Abstract
Chagas disease, an infection caused by the protozoan Trypanosoma cruzi and transmitted by the Reduuvid insect vector, remains a major cause of morbidity in Central and South America over a century after its discovery in 1909. Though major advances in preventing the spread of this disease have been made in recent decades, millions of individuals remain chronically infected due to prior exposure to T. cruzi and are at risk for future complications from the disease. Dermatologic manifestations of acute infection may include localized swelling at the site of inoculation (chagoma), conjunctivitis (Romaña’s sign), and a generalized morbilliform eruption (schizotrypanides). Reactivation of quiescent infection in immunocompromised hosts due to the acquired immunodeficiency syndrome or organ transplantation can present with fever and skin lesions including panniculitis. The wide-spread emigration of chronic carriers of T. cruzi to North America, Europe, and Australia makes it imperative that dermatologists worldwide be familiar with this entity to ensure proper diagnosis and treatment.
Introduction
Chagas disease is an infection caused by the protozoan parasite Trypanosoma cruzi.1 Endemic to large portions of Latin America, with the exception of the Caribbean, this disease in recent decades has increasingly been diagnosed worldwide because of global travel and large-scale emigration from Latin America to North America, Europe, and Australia.2 In addition, Chagas disease is now appreciated to be an opportunistic infection in immunocompromised individuals, including those with human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS).3 Both acute natural infection and recrudescence of infection observed in immunocompromised individuals may present with cutaneous lesions. This article reviews the pathogenesis, diagnosis, and treatment of Chagas disease, with an emphasis on its cutaneous manifestations.
Life cycle
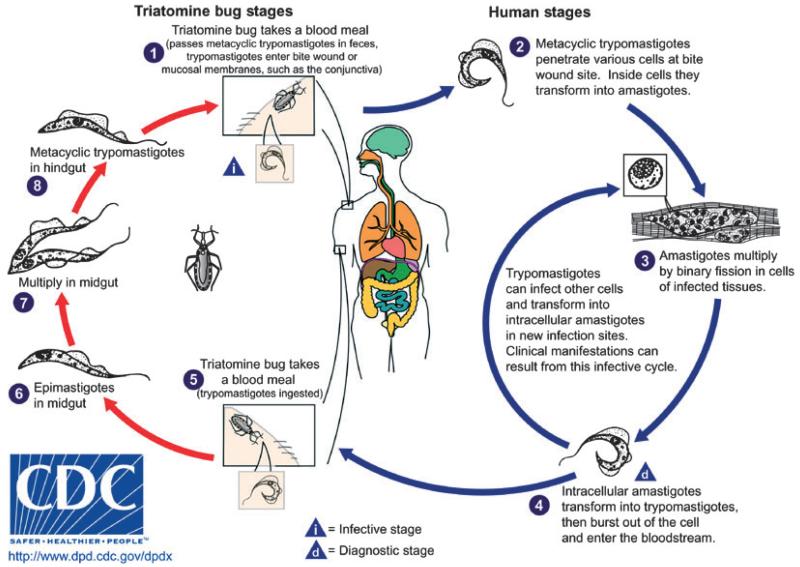
The parasite has a complex life cycle (Fig. 1). Over 100 species of Triatominae (Hemiptera: Reduviidae) can serve as insect vectors for T. cruzi. These vectors are found throughout Latin America and even in the southern USA, especially in rural areas.4 During a blood meal from an infected mammalian host, the insect vector ingests blood form trypomastigotes, which undergo transformation to epimastigotes. Within 3–4 weeks, infective, non-dividing metacyclic trypomastigotes present in the hindgut of the vector are deposited with the feces of the vector during subsequent blood meals.5 Transmission to the new host occurs when the parasite-laden feces contaminate oral or nasal mucous membranes, the conjunctiva, and other surfaces.6 The trypomastigotes enter a host cell and transform into intracellular amastigotes, which then multiply by binary fission and ultimately transform into blood form trypomastigotes, which are released as the host cell ruptures. These trypomastigotes infect adjacent cells or disseminate via the lymphatics and the bloodstream and infect new cells.5 Although any nucleated mammalian cell can be parasitized, the cells of the cardiovascular, reticuloendothelial, nervous, and muscular systems as well as adipose tissue are favored.7 Another mode of transmission is via blood transfusion. Although transfusion-associated Chagas disease was always less common than insect-transmitted Chagas disease, the frequently severe nature of transfusion-associated Chagas disease necessitated the implementation of blood donor screening programs. Subsequently, the incidence of transfusion-associated Chagas disease in endemic countries has declined dramatically in recent years.8 The parasite is transmitted congenitally in 1–10% of pregnancies in women who are carriers,9 posing an unavoidable risk for transmission in both endemic and non-endemic regions. Disease severity in neonates with congenitally acquired Chagas disease can range from asymptomatic acquisition10 to fulminant disease culminating in death.11 Ingestion of food or drink contaminated with trypomastigotes has recently been identified as the cause of several recent large-scale outbreaks of acute Chagas disease.12,13 Other modes of transmission include organ transplantation14 and laboratory accident.15
Figure 1.
