Abstract
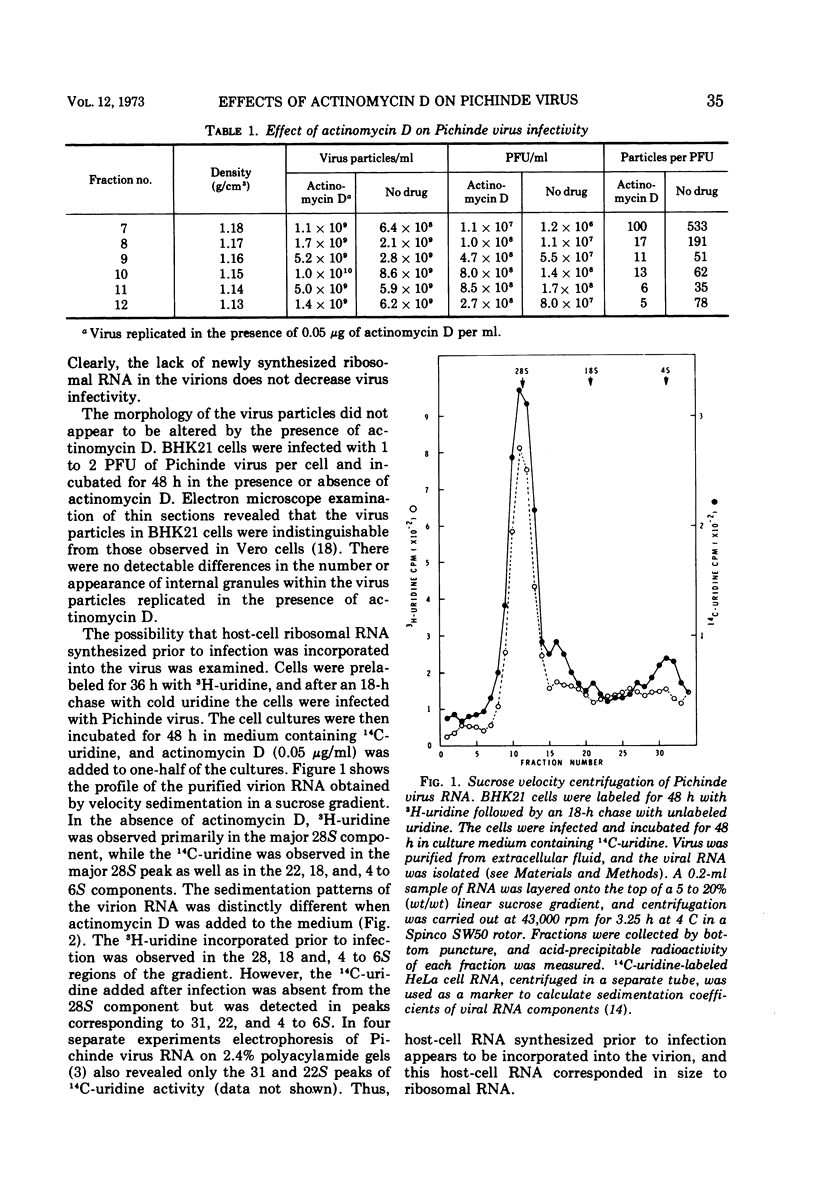
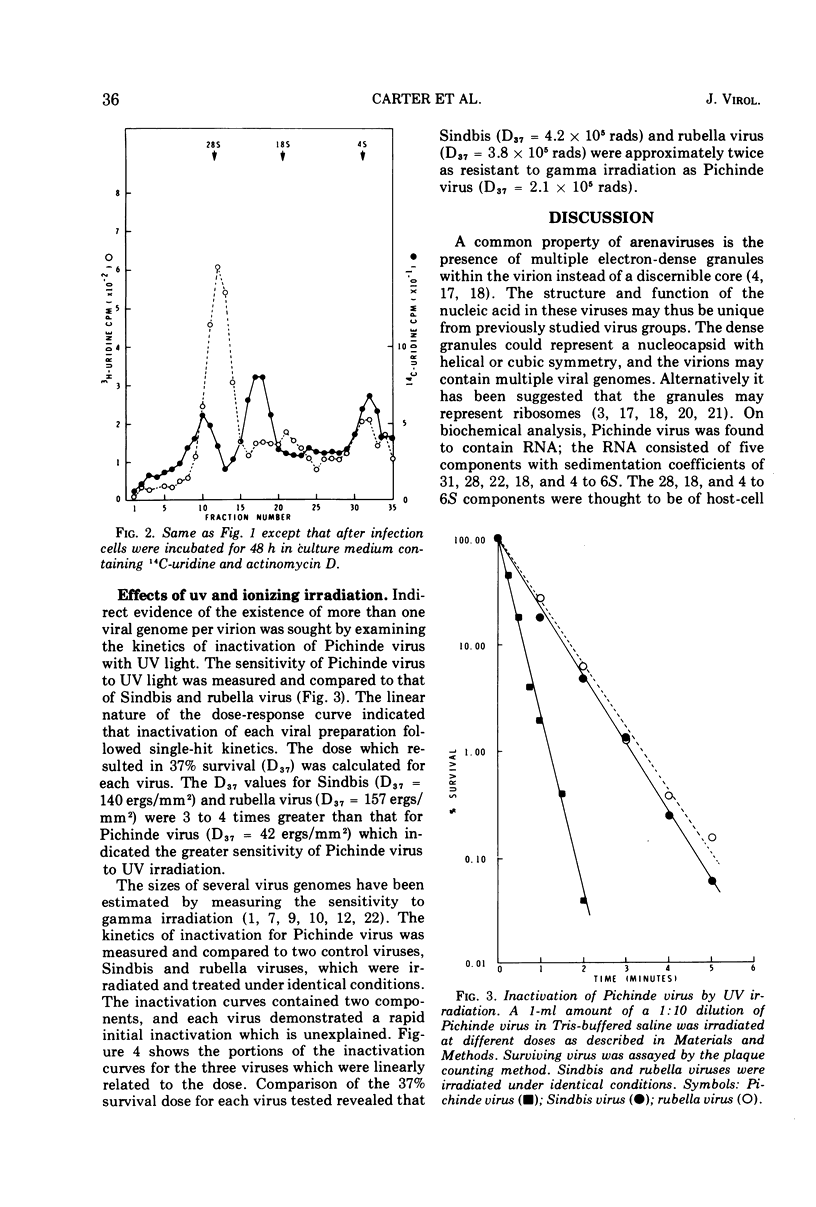
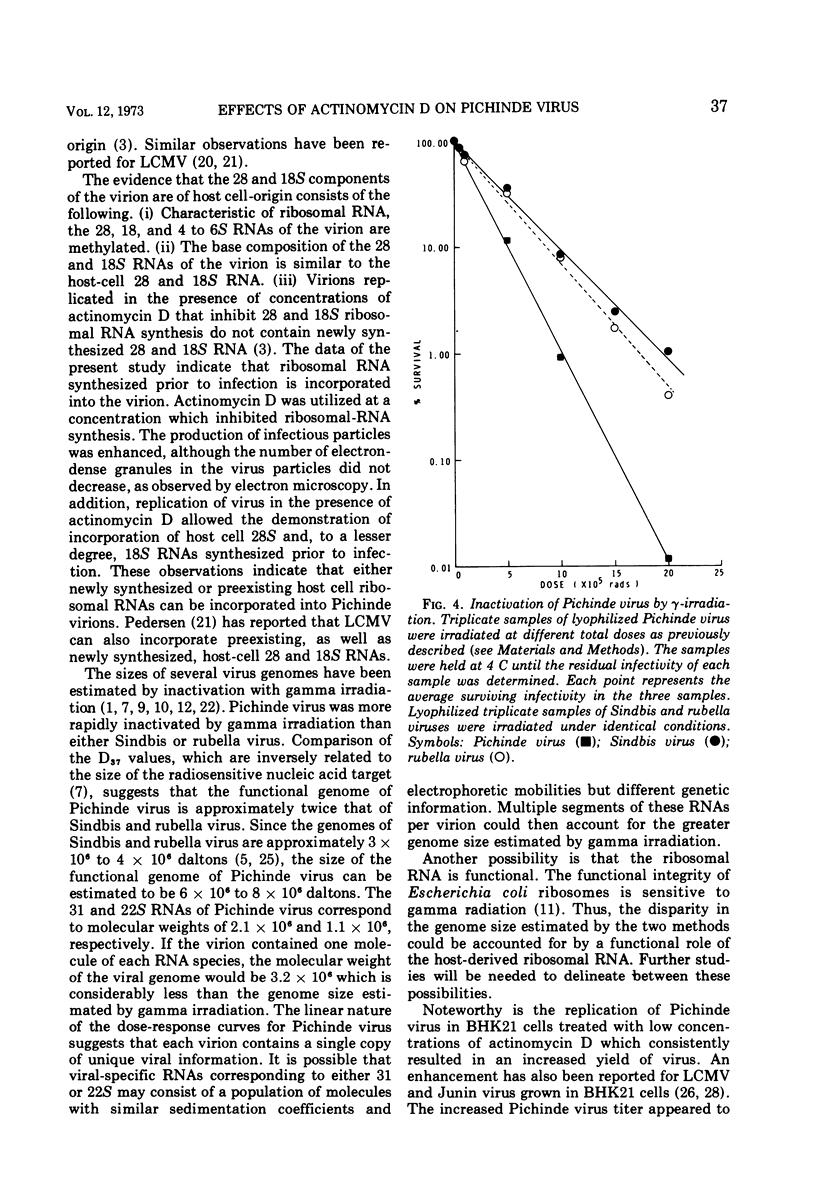
Actinomycin D (0.05 μg/ml) suppresses the synthesis of ribosomal RNA of baby hamster kidney (BHK21) cells. The production of infectious Pichinde virus was enhanced in the presence of actinomycin D, although the production of virus particles was not substantially different from cultures inoculated in the absence of the drug. By prelabeling BHK21 cells with 3H-uridine and then allowing the virus to replicate in the presence of actinomycin D, it was possible to show that ribosomal RNA synthesized prior to infection was incorporated into the virion. A single-hit kinetics of inactivation of Pichinde virus was observed with ultraviolet light, suggesting that the virus contains only a single copy of genome per virion. Comparison of the inactivation kinetics by gamma irradiation of Pichinde virus with Sindbis and rubella virus indicated that the radiosensitive genome of Pichinde virus was about 6 × 106 to 8 × 106 daltons. This value is greater than the 3.2 × 106 daltons which was estimated by biochemical analysis. One possible explanation considered is that the ribosomal RNA of host cell origin is functional and accounts for the differences in genome size estimated by the two methods.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basilico C., Di Mayorca G. Radiation target size of the lytic and the transforming ability of polyoma virus. Proc Natl Acad Sci U S A. 1965 Jul;54(1):125–127. doi: 10.1073/pnas.54.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M. F., Biswal N., Rawls W. E. Characterization of nucleic acid of pichinde virus. J Virol. 