001). The simplest interpretation of these observations would be that sperm possess an intracellular store (or stores) of Ca2+ that can be mobilised by treatment with thapsigargin. Mobilised Ca2+ may sometimes be insufficient to cause a detectable elevation of [Ca2+]i, but nevertheless can induce Ca2+ influx through store-operated (capacitative) Ca2+ channels (see section 5 below).
The mechanism by which thapsigargin mobilises stored Ca2+ in sperm is not clear. Rossato et al (2001) reported effects of the drug on Ca2+ handling by human sperm at 10-100 nM and Meizel & Turner (1993) observed dose-dependent induction of acrosome reaction at similar doses. These observations are consistent with studies on somatic cells where 50% block of SERCA activity (or 50% maximal Ca2+-store mobilisation) occurs at <100 nM and often <10 nM thapsigargin (Treiman et al, 1998; Wootton & Michelangeli, 2006). However, most studies on the effects of thapsigargin on sperm Ca2+ signalling have used micromolar doses (1-20 μM), with negligible effects being observed at does ≤ 5 μM (e.g. Dragileva et al, 1999; Williams & Ford, 2003; Harper et al, 2005). Cyclopiazonic acid (CPA), another widely used SERCA inhibitor, mobilised Ca2+ in human sperm at high doses (maximal effect at 100 μM; Rossato et al, 2001) but completely fails to mobilise Ca2+ at lower doses (Williams & Ford, 2003; Harper et al, 2005) that could be considered both saturating and specific (Wootton & Michelangeli, 2006). Thus, though it appears that SERCA (at least SERCA2) is expressed in mammalian sperm (see section 3.1.3 above), many of the reported effects of thapsigargin and CPA on Ca2+ stores in intact sperm may reflect non-specific actions at non-SERCA sites.
Work in our own laboratory has provided evidence for participation of stored Ca2+ in complex [Ca2+]i signals that occur in human sperm stimulated with progesterone or NO, both products of the female tract and cumulus-oocyte complex (Publicover et al, 2007). [Ca2+]i oscillations occur in the sperm neck and midpiece of up to 50% of cells stimulated with these agonists. Oscillations are resistant to reduction of [Ca2+]o to micromolar levels (5-10 μM) but buffering of [Ca2+]o with EGTA, which rapidly depletes cytoplasmic Ca2+, causes arrest of oscillations within one or two cycles (Harper et al, 2004; Kirkman-Brown et al, 2004; Machado-Oliveira et al, 2008). Pharmacological manipulations suggest that CICR, through activation of RyRs, underlies these oscillations and that InsP3 generation is not required (Harper et al, 2004). These oscillations in [Ca2+]i are resistant to thapsigargin at concentrations up to 10 μM, but are inhibited by bis-phenol, which blocks activity of SPCAs (Harper et al, 2005).
4. Location and identity of the Ca2+ storage organelle(s) in sperm
Localisation of the components of intracellular Ca2+ storage organelles (pumps and channels) shows two concentrations of staining in sperm, one over the anterior head and the other over the sperm neck and midpiece (section 3.1). Antimonate staining to identify calcium deposits within human sperm showed a similar distribution (Chandler & Battersby, 1976). Thus at least two organelles, in different parts of the cell, serve as Ca2+ stores in sperm. De Blas et al (2002) used human sperm permeabilised with streptolysin O and labelled with fluo3 directly to visualise Ca2+ stores. Fluorescence (indicating the presence of Ca2+-containing organelles) was again observed in the acrosomal region and at the midpiece. Acrosomal fluorescence was significantly reduced when the cells were exposed either to BAPTA-am (a membrane-permeant Ca2+ chelator) or to a combination of Br-A23187 (Ca2+ ionophore) and EGTA, but labelling in the sperm midpiece showed less sensitivity to these treatments. Herrick at al (2005) were able to observe Ca2+ stores in intact, live mouse sperm by exploiting the ability of manganese to quench fluorescence of the Ca2+ reporter fura-2. Cells were loaded with fura-2 then exposed to manganese, which entered the cytoplasm, thus quenching fluorescence of the dye, but was excluded from intracellular organelles. As in permeabilised cells, fluorescence was localised to the acrosomal and neck/midpiece regions. This pattern of labelling can also be observed when intact (non-permeabilised) mouse or human sperm are loaded with a low-affinity Ca2+ dye. In this case the dye highlights the high Ca2+ concentrations inside the Ca2+ storage organelles but does not fluoresce significantly at the much lower Ca2+ concentration in the cytoplasm (Herrick et al, 2005; Morris, J, unpublished data; fig. 2a). Intriguingly, immunolocalisation studies indicate that the ‘toolkits’ of these two stores may differ (section 3.1), such that mechanisms for Ca2+ mobilisation and accumulation at the two sites within the cell may be discrete (section 6.3 below; Publicover et al, 2007).
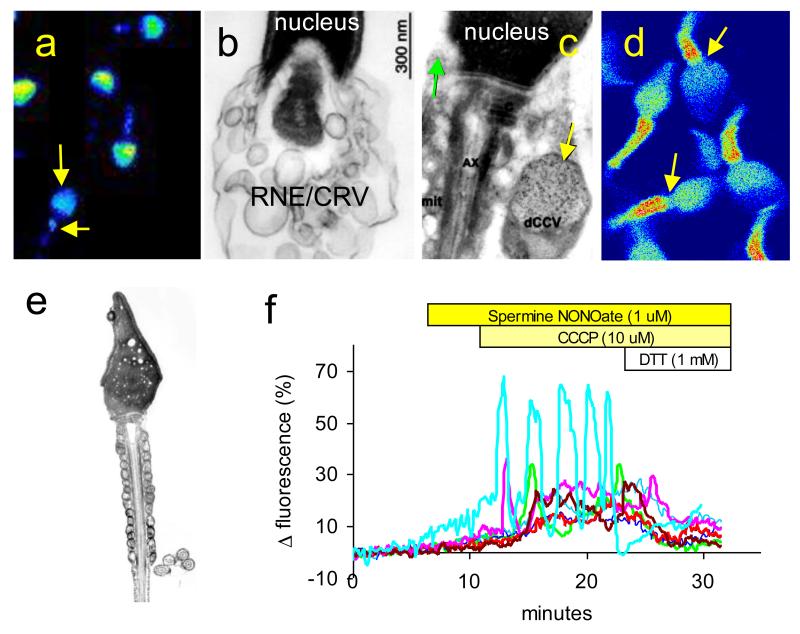
Figure 2.
Intracellular Ca2+ storage sites in mammalian sperm. a: Pseudocolour image of MagFluo-4 loaded sperm showing (warm colours show high [Ca2+]). This low-affinity Ca2+ indicator highlights the high concentrations of Ca2+ in the acrosomal store and at the sperm neck/midpiece (arrows). b: Electron micrograph showing posterior of the nucleus and the cytoplasmic droplet of a bovine sperm. Large numbers of membranous cisternae (parts of RNE and/or a discrete population of calreticulin containing vesicles – RNE/CRV) can be seen in this region. c: Electron micrograph showing posterior of the nucleus and the cytoplasmic droplet of a human sperm. Section has been labelled with gold-conjugated anti-calreticulin antibodies. Green arrow shows small calreticulin-containing vesicle in anterior of cytoplasmic droplet. Yellow arrow shows large calreticulin-containing vesicle in the cytoplasmic droplet adjoining the midpiece. d: Pseudocolour confocal image of Fluo-3 labelled human sperm (warm colours show high [Ca2+]). In cells from this donor there was a concentration of fluorescence in the mitochondrial midpiece, occurring as two ‘stripes’ of highly fluorescent points arranged exactly as the mitochondria appear in electron microscope sections (e). There is a clear gap (arrows) between the anterior end of the fluorescent ‘stripe’s and the sperm head, which is the position of the RNE and calreticulin-containing vesicles. f: Ca2+ oscillations at the sperm neck/midpiece do not require the mitochondrial membrane potential. Plots shows fluorescence from seven human sperm loaded with the Ca2+ indicator Oregon Green BAPTA 1. The NO• donor spermine NONOate causes protein S-nitrosylation, which sensitises the Ca2+ store in the neck/midpiece causing a slow elevation of [Ca2+]i (Machado-Oliveira et al, 2008). Upon application of the mitochondrial uncoupler CCCP, to collapse the mitochondrial inner membrane potential, there is a pronounced rise in [Ca2+]i (probably due to release of mitochondrial Ca2+) which is then followed in some cells by Ca2+ transients and oscillations (green, pink, brown and turquoise traces) in the sperm neck/midpiece. These transients and oscillations reflect cyclical release and re-uptake of Ca2+ stored at the sperm neck/midpiece by a mechanism that does not require an intact mitochondrial membrane potential. When dithiothreitol (DTT) is applied, reversing protein S-nitrosylation and removing the sensitising effect of NO on the Ca2+ store (Machado-Oliveira et al, 2008), the cells recover despite the continued presence of the mitochondrial uncoupler. Panel b from Ho and Suarez (2003) with permission, panel c from Naaby-Hanset et al (2001) with permission.