The triatomine and human stages of the life cycle of Trypanosoma cruzi (courtesy of CDC)
Acute chagas disease
Most patients with acute infection are asymptomatic or have mild symptoms. After an incubation period of 1–2 weeks, a minority of patients will experience a non-specific febrile illness. A newly infected individual may develop fever, chills, nausea, vomiting, diarrhea, rash, meningeal irritation, conjunctivitis, lymphadenopathy, or hepatosplenomegaly.16 Acute infection may be accompanied by anemia, thrombocytopenia, or elevated liver enzymes. Blood form trypomastigotes can be observed in wet preparations of blood and cerebrospinal fluid (CSF) in many patients.17 Eosinophilia is not associated with acute infection.18 Serological tests for T. cruzi-specific antibodies can be negative during acute infection.16 Although cardiac involvement in acute infection appears to be universal, myocarditis, cardiomegaly, and congestive heart failure only develop in a small percentage of acutely infected patients.19 The presence of arrhythmias or bundle branch blocks portends a poor prognosis.20 The mortality rate of acutely naturally infected patients, often children, is less than 2%, and the common mode of death is acute myocarditis or meningoencephalitis.21
There are several well-described dermatological manifestations of acute Chagas disease.
Chagoma
A chagoma is a red, indurated swelling at the site of inoculation,22 which develops in the weeks after the initial bite and persists for weeks afterward. If a biopsy is obtained, pathology demonstrates intracellular amastigotes and lymphocytes.23
Romaña’s sign
This classic sign of acute Chagas disease occurs due to the deposition of parasite-laden feces into the conjunctival sac. The patient may rub the eye after scratching at a freshly infected bite wound, transferring trypomastigotes to the conjunctiva. The patient subsequently develops eyelid edema and conjunctivitis, which may be associated with lymphadenitis or even pre-septal cellulitis.24
Schizotrypanides
A small minority of patients will develop a diffuse morbilliform eruption in the weeks after acute inoculation. Biopsy demonstrates dilation of the subpapillary plexus with swelling of the endothelial cells and edema of the surrounding connective tissue and lymphocytic perivascular infiltrates. Parasites are not present in the lesions.23
Indeterminate stage
In most patients, an immune response develops, the parasitemia wanes, and signs and symptoms resolve completely within a few months. These individuals then enter the indeterminate phase of infection, which is characterized by the presence of specific antibodies in the absence of clinical manifestations.21 To be diagnosed with indeterminate stage disease, patients must demonstrate no clinical symptoms related to T. cruzi infection. Furthermore, electrocardiogram (ECG) testing and cardiac and gastrointestinal imaging must be normal. However, patients in the indeterminate stage remain parasitemic.9 This phase may last from months to an entire lifetime.21
Chronic stage
Over a period of decades, 15–30% of patients with indeterminate stage Chagas disease will develop a chronic complication of T. cruzi infection, thereby progressing to the chronic stage of Chagas disease. The most common manifestations of chronic Chagas disease are cardiac and gastrointestinal.22
Chronic chagasic heart disease varies widely in its manifestations, ranging from asymptomatic ECG abnormalities to congestive heart failure, arrhythmias, and/or thromboembolic events.25 The severity of the disease is dependent on the duration of illness as well as the location and nature of cardiac lesions.26 Dilated congestive cardiomyopathy is an important manifestation and usually occurs years or even decades after a person first becomes infected. Apical aneurysm of the left ventricle is one of the hallmarks of chronic chagasic cardiomyopathy. The destruction of conduction tissue results in conduction abnormalities. The most common ECG abnormality is a right bundle branch block that may also be associated with an anterior fascicular block. A recently developed system for staging chagasic cardiac disease is presented in Table 1. Management of cardiac complications in patients with Chagas disease differs little from that in patients with cardiac problems from other causes.25
Table 1.
