Abstract
Selectins mediate circulatory leukocyte trafficking to sites of inflammation and trauma, and the extracellular microenvironments at these sites often become acidic. In this study, we investigated the influence of slightly acidic pH on the binding dynamics of selectins (P-, L-, and E-selectin) to P-selectin glycoprotein ligand-1 (PSGL-1) via computational modeling (molecular dynamics) and experimental rolling assays under shear in vitro. The P-selectin/PSGL-1 binding is strengthened at acidic pH, as evidenced by the formation of a new hydrogen bond (seen computationally) and the observed decrease in the rolling velocities of model cells. In the case of L-selectin/PSGL-1 binding dynamics, the binding strength and frequency increase at acidic pH, as indicated by the greater cell-rolling flux of neutrophils and slower rolling velocities of L-selectin-coated microspheres, respectively. The cell flux is most likely due to an increased population of L-selectin in the high-affinity conformation as pH decreases, whereas the velocities are due to increased L-selectin/PSGL-1 contacts. In contrast to P- and L-selectin, the E-selectin/PSGL-1 binding does not exhibit significant changes at acidic pH levels, as shown both experimentally and computationally.
Introduction
The localization of circulating leukocytes to sites of injury or inflammation is a multistep adhesion cascade (1,2) involving 1), the initial tethering and rolling of leukocytes along the vasculature wall; 2), integrin-mediated firm adhesion; and 3), the extravasation of cells into tissues (3). Selectins (P-, L-, and E-selectin) are membrane adhesion molecules that facilitate the initial tethering and rolling of leukocytes. Although selectin-mediated cell rolling is ubiquitously observed for leukocytes, accumulating evidence suggests that circulating tumor cells (CTCs) also exhibit selectin-mediated rolling (4,5).
Selectins are differentially expressed on many cell types. P-selectin is stored in the α-granules of platelets and the Weibel-Palade bodies of endothelial cells, and upon activation of the endothelial cells is rapidly transported to the surface (6,7). E-selectin expression is limited to the endothelial lining (8), and in addition to mediating leukocyte rolling during inflammation, it has also been shown to assist human CD34+ hematopoietic cell homing to the bone marrow (9). Whereas P- and E-selectin are endothelial selectins, L-selectin is expressed on most circulating leukocytes, with the exception of several subpopulations of memory cells (10). The presenting architectures of selectins are highly conserved. Their structures consist of a C-type lectin domain at the N-terminus, an epidermal growth factor (EGF)-like module, two to nine short consensus repeats, a transmembrane region, and a cytoplasmic tail (11). Selectin ligands consist of a structurally diverse array of surface glycoproteins (e.g., GlyCAM-1, CLA, HCELL, and PSGL-1) (12–14). These ligands require post-translational N- and/or O-linked glycosylation of their backbone with a tetrasaccharide carbohydrate called sialyl Lewis X (sLeX) to gain selectin-binding activity. sLeX and its derivative sLeA are the most basic binding determinants for all three selectins. In this study, we focus on selectin/PSGL-1 binding because PSGL-1, a glycoprotein heavily decorated with sLeX, is a common vascular selectin ligand that can readily bind to all three selectins.
The physiologic pH level in extracellular microenvironments can significantly decrease due to various pathological and physiological phenomena. For example, anaerobic lactate buildup and inadequate extraction of metabolites during prolonged ischemia can decrease the extracellular pH level, a condition known as acidosis (15,16). Studies have shown that acidosis occurs in the early stages of wound healing (17,18), which also involves periods of elevated leukocyte and platelet recruitment. Serrano et al. (19) showed that under acidic conditions, neutrophil adhesion to the vascular endothelium is enhanced. Acidic conditions also promote angiogenesis of injured tissues (20). Furthermore, tumor microenvironments are often acidic, with pH levels as low as 6.2 (21,22). In addition, because the recruitment of leukocytes to targeted tissues is selectin mediated, these observations suggest that acidic pH might alter selectin/ligand-binding mechanics, resulting in a change in cell-binding dynamics compared with the homeostasis pH level of 7.4.
In this study, we investigated the influence of acidic extracellular pH on P-, L-, and E-selectin interactions with PSGL-1 and sLex. We used molecular dynamics (MD) and steered MD (SMD) to predict changes in adhesion characteristics while performing cell-rolling assays with selectin-coated capillary tubing to characterize the effects of pH on cell rolling and adhesion in vitro.
Materials and Methods
MD
The lectin and EGF crystal structures of P-selectin bound to PSGL-1 (1G1S (23)), E-selectin (1G1T (23)), and L-selectin (3CFW) were obtained from the Protein Data Bank for use as starting atomic coordinates. Free-dynamics and SMD simulations were performed using the YASARA (http://yasara.org) package of MD programs with the YAMBER3 self-parameterizing force field (24), and therefore no external force field parameters were specified. For all types of simulations, the temperature and pressure were held constant at 300 K and 1 atm, respectively. Periodic boundary conditions, the particle mesh Ewald method for electrostatic interactions (25), and the recommended 7.86 Å force cutoff for long-range interactions were also used.