4.1 The acrosomal store
There is no dispute that the storage organelle in the acrosomal region of the sperm head is the acrosome (fig 1a) itself. Ca2+ release channels (InsP3Rs) in this region occur primarily (possibly exclusively) in the outer acrosomal membrane (see section 3.1). Thus the acrosomal store regulates Ca2+ concentration in the peri-acrosomal cytoplasm, its pumps and channels being lost during acrosome reaction when compound fusion occurs between the outer acrosomal membrane and the overlying plasmalemma.
4.2 Ca2+ storage at the sperm neck/midpiece
The identity of the Ca2+ storage organelle in the neck/midpiece region of the sperm is less clear. Suarez and colleagues have demonstrated the presence of InsP3Rs and calreticulin (a Ca2+ binding and storage protein) at the neck region of bovine and hamster sperm, in the region occupied by the redundant nuclear envelope (RNE; figs. 1b, 2b) the ‘excess’ nuclear membrane that accumulates due to nuclear condensation and is packaged at the sperm neck. Since this membrane is continuous with the ER in the immature cell, it may even include vestiges of functional ER membrane. Staining for nuclear pore complex proteins (markers for the RNE) only partial overlapped that for InsP3Rs (Ho & Suarez, 2003). Immunogold labelling of electron microscope sections showed that calreticulin and InsP3Rs were associated with membrane cisternae that did not contain nuclear pores and were apparently a separate compartment of the RNE (Ho & Suarez, 2001, 2003). No staining was associated with mitochondria. Pharmacological manipulations designed to activated these receptors (e.g. thimerosal) or to inhibit intracellular Ca2+ pumps (5-20 μM thapsigargin) mobilised Ca2+ in the region of the sperm neck and had functional effects (see below) on motility (Ho & Suarez, 2001, 2003). Naaby-Hansen et al (2001) observed co-localisation of InsP3Rs and calreticulin in both the acrosome and neck region of human sperm. Immunogold staining for calreticulin showed that this protein was present in the acrosome (particularly at the equatorial segment) and also in vesicles in the sperm neck (adjacent to the nucleus) and in the cytoplasmic droplet (fig. 2c). These vesicles were closely apposed to the plasma membrane (Naaby-Hansen et al 2001).
A further candidate for intracellular storage of Ca2+ in the neck/midpiece region of sperm is accumulation and release by mitochondria (see section 2.2.3). Mitochondria of mammalian sperm have been shown to take up Ca2+ in situ (Storey & Keyhani, 1973; 1974; Babcock et al, 1976; Vijayaraghavan & Hoskins, 1990). In mouse sperm the contribution of mitochondrial Ca2+ buffering was marginal under resting conditions but became more significant when plasma membrane Ca2+ pumps were inhibited, conditions under which resting [Ca2+]i may be elevated (Wennemuth et al, 2003). Occasionally we have observed strong fluorescence, apparently localized to the mitochondria, in human sperm labeled with Ca2+-reporting dyes (fig. 2d,e), suggesting that these organelles were accumulating large amounts of Ca2+. This was particularly characteristic of one donor who was known to be fertile and did not appear to be associated with reduced cell viability or function. ‘Conventional’ mitochondrial Ca2+ uptake and release does not contribute significantly to the store-mediated Ca2+-oscillations that occur in the posterior head and midpiece of human sperm stimulated with low doses of progesterone or with NO. Uncoupling of mitochondrial respiration (with 2,4 dinitrophenol or CCCP) does not inhibit these [Ca2+]i oscillations and can even activate them when the store in this region has been sensitized by NO•. (Machado-Oliveira et al, 2008; fig. 2f). An intriguing possibility is that the sperm mitochondrial inner membrane bears Ca2+ ATPases which permit Ca2+ accumulation supported by glycolytically-generated ATP. SPCA clearly localizes to the giant mitochondrion of sea urchin sperm (Gunaratne & Vacquier, 2006) and staining of human sperm for SPCA often shows both a concentration at the sperm neck and a more extended area of staining throughout the midpiece (fig. 1d-e). Furthermore, localization of stim 1, a marker of Ca2+ stores (see section 5 below), also stains the length of the midpiece. Two distinct ‘stripes’ of staining are often discernible in stim 1 stained cells, consistent with localization to the mitochondria and similar to the pattern of staining seen when mitochondria are Ca2+-loaded (fig. 3). However, it should be noted that, in sperm of the sea urchin Stronglocentrotus purpuratus, mitochondrial inhibitors and uncouplers cause mobilization of stored Ca2+ followed by sustained Ca2+ influx, apparently due to mobilization of mitochondrial Ca2+ and consequent activation of store-operated Ca2+ channels (Ardon et al, 2009).
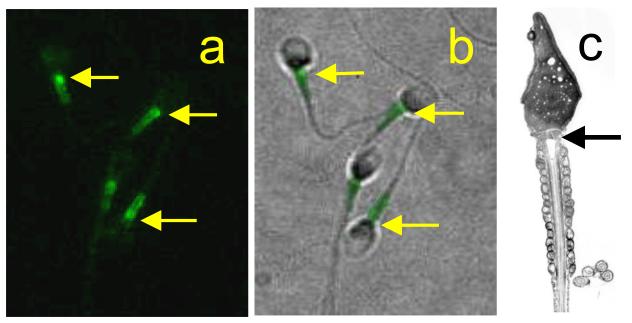
Figure 3.
Stim 1 is expressed at the sperm neck and midpiece. a: Immunolocalisation of stim 1 in human sperm. b shows immunofluorescence of stim 1 overlayed on a phase image of the same cells. The fluorescence appears as two ‘stripes’ (as is seen in Ca2+-loaded mitochondria; fig. 2d), with a brighter spot in the region of the RNE and calreticulin-containing vesicles (arrows) anterior to the mitochondria (arrow in c).
5. Store-operated Ca2+ channels in sperm
A common observation in somatic cells is that mobilisation of stored Ca2+ (leading to a fall in [Ca2+] inside the storage organelle) causes activation of Ca2+ influx though store-operated Ca2+ channels at the plasma membrane. This process is called ‘store-operated’ or ‘capacitative’ Ca2+ entry. It appears that store-operated influx encompasses ‘a family of Ca2+-permeable channels, with different properties in different cells’ (Parekh & Putney, 2005). Evidence in support of the occurrence of store operated Ca2+ influx in mammalian and sea urchin sperm has been reported by a number of laboratories, all of whom observed increased influx of Ca2+ into sperm in response to manoeuvres designed to mobilise stored Ca2+ (Blackmore, 1993; Dragileva et al, 1999, O’Toole et al, 2000, Rossato et al, 2001, Hirohashi & Vacquier, 2003; Williams & Ford, 2003; Espino et al, 2009; Ardon et al, 2009).