1973 Jan;11(1):61–68. doi: 10.1128/jvi.11.1.61-68.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton A. J., Rowe W. P., Smith G. H., Wilsnack R. E., Pugh W. E. Morphological and cytochemical studies on lymphocytic choriomeningitis virus. J Virol. 1968 Dec;2(12):1465–1478. doi: 10.1128/jvi.2.12.1465-1478.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobos P., Faulkner P. Molecular weight of Sindbis virus ribonucleic acid as measured by polyacrylamide gel electrophoresis. J Virol. 1970 Jul;6(1):145–147. doi: 10.1128/jvi.6.1.145-147.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa M. E., Rawls W. E. Biological markers for differentiation of herpes-virus strains of oral and genital origin. J Gen Virol. 1969 Mar;4(2):259–267. doi: 10.1099/0022-1317-4-2-259. [DOI] [PubMed] [Google Scholar]
- HOPPS H. E., BERNHEIM B. C., NISALAK A., TJIO J. H., SMADEL J. E. BIOLOGIC CHARACTERISTICS OF A CONTINUOUS KIDNEY CELL LINE DERIVED FROM THE AFRICAN GREEN MONKEY. J Immunol. 1963 Sep;91:416–424. [PubMed] [Google Scholar]
- JORDAN R. T., KEMPE L. L. Inactivation of some animal viruses with gamma radiation from cobalt-60. Proc Soc Exp Biol Med. 1956 Feb;91(2):212–215. doi: 10.3181/00379727-91-22215. [DOI] [PubMed] [Google Scholar]
- Kenny M. T., Albright K. L., Emery J. B., Bittle J. L. Inactivation of rubella virus by gamma radiation. J Virol. 1969 Dec;4(6):807–810. doi: 10.1128/jvi.4.6.807-810.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latarjet R., Cramer R., Montagnier L. Inactivation, by UV-, x-, and gamma-radiations, of the infecting and transforming capacities of polyoma virus. Virology. 