For equilibration simulations, each selectin was individually solvated in a water box and neutralized by adding Ca2+ and Cl− ions to a concentration of ∼50 mM calcium. To allow for free protein rotation, the water boxes were defined as cubes with lengths measuring ∼10 Å away from the two farthest atoms in each complex. The P-selectin (subunit A), E-selectin, and L-selectin water cube lengths were ∼100 Å, 80 Å, and 80 Å, respectively. The water boxes were allowed to adjust slightly to constrain the water density to 0.997 g/L. The conformational stresses were then removed via short steepest-descent minimizations followed by simulated annealing until sufficient convergences were reached. To simulate the effect of pH, changes in pKa values for the amino acids were computed by the fast empirical pKa prediction method as implemented by YASARA (26). At the physiologically neutral pH condition, amino acids with pKa values ≥ 7.4 were protonated, and at physiologically relevant acidic pH conditions, amino acids with pKa values ≥ 6.2 were protonated. Free-dynamics simulations were then run for each selectin in both neutral and acidic conditions for 10 ns time periods.
Predicted structures of PSGL-1 bound to L- and E-selectin were obtained by aligning the L-selectin (3CFW) and E-selectin (1G1T) crystal structures on the P-selectin/PSGL-1 complex (1G1S, subunit a) via the MUSTANG algorithm (27). This approach is similar to that used in previous studies of L-selectin/PSGL-1 binding dynamics (28,29). After the structures were overlaid, free-dynamics simulations were run for the L-selectin/PSGL-1 and E-selectin/PSGL-1 complexes at physiologically neutral pH conditions and the above MD parameters for 10 ns time periods. The equilibrated complex structures were then run for another 10 ns after the protonation of relevant amino acids in neutral and acidic conditions.
Following previous SMD studies on the P-selectin/PSGL-1 complex (30), the Gly-147 residue was held frozen as the Cα atom of the Pro-18 residue was pulled through a spring with a spring constant of 70 pN/Å and a speed of 5 Å/ns. To reduce the computational cost of these simulations, the simulation cells were changed to a water box of 140 × 50 × 60 Å3 and were also neutralized by adding CaCl2 to a concentration of ∼50 mM Ca. To further prevent the EGF domain from unfolding during SMD simulations with the YAMBER3 force field, the Gln-130 residue was also held frozen. SMD simulations were run until the PSGL-1 completely dissociated from the P-selectin binding site. The same conditions were also used for L-selectin/PSGL-1 SMD simulations, except that the Gly-129 residue of the EGF domain was held frozen instead of residue 130. All SMD simulations were run in triplicate to verify the dissociation times.
Reagents and antibodies
RPMI 1640 cell culture media, fetal bovine serum (FBS), penicillin-streptomycin, phosphate-buffered saline (PBS), and Hank’s balanced salt solution (HBSS) were purchased from Invitrogen (Grand Island, NY). Recombinant human P-, L-, and E-selectin/IgG chimera were purchased from R&D Systems (Minneapolis, MN).
Cell lines and cell culture
The acute myeloid leukemic KG1a cell line (ATCC number CCL-264.1) was purchased from ATCC (Manassas, VA) and cultured in RPMI 1640 media supplemented with 2 mM L-glutamine, 25 mM HEPES, 10% (v/v) FBS, and 100 U/mL penicillin-streptomycin at 37°C and 5% CO2.
Neutrophil isolation
Human peripheral blood was collected from healthy adult donors after they provided informed consent. Neutrophils were isolated using 1-Step Polymorphs (Accurate Chemical and Scientific; Westbury, NY) according to a previously described protocol (31). Isolated neutrophils were resuspended at a concentration of 1 × 106 cells/mL in HBSS buffer supplemented with 0.5% (wt/vol) human serum albumin, 10 mmol/L HEPES, and 2 mmol/L CaCl2. In specified experiments, 35 μmol of TAPI-0 was added to mitigate mechanical shedding of L-selectin (32). Experiments involving neutrophils were completed within 6 h after the neutrophils were extracted from the donor.
Microtube functionalization
Micro-Renathane (MRE) tubes (300 μm i.d. and 50 cm long; Braintree Scientific, Braintree, MA) were sterilized with 75% ethanol for 15 min. After three washes with PBS (Ca++ and Mn++ free), the microtubes were incubated with Protein G (2 μg/ml PBS) for 1 h. Next, the inner luminal surface was functionalized with recombinant human P-selectin, E-selectin, or PSGL-1 at specified concentrations for 2 h. The microtubes were then incubated with dry milk powder (5% w/v) in PBS for 1 h to prevent nonspecific adhesion (33). For control experiments, microtubes were prepared as indicated above except that the adhesion molecule was replaced with BSA.
Microsphere functionalization
SuperAvidin-coated microspheres (CP01N/10216) and Protein-A-coated microspheres (CP02N/10279) were purchased from Bangs Laboratories (Fishers, IN). Microspheres were washed three times with PBS buffer. Next, the microspheres were incubated with adhesion proteins at specified concentrations for 1 h with gentle mixing every 15 min. Finally, the microspheres were washed twice and resuspended in buffer (1 × 106 microspheres/ml) at varying pH levels.
Microtube flow experiment
Cells or microspheres suspended in flow buffer (PBS supplemented with 2 mmol Ca++) at varying pH were perfused through the microtubes using a syringe pump at specified wall shear stresses. Videos were recorded for 1 min at random locations along the length of the microtube after 5 min of perfusion.