Some members of the canonical transient receptor potential (TRPC) channel superfamily have been suggested as candidate store-operated channels in somatic cells (Birnbaumer et al, 1996; Abramowitz & Birnbaumer, 2009). In mouse sperm several TRPC channels are expressed and have been shown to be localised over the anterior sperm head (Jungnickel et al., 2001; Castellano et al., 2003; Sutton et al., 2004; Stamboulian et al., 2005). Channels incorporating TRPC2 play a role in the zona pellucida-induced Ca2+ influx that leads to acrosome reaction (Jungnickel et al, 2001), though whether activation of these channels is a response to Ca2+ store depletion is still not clear (Florman et al, 2008; see section 6.1). More recently the proteins of the stim and orai families have been proposed to play key roles in capacitative Ca2+ influx. Stim 1 is the putative sensor for detection of Ca2+ store status and orai 1 is thought to form the Ca2+-permeable membrane channel (Strange et al, 2007; Wang et al, 2008). These proteins may also combine/interact with TRPCs to form and regulate store-operated channels (Abramowitz & Birnbaumer, 2009; Kim et al, 2009). The role(s) of these channels in sperm are far from clear but there is evidence to suggest that they may be important in acrosome reaction in mammalian and echinoderm sperm (O’Toole et al, 2000; Gonzalez-Martinez et al, 2001; see section 6.1). We have examined the expression in human sperm of stim and orai (Nash, K & Lefievre, L unpublished data). Both western blotting and immunolocalisation confirm the presence of these proteins and suggest that they are present primarily at the sperm neck and midpiece, though lower levels of expression over the acrosomal region may also occur (fig. 3).
6. Roles of Ca2+ stores in sperm
Since at least two intracellular Ca2+ stores are present in sperm, in different locations and potentially with different mechanisms of filling and mobilisation (Publicover et al, 2007), it is likely that these organelles have different roles in the regulation of sperm function.
6.1 The acrosomal store
The acrosomal store is strongly implicated in regulation of exocytosis of the acrosomal vesicle itself (acrosome reaction). Mouse sperm stimulated with zona pellucida glycoprotein ZP3 show a large, transient influx of Ca2+ (Arnoult et al, 1999) that may reflect activation of a T-type voltage-operated Ca2+ channel, though the identity of this channel is not yet established (Florman et al, 2008). In parallel to this Ca2+ influx there is believed to be a G-protein dependent elevation of pHi and also activation of PLC leading to generation of InsP3 (Florman et al, 2008). Male mice that are null for phospholipase Cδ4 show reduced fertility associated with failure of the sperm to undergo acrosome reaction upon binding to the zona pellucida (Fukami et al, 2001). Further investigation of sperm from these animals showed that they were unable to generate a [Ca2+]i signal in response to solubilised zona pellucida, whereas the cells could respond normally to 5 μM thapsigargin (Fukami et al, 2003). DeBlas et al (2002) used streptolysin-O treated (permeabilised) human sperm directly to observe the status of the acrosomal Ca2+ store (see section 4 above). Using this approach they were able to show that mobilisation of acrosomal Ca2+ through InsP3-sensitive channels was required for induction of acrosome reaction by the small GTPase Rab3A. Herrick et al (2005) used labelling of Ca2+ stores in intact mouse sperm (see section 4 above) to show a clear association between mobilisation of acrosomal Ca2+ (by 20 μM thapsigargin) and acrosome reaction. In cells bathed in medium containing no added Ca2+ and supplemented with 5 mM EGTA thapsigargin was still effective in inducing acrosome reaction. They concluded that mobilisation of the acrosomal store can be sufficient to induce acrosome reaction, such that the acrosome can be viewed as a Ca2+-storage organelle that is capable of regulating its own secretion (Herrick et al, 2005).
After the initial activation of signalling that occurs upon contact with the egg vestments, there is a sustained influx of Ca2+ which is apparently required for acrosome reaction, both in mouse and sea urchin sperm (O’Toole et al, 2000; Gonzalez-Martinez et al, 2001). In mouse sperm plasma membrane channels incorporating TRPC2 subunits are implicated in this process (Jungnickel et al, 2001), potentially being activated by a store operated mechanism (O’Toole et al, 2000), though other mechanisms of activation are also possible (Florman et al, 2008). A role for store operated Ca2+-influx in induction of acrosome reaction is consistent with observations that treatment of mammalian sperm with thapsigargin (to mobilise stored Ca2+) causes both elevation of [Ca2+]i and acrosome reaction (Blackmore; 1993; Meizel & Turner; 1993; Dragileva et al, 1999; Rossato et al, 2001; Williams & Ford, 2003), both these effects being dependent upon influx of extracellular Ca2+. Since store operated Ca2+ influx may involve a combination of TRPC and orai subunits (section 5), orai may also participate in this process. Store-operated Ca2+ influx is also implicated in acrosome reaction in sea urchin spermatozoa stimulated with egg jelly (Gonzalez-Martinez et al, 2001).
Models for activation of SNARE (membrane fusion) proteins during acrosome reaction typically incorporate a two-stage Ca2+ signal, but mobilisation of stored Ca2+ is the final ‘trigger’ of acrosome reaction (e.g. Mayorga et al, 2007; Zarelli et al, 2009). Requirement for store-operated Ca2+ influx downstream of mobilisation of the acrosomal store is thus not absolutely established. However, it should be noted that in experimental situations mobilisation of stored Ca2+ might be exaggerated, generating a [Ca2+]i signal that is larger and more effective than that occurring upon zona pellucida binding.
Recently the model (described above) for activation of sperm Ca2+ signalling by zona pellucida has been challenged. Xia and Ren (2009) reported that, in epididymal mouse sperm, the only functional plasma membrane Ca2+ channels were formed by CatSpers, a family of sperm-specific, plasma membrane ion channel subunits. CatSpers are localised to the principal piece of the flagellum, where they form weakly voltage-sensitive, Ca2+-permeable channels that are activated by elevated pHi and mediate hyperactivation (Navarro et al, 2008). Solubilised zona pellucida induced a [Ca2+]i elevation in 66% of sperm that initiated (within 20 seconds of stimulation) at the principal piece and then spread forward, taking almost 3 seconds to reach the sperm head (Xia & Ren, 2009). In 37% of cells a second (delayed) response occurred a few minutes after stimulation. Zona pellucida could not induce the first elevation of [Ca2+]i in any CatSper null sperm, but the delayed response occurred in 18% of these cells. Since zona pellucida receptors are likely to be in the sperm head activation of CatSpers in the principal piece of the flagellum is probably indirect, possibly via zona pellucida-induced elevation of pHi. CatsSper null sperm were able to undergo acrosome reaction in response to stimulation with zona pellucida, leading the authors to speculate that it was the delayed phase of the Ca2+ signal (possibly Ca2+ store generated) that induced acrosome reaction. The spread of elevated [Ca2+]i from the principal piece into the head of wild-type cells is probably an active process (Xia & Ren, 2009) and may well reflect mobilisation of Ca2+ stores by CICR. However, this model cannot easily be reconciled with the key role of voltage operated Ca2+ channels in the established model described above, for which there is a considerable body of evidence. Clearly there is a need for further work in this area.
6.2 Effects of mobilisation of Ca2+ stored at the neck/midpiece
The store located in the region of the sperm neck functions as a regulator of sperm motility. Ho & Suarez showed that manoeuvres designed to mobilise this store (application of thapsigargin or the InsP3R agonist thimerosal) caused elevation of [Ca2+]i in the neck region and hyperactivation in bovine sperm, these effects being independent of [Ca2+]o (Ho & Suarez; 2001, 2003). Assessment of mitochondrial function and pharmacological manipulation of the mitochondrial Na+/Ca2+ exchanger indicated that the observed effects did not reflect activity of conventional mitochondrial Ca2+ uptake and release mechanisms (Ho & Suarez, 2003). They went on to show a similar effect of store mobilisation in mouse sperm from both wild type mice and also in a proportion of sperm from mice null for CatSpers (Marquez et al, 2007).
