Abstract
Trypanosoma cruzi the cause of Chagas disease persists in tissues of infected experimental animals and humans. Here we demonstrate the persistence of the parasite in adipose tissue from of three of 10 elderly seropositive patients with chronic chagasic heart disease. Nine control patients had no parasites in the fat. We also demonstrate that T. cruzi parasitizes primary adipocytes in vitro. Thus, in humans as in mice the parasite may persist in adipose tissue for decades and become a reservoir of infection.
Keywords: Chagas disease, Trypanosoma cruzi, Adipose tissue, Adipocyte
1. Introduction
Chagas disease caused by infection with Trypanosoma cruzi is an important cause of heart disease in endemic areas of Latin America [1]. It is being recognized with increasing frequency among persons emigrating from endemic regions to non-endemic regions of North America, Europe, Australia and Japan [2]. Chagas disease may also undergo reactivation during periods of immunosuppression such as during the administration of immunosuppressive therapy or HIV/AIDS [3,4]. Parasites have been demonstrated to persist in heart tissue of the mammalian host [5].
Shoemaker et al. [6,7], and Andrade and Silva [8] demonstrated that T. cruzi parasitized adipose tissues and specifically the adipocyte in mice. Buckner et al. [9] also demonstrated parasites in adipose tissue of mice employing a transfected strain of T. cruzi strain expressing Escherichia coli β-galactosidase which makes the parasite visible when stained with X-Gal. Our laboratory group made similar observations, but also examined the consequences of these observations on the pathogenesis of T. cruzi infection in a mouse model with implications for human disease. For example, we demonstrated that T. cruzi may persist in adipose tissue for a year after infection in a mouse model [10] suggesting that adipose tissue and the adipocyte are reservoirs from which the parasite could cause recrudescence of infection especially during immunosuppressive states.
Since we have clearly demonstrated that T. cruzi persists in adipose tissue in a mouse model we investigated whether the same was true in infected humans. Here we demonstrate that human adipose tissue is also a reservoir for this parasite. To our knowledge, this represents the first report of the persistence of T. cruzi in human adipose tissue.
2. Materials and methods
2.1. Studies on human adipose tissue
Patients presented to the Cardiology Service of the Clinical Hospital of the Federal University of Minas Gerais, Brazil for pacemaker placement. The patients presented with a variety of conduction abnormalities requiring pacemaker placement. Ten patients tested positive on two serological tests for Chagas disease and composed the infected group (see Table 1). An additional nine serologically negative patients comprised the control group. Discarded subcutaneous adipose tissue was obtained from patients in both groups and subjected to PCR analysis.
Table 1.
Adipose tissue PCR results.
| Patient Groups | Gender | Age (years) | PCR-positive | PCR negative |
|---|---|---|---|---|
| Control (n = 9) | 2M/7F | 73.6 ± 6 | 0 | 9 |
| Chagas disease (n = 10) |
5M/5F | 67.3 ± 4.8 | 3* | 7 |
M- male; F- female; ages are presented as mean age ± SEM.
P < 0.05 (by Chi Square and Fischer’s Exact Test).
We performed PCR amplification of ~330 bp fragment derived from the variable regions of minicircle kinetoplast DNA (kDNA), as previously described [11]. PCR-positive tissues were further genotyped to identify T. cruzi discrete taxonomic units (DTUs) I to VI. To achieve this, a triple step assay [12] associated to a nested PCR protocol was applied in order to improve T. cruzi genotyping directly in infected tissues. Initially we used a PCR-RFLP of the cytochrome oxidase subunit II gene (COII), which allows the discrimination of T. cruzi I (haplotypes A) and T. cruzi II (haplotype C) from the others T. cruzi III-VI (haplotype B) [13]. T. cruzi populations belonging to T. cruzi I: Col1.7G2 clone (mitochondrial haplotype A - 30, 81 and 264bp); T. cruzi II: JG (mitochondrial haplotype C - 81, 82 and 212 bp); and T. cruzi VI: CL Brener clone (haplotype B - 81 and 294 bp) were used as RFLP-COII standard patterns. A second step, consisting of amplification of the intergenic region of spliced-leader genes (SL-IR), was then applied to the mitochondrial haplotypes B strains resulting in two distinct clusters, one formed by T. cruzi III-IV (amplicons of 150 bp) and another by T. cruzi V–VI (amplicons of 200 bp) [14]. The final step consisted of rDNA 24Sα PCR that allowed the differentiation of T. cruzi III (110 bp), T. cruzi IV (~119 bp), T. cruzi V (110 and 125 bp) and T. cruzi VI (125 bp) [15]. The adipose tissue samples were thoroughly washed in PBS. These studies were approved by the Intuitional Review Boards of the Federal University of Minas Gerais, Brazil and the Albert Einstein College of Medicine, New York, USA.