Staging of chagasic cardiomyopathy, according to the Brazilian Expert Consensus in Chagas disease
| Stage | ECG | Echocardiogram | Symptomatic heart failure |
|---|---|---|---|
| A | Abnormal | Normal | Absent |
| B1 | Abnormal | Abnormal Left ventricular ejection fraction >45% |
Absent |
| B2 | Abnormal | Abnormal Left ventricular ejection fraction <45% |
Absent |
| C | Abnormal | Abnormal | Treatable |
| D | Abnormal | Abnormal | Refractory |
ECG, electrocardiogram
Gastrointestinal symptoms develop in 6% of patients with Chagas disease. The most common manifestations are those related to the megasyndrome. This results from damage to the autonomic ganglia leading to denervation of the tubular structures of the gastrointestinal tract. In the esophagus, this process leads to megaesophagus associated with achalasia. This is usually associated with dysphagia, weight loss, and characteristic radiological findings. These patients may also experience chronic aspiration. Management options are similar to those in idiopathic achalasia. Patients with megacolon due to denervation of the colon may experience constipation, which can be severe and unremitting. Management varies from dietary changes and enemas in minor cases to surgical or endoscopic intervention in more severe cases.27
Reactivation
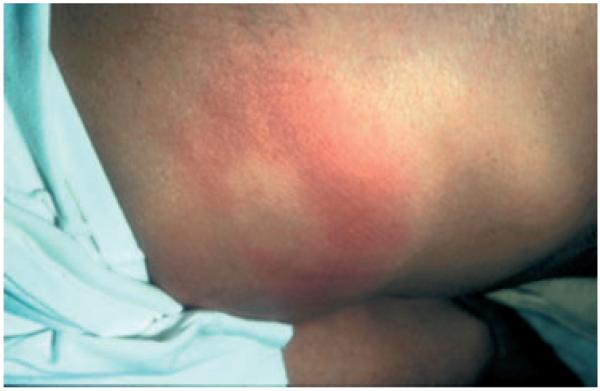
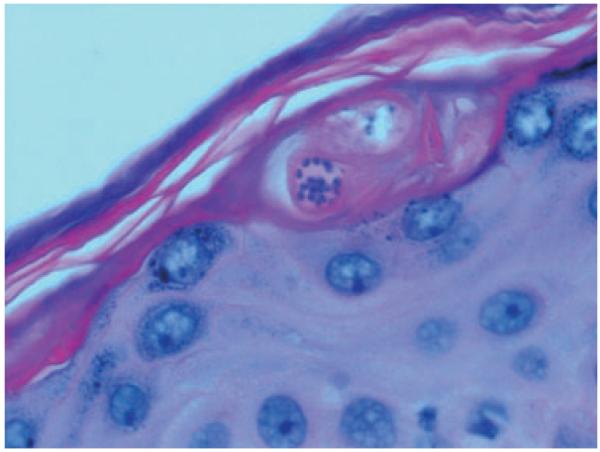
Patients with indeterminate or chronic Chagas disease who develop an immunocompromised state may experience reactivation of the infection. Reactivation syndromes may present as acute myocarditis or meningoencephalitis. Not uncommonly, however, patients present with fever and cutaneous lesions.28 The cutaneous lesions are most commonly erythematous nodules or plaques. The diagnosis is made by visualizing intracellular amastigotes on pathology from skin biopsy specimens (Figs. 2-4). Reactivation with cutaneous manifestations has been described following kidney transplantation,29,30 bone marrow transplantation,28 cardiac transplantation,31,32 and liver transplantation.33 A similar presentation has been described in patients with HIV/AIDS.3
Figure 2.
Erythematous plaque in a renal transplantation patient with reactivation Chagas disease (Gallerano, 2007)
Figure 4.