It appears that stored Ca2+ in the neck/midpiece region of human sperm acts similarly. In these cells, treatments which induce Ca2+ influx can ‘switch on’ cyclical mobilisation of this store (causing cytoplasmic [Ca2+]i oscillations) apparently due to a form of CICR (Harper et al, 2005; Kirkman-Brown et al, 2004; Bedu-Addo et al, 2005; section 3.2). In many of the cells that show oscillations an increased excursion of the flagellum, often associated with asymmetrical bending of the midpiece, occurs during the [Ca2+]i peaks. Flagellar activity ‘relaxes’ during the intervening troughs (Harper et al, 2004; Bedu-Addo et al, 2005; Machado-Oliveira et al, 2008; fig. 4). 4-aminopyridine, an extremely potent inducer of hyperactivation in human sperm (Gu et al, 2004) causes reversible, repeatable mobilisation of Ca2+ stored in the neck/midpiece. In many cells, Ca2+ mobilisation is accompanied by (and apparently induces) sustained, asymmetric bending of the proximal flagellum, whilst, the distal flagellum continues to beat. Upon removal of 4-aminopyridine [Ca2+]i falls and the flagellar bend ‘relaxes’ (Costello, S. unpublished data). Investigations of the Ca2+ dependence of 4-AP-induced hyperactivation clearly show that, as in mouse and bovine sperm, mobilisation of stored Ca2+ is sufficient to initiate hyperactivation, but also suggest that store-operated Ca2+ influx contributes to maintenance of this mode of motility. Castellano et al (2003) observed that blockers of store operated channels caused inhibition of motility in human sperm.
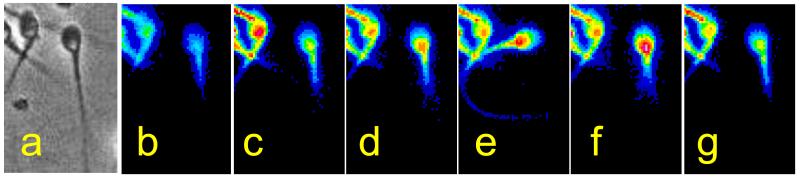
Figure 4.
Mobilisation of stores Ca2+ at the sperm neck/midpiece leads to modulation of flagellar activity. a shows phase image of immobilised, Oregon Green BAPTA 1-labelled human sperm. b-g are a series of pseudocoloured fluorescence images (taken at 10 s intervals) of the same cell during a Ca2+ transient induced by treatment with progesterone. The cell was bathed in medium with no added Ca2+. Ca2+ is liberated at the sperm neck and spreads into both the posterior head and the flagellum. During the Ca2+ peak a pronounced bend occurs in the proximal flagellum (e) and excursion of the flagellum increases (d,f).
6.3 Separation of store-regulated activities
Ca2+ mobilisation from the acrosome and from the store(s) in the sperm neck/midpiece regulate different activities. It is, therefore, important that they can be controlled separately. In mammals acrosome reaction is believed to occur at the surface of the zona pellucida. Sperm-zona pellucida interaction activates signalling cascades leading to acrosomal exocytosis (Florman et al, 2008; fig. 5)). The acrosomal content then disperses slowly (Harper et al, 2008), its content probably aiding penetration of the zona pellucida matrix. Though sperm in the early stages of acrosome reaction may bind to and go on to penetrate the zona pellucida (Buffone et al, 2008), it is likely that those that undergo acrosome reaction prematurely will be severely compromised in their ability to fertilise. It is therefore vital that stimuli that mobilise Ca2+ stored in the midpiece/neck region, for regulation of motility, should not ‘accidently’ activate the acrosomal store. In human sperm stimulated with progesterone, large [Ca2+]i oscillations at the sperm neck and consequent regulation of flagellar activity cause no detectable increase in the occurrence of acrosome reaction (Harper et al, 2004). Also, in an elegant study on hamster sperm, Suarez and Dai (1995) observed that ‘[Ca2+]i had increased to a greater extent in the midpiece than in the head in hyperactivated sperm, while the reverse was true for acrosome-reacted sperm’.
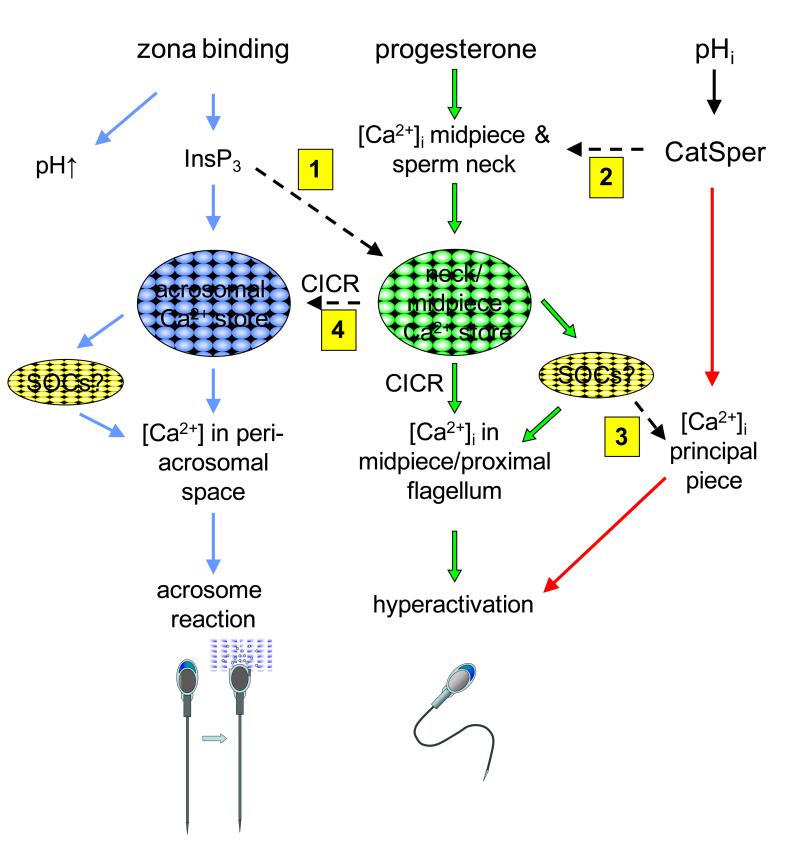
Figure 5.
Model for roles and interactions of Ca2+ stores in mammalian sperm. Zona binding (pathway shown in blue) induces generation of InsP3, mobilisation of the acrosomal store and activation of store operated Ca2+ influx, leading to elevated [Ca2+]i in the peri-acrosomal space and acrosome reaction. Progesterone (and probably other stimuli which cause Ca2+ influx; pathway shown in green) mobilise Ca2+ stored at the sperm neck/midpiece by Ca2+-induced Ca2+ release (CICR) and (probably) activation of store operated Ca2+ influx, causing elevation of Ca2+ in the midpiece/proximal flagellum leading to regulation of flagellar activity and hyperactivation. CatSper channels in the principal piece of the flagellum are activated by increased pHi, causing influx of Ca2+, elevation of [Ca2+]i and hyperactivation (shown in red). Dashed arrows (numbered) show potential crosstalk between these pathways. 1: InsP3 generated downstream of zona binding may activate InsP3Rs at the sperm neck, leading to Ca2+ mobilisation and hyperactivation. 2: Ca2+ influx through CatSpers in the principal piece may affect [Ca2+]i at the sperm neck/midpiece, mobilising stores Ca2+ by CICR. 3: SOCs in the principal piece may be activated downstream of store mobilisation. 4: Ca2+ mobilisation in the neck/midpiece may spread forward into the head, potentially mobilising acrosomal Ca2+ by CICR.
How might this be achieved? The store(s) in the sperm neck/midpiece appears to be mobilised by CICR. In human sperm a minimal level of Ca2+ influx at the plasma membrane is required to support cyclical Ca2+ mobilisation (Ca2+ oscillations) but pharmacological blockade of InsP3Rs (with 2-APB) or of phospholipase C (with U73122 or neomycin) has no effect. Thus this activity does not seem to require agonist-stimulated generation of InsP3. In fact, after stimulation with progesterone to induce [Ca2+]i oscillations, many cells continue to oscillate after removal of the agonist, presumably because, in these cells, Ca2+ ‘leak’ at the plasmalemma can support CICR once it has been initiated (Harper et al, 2004). The putative expression of RyRs in the sperm neck/midpiece (section 3.1.2) is consistent with Ca2+ mobilisation by CICR, but why are InsP3Rs (section 3.1.1) also expressed here? Firstly, InsP3Rs may play a role in CICR. It is known that these receptors can support this process provided that an adequate ‘background’ level of InsP3 is present (Berridge, 1993). Such a background level of InsP3 may be present in capacitated sperm, particularly since hydrolysis of phosphatidylinositol 4,5-bisphosphate to generate InsP3 may be activated by elevation of [Ca2+]i (Thomas & Meizel, 1989). Secondly, during the burst of InsP3 generation that follows zona pellucida binding and mediates emptying of the acrosomal store, the store in the midpiece/sperm neck may be strongly activated through its InsP3Rs. In addition, zona binding mat activate CatSpers (Xia & Ren, 2009; section 6.1) If either or both these processes occur, arrival of the sperm at the zona pellucida will initiate a combination of acrosome reaction and intense hyperactivation to facilitate penetration of the zona pellucida (fig. 5). In this context, it is noteworthy that hyperactivation is intensified in acrosome reacted hamster sperm (induced by zona pellucida) and that in these cells [Ca2+]i is increased in the flagellum (Suarez & Dai, 1995).