2.2. In vitro studies
Primary adipocytes were isolated under sterile condition from epididymal white adipose tissue of Balb/c mice as previously described [16]. Subsequently, they were maintained in primary culture and incubated with trypomastigotes of the Y strain of T. cruzi for 4 h at a multiplicity of infection of 25:1. The adipocytes were washed in PBS and incubated for an additional 12 h in DMEM containing 5 mmol/L glucose, 25 mmol/L HEPES, 2% fetal bovine serum, 20 U/mL penicillin, 20 mg/mL streptomycin, and 1% BSA fresh medium. Presence of extracellular parasites was evaluated using an inside/outside immunofluorescence assay as described previously [17]. Briefly, cell suspension was incubated with anti-T. cruzi polyclonal antibody, washed and incubated with secondary antibody (goat anti-rabbit) labeled with red-fluorescent Alexa Fluor 546 (Invitrogen). Adipocyte and parasite DNA were stained for 1 min with blue-fluorescent DAPI (Sigma–Aldrich, St Louis, MO). Adipocytes were placed in blade and examined on a Ziess Axioplan microscope equipped with an Axiocam HRC camera controlled by Axiovision Software (Zeiss).
3. Results
3.1. Parasite kDNA detected in adipose tissue
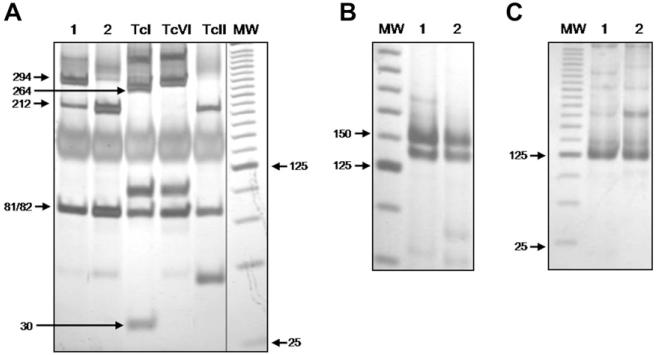
T. cruzi kDNA was detected in the adipose tissue in only 3 of the 10 Chagas patients and in two of these patients the organism was genotyped (Fig. 1). The PCR-RFLP profiles of COII genes revealed the presence of T. cruzi II in both samples (Fig. 1A). In one patient, in addition to a band pattern characteristic for T. cruzi II (81/82 + 212 bp) there was an extra fragment (294 bp) characteristic of T. cruzi III-VI populations. The subsequent steps of our genotyping strategy resulted in SL-IR and 24Sα rDNA amplicons of 150 and 125 bp, respectively (Fig. 1B and C). Taken together these findings confirm the occurrence of a mixed infection in this patient consisting of T. cruzi II and T. cruzi VI major lineages. In the three patients whose adipose tissue tested positive for T. cruzi by PCR the peripheral blood was negative by PCR.
Fig. 1.
Genetic characterization of T. cruzi in adipose tissue. (A) PCR-RFLP profiles of the mitochondrial COII gene revealed on silver stained 6% polyacrylamide gel electrophoresis. Amplicons digestion with Alu I generates three RFLP patterns for T. cruzi strains: restriction fragments of 264, 81, and 30 bp are classified as T. cruzi I (control, Col1.7G2 clone - lane 3), restriction fragments of 294 and 81 bp are classified as T. cruzi III-VI (control, CL Brener clone - T. cruzi VI - lane 4), and restriction fragments of 212 and 81 bp are classified as T. cruzi II (control, JG strain - lane 5); (B) spliced-leader gene (SL-IR) profiles and (C) rDNA 24Sα profiles on silver stained 6% polyacrylamide gel. (1) patient 1; (2) patient 2; (MW) molecular weight: a 25 bp DNA ladder (Invitrogen) is used as gel standard.