Data acquisition
Videos of the rolling cells were captured using a microscope-linked Hitachi CCD camera KP-M1AN (Hitachi, Japan) and a Sony DVD Recorder DVO-1000MD (San Diego, CA). Rolling velocity was determined using ImageJ (U.S. National Institutes of Health, Bethesda, MD). Rolling cells were defined as any cell translating along the tube surface for >2 s at a velocity < 50% of the free stream velocity of a noninteracting cell near the tube wall. Rolling flux was determined by counting the number of cells that entered the field of view over a period of 1 min.
Statistical analysis
Rolling velocity and rolling flux were plotted and analyzed using Prism (GraphPad Software, San Diego, CA). Two-tailed unpaired t-tests were used to analyze results.
Results and Discussion
P-selectin/PSGL-1 adhesion at acidic pH—SMD analysis
Fig. 1 depicts the equilibrated P-selectin/PSGL-1 complex structures at physiologically neutral pH (A) and acidic pH (B), and the histidine pKa values for P-selectin bound (pKa∗) and unbound (pKa) from PSGL-1 are shown in Table 1. When P-selectin was not bound to PSGL-1, the predicted histidine pKa values were <5.8, suggesting that no significant change in histidine protonation states or protein conformation occurs in slightly acidic pH environments. On the other hand, when P-selectin was bound to PSGL-1, the predicted H114 pKa value increased from 5.0 to 7.1, thus allowing H114 to be protonated in slightly acidic environments. As a result, a hydrogen bond between H114 and Y7 (on PSGL-1) was formed after H114 protonation, which would suggest an increase in binding energy and possibly longer interaction lifetimes.
Figure 1.
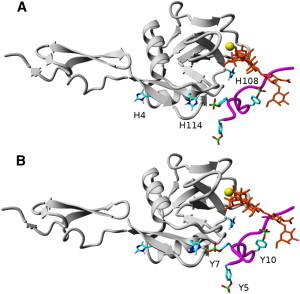
(A) Equilibrated PSGL-1 (magenta) and sLeX (orange) structures bound to P-selectin (gray) at physiologically neutral pH. (B) Equilibrated P-selectin/PSGL-1 structure at physiologically acidic pH, where the H114/Y7 contact forms a hydrogen bond. All hydrogens except for those on the imidazoles of histidines are omitted for clarity, and the yellow sphere is the calcium ion. All figures available online in color.
Table 1.
Predicted histidine pKa values for unbound P-selectin (left) and P-selectin bound to PSGL-1 (right)
| Residue | pKa | pKa∗ |
|---|---|---|
| H4 | 5.0 | 6.1 |
| H108 | 5.8 | 5.9 |
| H114 | 5.0 | 7.1 |
To examine the effect of H114 protonation on the binding lifetime of the P-selectin/PSGL-1 complex, we used SMD to manually dissociate PSGL-1 from P-selectin. Representative SMD force versus time graphs are shown in Fig. 2 to illustrate variations between neutral and acidic environments. Interestingly, there was a remarkable difference in the dissociation dynamics depending on the protonation state of H114. At the start of the simulation (Fig. 1), the P-selectin/PSGL-1 complex conformations were nearly identical where clear H114/Y7 and R85/Y10 contacts were present, as suggested by Somers et al. (23). However, after 6 ns in neutral conditions, R85 had already dissociated from Y10 to form a hydrogen bond to sLeX (Fig. 2 B), whereas in acidic conditions the R85/Y10 contact was preserved (Fig. 2 D), most likely due to the increased interaction energy from the hydrogen-bonded H114/Y7 contact. Moreover, Y5 was hydrogen-bonded to both K8 and the P-selectin backbone, resulting in a total of four contacts in acidic conditions, compared with two contacts in neutral conditions. As PSGL-1 was pulled away from P-selectin in neutral conditions, the Y5 and Y7 amino acids underwent several sliding-rebinding events involving K8, K112, and the P-selectin backbone, resulting in a force plateau from 8.5 to 10.5 ns. Subsequent to the numerous sliding-rebinding events, Y5 was pulled too far to contact P-selectin significantly (Fig. 2 C). In contrast, at ∼9 ns in acidic conditions, there was a rupture of all contacts (except for coordination bonding), signified by a dramatic decrease in the pulling force. The R85 and Y7 contacts were then bonded to sLeX and H114, respectively, while Y5 was kept within the binding range of K8 and K112 (Fig. 2 E). On average, the protonation of H114 increased the binding lifetime by >1 ns, and therefore cells expressing PSGL-1 were predicted to roll more slowly at slightly acidic pH than at physiologically neutral pH on P-selectin surfaces.
Figure 2.