Intracellular amastigotes visualized on an H&E stain from a skin biopsy specimen from the patient in Fig. 3 (courtesy of Eva Parker)
Epidemiology
The epidemiology of this disease has changed over the course of the last several decades. When initially discovered, Chagas disease was a disease endemic to the rural poor who lived in substandard housing, allowing close contiguity with the insect vector. Subsequently, blood transfusions became a major avenue of transmission of T. cruzi infection in major cities in endemic countries prior to the institution of blood donor screening.4 The Southern Cone Initiative, supported by major international bodies, has significantly reduced the transmission of the infection through vector control programs.21 Thus, Chile, Uruguay, and large portions of Brazil are now transmission-free, although it is worth noting that the gains achieved in Brazil have occurred despite the persistent presence of wild insect vectors capable of transmitting the parasite. In addition, there has been a decrease in the proportion of children in endemic countries who demonstrate antibodies against T. cruzi.6 Nevertheless, an estimated 8 million1 to 16-18 million21 people worldwide have already been infected with T. cruzi, and cases of chronic reactivation and congenital Chagas disease will continue to present for decades. Caring for this cohort of infected people will remain a major challenge for Latin American countries due to economic and geographic barriers to the provision of health care to large proportions of this population. Furthermore, the United States, Europe, and Australia have seen large-scale immigration from Latin America in recent years. An estimated 325,671 immigrants in the USA are infected with T. cruzi, as are an estimated 86,947 in Spain, 5553 in Canada, and 3088 in Australia.2 This cohort is at risk of developing the complications of chronic or reactivation Chagas disease while residing in countries where physicians are not familiar with this disease. Furthermore, although blood donor screening for Chagas disease has recently been instituted in the USA8 and other countries with significant numbers of at-risk potential donors,34 previous recipients of blood transfusions or organs in those countries remain at risk of developing Chagas disease, even without a history of travel to endemic areas. Furthermore, the vector is found in the southern USA, and several cases of natively acquired Chagas disease have been reported.35 One study in Arizona, a state in which both Reduviid bugs and their sylvatic reservoir are plentiful, found that 41.5% of triatomine insects collected by volunteers demonstrated polymerase chain reaction (PCR) evidence of active T. cruzi infection.36
Laboratory diagnosis
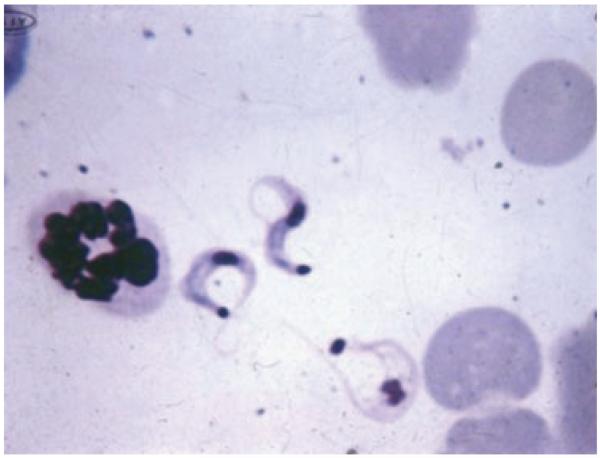
The diagnosis of acute T. cruzi infection is usually made by observing trypomastigotes in wet mounts of blood or CSF or in Giemsa-stained slides (Fig. 5).4 Inoculation of blood into special media37 or into mice38 may be required, though these methods may not be useful in aiding management decisions in acutely infected patients because parasites may not be seen for several weeks. PCR technology is thought to be the most sensitive method for detecting acute and congenital infection.39 If acute infection is suspected in an immunocompromised patient, an examination of tissue may be needed. Parasites may be observed in pericardial fluid,40 bone marrow,41 brain,42 skin,31 or lymph nodes.43 The diagnosis of chronic Chagas disease is usually based on detecting specific antibodies. Several serological assays are employed, including indirect immunofluorescence (IFA) and enzyme-linked immunosorbent assay (ELISA). Serological assays are used widely for clinical diagnosis and screening donated blood, as well as in epidemiological studies. The diagnosis should only be made if antibody presence is confirmed with two different serological modalities or if one modality is used to confirm the presence of at least two antigens;44 commercial kits combining multiple serological modalities or antigens have been developed.45
Figure 5.
Trypomastigotes in a Giemsa smear (×650) prepared from the blood of an infected mouse
Treatment
The two established medications for the treatment of Chagas disease are benznidazole and nifurtimox, which are nitroheterocyclic compounds that have been available for decades. These drugs are only available in the USA from the Centers for Disease Control and Prevention. Benznidazole is the preferred agent due to its superior side effect profile and is given in doses of 5 mg/kg/d for 60 days.46 Common side effects include rash (ranging from photosensitivity to severe exfoliative dermatitis), peripheral neuropathy, and bone marrow suppression. An initial complete blood count, hepatic function profile, and serum chemistries should be obtained upon initiation of treatment. Further complete blood counts should be obtained every two weeks while on therapy.44
Nifurtimox is given in dosages of 8–10 mg/kg/d in adults, 10–12.5 mg/kg/d in adolescents, and 15–20 mg/kg/d in children in four divided doses. The appropriate duration of therapy is 90–120 days.46 The most common side effects are gastrointestinal and neurological (including mood changes and peripheral neuropathy). While on therapy, patients should be monitored with a complete blood count, hepatic function profile, and serum chemistries, initially, after 4–6 weeks of treatment, and upon completion. The patient’s weight and the presence or absence of peripheral neuropathy should be noted every two weeks.44
Which patients benefit from pharmacological treatment remains the subject of clinical trials. Well-accepted indications include acute infection, congenital infection, infection of any stage in a child, accidental laboratory exposure, and reactivation in an immunocompromised host.47 Although some studies have shown the potential for benefit in treating chronic or asymptomatic Chagas disease,48 evidence for clear benefit is currently lacking.49,50
Conclusion
Chagas disease is a systemic infection of life-long duration that is of increasing global interest. Dermatologists should be aware of the acute signs and chronic manifestations of this disease. A high index of suspicion should be maintained for reactivation of this entity in immunosuppressed patients with an appropriate previous exposure history or a history of having lived in or traveled to an endemic area.