1967 Sep;33(1):104–111. doi: 10.1016/0042-6822(67)90098-0. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- McCombs R. M., Melnick M. B., Brunschwig J. P. Biophysical studies of vesicular stomatitis virus. J Bacteriol. 1966 Feb;91(2):803–812. doi: 10.1128/jb.91.2.803-812.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifune K., Carter M., Rawls W. Characterization studies of the Pichinde virus-a member of the arenavirus group. Proc Soc Exp Biol Med. 1971 Feb;136(2):637–644. doi: 10.3181/00379727-136-35330. [DOI] [PubMed] [Google Scholar]
- Mifune K., Desmyter J., Rawls W. E. Effect of exogenous interferon on rubella virus production in carrier cultures of cells defective in interferon production. Infect Immun. 1970 Aug;2(2):132–138. doi: 10.1128/iai.2.2.132-138.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F. A., Webb P. A., Johnson K. M., Whitfield S. G., Chappell W. A. Arenoviruses in Vero cells: ultrastructural studies. J Virol. 1970 Oct;6(4):507–518. doi: 10.1128/jvi.6.4.507-518.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F. A., Webb P. A., Johnson K. M., Whitfield S. G. Morphological comparison of Machupo with lymphocytic choriomeningitis virus: basis for a new taxonomic group. J Virol. 1969 Oct;4(4):535–541. doi: 10.1128/jvi.4.4.535-541.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen I. R. Density gradient centrifugation studies on lymphocytic choriomeningitis virus and on viral ribonucleic acid. J Virol. 1970 Oct;6(4):414–420. doi: 10.1128/jvi.6.4.414-420.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen I. R. Different classes of ribonucleic acid isolated from lymphocytic choriomeningitis virus. J Virol. 