3.2. Primary adipocytes are infected by T. cruzi
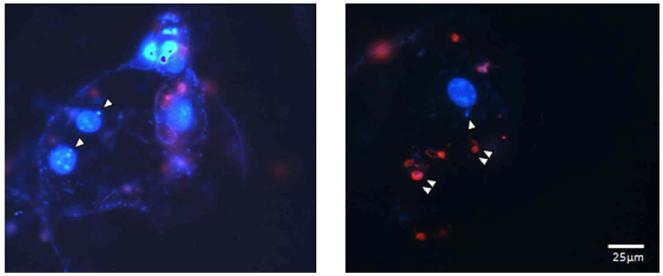
Previous studies by our laboratory group demonstrated infection of 3 TC-derived adipocytes in vitro by trypomastigotes [18]. Since we could not obtain the primary adipocytes from human subjects we obtained them from mice. Fig. 2 shows amastigotes (white single arrows) located close to the nucleus of adipocytes indicating that trypomastigotes are capable of infecting primary adipocytes.
Fig. 2.
Immunofluorescence images of primary adipocytes exposed to trypomastigotes. Isolated adipocytes from epididymal white adipose tissue of a Balb/c mouse were infected with trypomastigotes of the Y strain of T. cruzi and performed immunofluorescence analysis. The single arrows indicate the intracellular parasites (blue) and the double arrows the extracellular parasites (red) as described in Materials and Methods. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
The adipocyte, once considered to be a static storage compartment for triglycerides is now appreciated to be an active endocrine cell playing a critical role in various metabolic and immune responses [19,20]. The adipocyte is the major component of adipose tissue and contributes to the pathogenesis of diabetes, obesity, the metabolic syndrome and both innate and adoptive immunity [21]. Adipocytes contribute to these functions by influencing systemic lipid homeostasis and also through the production and release of a host of adipocyte-specific and adipocyte-enriched hormonal factors and inflammatory mediators, including adipokines. It is now well-established that T. cruzi persists in the tissues of chronically infected mice and that this persistence is important in the pathogenesis of Chagas disease.
The present report confirms what we have observed in the mouse model that the parasite persists in adipose tissue. Three of 10 patients who were seropositive for T. cruzi infection and had a cardiomyopathy and conduction defects that required pacemaker placement had evidence of T. cruzi as determined by PCR. The adipose tissue obtained from seronegative patients had no evidence of T. cruzi by the same test. The adipose tissue obtained was from the anterior chest area. It may be that adipose tissue obtained from visceral fat pads would have had a higher yield. The PCR for T. cruzi was positive in three patients. In those patients there was no amplification of T. cruzi DNA in the peripheral blood indicating that there was no blood contamination of the tissue sample.
Several groups have demonstrated that T. cruzi parasitizes adipose tissue in murine models. We were the first to investigate the consequences of this infection on adipose tissue and on cultured adipocytes [10,18]. In these investigations we demonstrated that when mice were infected adipose tissue was an early target and that the parasite persisted well into the chronic stage as demonstrated by electron microscopy and real-time PCR [10]. Additionally, infection was accompanied by an upregulation of inflammatory mediators including, cytokines and chemokines which began early in infection and persisted [10]. Since adipose tissue is a heterogeneous tissue composed of many cell types we also infected cultured adipocytes derived from fibroblasts and found that there was an upregulation of many inflammatory mediators [18]. In the current report we demonstrate that primary adipocytes can be infected with trypomastigotes of T. cruzi.
Recently, it was reported that Rickettsia prowazekii, the cause of Brill-Zinsser Disease, the relapsing form of epidemic typhus, resides in adipocytes and adipose tissue and may be a reservoir from which the infection can recrudesce decades later [22]. Similarly, Mycobacterium tuberculosis persists without replication within adipocytes and naturally infected humans with latent or active tuberculosis could have the M. tuberculosis in the adipose tissue [23]. Interestingly, the malaria parasite has been demonstrated in the vasculature of adipose tissue [24].
The persistence of T. cruzi in adipose tissue beyond the acute phase of infection results in a chronic inflammatory state which may influence the development of heart disease and diabetes. As noted adipose tissue may also be a reservoir from which there may be a recrudescence of infection especially during periods of immunosuppression. Additionally, periods of lipoatrophy as a result of HIV/AIDS and/or its treatment [25] may result in a release of parasites into the circulation.
