Abstract
Benjamin Franklin, mostly known for his participation in writing The Declaration of Independence and work on electricity, was also one of the first scientists to seek to understand the properties of oil monolayers on water surfaces. During one of his many voyages across the Atlantic Ocean, Franklin observed that oil had a calming effect on waves when poured into rough ocean waters. Though at first taking a backseat to many of his other scientific and political endeavors, Franklin went on to experiment with oil, spreading monomolecular films on various bodies of water, and ultimately devised a concept of particle repulsion that is indirectly related to the hydrophobic effect. His early observations inspired others to measure the dimensions of oil monolayers, which eventually led to the formulation of the contemporary lipid bilayer model of the cell membrane.
Main Text
As a Founding Father of the United States of America, statesman, philosopher, diplomat, inventor, and scientist, Benjamin Franklin (1706–1790) had an amazing life (1,2). Born in Boston, Franklin moved to Philadelphia at the age of 17 and began working in local print houses. He was soon heralded as a favorite son of Philadelphia because of his literacy, devotion to learning, community service, and leadership (Fig. 1). His experiments on the electric properties of lightning are much renowned; however, less well known are his studies involving oil monolayers on water surfaces and hydrophobic forces. As told in the book Ben Franklin Stilled Waves: An Informal History of Pouring Oil on Water with Reflections on the Ups and Downs of Scientific Life in General by the late Charles Tanford (3), a former president of the Biophysical Society (1979–1980), Franklin’s experiments on oil monolayers were the first of their kind and eventually led to the formulation of the lipid bilayer model of the biological membrane.
Figure 1.

Benjamin Franklin of Philadelphia, L.L.D., F.R.S., in between 1763 and 1785, during the period when he carried out his experiments of oil on water (by Edward Fisher, 1730–ca.1785). Print shows Benjamin Franklin, three-quarter-length portrait, seated at desk, looking to his right at an electrical device; in his left hand are papers upon which he is taking notes, and visible through a window to his left is lightning striking a building (Courtesy of the Library of Congress, Washington, DC).
Oil on the Sea
In 1757, Franklin was sent by the American House of Assembly of Philadelphia to Great Britain to petition King George II against the policies and activity of the Penn family, the proprietors of Pennsylvania. Soon after leaving New York harbor, the fleet of 96 ships encountered windy weather, sending them ferociously rocking over the waves. Franklin noticed that two of the ships in the fleet were sailing much more smoothly than the rest and inquired from the Captain a reason for the anomalous smooth sailing (4). “The cooks … have, I suppose, been just emptying their greasy water through the scuppers, which has greased the sides of those ships a little,” the captain told him in a matter-of-fact tone. (Apparently, pouring olive oil on rough water was known since the Classical Era to have a calming effect and was a common way for seamen to weather storms (5), although the practise was connected with magic and fanciful explanations. Plutarch attributed to Aristotle that “the oil produces calm by smoothing the water surface so that the wind can slip over it without making an impression” (6).) The incident piqued Franklin’s curiosity, perhaps partially because it reminded him of the wax he played with as a 10-year-old apprentice in his father’s soap-making shop. However, he did not quite agree with the captain’s rationale that ship-greasing was the cause of the water-calming effect, but was unable at the time to think of another explanation.
During later trips he observed the phenomenon again and again and like any good scientist, Franklin performed a literature search to find anything he could about the phenomenon and its underlying cause (4): “I at times revolved in my mind, and wondered to find no mention of them in our books of experimental philosophy.” Therefore, Franklin “resolved to make some experiment of the effect of oil on water, when I should have opportunity.”
Monolayer of Oil on a Lake
Over the next decade, Franklin continued his distinguished work on lightning for which he was eventually awarded the Copley Medal, a first for any scientific work carried out in North America. Previous winners of this most prestigious honor from the Royal Society included Franklin’s long-time hero, Isaac Newton. Possibly because he was encouraged by his recent successes or the opportunity finally arose, in 1769, the same year Franklin published his book, Experiments and Observations on Electricity, made at Philadelphia in America, he decided to revisit his questions on the water-calming effect of oil that he had been pondering for over a decade.