7. Outlook
Only 10 years ago the presence of Ca2+ stores in sperm was a matter for debate (Publicover & Barratt, 1999). The presence of these stores is now well established and there is little doubt that they enable the cell to generate Ca2+-signals that vary in size, ‘shape’ and location within the cell, permitting discrete control of different Ca2+-regulated functions. However, there are many aspects of the activation and control of store mobilisation of which we are still ignorant and on which future work should be focussed.
The identity and characteristics of the store located at the sperm neck/midpiece is far from clear. It is likely that Ca2+ storage here comprises more than one structure. Furthermore, the nature of the pumps and channels that are functional in this region is disputed. There is evidence for expression and or function of SERCAs, SPCAs, InsP3Rs and RyRs in the Ca2+ stores of the neck/midpiece (see sections 3.2 and 4.2) and it may be that these Ca2+ handling ‘tools’ are all expressed in this region of the cell but used in discrete ways to regulate functionally separate Ca2+ storage compartments.
Another area of great interest is the question of whether Ca2+ stores in sperm are functional in freshly ejaculated cells. Is delay of filling of the store(s) or delay of store ‘priming’ (development of sensitivity to stimulation) a mechanism by which premature activation of Ca2+-regulated processes is controlled? It has been suggested recently that Ca2+ mobilisation from the RNE might be regulated during capacitation by activity of Src kinase, which is localised to this region of human sperm and is activated during capacitation (Varano et al, 2008). Furthermore, residence in the female tract may affect sensitivity of Ca2+ mobilisation. For instance, NO•, which is generated by endothelial cells of the oviduct, sensitises Ca2+ mobilisation from the store in the neck/midpiece of human sperm (Machado-Oliveira et al, 2008).
Finally, the relationship between mobilisation of stored Ca2+, influx of Ca2+ at the sperm plasma membrane, hyperactivation and acrosome reaction must be elucidated. CatSper channels are required for normal hyperactivation of mouse sperm. Cells null for these channels cannot hyperactivate and the mice are sterile (Navarro et al, 2008). More recently they have been implicated in acrosome reaction (see section 6.1). Since stored Ca2+, at least at the neck/midpiece of human sperm, can be mobilised by CICR, Ca2+-influx through CatSpers may recruit stored Ca2+, in addition to Ca2+ entering through the plasma membrane (fig. 5). The observations of Xia and colleagues (Xia et al, 2007; Xia & Ren, 2009) that the elevation of [Ca2+]i that occurs upon opening of CatSper channels can propagate to the sperm head is consistent with this suggestion. Direct pharmacological mobilisation of stored Ca2+ can itself induce hyperactivation in wild type mouse sperm bathed in Ca2+-free medium and also in a proportion of sperm from mice null for CatSpers (Marquez et al, 2007). Thus store mobilisation alone is apparently sufficient to induce hyperactivation (fig. 5). An important part of the function of CatSper channels in supporting hyperactivation may be to induce CICR at the sperm neck/midpiece.
Recent findings have revealed unexpected sophistication in the Ca2+ signalling capability of sperm (Publicover et al, 2007). It may be that there is considerably more to functioning of the Ca2+ store in sperm than we currently know.
Acknowledgements
Our thanks to Gordon Milne for his expert help with the electron microscopy
Funding LL and CWF were supported by the Wellcome Trust (grant # 078905), SC and KN were in receipt of BBSRC studentships, G M-O was in receipt of a studentship from Fundação para a Ciência e Tecnologia (FCT) Portugal (SFRH/BD/17780/2004)
References
- Abramowitz J, Birnbaumer L. Physiology and pathphysiology of canonical transient receptor potential channels. The FASEB Journal. 2009;23:297–328. doi: 10.1096/fj.08-119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardón F, Rodríguez-Miranda E, Beltrán C, Hernández-Cruz A, Darszon A. Mitochondrial inhibitors activate influx of external Ca2+ in sea urchin sperm. Biochimica et Biophysica Acta. 2009;1787:15–24. doi: 10.1016/j.bbabio.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Arnoult C, Kazam IG, Visconti PE, Kopf GS, Villaz M, Florman HM. Control of the low voltage-activated calcium channel of mouse sperm by egg ZP3 and by membrane hyperpolarization during capacitation. Proceedings of the National Academy of Sciences of the USA. 1999;96:6757–6762. doi: 10.1073/pnas.96.12.6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock DF, First NL, Lardy HA. Action of ionophore A23187 at the cellular level. Separation of effects at the plasma and mitochondrial membranes. The Journal of Biological Chemistry. 1976;251:3881–3886. [PubMed] [Google Scholar]
- Baldi E, Luconi M, Bonaccorsi L, Maggi M, Francavilla S, Gabriele A, Properzi G, Forti G. Nongenomic progesterone receptor on human spermatozoa: biochemical aspects and clinical implications. Steroids. 1999;64:143–148. doi: 10.1016/s0039-128x(98)00100-7. [DOI] [PubMed] [Google Scholar]
- Bedu-Addo K, Lefievre L, Moseley FL, Barratt CL, Publicover SJ. Bicarbonate and bovine serum albumin reversibly ‘switch’ capacitation-induced events in human spermatozoa. Molecular Human Reproduction. 2005;11:683–691. doi: 10.1093/molehr/gah226. [DOI] [PubMed] [Google Scholar]
- Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiological Reviews. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Irvine RF. Inositol phosphates and cell signalling. Nature. 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Lipp P. Calcium -a life and death signal. Nature. 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- Bianchi K, Rimessi A, Prandini A, Szabadkai G, Rizzuto R. Calcium and mitochondria: mechanisms and functions of a troubled relationship. Biochimica et Biophysica Acta. 2004;1742:119–131. doi: 10.1016/j.bbamcr.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L, Zhu X, Jiang M, Boulay G, Peyton M, Vannier B, Brown D, Platano D, Sadeghi H, Stefani E, et al. On the molecular basis and regulation of cellular capacitative calcium entry: roles for Trp proteins. Proceeding of the National Academy of Science. 1996;93:15195–15202. doi: 10.1073/pnas.93.26.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore PF. Thapsigargin elevates and potentiates the ability of progesterone to increase intracellular free calcium in human sperm: possible role of perinuclear calcium. Cell Calcium. 1993;14:53–60. doi: 10.1016/0143-4160(93)90018-2. [DOI] [PubMed] [Google Scholar]
- Bootman MD, Collins TJ, Peppiatt CM, Prothero LS, MacKenzie L, De Smet P, Travers M, Tovey SC, Seo JT, Berridge MJ, Ciccolini F, Lipp P. Calcium signaling – an overview. Seminars in Cell and Developmental Biology. 2001;12:3–10. doi: 10.1006/scdb.2000.0211. [DOI] [PubMed] [Google Scholar]
- Breitbart H. Intracellular calcium regulation in sperm capacitation and acrosomal reaction. Molecular and Cellular Endocrinology. 2002;187:139–144. doi: 10.1016/s0303-7207(01)00704-3. [DOI] [PubMed] [Google Scholar]