Questions (See answers on page 508)
- According to current expert consensus, which patient should definitely receive anti-Chagas therapy?
- A 42-year-old asymptomatic man from Honduras identified as seropositive during a blood drive
- A 65-year-old female from Brazil with end-stage Chagas cardiomyopathy
- A 40-year-old female from Mexico with acute leukemia and positive Chagas serology
- A 45-year-old phlebotomist with accidental exposure to T. cruzi-infected blood
- During nifurtimox therapy, one should monitor the patient’s physical exam for the development of which of the following?
- Peripheral neuropathy
- Hypopigmented macules
- Pulmonary edema
- Icterus
- Which of the following has been identified as the cause of recent large-scale outbreaks of acute Chagas disease in Latin America?
- Blood transfusions
- Contaminated food or drink
- Invasions by the insect vector into non-native habitat
- Fecal–oral transmission
- Which of the following laboratory abnormalities is NOT typical of acute Chagas disease?
- Anemia
- Thrombocytopenia
- Elevated liver enzymes
- Eosinophilia
- Which of the following findings conveys the worst prognosis in acute Chagas disease?
- The presence of trypanosomes in CSF obtained via lumbar puncture
- A new bundle branch block on ECG
- The presence of a chagoma on the right arm
- Fever
- What proportion of patients with positive T. cruzi serologies but no symptoms will progress to symptomatic chronic Chagas disease?
- 0–10%
- 15–30%
- 40–60%
- 80–100%
- Which of the following is NOT an appropriate method to use in a patient with suspected acute Chagas disease?
- Serological testing
- Direct smear
- PCR
- Inoculation into mice
- Which of the following patients meets diagnostic criteria for indeterminate Chagas disease?
- A 45-year-old man from Bolivia with no symptoms, a right bundle branch block on ECG, and both a positive T. cruzi IFA and positive ELISA
- A 40-year-old woman from Paraguay who reports developing Romaña’s sign as a child and now has developed refractory constipation
- A 60-year-old male from Brazil with no symptoms, normal ECG, and both a positive T. cruzi IFA and positive ELISA
- A 50-year-old man from Venezuela with both a positive T. cruzi IFA and positive ELISA with newly-diagnosed dilated cardiomyopathy
- A 40-year-old male from rural Guatemala develops fevers and several erythematous nodules several months after liver transplantation. Which of the following would be the most appropriate method to evaluate for reactivation Chagas disease?
- Serum ELISA
- Serum PCR
- Skin biopsy for pathology
- Inoculation of blood into mice
- A 40-year-old Caucasian male in the USA develops biopsy-proven cutaneous reactivation of Chagas disease after undergoing renal transplantation. Which of the following would NOT be a possible method of his having contracted the disease?
- Receipt of a blood transfusion 15 years previously
- Exposure to Reduviid bugs as a child in rural Tennessee
- Consumption of raw guava juice during a previous trip to Brazil 20 years previously
- Sexual contact with a woman who subsequently developed Chagas cardiomyopathy
Answers to questions
d.
a.
b.
d.
b.
b.
a.
c.
c.
d.
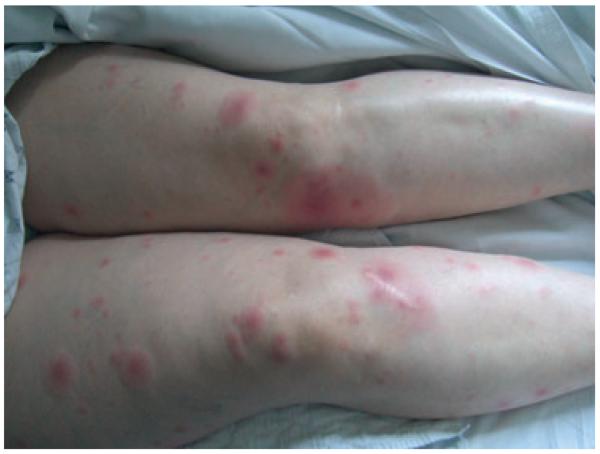
Figure 3.