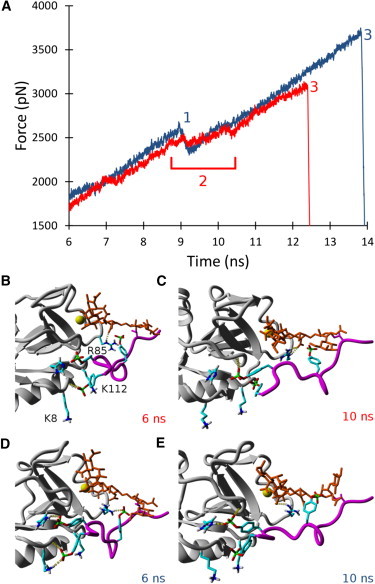
(A) Representative SMD force versus time graphs depicting the dissociation of PSGL-1 from P-selectin in physiologically neutral (red) and acidic (blue) conditions. Time point 1 represents the rupture of all amino acid contacts, except for coordination bonding, in the acidic case. Time point 2 represents a long force plateau in the neutral case, where several sliding and rebinding events occur. Time point 3 represents the complete rupture of all bonds, leading to the dissociation of PSGL-1 from P-selectin in both conditions. (B) Representative P-selectin/PSGL-1 structure in neutral conditions for time periods < ∼6 ns, where R85 forms a hydrogen bond with sLeX, and Y5 forms a hydrogen bond with the backbone structure of P-selectin. (C) Representative structure in neutral conditions for a time period > ∼10 ns, where Y5 has no contacts and Y7 forms a contact with the backbone structure of P-selectin. (D) Representative structure in acidic conditions for time periods < ∼6 ns, where Y5 has two hydrogen bonds with K8 and the backbone structure of P-selectin, Y7 forms a hydrogen bond to H114, and Y10 is hydrogen bound to R85. (E) Representative structure in acidic conditions for time periods > ∼10 ns, where Y7 is still hydrogen bound to H114 and forms a hydrogen bond with the P-selectin backbone structure, and R85 is now hydrogen bound to sLeX.
P-selectin/PSGL-1 adhesion at acidic pH—experimental rolling analysis
To further substantiate our SMD results, we investigated P-selectin/PSGL-1 complex binding dynamics in acidic environments in vitro by perfusing KG1a cell suspensions through P-selectin-coated microtubes at varying pH levels (6.2–7.4) with a flow rate of 0.111 ml/min, an equivalent shear stress of 7 dyn/cm2 (Fig. 3 A). Cell rolling was confirmed to be specifically mediated by P-selectin/ PSGL-1 binding, because all interactions were abrogated when cells were perfused in Ca2+-free buffer containing 5 mM of EDTA (data not shown). As the buffer pH level decreased from 7.4 to 6.8, KG1a cells rolled more slowly, with the average rolling velocity reaching a minimum of 4.25 ± 0.21 μm/s at pH 6.8, suggesting longer P-selectin/PSGL-1 interaction lifetimes at slightly acidic pH levels. Conversely, pH levels below 6.8 decreased the binding interaction lifetime of P-selectin to PSGL-1, which was manifested by an increase in rolling velocity. Rolling-assay experiments were also performed with PSGL-1 coated microspheres as shown in Fig. 3 B. The microspheres exhibited rolling characteristics similar to those of KG1a cells: the average rolling velocity decreased at slightly acidic pH, with a minimum at pH 6.6, and increased from the minimum at pH levels below 6.6. This increase in rolling velocity may be a consequence of the on-rate kinetics. Microspheres that were functionalized with carbohydrate sLeX (effectively eliminating the putative PSGL-1 backbone responsible for hydrogen binding) did not exhibit enhanced binding lifetime in slightly acidic environments (Fig. 3 C).
Figure 3.
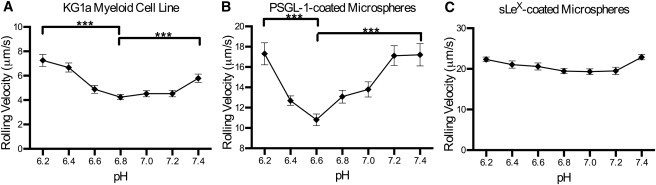
(A) 106 cells/ml of KG1a cells were perfused through MRE tubing functionalized with 2 μg/ml of recombinant human P-selectin/Fc at a wall shear stress of 7 dyn/cm2. (B and C) Protein-A-coated microspheres (106 beads/ml) functionalized with 14.47 μg/mg particles of recombinant human PSGL-1/Fc (B), or with 0.017 μg/mg particles of sialyl Lewis-x-PAA-biotin (C) were perfused through MRE tubing functionalized with 10 μg/ml recombinant human P-selectin/Fc at a wall shear stress of 1 dyn/cm2. Experiments were recorded for 1 min each at three randomly selected locations along the tube (unpaired t-test was performed; errors are mean ± SE; ∗∗∗ p < 0.0001; n = 3).
Circulating myeloid cells express PSGL-1 on their surfaces, which serve as a counterreceptor for P-selectin (34). As previously mentioned, P-selectin/ligand binding facilitates the tethering and eventual rolling of myeloid cells on the activated endothelial lining during physiological events such as injury to the vessel wall and inflammation. These phenomena are often coupled with a decrease in pH level in the microenvironment where adhesion occurs (35). The results of both the computational and experimental studies presented here suggest that in slightly acidic environments, the binding of the P-selectin/PSGL-1 complex is enhanced due to the formation of a hydrogen bond between the protonated H114 (P-selectin) and Y7 (PSGL-1) residues. Our findings suggest that during inflammation, a drop in pH may have a contributory effect on recruiting circulating myeloid cells by increasing the binding strength and lifetime between P-selectin to PSGL-1, which slows cell rolling velocities at and around the target site.