While staying in the Clapham Common area in south London during another trip to Great Britain, he and his merchant friend Christopher Baldwin went to lake Mount Pond, located near Baldwin’s home (7). There they started the experiment that is best described by Franklin’s own words (4):
“At the length being at Clapham where there is, on the common, a large pond, which I observed to be one day very rough with the wind, I fetched out a cruet of oil, and dropt a little of it on the water. I saw it spread itself with surprising swiftness upon the surface … and there the oil, though not more than a teaspoonful, produced an instant calm over a space several yards square, which spread amazingly, and extended itself gradually till it reached the lee side, making all that quarter of the pond, perhaps half an acre, as smooth as a looking-glass. … [The oil layer was] so thin as to produce the prismatic colors, for a considerable space, and beyond them so much thinner as to be invisible, except in its effect of smoothing the waves at a much greater distance.”
Franklin had actually discovered a layer of oil that was a single-molecule-thick!
As a good experimentalist, Franklin did not forget to repeat his experiment at other locations and under different conditions, and was able to reproduce his results (4). “After this, I contrived to take with me, whenever I went into the country, a little oil in the upper hollow joint of my bamboo cane, with which I might repeat the experiment as opportunity should offer; and I found it constantly to succeed.”
The Hydrophobic Force
Franklin actually considered the underlying forces that caused the oil to spread on the water surface (4):
“In these experiments, one circumstance struck me with particular surprise. This was the sudden, wide, and forcible spreading of a drop of oil on the face of water, which I do not know that anybody has hitherto considered. If a drop of oil is put on a polished marble table, or on a looking-glass that lies horizontally; the drop remains in its place, spreading very little.”
In contrast, on a water surface, Franklin observed something totally different:
“If there be a mutual repulsion between the particles of oil, and no attraction between oil and water, oil dropt on water will not be held together by adhesion to the spot whereon it falls; …it will be at liberty to expand itself; and it will spread on a surface that, besides being smooth to the most perfect degree of polish, prevents, perhaps by repelling the oil, all immediate contact, keeping it at a minute distance from itself; and the expansion will continue, till the mutual repulsion between the particles of the oil is weakened and reduced to nothing by their distance.”
Importantly, Franklin further observed, “there seems to be no natural repulsion between water and air, such as to keep them from coming into contact with each other.”
Clearly, Franklin understood that oil “particles” could move freely at the interface between water and air and that they reduced the tension between the two bulk phases. The concept of molecules, which among others was promoted by John Dalton in the early 1800s, was of course not yet known during Franklin’s times and even the ideal gas law attributed to Emile Clapeyron based on Amedeo Avogadro’s law of 1811 was only formulated in 1834. What Franklin actually observed was a monomolecular layer of oil, which eventually expanded into a two-dimensional gas of oil molecules at the air-water interface. When expanded into the more condensed liquid monolayer state, Franklin observed a reduction of surface tension, which caused the oil’s wave-calming effect and which is at the source of the hydrophobic forces or hydrophobic effect as they were realized later. Therefore, Franklin’s experiment may well be the first experiment on the nature of the hydrophobic effect!
Franklin was eager to understand such forces (4). “The quantity of this force, and the distance to which it will operate, I have not yet ascertained; but I think it a curious inquiry and I wish to understand whence it arises.” Of course, the underlying molecular nature of the hydrophobic effect was, understandably, to remain unclear for another 150 years (8,9).
Measurements of Monolayer Thickness
Franklin did not go on to calculate the thickness of the oil monolayer, although he did mention “particles spreading on the water surface” (4). Had he done this he would have predated the first measurement of the physical dimensions of a molecule by over 100 years. The fact that he did not attempt the calculation is a bit puzzling, however, for Franklin must have had the conceptual computational knowledge required. In Philadelphia, he once calculated the audience size for a popular priest, who had a clear and very loud voice, by measuring the furthermost distance one could hear his voice and by estimating the surface area that one person occupies (1). Franklin found that he could still distinctly hear the preacher’s voice up to a distance of 200 feet. He then determined that by “imagining then a semi-circle, of which my distance should be the radius, and that it were fill’d with auditors, to each of whom I allow’d two square feet, I computed that he might well be heard by more than thirty thousand.” Both calculations are the same type of close-packing problems that occur on a two-dimensional flat surface. However, the actual experimental measurements of a molecule’s dimensions would have to wait until a British Lord and a German amateur scientist entered the scene (3,7,10).