Acknowledgments
This work was supported by a pilot grant from the Diabetes Research Center, Albert Einstein College of Medicine, and NIH Grant AI-76248 (HBT) and grants from Conselho Nacional de Desenvolvimento Científico e Tecnologico (CNPq, Brazil - MMT) and Fundação de Amparo a Pesquisas do Estado de Minas Gerais (FAPEMIG, Brazil-MMT).
References
- [1].Tanowitz HB, Machado FS, Jelicks LA, Shirani J, de Carvalho AC, Spray DC, Factor SM, Kirchhoff LV, Weiss LM. Perspectives on Trypanosoma cruzi-induced heart disease (Chagas disease) Prog. Cardiovasc. Dis. 2009;51:524–539. doi: 10.1016/j.pcad.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gascon J, Bern C, Pinazo MJ. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop. 2010;115:22–27. doi: 10.1016/j.actatropica.2009.07.019. [DOI] [PubMed] [Google Scholar]
- [3].Sartori AM, Ibrahim KY, Nunes Westphalen EV, Braz LM, .Oliveira OC, Jr., Gakiya E, Lopes MH, Shikanai-Yasuda MA. Manifestations of Chagas disease (American trypanosomiasis) in patients with HIV/AIDS. Ann. Trop. Med. Parasitol. 2007;101:31–50. doi: 10.1179/136485907X154629. [DOI] [PubMed] [Google Scholar]
- [4].Fiorelli AI, Santos RH, Oliveira JL, Jr., Lourenco-Filho DD, Dias RR, Oliveira AS, da Silva MF, Ayoub FL, Bacal F, Souza GE, Bocchi EA, Stolf NA. Heart transplantation in 107 cases of Chagas’ disease. Transplan. Proc. 2011;43:220–224. doi: 10.1016/j.transproceed.2010.12.046. [DOI] [PubMed] [Google Scholar]
- [5].Zhang L, Tarleton RL. Parasite persistence correlates with disease severity and localization in chronic Chagas’ disease. J. Inf. Dis. 1999;180:480–486. doi: 10.1086/314889. [DOI] [PubMed] [Google Scholar]
- [6].Shoemaker JP, Hoffman RV., Jr. Trypanosoma cruzi: possible stimulatory factor(s) on brown adipose tissue of mice. Exp. Parasitol. 1974;35:272–274. doi: 10.1016/0014-4894(74)90033-2. [DOI] [PubMed] [Google Scholar]
- [7].Shoemaker JP, Hoffman RV, Jr., Huffman DG. Trypanosoma cruzi: preference for brown adipose tissue in mice by the Tulahuen strain. Exp. Parasitol. 1970;27:403–407. doi: 10.1016/0014-4894(70)90045-7. [DOI] [PubMed] [Google Scholar]
- [8].Andrade ZA, Silva HR. Parasitism of adipocytes by Trypanosoma cruzi. Mem. Inst. Oswaldo Cruz. 1995;90:521–522. doi: 10.1590/s0074-02761995000400018. [DOI] [PubMed] [Google Scholar]
- [9].Buckner FS, Wilson AJ, Van Voorhis WC. Detection of live Trypanosoma cruzi in tissues of infected mice by using histochemical stain for beta-galactosidase. Infect. Immun. 1999;67:403–409. doi: 10.1128/iai.67.1.403-409.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Combs TP, Mukherjee Nagajyothi S., de Almeida CJ, Jelicks LA, Schubert W, Lin Y, Jayabalan DS, Zhao D, Braunstein VL, Landskroner-Eiger S, Cordero A, Factor SM, Weiss LM, Lisanti MP, Tanowitz HB, Scherer PE. The adipocyte as an important target cell for Trypanosoma cruzi infection. J. Biol. Chem. 2005;280:24085–24094. doi: 10.1074/jbc.M412802200. [DOI] [PubMed] [Google Scholar]
- [11].Andrade LO, Machado CR, Chiari E, Pena SD, Macedo AM. Differential tissue distribution of diverse clones of Trypanosoma cruzi in infected mice. Mol. Biochem. Parasitol. 1999;100:163–172. doi: 10.1016/s0166-6851(99)90035-x. [DOI] [PubMed] [Google Scholar]