L-selectin/PSGL-1 adhesion at acidic pH—MD analysis
Given the good agreement between theory and experiment for P-selectin, a similar computational analysis was performed for L-selectin, as shown in Figs. 4 and 5. L-selectin contains three histidine residues that potentially can be protonated. The H4 and H130 residues had predicted pKa values of 6.9 and 6.8 (Table 2), respectively, when not bound to PSGL-1, and therefore likely change protonation states in slightly acidic conditions. The main effect of protonating these two residues in acidic conditions was the dissociation of the hydrogen bond between the N138 residue (of the EGF domain) and the lectin domain. This hydrogen bond is important, because Phan et al. (36) found that a single point mutation of N138 to glycine increased cell flux, decreased cell rolling velocities, and increased the duration of cell-tethering events. They suggested that these changes were due to a greater L-selectin population in the extended (high-affinity) conformation.
Figure 4.
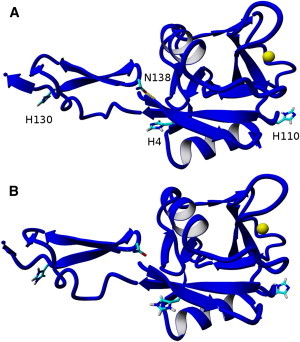
(A) Equilibrated L-selectin (blue) at physiologically neutral pH, where N138 is hydrogen bound to the backbone structure of L-selectin. (B) Equilibrated L-selectin structure at physiologically acidic pH, where N138 is no longer close enough to form a hydrogen bond. All hydrogens except for those on the imidazoles of histidines are omitted for clarity, and the yellow sphere is the calcium ion.
Figure 5.
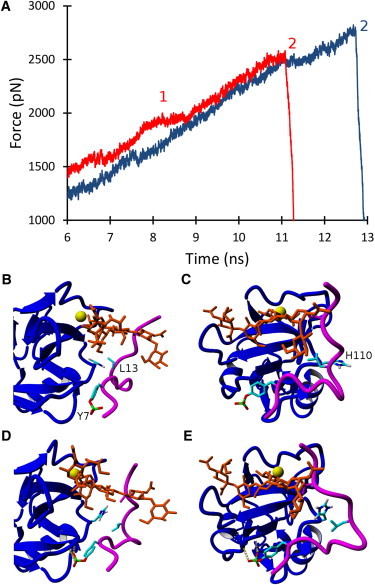
(A) Representative SMD force versus time graphs depicting the dissociation of PSGL-1 from L-selectin in physiologically neutral (red) and acidic (blue) conditions. Time interval 1 represents a long force plateau in the neutral case, where several sliding and rebinding events occur. Time point 2 represents the complete rupture of all bonds, leading to the dissociation of PSGL-1 from L-selectin in both conditions. (B and C) Equilibrated PSGL-1 (magenta) and sLeX (orange) structures bound to L-selectin (blue) at physiologically neutral pH. (D and E) Equilibrated PSGL-1/L-selectin structure at physiologically acidic pH, where Y7 forms contacts with the L-selectin backbone and L13 contacts H110 via a stacking interaction.
Table 2.
Predicted histidine pKa values for unbound L-selectin (left) and L-selectin bound to PSGL-1 (right)
| Residue | pKa | pKa∗ |
|---|---|---|
| H4 | 6.9 | 7.1 |
| H110 | 4.6 | 6.5 |
| H130 | 6.8 | 6.9 |
The predicted L-selectin/PSGL-1 complex showed an increase of the H110 pKa value from 4.6 (unbound) to 6.5 (bound), and was also expected to be protonated in slightly acidic conditions. The protonation of H110 dramatically changed the PSGL-1 conformation, yielding a stacking interaction between H110 and L13, and increased the likelihood of hydrogen bonding between Y7 and the L-selectin backbone (Fig. 5). SMD simulations demonstrated that the protonation of H110 increased the L-selectin/PSGL-1 binding lifetime by >1.5 ns, as shown by the representative SMD force versus time graphs in Fig. 5. A similar result was discovered via the mutation of A108 of L-selectin (an amino acid neighboring H110) to histidine, where the mutation was proposed to increase the stacking interactions of L-selectin and PSGL-1, resulting in an increased binding interaction (28). Therefore, an acidic pH environment should produce considerable changes in the rolling profiles of cells expressing L-selectin when perfused over PSGL-1-coated surfaces via a change in selectin conformation and an increased selectin/PSGL-1 binding interaction.
L-selectin/PSGL-1 adhesion at acidic pH—experimental rolling analysis
Rolling-assay experiments with isolated human neutrophils perfused through PSGL-1-coated microtubes showed that the neutrophils maintained a statistically constant rolling profile at pH levels ranging from 7.4 down to 6.6, but steadily increased as the pH level became more acidic than 6.6 (Fig. 6 A). Interestingly, pH had an effect on the number of neutrophils that adhered and rolled on the PSGL-1-coated surface, causing the cell flux to increase in more acidic conditions over a range of shear stresses (Fig. 6 C). This suggests that slightly acidic pH may increase the binding frequency of L-selectin to PSGL-1, as manifested by a higher rolling flux at lower pH. Rolling-assay experiments using L-selectin-coated microspheres perfused over PSGL-1-coated surfaces were also conducted (Fig. 6 B). Interestingly, the microspheres rolled more slowly at pH 7.2 than at 7.4, and remained at this depressed velocity at pH levels as low as 6.4 before they began to increase to higher velocities at pH levels below 6.4, indicating stronger L-selectin/PSGL-1 interactions at slightly acidic pH. In contrast to a uniformly coated microbead, the surface of neutrophils primarily express L-selectin on the tips of microvilli (31,37). Neutrophils use these L-selectin-decorated microvilli to attach to the targeted surface expressing L-selectin receptors. Furthermore, neutrophils can passively deform and modulate the number of microvilli attached to the substrate in accordance with external stimuli to allow maximum stability—a mechanism that is unavailable to microspheres (38,39). These observations may explain why the effect of pH on L-selectin/PSGL-1 interactions is more pronounced on rolling microspheres than on neutrophils. However, the rolling flux for the microspheres exhibited the same behavior as neutrophils: at lower pH conditions, a greater flux of rolling microspheres was observed on PSGL-1-coated surfaces (data not shown).