Lower extremity plaques in a cardiac transplantation patient with reactivation Chagas disease in North America (courtesy of Eva Parker)
Acknowledgments
We would like to acknowledge Verónica Gallerano and colleagues from the University of Córdoba and Eva Parker of Northwestern University for the use of their pictures. This work was supported in part by National Institutes of Health grant AI-076248 to Herbert Tanowitz.
Footnotes
Conflicts of interest: None to report.
References
- 1.Salvatella R. Current Status of Chagas Disease. Pan American Health Organization; Washington, DC: 2006. [Google Scholar]
- 2.Schmunis GA, Yadon ZE. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop. 2010;115:14–21. doi: 10.1016/j.actatropica.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Sartori AM, Sotto MN, Braz LM, et al. Reactivation of Chagas disease manifested by skin lesions in a patient with AIDS. Trans R Soc Trop Med Hyg. 1999;93:631–632. doi: 10.1016/s0035-9203(99)90077-9. [DOI] [PubMed] [Google Scholar]
- 4.Diaz JH. Recognizing and reducing the risks of Chagas disease (American trypanosomiasis) in travelers. J Travel Med. 2008;15:184–195. doi: 10.1111/j.1708-8305.2008.00205.x. [DOI] [PubMed] [Google Scholar]
- 5.Tyler KM, Engman DM. The life cycle of Trypanosoma cruzi revisited. Int J Parasitol. 2001;31:472–481. doi: 10.1016/s0020-7519(01)00153-9. [DOI] [PubMed] [Google Scholar]
- 6.Moncayo A, Ortiz Yanine MI. An update on Chagas disease (human American trypanosomiasis) Ann Trop Med Parasitol. 2006;100:663–677. doi: 10.1179/136485906X112248. [DOI] [PubMed] [Google Scholar]
- 7.Tanowitz HB, Machado FS, Jelicks LA, et al. Perspectives on Trypanosoma cruzi-induced heart disease (Chagas disease) Prog Cardiovasc Dis. 2009;51:524–539. doi: 10.1016/j.pcad.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bern C, Montgomery SP, Katz L, et al. Chagas disease and the US blood supply. Curr Opin Infect Dis. 2008;21:476–482. doi: 10.1097/QCO.0b013e32830ef5b6. [DOI] [PubMed] [Google Scholar]
- 9.Prata A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis. 2001;1:92–100. doi: 10.1016/S1473-3099(01)00065-2. [DOI] [PubMed] [Google Scholar]
- 10.Freilij H, Altcheh J. Congenital Chagas disease: diagnostic and clinical aspects. Clin Infect Dis. 1995;21:551–555. doi: 10.1093/clinids/21.3.551. [DOI] [PubMed] [Google Scholar]
- 11.Flores-Chavez M, Faez Y, Olalla J, et al. Fatal congenital Chagas’ disease in a non-endemic area: a case report. Cases J. 2008;1:302. doi: 10.1186/1757-1626-1-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alarcón de Noya B, Díaz-Bello Z, Colmenares C, et al. Large urban outbreak of orally acquired acute chagas disease at a school in Caracas, Venezuela. J Infect Dis. 2010;201:1308–1315. doi: 10.1086/651608. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Morales AJ. Chagas disease: an emerging food-borne entity? J Infect Dev Ctries. 2008;2:149–150. [PubMed] [Google Scholar]
- 14.CDC Chagas disease after organ transplantation – United States, 2001. MMWR Morb Mortal Wkly Rep. 2002;51:210–212. [PubMed] [Google Scholar]
- 15.Hofflin JM, Sadler RH, Araujo FG, et al. Laboratory-acquired Chagas disease. Trans R Soc Trop Med Hyg. 1987;81:437–440. doi: 10.1016/0035-9203(87)90162-3. [DOI] [PubMed] [Google Scholar]
- 16.Anez N, Carrasco H, Parada H, et al. Acute Chagas’ disease in western Venezuela: a clinical, seroparasitologic, and epidemiologic study. Am J Trop Med Hyg. 1999;60:215–222. doi: 10.4269/ajtmh.1999.60.215. [DOI] [PubMed] [Google Scholar]
- 17.Hoff R, Teixeira RS, Carvalho JS, Mott KE. Trypanosoma cruzi in the cerebrospinal fluid during the acute stage of Chagas’ disease. N Engl J Med. 1978;298:604–606. doi: 10.1056/NEJM197803162981106. [DOI] [PubMed] [Google Scholar]