- Breitbart H, Spungin B. The biochemistry of the acrosome reaction. Molecular Human Reproduction. 1997;3:195–202. doi: 10.1093/molehr/3.3.195. [DOI] [PubMed] [Google Scholar]
- Buffone MG, Foster JA, Gerton GL. The role of the acrosomal matrix in fertilization. International Journal of Developmental Biology. 2008;52:511–522. doi: 10.1387/ijdb.072532mb. [DOI] [PubMed] [Google Scholar]
- Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, Lin P, Xiao R, Wang C, Zhu Y, Lin Y, Wyatt CN, Parrington J, Ma J, Evans AM, Galione A, Zhu MX. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano LE, Treviño CL, Rodríguez D, Serrano CJ, Pacheco J, Tsutsumi V, Felix R, Darszon A. Transient receptor potential (TRPC) channels in human sperm: expression, cellular localization and involvement in the regulation of flagellar motility. FEBS Lett. 2003;541:69–74. doi: 10.1016/s0014-5793(03)00305-3. [DOI] [PubMed] [Google Scholar]
- Chandler JA, Battersby S. X-ray microanalysis of zinc and calcium in ultrathin sections of human sperm cells using the pyroantimonate technique. The Journal of Histochemistry and Cytochemistry. 1976;24:740–748. doi: 10.1177/24.6.59774. [DOI] [PubMed] [Google Scholar]
- Churchill GC, Okada Y, Thomas JM, Genazzani AA, Patel S, Galione A. NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell. 2002;111:703–708. doi: 10.1016/s0092-8674(02)01082-6. [DOI] [PubMed] [Google Scholar]
- Darszon A, Labarca P, Nishigaki T, Espinosa F. Ion channels in sperm physiology. Physiological Reviews. 1999;79:481–510. doi: 10.1152/physrev.1999.79.2.481. [DOI] [PubMed] [Google Scholar]
- Darszon A, Nishigaki T, Wood C, Treviño CL, Felix R, Beltrán C. Calcium channels and Ca2+ fluctuations in sperm physiology. International Review of Cytology. 2005;243:79–172. doi: 10.1016/S0074-7696(05)43002-8. [DOI] [PubMed] [Google Scholar]
- De Blas G, Michaut M, Trevino CL, Tomes CN, Yunes R, Darszon A, Mayorga LS. The intraacrosomal calcium pool plays a direct role in acrosomal exocytosis. The Journal of Biological Chemistry. 2002;277:49326–49331. doi: 10.1074/jbc.M208587200. [DOI] [PubMed] [Google Scholar]
- Dong Z, Saikumar P, Weinberg JM, Venkatachalam MA. Calcium in cell injury and death. Annu Rev Pathol. 2006;1:405–434. doi: 10.1146/annurev.pathol.1.110304.100218. [DOI] [PubMed] [Google Scholar]
- Dragileva E, Rubinstein S, Breitbart H. Intracellular Ca2+-Mg2+-ATPase regulates calcium influx and acrosomal exocytosis in bull and ran spermatozoa. Biology of Reproduction. 1999;61:1226–1234. doi: 10.1095/biolreprod61.5.1226. [DOI] [PubMed] [Google Scholar]
- Echevarria D, Vieira C, Gimeno L, Martinez S. Neuroepithelial secondary organizers and cell fate specification in the developing brain. Brain Research Reviews. 2003;43:179–191. doi: 10.1016/j.brainresrev.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Espino J, Mediero M, Lozano GM, Bejarano I, Ortiz A, García JF, Pariente JA, Rodríguez AB. Reduced levels of intracellular calcium releasing in spermatozoa from asthenozoospermic patients. Reproductive Biology and Endocrinology. 2009;7:11. doi: 10.1186/1477-7827-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix R. Molecular physiology and pathology of Ca2+-conducting channels in the plasma membrane of mammalian sperm. Reproduction. 2005;129:251–62. doi: 10.1530/rep.1.00478. [DOI] [PubMed] [Google Scholar]
- Florman HM, Darszon A. A sustained increase in intracellular Ca2+ is required for the acrosome reaction in sea urchin sperm. Developmental Biology. 2001;236:220–229. doi: 10.1006/dbio.2001.0323. [DOI] [PubMed] [Google Scholar]
- Florman HM, Jungnickel MK, Sutton KA. Regulating the acrosome reaction. International Journal of Developmental Biology. 2008;52:503–510. doi: 10.1387/ijdb.082696hf. [DOI] [PubMed] [Google Scholar]
- Fukami K, Nakao K, Inoue T, Kataoka Y, Kurokawa M, Fissore RA, Nakamura K, Katsuki M, Mikoshiba K, Yoshida N, Takenawa T. Requirement of phospholipase Cdelta4 for the zona pellucida-induced acrosome reaction. Science. 2001;292:920–923. doi: 10.1126/science.1059042. [DOI] [PubMed] [Google Scholar]
- Fukami K, Yoshida M, Inoue T, Kurokawa M, Fissore RA, Yoshida N, Mikoshiba K, Takenawa T. Phospholipase Cdelta4 is required for Ca2+ mobilization essential for acrosome reaction in sperm. Journal of Cell Biology. 2003;161:79–88. doi: 10.1083/jcb.200210057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko JV, Maruyama Y, Yano K, Dolman NJ, Tepikin AV, Petersen OH, Gerasimenko OV. NAADP mobilizes Ca2+ from a thapsigargin-sensitive store in the nuclear envelope by activating ryanodine receptors. Journal of Cell Biology. 2003;163:271–282. doi: 10.1083/jcb.200306134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Martinez MT, Galindo BE, de De La Toore L, Zapata O, Rodriquez E, Gu Y, Kirkman-Brown JC, Korchev Y, Barratt CL, Publicover SJ. Multi-state, 4-aminopyridine-sensitive ion channels in human spermatozoa. Developmental Biology. 2004;274:308–317. doi: 10.1016/j.ydbio.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Gunaratne HJ, Vacquier VD. Evidence for a secretory pathway Ca2+-ATPase in sea urchin spermatozoa. FEBS Letters. 2006;580:3900–3904. doi: 10.1016/j.febslet.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Yule DI, Gunter KK, Eliseev RA, Salter JD. Calcium and mitochondria. FEBS Letters. 2004;567:96–102. doi: 10.1016/j.febslet.2004.03.071. [DOI] [PubMed] [Google Scholar]
- Harper CV, Barratt CL, Publicover SJ. Stimulation of human spermatozoa with progesterone gradients to stimulate approach to the oocyte. Induction of [Ca2+]i oscillations and cyclical transitions in flagellar beating. Journal of Biological Chemistry. 2004;279:46315–46325. doi: 10.1074/jbc.M401194200. [DOI] [PubMed] [Google Scholar]
- Harper CV, Cummerson JA, White MR, Publicover SJ, Johnson PM. Dynamic resolution of acrosomal exocytosis in human sperm. Journal of Cell Science. 2008;121:2130–2135. doi: 10.1242/jcs.030379. [DOI] [PubMed] [Google Scholar]
- Harper C, Wootton L, Michelangeli F, Lefievre L, Barratt C, Publicover S. Secretory pathway Ca2+-ATPase (SPCA1) Ca2+ pumps, not SERCAs, regulate complex [Ca2+]i signals in human spermatozoa. Journal of Cell Science. 2005;15:1673–1685. doi: 10.1242/jcs.02297. [DOI] [PubMed] [Google Scholar]
- Herrick SB, Schweissinger DL, Kim SW, Bayan KR, Mann S, Cardullo RA. The acrosomal vesicle of mouse sperm is a calcium store. Journal of Cellular Physiology. 2005;202:663–671. doi: 10.1002/jcp.20172. [DOI] [PubMed] [Google Scholar]
- Hirohashi N, Vacquier VD. Store-operated calcium channels trigger exocytosis of the sea urchin sperm acrosomal vesicle. Biochemical and Biophysical Research Communications. 2003;304:285–292. doi: 10.1016/s0006-291x(03)00587-4. [DOI] [PubMed] [Google Scholar]
- Ho HC, Suarez SS. An inositol 1,4,5-trisphosphate receptor-gated intracellular Ca2+ store is involved in sperm hyperactivated motility. Biology of Reproduction. 2001;65:1606–1615. doi: 10.1095/biolreprod65.5.1606. [DOI] [PubMed] [Google Scholar]
- Ho HC, Suarez SS. Characterization of the intracellular calcium store at the base of the sperm flagellum that regulates hyperactivated motility. Biology of Reproduction. 2003;68:1590–1596. doi: 10.1095/biolreprod.102.011320. [DOI] [PubMed] [Google Scholar]