Figure 6.
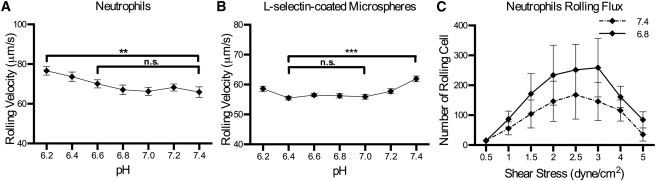
(A and B) Human neutrophils (106 cells/ml) (A) or protein-A-coated microspheres functionalized with 14.47 μg/mg particles of recombinant human L-selectin/Fc (B) were perfused through MRE tubing functionalized with 10 μg/ml of recombinant human PSGL-1/Fc at a wall shear stress of 2 dyn/cm2. (C) Human neutrophils (106 cells/ml) were perfused in buffer of indicated pH through MRE tubing functionalized with 10 μg/ml recombinant human PSGL-1/Fc, and the number of rolling cells through the cross-sectional area of the tube was recorded for varying shear stresses (unpaired t-test; errors are mean ± SE; ∗∗ p < 0.0012, ∗∗∗ p < 0.0001; n = 3).
E-selectin/PSGL-1 adhesion at acidic pH—MD analysis
To complete our study of selectins, we performed a pKa analysis of E-selectin as shown in Fig. 7. In this case, only two histidine residues were present, where the H25 predicted pKa was 6.8 (Table 3) and could change protonation states in slightly acidic conditions. However, H25 is not near the calcium ion and was not observed to increase amino acid contacts between E-selectin and PSGL-1 (structure not shown). Moreover, the N138 residue did not contact the lectin domain in either neutral or acidic conditions. Hence, pH is not expected to affect the binding strength or the binding affinity of PSGL-1 to E-selectin, and no significant change in rolling profiles is expected for cells expressing PSGL-1 when perfused over E-selectin-coated surfaces.
Figure 7.
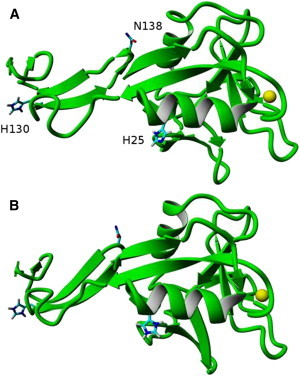
(A) Equilibrated E-selectin (green) at physiologically neutral pH. (B) Equilibrated E-selectin structure at physiologically acidic pH. All hydrogens except for those on the imidazoles of histidines are omitted for clarity, and the yellow sphere is the calcium ion.
Table 3.
Predicted histidine pKa values for unbound E-selectin (left) and E-selectin bound to PSGL-1 (right)
| Residue | pKa | pKa∗ |
|---|---|---|
| H25 | 6.8 | 6.6 |
| H130 | 7.8 | 7.4 |
E-selectin/PSGL-1 adhesion at acidic pH—experimental rolling analysis
Fig. 8 A displays the rolling velocity profile for KG1a cells perfused through E-selectin-coated microtubes, where only a steady rise in rolling velocities was observed as the pH level became more acidic. However, the PSGL-1-coated microspheres did not demonstrate any significant change in rolling velocity at any pH level (Fig. 8 B). These data, along with the computational predictions, indicate that the pH range investigated in this study does not significantly affect E-selectin/PSGL-1 binding dynamics.
Figure 8.
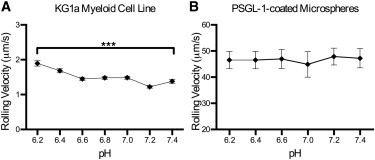
(A and B) KG1a cells (106 cells/ml) (A) or protein-A-coated microspheres functionalized with 14.47 μg/mg particles of recombinant human E-Sel/Fc (B) were perfused through MRE tubing functionalized with 2 μg/ml of recombinant human E-selectin/Fc at a wall shear stress of 7 dyn/cm2 (unpaired t-test; errors are mean ± SE; ∗∗∗ p < 0.0001; n = 3).