- 18.Page KR, Zenilman J. Eosinophilia in a patient from South America. JAMA. 2008;299:437–444. doi: 10.1001/jama.2008.21. [DOI] [PubMed] [Google Scholar]
- 19.Parada H, Carrasco HA, Añez N, et al. Cardiac involvement is a constant finding in acute Chagas’ disease: a clinical, parasitological and histopathological study. Int J Cardiol. 1997;60:49–54. doi: 10.1016/s0167-5273(97)02952-5. [DOI] [PubMed] [Google Scholar]
- 20.Garzon SA, Lorga AM, Nicolau JC. Electrocardiography in Chagas heart disease. Sao Paulo Med J. 1995;113:802–813. doi: 10.1590/s1516-31801995000200011. [DOI] [PubMed] [Google Scholar]
- 21.WHO Expert Committee . Control of Chagas Disease. World Health Organization; Brasilia, Brazil: 2002. [PubMed] [Google Scholar]
- 22.Patel S, Sethi A. Imported tropical diseases. Dermatol Ther. 2009;22:538–549. doi: 10.1111/j.1529-8019.2009.01275.x. [DOI] [PubMed] [Google Scholar]
- 23.Ortiz Medina A, Pons S. Manifestaciones cutáneas de la enfermedad de Chagas. Cuarta parte: Histopatología. Med Cutan Ibero Lat Am. 1974;2:391–397. [PubMed] [Google Scholar]
- 24.Lupi O, Bartlett BL, Haugen RN, et al. Tropical dermatology: tropical diseases caused by protozoa. J Am Acad Dermatol. 2009;60:897–925. doi: 10.1016/j.jaad.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Biolo A, Ribeiro A, Clausell N. Chagas cardiomyopathy – where do we stand after a hundred years? Prog Cardiovasc Dis. 2010;52:300–316. doi: 10.1016/j.pcad.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Rassi A, Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 27.Matsuda N, Miller S, Evora P. The chronic gastrointestinal manifestations of Chagas disease. Clinics (Sao Paulo) 2009;64:1219–1224. doi: 10.1590/S1807-59322009001200013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altclas J, Sinagra A, Jaimovich G, et al. Reactivation of chronic Chagas’ disease following allogeneic bone marrow transplantation and successful pre-emptive therapy with benznidazole. Transpl Infect Dis. 1999;1:135–137. doi: 10.1034/j.1399-3062.1999.010207.x. [DOI] [PubMed] [Google Scholar]
- 29.La Forgia MP, Pellerano G, de las Mercedes Portaluppi M, et al. Cutaneous manifestation of reactivation of Chagas disease in a renal transplant patient: long-term follow-up. Arch Dermatol. 2003;139:104–105. doi: 10.1001/archderm.139.1.104. [DOI] [PubMed] [Google Scholar]
- 30.Gallerano V, Consigli J, Pereyra S, et al. Chagas’ disease reactivation with skin symptoms in a patient with kidney transplant. Int J Dermatol. 2007;46:607–610. doi: 10.1111/j.1365-4632.2007.03127.x. [DOI] [PubMed] [Google Scholar]
- 31.Tomimori-Yamashita J, Deps PD, Almeida DR, et al. Cutaneous manifestation of Chagas’ disease after heart transplantation: successful treatment with allopurinol. Br J Dermatol. 1997;137:626–630. doi: 10.1111/j.1365-2133.1997.tb03800.x. [DOI] [PubMed] [Google Scholar]
- 32.Hall CS, Fields K. Cutaneous presentation of Chagas’ disease reactivation in a heart-transplant patient in Utah. J Am Acad Dermatol. 2008;58:529–530. doi: 10.1016/j.jaad.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Tuculet CN, Parra IH, Enz P, et al. Enfermedad de Chagas: manifestación cutánea en un paciente con trasplante hepático. Reactivación. Med Cutan Ibero Lat Am. 2007;35:25–28. [Google Scholar]
- 34.El Ghouzzi M-H, Boiret E, Wind F, et al. Testing blood donors for Chagas disease in the Paris area, France: first results after 18 months of screening. Transfusion. 2010;50:575–583. doi: 10.1111/j.1537-2995.2009.02476.x. [DOI] [PubMed] [Google Scholar]