Lord Rayleigh (1842–1919), then Professor of Natural Science at the Royal Institute in London, was a physicist with many interests who made contributions to multiple areas of science, from optics and electromagnetism, to photography and liquid capillarity. He found the question of oil spreading on a water surface to be “of great interest which attaches to the determination of molecular magnitudes, [and] the matter seemed well worthy of investigation” (11). In March, 1890 he published his experiments on the thickness of an olive oil monolayer performed in a round sponge-bath (Fig. 2). He found that 0.81 mg of olive oil was just enough to cover the entire surface area of the bath with a diameter of 84 cm. Using a density of 0.9 g/mL, Lord Rayleigh obtained 16.3 Å as the thickness of the olive oil monolayer (5,11).
Figure 2.
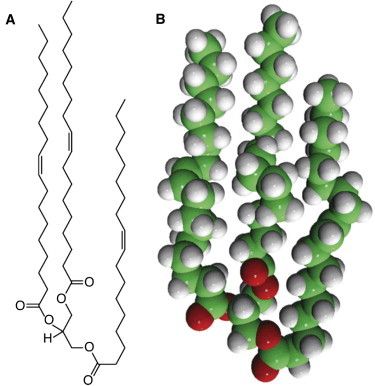
Structure of a major component of olive oil, triolein (1,2,3-(9Z-octadecenoyl)-glycerol). Three oleate chains are ester-linked to a glycerol moiety at the bottom, which contacts the water surface when spread as a monolayer. (A) Chemical structure. (B) Space-filling model.
The accuracy of Rayleigh’s measurements improved significantly when a self-taught scientist, Agnes Pockels (1862–1935), approached Lord Rayleigh when she saw his 1890 paper in the Royal Proceedings. Pockels was born in Venice but grew up in Lower Saxony, Germany. Even though she had no formal training and suffered various health problems during her life, Pockels developed a strong interest in surface chemistry and physics (3,10). Working totally on her own, at the age of 20 she invented a tin trough with a sliding barrier that was used to measure surface tension by means of the force required to pull a small disk (a button) from contact with the surface. This trough is considered to be a precursor of the more famous Langmuir trough of 1917, as also acknowledged by Langmuir himself. When Pockels sent to Lord Rayleigh her experimental results that she obtained independently and literally at her kitchen bench, he immediately arranged for their publication in the journal Nature (12,13). Pockels’ original experiments were actually carried out between 1882 and 1890, i.e., they predated Lord Rayleigh’s experiments (10). Pockels measured the first pressure-area diagrams of lipid monolayers with her device. By identifying the drop in surface tension when a specified amount of oil was applied, she calculated the thickness of the oil film to be 13 Å. Guided by Agnes Pockels’ findings, Lord Rayleigh subsequently improved his own measurements of molecules in surface films (14). These accomplishments by the famous future Nobel Laureate Lord Rayleigh and Miss Pockels, a self-educated scientist who as a woman was denied higher education and never held any academic position, using very simple techniques, were extraordinarily ahead of their time. They measured sizes of molecules even before the discovery of x-rays, which would be used decades later to accurately measure molecular dimensions of lipid films as well as many other molecules. (Agnes Pockels’ accomplishments were recognized by the German academic establishment only much later. In 1932, shortly before her 70th birthday, she was awarded her well deserved if belated honorary doctorate from the University of Braunschweig in her hometown (10).)
Then entered on the scene the great American chemist and physicist and future Nobel Laureate, Irving Langmuir (1881–1957). Langmuir was working at the General Electric Research Laboratory in Schenectady, New York, where he initially worked on the improvement of light bulbs by filling them with gases. His work on the surface chemistry of gas adsorption to metals (motivated by understanding the properties of tungsten wires in gas-filled light bulbs) and his famous experimental and theoretical work on the physical chemistry of adsorption also piqued his interest in the chemistry of oil films. By inventing the Langmuir trough of similar design to that of Miss Pockels with the addition of a pressure-measuring device attached to a fixed barrier, he was able to accurately measure the effect of various compounds on water’s surface tension (15,16). This in turn provided a way to investigate both the water-oil interaction and the properties of oil monolayers at the air-water interface, including the monolayer thickness and cross-sectional areas of many amphiphilic molecules including membrane lipids.