Conclusions
Acidic extracellular pH does indeed alter the adhesion dynamics of certain selectins to PSGL-1, which causes differential rolling and adhesion characteristics of cells dependent on selectin-mediated rolling. P-selectin/PSGL-1 binding was enhanced at slightly acidic pH due to the formation of a new hydrogen bond between H114 (P-selectin) and Y7 (PSGL-1). As a result, the P-selectin/PSGL-1 binding lifetime increased, and, correspondingly, cell rolling velocities on P-selectin-coated surfaces were experimentally shown to decrease. When cells expressing L-selectin were perfused through PSGL-1-coated microtubes, cell flux increased at slightly acidic pH, whereas rolling velocities remained unchanged. The cell flux is primarily due to the altered structure of L-selectin at acidic pH, where the hydrogen bond between residue N138 (EGF domain) and the lectin-binding domain is dissociated as a result of protonating the H4 and H130 residues. The dissociation of this hydrogen bond allows L-selectin to assume a more flexible conformation (high affinity) that results in improved frequency of binding to PSGL-1. On the other hand, residue H110 is also predicted to be protonated when bound to L-selectin in acidic conditions that increase contacts and strengthen the binding interaction. We only observed this effect (i.e., slower rolling velocity) when we eliminated cellular effects by using L-selectin-coated microspheres. A previous study by Serrano et al. (19) also showed that neutrophil recruitment is enhanced in acidic conditions due to the upregulation of CD18 by the neutrophils. In this study, we showed that before firm adhesion facilitated by the CD18/ICAM-1 interaction occurs, acidic pH also enhances neutrophil recruitment in the initial step of the adhesion cascade through the mediation of L-selectin/PSGL-1 binding dynamics. In contrast to P- and L-selectin, E-selectin/PSGL-1 binding was not significantly affected by acidic pH. In conclusion, both theoretical predictions and experimental observations indicate that the extracellular pH level modulates selectin adhesion under flow. Thus, the extracellular pH level may serve as an external signaling stimulus for leukocytes trafficking during the inflammatory response. These results further suggest an additional axis that can be used to control applications such as the selectin-based isolation of CTCs and hematopoietic stem cells (33,40).
Acknowledgments
This work was funded by the National Institutes of Health (grants HL018128 and HL097971).
The authors thank Jeff Mattison for blood collection work.
References
- 1.Ley K., Gaehtgens P., Rosen S.D. Lectin-like cell adhesion molecule 1 mediates leukocyte rolling in mesenteric venules in vivo. Blood. 1991;77:2553–2555. [PubMed] [Google Scholar]
- 2.Alon R., Rossiter H., Kupper T.S. Distinct cell surface ligands mediate T lymphocyte attachment and rolling on P and E selectin under physiological flow. J. Cell Biol. 1994;127:1485–1495. doi: 10.1083/jcb.127.5.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Springer T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 4.Weston B.W., Hiller K.M., Cusack J.C., Jr. Expression of human α(1,3)fucosyltransferase antisense sequences inhibits selectin-mediated adhesion and liver metastasis of colon carcinoma cells. Cancer Res. 1999;59:2127–2135. [PubMed] [Google Scholar]
- 5.Geng Y., Marshall J.R., King M.R. Glycomechanics of the metastatic cascade: tumor cell-endothelial cell interactions in the circulation. Ann. Biomed. Eng. 2012;40:790–805. doi: 10.1007/s10439-011-0463-6. [DOI] [PubMed] [Google Scholar]
- 6.Harrison P., Cramer E.M. Platelet α-granules. Blood Rev. 1993;7:52–62. doi: 10.1016/0268-960x(93)90024-x. [DOI] [PubMed] [Google Scholar]
- 7.McEver R.P., Moore K.L., Cummings R.D. Leukocyte trafficking mediated by selectin-carbohydrate interactions. J. Biol. Chem. 1995;270:11025–11028. doi: 10.1074/jbc.270.19.11025. [DOI] [PubMed] [Google Scholar]
- 8.Ley K., Kansas G.S. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat. Rev. Immunol. 2004;4:325–335. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- 9.Katayama Y., Hidalgo A., Frenette P.S. PSGL-1 participates in E-selectin-mediated progenitor homing to bone marrow: evidence for cooperation between E-selectin ligands and α4 integrin. Blood. 2003;102:2060–2067. doi: 10.1182/blood-2003-04-1212. [DOI] [PubMed] [Google Scholar]
- 10.Kansas G.S., Wood G.S., Engleman E.G. Functional characterization of human T lymphocyte subsets distinguished by monoclonal anti-leu-8. J. Immunol. 1985;134:2995–3002. [PubMed] [Google Scholar]
- 11.McEver R.P. Role of selectins in leukocyte adhesion to platelets and endothelium. Ann. N. Y. Acad. Sci. 1994;714:185–189. doi: 10.1111/j.1749-6632.1994.tb12043.x. [DOI] [PubMed] [Google Scholar]
- 12.Varki A. Selectin ligands. Proc. Natl. Acad. Sci. USA. 1994;91:7390–7397. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kansas G.S. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 14.Dimitroff C.J., Lee J.Y., Sackstein R. A distinct glycoform of CD44 is an L-selectin ligand on human hematopoietic cells. Proc. Natl. Acad. Sci. USA. 2000;97:13841–13846. doi: 10.1073/pnas.250484797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitakaze M., Weisfeldt M.L., Marban E. Acidosis during early reperfusion prevents myocardial stunning in perfused ferret hearts. J. Clin. Invest. 1988;82:920–927. doi: 10.1172/JCI113699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orchard C.H., Kentish J.C. Effects of changes of pH on the contractile function of cardiac muscle. Am. J. Physiol. 1990;258:C967–C981. doi: 10.1152/ajpcell.1990.258.6.C967. [DOI] [PubMed] [Google Scholar]