- Humbert J-P, Matter NM, Artault J-C, Köppler P, Malviya AN. Inositol 1,4,5-Trisphosphate Receptor Is Located to the Inner Nuclear Membrane Vindicating Regulation of Nuclear Calcium Signaling by Inositol 1,4,5-Trisphosphate. J. Biol. Chem. 1996;271:478–485. doi: 10.1074/jbc.271.1.478. [DOI] [PubMed] [Google Scholar]
- Jeong SY, Seol DW. The role of mitochondria in apoptosis. BMB Reports. 2008;41:11–22. doi: 10.5483/bmbrep.2008.41.1.011. [DOI] [PubMed] [Google Scholar]
- Jimenez-Gonzalez C, Michelangeli F, Harper CV, Barratt CL, Publicover SJ. Calcium signalling in human spermatozoa: a specialized ‘toolkit’ of channels, transporters and stores. Human Reproduction Update. 2006;12:253–267. doi: 10.1093/humupd/dmi050. [DOI] [PubMed] [Google Scholar]
- Jungnickel MK, Marrero H, Birnbaumer L, Lemos JR, Florman HM. Trp2 regulates entry of Ca2+ into mouse sperm triggered by egg ZP3. Nature Cell Biology. 2001;3:499–502. doi: 10.1038/35074570. [DOI] [PubMed] [Google Scholar]
- Kim MS, Zeng W, Yuan JP, Shin DM, Worley PF, Muallem S. Native store-operated Ca2+ influx requires the channel function of orai1 and TRPC1. Journal of Biological Chemistry. 2009;284:9733–9741. doi: 10.1074/jbc.M808097200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnear NP, Boittin FX, Thomas JM, Galione A, Evans AM. Lysosome-sacroplasmic reticulum junctions. A trigger zone for calcium signaling by nicotinic acid adenine dinucleotide phosphate and endothelin-1. Journal of Biological Chemistry. 2004;279:54319–54326. doi: 10.1074/jbc.M406132200. [DOI] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- Kirkman-Brown JC, Barratt CL, Publicover SJ. Slow calcium oscillations in human spermatozoa. The Biochemical Journal. 2004;378:827–832. doi: 10.1042/BJ20031368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krausz C, Bonaccorsi L, Luconi M, Fuzzi B, Criscuoli L, Pellegrini S, Forti G, Baldi E. Intracellular calcium increase and acrosome reaction in response to progesterone in human spermatozoa are correlated with in-vitro fertilization. Human Reproduction. 1995;10:120–124. doi: 10.1093/humrep/10.1.120. [DOI] [PubMed] [Google Scholar]
- Kuoda Y, Kaneko S, Yoshimura Y, Nozawa S, Mikoshiba K. Are there inositol 1,4,5-triphosphate (IP3) receptors in human sperm? Life Science. 1999;65:135–143. doi: 10.1016/s0024-3205(99)00230-1. [DOI] [PubMed] [Google Scholar]
- Lanini L, Bachs O, Carafoli E. The calcium pump of the liver nuclear membrane is identical to that of the endoplasmic reticulum. Journal of Biological Chemistry. 1992;267:11548–11552. [PubMed] [Google Scholar]
- Lawson C, Dorval V, Goupil S, Leclerc P. Identification and localisation of SERCA 2 isoforms in mammalian sperm. Molecular Human Reproduction. 2007;13:307–316. doi: 10.1093/molehr/gam012. [DOI] [PubMed] [Google Scholar]
- Lefievre L, Chen Y, Conner SJ, Scott JL, Publicover SJ, Ford WC, Barratt CL. Human spermatozoa contain multiple targets for protein S-nitrosylation: an alternative mechanism of the modulation of sperm function by nitric oxide? Proteomics. 2007;7:3066–3084. doi: 10.1002/pmic.200700254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Oliveira G, Lefievre L, Ford C, Herrero MB, Barratt C, Connolly TJ, Nash K, Morales-Garcia A, Kirkman-Brown J, Publicover S. Mobilisation of Ca2+ stores and flagellar regulation in human sperm by S-nitrosylation: a role for NO synthesised in the female reproductive tract. Development. 2008;135:3677–3686. doi: 10.1242/dev.024521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez B, Ignotz G, Suarez SS. Contributions of extracellular and intracellular Ca2+ to regulation of sperm motility: Release of intracellular stores can hyperactivate CatSper1 and CatSper2 null sperm. Developmental Biology. 2007;303:214–221. doi: 10.1016/j.ydbio.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayora LS, Tomes CN, Belmonte SA. Acrosomal exocytosis, a special type of regulated secretion. IUBMB Life. 2007;59:286–292. doi: 10.1080/15216540701222872. [DOI] [PubMed] [Google Scholar]
- Meizel S, Turner KO. Initiation of the human sperm acrosome reaction by thapsigargin. The Journal of Experimental Zoology. 1993;267:350–355. doi: 10.1002/jez.1402670312. [DOI] [PubMed] [Google Scholar]
- Michelangeli F, Ogunbayo OA, Wootton LL. A plethora of interacting organellar Ca2+ stores. Current Opinion in Cell Biology. 2005;17:135–140. doi: 10.1016/j.ceb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Michelangeli F, Mezna M, Tovey S, Sayers LG. Pharmacological modulators of the inositol 1,4,5-trisphosphate receptor. Neuropharmacology. 1995;34:1111–1122. doi: 10.1016/0028-3908(95)00053-9. [DOI] [PubMed] [Google Scholar]
- Missiaen L, Van Acker K, Van Baelen K, Raeymaekers L, Wuytack F, Parys JB, De Smedt H, Vanoevelen J, Dode L, Rizzuto R, et al. Calcium release from the Golgi apparatus and the endoplasmic reticulum in HeLa cells stably expressing targeted aequorin to these compartments. Cell Calcium. 2004;36:479–487. doi: 10.1016/j.ceca.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Naaby-Hansen S, Wolkowicz MJ, Klotz K, Bush LA, Westbrook VA, Shibahara H, Shetty J, Coonrod SA, Reddi PP, Shannon J, et al. Co-localization of the inositol 1,4,5-trisphosphate receptor and calreticulin in the equatorial segment and in membrane bounded vesicles in the cytoplasmic droplet of human spermatozoa. Molecular Human Reproduction. 2001;7:923–933. doi: 10.1093/molehr/7.10.923. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Michikawa Y, Baba T, Okinaga S, Arai K. Calreticulin is present in the acrosome of spermatids of rat testis. Biochemical and Biophysical Research Communications. 1992;186:668–673. doi: 10.1016/0006-291x(92)90798-p. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Moriya M, Baba T, Michikawa Y, Yamanobe T, Arai K, Okinaga S, Kobayashi T. An endoplasmic reticulum protein, calreticulin, is transported into the acrosome of rat sperm. Experimental Cell Research. 1993;205:101–110. doi: 10.1006/excr.1993.1063. [DOI] [PubMed] [Google Scholar]
- Navarro B, Kirichok Y, Chung JJ, Clapham DE. Ion channels that control fertility in mammalian spermatozoa. International Journal of Developmental Biology. 2008;52:607–613. doi: 10.1387/ijdb.072554bn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole CM, Arnoult C, Darzon A, Steinhardt RA, Florman HM. Ca2+ entry through store-operated channels in mouse sperm is initiated by egg ZP3 and drives the acrosome reaction. Molecular Biology of the Cell. 2000;11:1571–1584. doi: 10.1091/mbc.11.5.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Naturex Reviews in Molecular Cell Biology. 2003;47:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Putney JW., Jr. Store-operated calcium channels. Physiological Reviews. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- Publicover SJ, Barratt CL. Voltage-operated Ca2+ channels and the acrosome reaction: which channels are present and what do they do? Human Reproduction. 1999;14:873–879. doi: 10.1093/humrep/14.4.873. [DOI] [PubMed] [Google Scholar]