Calming Water Waves
Following Franklin’s experiments, thoughts about the underlying reasons for the calming effect of oil on water waves progressed in the late 19th century. A Scottish meteorologist named John Aitken expanded on Franklin’s observation and proposed that it is “not the bite, grip or friction of the air on the surface” that was reduced when oil was poured on water as had been believed by many of his predecessors, but that it is the surface tension on the water surface, or the lack of it upon the spreading of oil, that accounts for the oil’s wave-calming effect (17,18). This is best explained by Lord Rayleigh (5):
“Let us consider small waves as propagated over the surface of clean water; as the waves advance, the surface of the water has to submit to periodic extensions and contractions. At the crest of the wave the surface is compressed, while at the trough it is extended. As long as the water is pure there is no force to oppose that, and the wave can be propagated without difficulty; but if the surface be contaminated, the contamination strongly resists the alternating stretching and contraction. It tends always, on the contrary, to spread itself uniformly; and the result is that the water refuses to lend itself to the motion which is required of it. The film of oil may be compared to an inextensible membrane floating on the surface of the water, and hampering its motion; and under these conditions it is not possible for the waves to be generated, unless the forces are very much greater than usual.”
From Monolayer to Bilayer and Beyond
Initially, Langmuir’s work attracted little attention from biologists, but one Dutch pediatrician, Evert Gorter, saw the important implications for Biology almost immediately (3,19). In 1925, he and his graduate student François Grendel published a short paper describing their measurements of the total area of lipids extracted from red blood cells by spreading the lipids as a monolayer on water (20). They compared this to the total surface area of the red blood cell membrane and obtained a ratio of two. This seminal work provided the first evidence that the cell membrane may consist of a phospholipid bilayer. Although their conclusions were correct, Gorter and Grendel were lucky. Due to technical limitations and the limited knowledge of biological membranes at the time, they made a few “mistakes,” which however canceled each other out (21). Contemporary lipid extraction procedures allowed them to quantitatively extract only about half of all lipids present in the red cell membrane. This underestimate was compensated by the chosen film balance surface pressure, which we now know was much too low to simulate the area/lipid in a bilayer. Aditionally, an underestimate of the red blood cell surface was counteracted by the fact that a substantial fraction of the total area of cell membranes is occupied by membrane proteins. Despite these shortcomings, the ratio of two is still true and the concept of the lipid bilayer was born.
In the 1940s, the newly-invented electron microscope provided the first picture of a cell at a magnification and resolution high enough that its membrane could be visualized. Improved resolution allowed researchers to discover the “tri-laminar” ultrastructure of membranes in the late 1950s, which however was initially incorrectly interpreted in terms of lipid and protein arrangements (22). In the 1960s, a wealth of classical x-ray diffraction experiments on lipids in excess water by Vittorio Luzzati firmly established that the lipids in biological membranes are organized in bilayer structures with liquid acyl chains (23). Based on a multitude of biochemical and functional experiments and additional electron microscopy, x-ray diffraction, and thermodynamic and spectroscopic data, the fluid mosaic model of the cell membrane eventually became widely accepted (24).
With the development of new concepts and novel technologies, our understanding of biological membranes is making rapid progress (25). But it is with the same curiosity that Benjamin Franklin displayed at sea and on a lake 250 years ago that the advance of science today is driven. Research on membrane biophysics, pioneered by Mr. Franklin, has gone a long way in answering fundamental questions in biology and medicine and, yet, still more discoveries lay ahead.
Acknowledgments
D.-N.W.’s work is supported by National Institutes of Health grants No. R01 DK073973, No. R01 GM093825, No. R01 MH083840, No. R01 DA019676, and No. U54 GM075026. L.K.T.’s work is supported by National Institutes of Health grants No. R01 GM51329, No. R01 AI30577, and No. P01 GM72694.
Footnotes
Note: This article is published on the occasion of the 57th Annual Meeting of the Biophysical Society, held February 2–6, 2013 in Philadelphia.
Contributor Information
Da-Neng Wang, Email: wang@saturn.med.nyu.edu.
Lukas K. Tamm, Email: lkt2e@virginia.edu.