- [12].D’Avila DA, Macedo AM, Valadares HM, Gontijo ED, de Castro AM, Machado CR, Chiari E, Galvao LM. Probing population dynamics of Trypanosoma cruzi during progression of the chronic phase in chagasic patients. J. Clin. Microbiol. 2009;47:1718–1725. doi: 10.1128/JCM.01658-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].de Freitas JM, Augusto-Pinto L, Pimenta JR, Bastos-Rodrigues L, Goncalves VF, Teixeira SM, Chiari E, Junqueira AC, Fernandes O, Macedo AM, Machado CR, Pena SD. Ancestral genomes, sex, and the population structure of Trypanosoma cruzi. PLoS Pathog. 2006;2:e24. doi: 10.1371/journal.ppat.0020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Burgos JM, Altcheh J, Bisio M, Duffy T, Valadares HM, Seidenstein ME, Piccinali R, Freitas JM, Levin MJ, Macchi L, Macedo AM, Freilij H, Schijman AG. Direct molecular profiling of mini-circle signatures and lineages of Trypanosoma cruzi bloodstream populations causing congenital Chagas disease. Int. J. Parasitol. 2007;37:1319–1327. doi: 10.1016/j.ijpara.2007.04.015. [DOI] [PubMed] [Google Scholar]
- [15].Souto RP, Fernandes O, Macedo AM, Campbell DA, Zingales B. DNA markers define two major phylogenetic lineages of Trypanosoma cruzi. Mol. Biochem. Parasitol. 1996;83:141–152. doi: 10.1016/s0166-6851(96)02755-7. [DOI] [PubMed] [Google Scholar]
- [16].Rodbell M. Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J. Biol. Chem. 1964;239:375–380. [PubMed] [Google Scholar]
- [17].Andrews NW, Hong KS, Robbins ES, Nussenzweig V. Stage-specific surface antigens expressed during the morphogenesis of vertebrate forms of Trypanosoma cruzi. Exp. Parasitol. 1987;64:474–484. doi: 10.1016/0014-4894(87)90062-2. [DOI] [PubMed] [Google Scholar]
- [18].Nagajyothi F, Desruisseaux MS, Thiruvur N, Weiss LM, Braunstein VL, Albanese C, Teixeira MM, de Almeida CJ, Lisanti MP, Scherer PE, Tanowitz HB. Trypanosoma cruzi infection of cultured adipocytes results in an inflammatory phenotype. Obesity. 2008;16:1992–1997. doi: 10.1038/oby.2008.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Halberg N, Wernstedt-Asterholm I, Scherer PE. The adipocyte as an endocrine cell. Endocrinol. Metab. Clin. North. Am. 2008;37:753–768. doi: 10.1016/j.ecl.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kaminski DA, Randall TD. Adaptive immunity and adipose tissue biology. Trends Immunol. 2010;31:384–390. doi: 10.1016/j.it.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Attie AD, Scherer PE. Adipocyte metabolism and obesity. J. Lipid Res. 2009;50(Suppl):S395–S399. doi: 10.1194/jlr.R800057-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bechah Y, Paddock CD, Capo C, Mege JL, Raoult D. Adipose tissue serves as a reservoir for recrudescent Rickettsia prowazekii infection in a mouse model. PLoS One. 2010;5:e8547. doi: 10.1371/journal.pone.0008547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Neyrolles O, Hernandez-Pando R, Pietri-Rouxel F, Fornes P, Tailleux L, Barrios Payan JA, Pivert E, Bordat Y, Aguilar D, Prevost MC, Petit C, Gicquel B. Is adipose tissue a place for Mycobacterium tuberculosis persistence? PLoS One. 2006;1:e43. doi: 10.1371/journal.pone.0000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Franke-Fayard B, Fonager J, Braks A, Khan SM, Janse CJ. Sequestration and tissue accumulation of human malaria parasites: can we learn anything from rodent models of malaria? PLoS Pathog. 2010;6:e1001032. doi: 10.1371/journal.ppat.1001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tershakovec AM, Frank I, Rader D. HIV-related lipodystrophy and related factors. Atherosclerosis. 2004;174:1–10. doi: 10.1016/S0021-9150(03)00246-6. [DOI] [PubMed] [Google Scholar]