- Publicover S, Harper CV, Barratt C. Ca2+]i signalling in sperm - making the most of what you’ve got. Nature Cell Biology. 2007;9:235–242. doi: 10.1038/ncb0307-235. [DOI] [PubMed] [Google Scholar]
- Rimessi A, Giorgi C, Pinton P, Rizzuto R. The versatility of mitochondrial calcium signals: from stimulation of cell metabolism to induction of cell death. Biochimica et Bioohysica Acta. 2008;1777:808–816. doi: 10.1016/j.bbabio.2008.05.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossato M, Di Virgilio F, Rizzuto R, Galeazzi C, Foresta C. Intracellular calcium store depletion and acrosome reaction in human spermatozoa: role of calcium and plasma membrane potential. Molecular Human Reproduction. 2001;7:119–128. doi: 10.1093/molehr/7.2.119. [DOI] [PubMed] [Google Scholar]
- Spungin B, Breitbart H. Calcium mobilization and influx during sperm exocytosis. The Journal of Cell Science. 1996;109:1947–1955. doi: 10.1242/jcs.109.7.1947. [DOI] [PubMed] [Google Scholar]
- Stamboulian S, Moutin MJ, Treves S, Pochon N, Grunwald D, Zorzato F, De Waard M, Ronjat M, Arnoult C. Junctate, an inositol 1,4,5-triphosphate receptor associated protein, is present in rodent sperm and binds TRPC2 and TRPC5 but not TRPC1 channels. Developmental Biology. 2005;286:326–37. doi: 10.1016/j.ydbio.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Storey BT, Keyhani E. Interaction of calcium ion with the mitochondria in rabbit spermatozoa. FEBS Letter. 1973;37:33–36. doi: 10.1016/0014-5793(73)80420-x. [DOI] [PubMed] [Google Scholar]
- Storey BT, Keyhani E. Energy metabolism of spermatozoa: III. Energy-linked uptake of calcium ion by the mitochondria of rabbit epididymal spermatozoa. Fertility and Sterility. 1974;25:976–984. [PubMed] [Google Scholar]
- Strange K, Yan X, Lorin-Nebel C, Xing J. Physiological roles of STIM1 and Orai1 homologs and CRAC channels in the genetic model organism Caenorhabditis elegans. Cell Calcium. 2007;42:193–203. doi: 10.1016/j.ceca.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez SS, Dai X. Intracellular calcium reaches different levels of elevation in hyperactivated and acrosome-reacted hamster sperm. Molecular Reproduction and Development. 1995;42:325–333. doi: 10.1002/mrd.1080420310. [DOI] [PubMed] [Google Scholar]
- Surroca A, Wolff D. Inositol 1,4,5-trisphosphate but not ryanodine-receptor agonists induces calcium release from rat liver Golgi apparatus membrane vesicles. The Journal of Membrane Biology. 2000;177:243–249. doi: 10.1007/s002320010008. [DOI] [PubMed] [Google Scholar]
- Sutton KA, Jungnickel MK, Wang Y, Cullen K, Lambert S, Florman HM. Enkurin is a novel calmodulin and TRPC channel binding protein in sperm. Developmental Biology. 2004;274:426–35. doi: 10.1016/j.ydbio.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Taylor CW. Why do hormones stimulate Ca2+ mobilization? Biochem Soc Trans. 1995;23:637–642. doi: 10.1042/bst0230637. [DOI] [PubMed] [Google Scholar]
- Tomes CN, McMaster CR, Saling PM. Activation of mouse sperm phosphatidylinositol-4,5 bisphosphate-phospholiapase C by zona pellucida is modulated by tyrosine phosphorylation. Molecular Reproduction and Development. 1996;43:196–204. doi: 10.1002/(SICI)1098-2795(199602)43:2<196::AID-MRD9>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Treiman M, Caspersen C, Christensen SB. A tool coming of age: thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca2+-ATPases. Trends in Pharmacological Science. 1998;19:131–135. doi: 10.1016/s0165-6147(98)01184-5. [DOI] [PubMed] [Google Scholar]
- Trevino CL, Santi CM, Beltran C, Hernandez-Cruz A, Darzon A, Lomeli H. Localisation of inositol trisphosphate and ryanodine receptors during mouse spermatogenesis: possible functional implications. Zygote. 1998;6:159–172. doi: 10.1017/s0967199498000094. [DOI] [PubMed] [Google Scholar]
- Varano G, Lombardi A, Cantini G, Forti G, Baldi E, Luconi M. Src activation triggers capacitation and acrosome reaction but not motility in human spermatozoa. Human Reproduction. 2008;23:2652–2662. doi: 10.1093/humrep/den314. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan S, Hoskins DD. Changes in the mitochondrial calcium influx and efflux properties are responsible for the decline in sperm calcium during epididymal maturation. Molecular Reproduction and Development. 1990;25:186–194. doi: 10.1002/mrd.1080250212. [DOI] [PubMed] [Google Scholar]
- Walensky LD, Snyder SH. Inositol 1,4,5-trisphosphate receptors selectively localized to the acrosome of mammalian sperm. Journal of Cell Biology. 1995;130:857–869. doi: 10.1083/jcb.130.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Deng X, Hewavitharana T, Soboloff J, Gill DL. Stim, ORAI and TRPC channels in the control of calcium entry signals in smooth muscle. Clinical and Experimential Pharmacology and Physiology. 2008;35:1127–1133. doi: 10.1111/j.1440-1681.2008.05018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenemuth G, Babcock DF, Hille B. Calcium clearance mechanisms of mouse sperm. The Journal of General Physiology. 2003;122:115–128. doi: 10.1085/jgp.200308839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KM, Ford WC. Effects of Ca-ATPase inhibitors on the intracellular calcium activity and motility of human spermatozoa. International Journal of Andrology. 2003;26:366–375. doi: 10.1111/j.1365-2605.2003.00438.x. [DOI] [PubMed] [Google Scholar]
- Wootton LL, Argent CC, Wheatley M, Michelangeli F. The expression, activity and localisation of the secretory pathway Ca2+-ATPase (SPCA1) in different mammalian tissues. Biochimica et Biophysica Acta. 2004;1664:189–197. doi: 10.1016/j.bbamem.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Wootton LL, Michelangeli F. The effects of the phenylalanine 256 to valine mutation on the sensitivity of sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA) Ca2+ pump isoforms 1, 2, and 3 to thapsigargin and other inhibitors. Journal of Biological Chemistry. 2006;281:6970–6976. doi: 10.1074/jbc.M510978200. [DOI] [PubMed] [Google Scholar]
- Xia J, Reigada D, Mitchell CH, Ren D. CATSPER channel-mediated Ca2+ entry into mouse sperm triggers a tail-to-head propagation. Biology of Reproduction. 2007;77:551–559. doi: 10.1095/biolreprod.107.061358. [DOI] [PubMed] [Google Scholar]
- Xia J, Ren D. Egg Coat Proteins Activate Calcium Entry into Mouse Sperm via CATSPER Channels. Biology of Reproduction. 2009;80:1092–1098. doi: 10.1095/biolreprod.108.074039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata O, Ralston J, Beltran C, Parys JB, Chen JL, Longo FJ, Darzon A. Inositol trisphosphate receptors in sea urchin sperm. Zygote. 1997;5:355–364. doi: 10.1017/s0967199400003932. [DOI] [PubMed] [Google Scholar]
- Zalk R, Lehnart SE, Marks AR. Modulation of the ryanodine receptor and intracellular calcium. Annual Review of Biochemistry. 2007;76:367–385. doi: 10.1146/annurev.biochem.76.053105.094237. [DOI] [PubMed] [Google Scholar]
- Zarelli VE, Rute MC, Roggero CM, Mayorga LS, Tomes CN. PTB1B dephosphorylates NSF and elicits SNARE complex disassembly during human sperm exocytosis. The Journal of Biological Chemistry. 2009;284:10491–10503. doi: 10.1074/jbc.M807614200. [DOI] [PMC free article] [PubMed] [Google Scholar]