References
- 1.Franklin B. Dover Publications; Mineola, NY: 1996. The Autobiography of Benjamin Franklin. [Google Scholar]
- 2.Isaacson W. Simon & Schuster; New York: 2003. Benjamin Franklin. [Google Scholar]
- 3.Tanford C. Oxford; New York: 2004. Ben Franklin Stilled the Waves—An Informal History of Pouring Oil on Water with Reflections on the Ups and Downs of Scientific Life in General. [Google Scholar]
- 4.Franklin B. Of the stilling of waves by means of oil. Philos. Trans. R. Soc. Lond. B. 1774;64:445–460. [Google Scholar]
- 5.Rayleigh L. On foam. Proc. R. Inst. G. B. 1890;13:85–97. [Google Scholar]
- 6.Scott J.C. The historical development of theories of wave-calming using oil. In: Hall A.R., Smith N., editors. History of Technology. Mansell; London: 1978. pp. 163–186. [Google Scholar]
- 7.Giles C.H. Franklin's teaspoonful of oil—studies in the early history of surface chemistry, part 1. Chem. Ind. 1969:1616–1624. [Google Scholar]
- 8.Hartley G.S. Hermann; Paris: 1936. Aqueous Solutions of Paraffin Chain Salts. A Study in Micelle Formation. [Google Scholar]
- 9.Tanford C. John Wiley; New York: 1973. The Hydrophobic Effect: Formation of Micelles and Biological Membranes. [Google Scholar]
- 10.Giles C.H., Forrester S.D. The origins of the surface film balance—studies in the early history of surface chemistry, part 3. Chem. Ind. 1971:43–53. [Google Scholar]
- 11.Rayleigh L. Measurements of the amount of oil necessary in order to check the motions of camphor upon water. Proc. R. Soc. Lond. 1890;47:364–367. [Google Scholar]
- 12.Pockels A. Surface tension. Nature. 1891;43:437–439. [Google Scholar]
- 13.Pockels A. On the relative contamination of the water-surface by equal quantities of different substances. Nature. 1892;46:418–419. [Google Scholar]
- 14.Rayleigh L. Investigations in capillarity: the size of drops—the liberation of gas from supersaturated solutions—colliding jets—the tension of contaminated water surfaces. Phil. Mag. & J. Sci. 1899;48:321–337. [Google Scholar]
- 15.Langmuir I. The constitution and fundamental properties of solids and liquids. II. Liquids. J. Amer. Chem. Soc. 1917;39:1848–1906. [Google Scholar]
- 16.Langmuir I. The shapes of group molecules forming the surfaces of liquids. Proc. Natl. Acad. Sci. USA. 1917;3:251–257. doi: 10.1073/pnas.3.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aitken J. On the effect of oil on a stormy sea. Proc. R. Soc. Edinb. 1882;12:56–75. [Google Scholar]
- 18.Giles C.H., Forrester S.D. Wave damping: the Scottish contribution studies in the early history of surface chemistry, part 2. Chem. Ind. 1970:80–87. [Google Scholar]
- 19.Tanford C. Amphiphile orientation: physical chemistry and biological function. Biochem. Soc. Trans. 1987;15(Suppl):1S–7S. [PubMed] [Google Scholar]
- 20.Gorter E., Grendel F. On bimolecular layers of lipoids on the chromocytes of the blood. J. Exp. Med. 1925;41:439–443. doi: 10.1084/jem.41.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zwaal R.F.A., Demel R.A., van Deenen L.L.M. The lipid bilayer concept of cell membranes. Trends Biochem. Sci. 1976;1:112–114. [Google Scholar]
- 22.Robertson J.D. The molecular structure and contact relationships of cell membranes. Prog. Biophys. Mol. Biol. 1960;10:343–418. [PubMed] [Google Scholar]
- 23.Luzzati V. X-ray diffraction studies of lipid-water systems. In: Chapman D., editor. Biological Membranes. Academic Press; New York: 1968. pp. 71–123. [Google Scholar]
- 24.Singer S.J., Nicolson G.L. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 25.Tamm, L. K. 2012. Biophysics of membranes. In Comprehensive Biophysics. E. H. Egelman, editor. Vol. 5, Membranes. Lukas Tamm, editor. Academic Press, New York. 1–2.