1973 Mar;11(3):416–423. doi: 10.1128/jvi.11.3.416-423.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen I. R. Lymphocytic choriomeningitis virus RNAs. Nat New Biol. 1971 Nov 24;234(47):112–114. doi: 10.1038/newbio234112a0. [DOI] [PubMed] [Google Scholar]
- Polatnick J., Bachrach H. L. Ionizing irradiation of foot-and-mouth disease virus and its ribonucleic acid. Arch Gesamte Virusforsch. 1968;23(1):96–104. doi: 10.1007/BF01242118. [DOI] [PubMed] [Google Scholar]
- Rawls W. E., Desmyter J., Melnick J. L. Virus carrier cells and virus-free cells in fetal rubella. Proc Soc Exp Biol Med. 1968 Nov;129(2):477–483. doi: 10.3181/00379727-129-33348. [DOI] [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- SMITH K. O., MELNICK J. L. A method for staining virus particles and identifying their nucleic acid type in the electron microscope. Virology. 1962 Jul;17:480–490. doi: 10.1016/0042-6822(62)90143-5. [DOI] [PubMed] [Google Scholar]
- Sedwick W. D., Sokol F. Nucleic acid of rubella virus and its replication in hamster kidney cells. J Virol. 1970 Apr;5(4):478–489. doi: 10.1128/jvi.5.4.478-489.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanwick T. L., Kirk B. E. Effect of actinomycin D on the yield of lymphocytic choriomeningitis virus in baby hamster kidney cells. Infect Immun. 1971 Oct;4(4):511–512. doi: 10.1128/iai.4.4.511-512.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapido H., Sanmartín C. Pichindé virus, a new virus of the Tacaribe group from Colombia. Am J Trop Med Hyg. 1971 Jul;20(4):631–641. [PubMed] [Google Scholar]