- 17.Lengheden A., Jansson L. pH effects on experimental wound healing of human fibroblasts in vitro. Eur. J. Oral Sci. 1995;103:148–155. doi: 10.1111/j.1600-0722.1995.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y., Kalén A., Wahlström O. Fibroblast proliferation due to exposure to a platelet concentrate in vitro is pH dependent. Wound Repair Regen. 2002;10:336–340. doi: 10.1046/j.1524-475x.2002.10510.x. [DOI] [PubMed] [Google Scholar]
- 19.Serrano C.V., Jr., Fraticelli A., Capogrossi M.C. pH dependence of neutrophil-endothelial cell adhesion and adhesion molecule expression. Am. J. Physiol. 1996;271:C962–C970. doi: 10.1152/ajpcell.1996.271.3.C962. [DOI] [PubMed] [Google Scholar]
- 20.Knighton D.R., Hunt T.K., Banda M.J. Oxygen tension regulates the expression of angiogenesis factor by macrophages. Science. 1983;221:1283–1285. doi: 10.1126/science.6612342. [DOI] [PubMed] [Google Scholar]
- 21.Gatenby R.A., Gillies R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 22.Brahimi-Horn M.C., Bellot G., Pouysségur J. Hypoxia and energetic tumour metabolism. Curr. Opin. Genet. Dev. 2011;21:67–72. doi: 10.1016/j.gde.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Somers W.S., Tang J., Camphausen R.T. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1. Cell. 2000;103:467–479. doi: 10.1016/s0092-8674(00)00138-0. [DOI] [PubMed] [Google Scholar]
- 24.Krieger E., Darden T., Vriend G. Making optimal use of empirical energy functions: force-field parameterization in crystal space. Proteins. 2004;57:678–683. doi: 10.1002/prot.20251. [DOI] [PubMed] [Google Scholar]
- 25.Essmann U., Perera L., Pedersen L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995;103:8577. [Google Scholar]
- 26.Krieger E., Nielsen J.E., Vriend G. Fast empirical pKa prediction by Ewald summation. J. Mol. Graph. Model. 2006;25:481–486. doi: 10.1016/j.jmgm.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Konagurthu A.S., Whisstock J.C., Lesk A.M. MUSTANG: a multiple structural alignment algorithm. Proteins. 2006;64:559–574. doi: 10.1002/prot.20921. [DOI] [PubMed] [Google Scholar]
- 28.Klopocki A.G., Yago T., McEver R.P. Replacing a lectin domain residue in L-selectin enhances binding to P-selectin glycoprotein ligand-1 but not to 6-sulfo-sialyl Lewis x. J. Biol. Chem. 2008;283:11493–11500. doi: 10.1074/jbc.M709785200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarangapani K.K., Qian J., Zhu C. Regulation of catch bonds by rate of force application. J. Biol. Chem. 2011;286:32749–32761. doi: 10.1074/jbc.M111.240044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lou J., Zhu C. A structure-based sliding-rebinding mechanism for catch bonds. Biophys. J. 2007;92:1471–1485. doi: 10.1529/biophysj.106.097048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ball C.J., King M.R. Role of c-Abl in L-selectin shedding from the neutrophil surface. Blood Cells Mol. Dis. 2011;46:246–251. doi: 10.1016/j.bcmd.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee D., Schultz J.B., King M.R. Mechanical shedding of L-selectin from the neutrophil surface during rolling on sialyl Lewis x under flow. J. Biol. Chem. 2007;282:4812–4820. doi: 10.1074/jbc.M609994200. [DOI] [PubMed] [Google Scholar]
- 33.Narasipura S.D., Wojciechowski J.C., King M.R. P-Selectin coated microtube for enrichment of CD34+ hematopoietic stem and progenitor cells from human bone marrow. Clin. Chem. 2008;54:77–85. doi: 10.1373/clinchem.2007.089896. [DOI] [PubMed] [Google Scholar]
- 34.McEver R.P., Zhu C. Rolling cell adhesion. Annu. Rev. Cell Dev. Biol. 2010;26:363–396. doi: 10.1146/annurev.cellbio.042308.113238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Etulain J., Negrotto S., Schattner M. Acidosis downregulates platelet haemostatic functions and promotes neutrophil proinflammatory responses mediated by platelets. Thromb. Haemost. 2012;107:99–110. doi: 10.1160/TH11-06-0443. [DOI] [PubMed] [Google Scholar]
- 36.Phan U.T., Waldron T.T., Springer T.A. Remodeling of the lectin-EGF-like domain interface in P- and L-selectin increases adhesiveness and shear resistance under hydrodynamic force. Nat. Immunol. 2006;7:883–889. doi: 10.1038/ni1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kishimoto T.K., Jutila M.A. Identification of a human peripheral lymph node homing receptor: a rapidly down-regulated adhesion molecule. Proc. Natl. Acad. Sci. 1990;87:2244–2248. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen S., Springer T.A. An automatic braking system that stabilizes leukocyte rolling by an increase in selectin bond number with shear. J. Cell Biol. 1999;144:185–200. doi: 10.1083/jcb.144.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pawar P., Jadhav S., Konstantopoulos K. Roles of cell and microvillus deformation and receptor-ligand binding kinetics in cell rolling. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H1439–H1450. doi: 10.1152/ajpheart.91536.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hughes A.D., Mattison J., King M.R. Microtube device for selectin-mediated capture of viable circulating tumor cells from blood. Clin. Chem. 2012;58:846–853. doi: 10.1373/clinchem.2011.176669. [DOI] [PubMed] [Google Scholar]