- 35.Dorn PL, Perniciaro L, Yabsley MJ, et al . Emerg Infect Dis. 2007;13:605–607. doi: 10.3201/eid1304.061002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reisenman CE, Lawrence G, Guerenstein PG, et al. Infection of kissing bugs with Trypanosoma cruzi, Tucson, Arizona, USA. Emerg Infect Dis. 2010;16:400–405. doi: 10.3201/eid1603.090648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiari E, Dias JC, Lana M, Chiari CA. Hemocultures for the parasitological diagnosis of human chronic Chagas’ disease. Rev Soc Bras Med Trop. 1989;22:19–23. doi: 10.1590/s0037-86821989000100004. [DOI] [PubMed] [Google Scholar]
- 38.Feilij H, Muller L, Gonzalez Cappa SM. Direct micromethod for diagnosis of acute and congenital Chagas’ disease. J Clin Microbiol. 1983;18:327–330. doi: 10.1128/jcm.18.2.327-330.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zulantay I, Apt W, Gil LC, et al. The PCR-based detection of Trypanosoma cruzi in the faeces of Triatoma infestans fed on patients with chronic American trypanosomiasis gives higher sensitivity and a quicker result than routine xenodiagnosis. Ann Trop Med Parasitol. 2007;101:673–679. doi: 10.1179/136485907X241415. [DOI] [PubMed] [Google Scholar]
- 40.Ferreira MS, Nishioka SdA, Silvestre MTA, et al. Reactivation of Chagas’ disease in patients with AIDS:report of three new cases and review of the literature. Clin Infect Dis. 1997;25:1397–1400. doi: 10.1086/516130. [DOI] [PubMed] [Google Scholar]
- 41.Fores R, Sanjuan I, Portero F, et al. Chagas disease in a recipient of cord blood transplantation. Bone Marrow Transplant. 2007;39:127–128. doi: 10.1038/sj.bmt.1705551. [DOI] [PubMed] [Google Scholar]
- 42.Pittella JE. Central nervous system involvement in Chagas disease: a hundred-year-old history. Trans R Soc Trop Med Hyg. 2009;103:973–978. doi: 10.1016/j.trstmh.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 43.Kirchhoff LV. American trypanosomiasis (Chagas’ disease) – a tropical disease now in the United States. N Engl J Med. 1993;329:639–644. doi: 10.1056/NEJM199308263290909. [DOI] [PubMed] [Google Scholar]
- 44.Bern C, Montgomery SP, Herwaldt BL, et al. Evaluation and treatment of Chagas disease in the United States. JAMA. 2007;298:2171–2181. doi: 10.1001/jama.298.18.2171. [DOI] [PubMed] [Google Scholar]
- 45.Gomes YM, Lorena VM, Luquetti AO. Diagnosis of Chagas disease: what has been achieved? What remains to be done with regard to diagnosis and follow up studies? Mem Inst Oswaldo Cruz. 2009;104(Suppl. 1):115–121. doi: 10.1590/s0074-02762009000900017. [DOI] [PubMed] [Google Scholar]
- 46.Kirchhoff LV. Trypanosoma species (American Trypanosomiasis, Chagas disease): biology of trypanosomes. In: Mandell GM, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett’s Principles & Practice of Infectious Diseases. 7th edn. Elsevier; Philadelphia: 2009. pp. 3481–3488. [Google Scholar]
- 47.Rodriques Coura J, de Castro SL. A critical review on Chagas disease chemotherapy. Mem Inst Oswaldo Cruz. 2002;97:3–24. doi: 10.1590/s0074-02762002000100001. [DOI] [PubMed] [Google Scholar]
- 48.Viotti R, Vigliano C, Lococo B, et al. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment. Ann Intern Med. 2006;144:724–734. doi: 10.7326/0003-4819-144-10-200605160-00006. [DOI] [PubMed] [Google Scholar]
- 49.Reyes PA, Vallejo M. Trypanocidal drugs for late stage, symptomatic Chagas disease (Trypanosoma cruzi infection) Cochrane Database Syst Rev. 2005;4:CD004102. doi: 10.1002/14651858.CD004102.pub2. [DOI] [PubMed] [Google Scholar]
- 50.Villar JC, Marin-Neto JA, Ebrahim S, Yusuf S. Trypanocidal drugs for chronic asymptomatic Trypanosoma cruzi infection. Cochrane Database Syst Rev. 2002;1:CD003463. doi: 10.1002/14651858.CD003463. [DOI] [PubMed] [Google Scholar]