Summary
When cells are activated by calcium-mobilizing agonists at low, physiological concentrations, the resulting calcium signals generally take the form of repetitive regenerative discharges of stored calcium, termed calcium oscillations [1]. These intracellular calcium oscillations have long fascinated biologists as representing a mode of digitized intracellular signaling. Recent work has highlighted the role of calcium influx as an essential component of calcium oscillations [2]. This influx occurs through a process known as store-operated calcium entry which is initiated by calcium sensor proteins in the endoplasmic reticulum, STIM1 and STIM2 [3]. STIM2 is activated by changes in endoplasmic reticulum calcium near the resting level, while a threshold of calcium depletion is required to activate STIM1 [4]. In this study, we show that, surprisingly, it is STIM1 and not STIM2 that is exclusively involved in calcium entry during calcium oscillations. The implication is that each oscillation produces a transient drop in endoplasmic reticulum calcium that is sufficient to transiently activate STIM1. This transient activation of STIM1 can be observed in some cells by total internal reflection fluorescence microscopy. This arrangement nicely provides a clearly defined and unambiguous signaling system, translating a digital calcium release signal into calcium influx that can signal to downstream effectors.
Results and Discussion
STIM2, but not STIM1, regulates basal Ca2+
We initially confirmed key observations regarding the actions of STIM1 and STIM2. As previously reported [4], when Ca2+ stores were gradually depleted, STIM2 moved to near plasma membrane areas sooner than STIM1 (Supplemental Figures 1A, B, 3C, D). Consistent with this finding, overexpression of STIM2, but not STIM1, resulted in constitutive Ca2+ entry (Supplemental Figure 1C – I). As reported [5], STIM2 overexpression had a partial inhibitory effect on store-operated Ca2+ entry (SOCE) activated by thapsigargin in cells transfected with 2 μg cDNA/well, but not with 0.5 μg cDNA/well. However, both plasmid concentrations caused an elevation in constitutive entry (Supplemental Figure 1G – I). Also as reported [2,4,6,7], knockdown of STIM1 by RNAi almost completely eliminated SOCE, while knockdown of STIM2 produced a minimal effect (Supplemental Figure 2).
It has been suggested that inhibition by overexpressed STIM2 might result from down-regulation of Orai1 [4]. However, by Western blot, we saw no difference in Orai1 levels between cells transfected with 0.5 and 2.0 μg cDNA/well (not shown).
Effect of STIM2 expression and knockdown on calcium oscillations
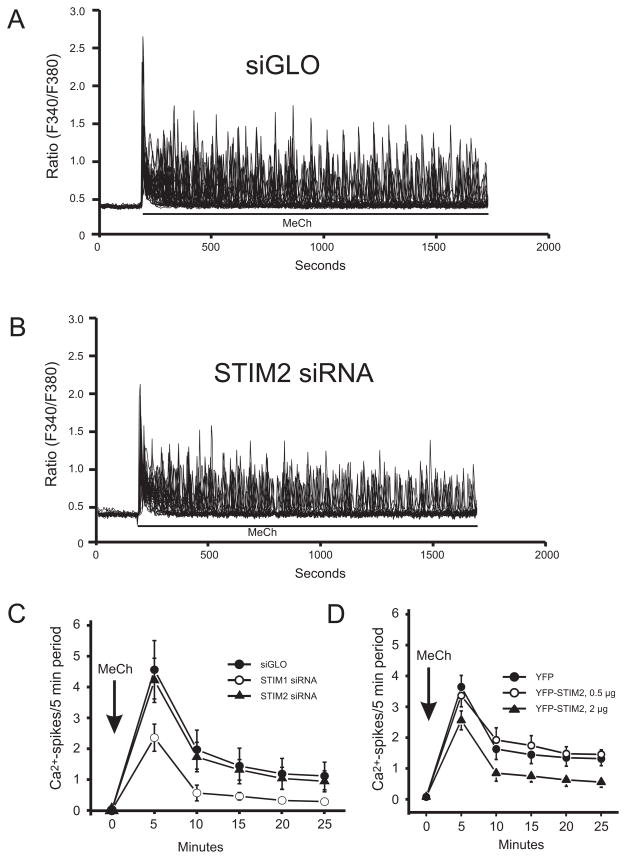
Activation of HEK293 cells with low concentrations of the PLC-linked agonist methacholine (MeCh) results in repetitive cytoplasmic calcium transients, or oscillations. An underlying entry of Ca2+ maintains these oscillations, and it has been suggested that this entry could be activated by arachidonic acid [8]. However, more recent work demonstrated that the entry associated with oscillations in HEK293 cells is SOCE [2,9]. Thus maintenance of MeCh-induced calcium oscillations depends on SOCE, but involves minimal depletion of ER calcium [9]. STIM2 is expressed in HEK293 cells (Supplemental Fig. 2), and thus with low agonist stimulation, a predominant role for STIM2 is expected. However, knockdown of STIM1 substantially reduced the frequency of sustained Ca2+ oscillations (Fig. 1C), to the same extent as removal of extracellular Ca2+ [2], while knockdown of STIM2 had no effect (Fig. 1A–C). Also, transient expression of STIM2 with 0.5 μg [cDNA] was without effect, while 2 μg [cDNA] inhibited oscillations (Fig. 1D), consistent with inhibition by STIM2 of SOCE (Supplemental Fig. 1).
Figure 1. Effects of STIM1 and STIM2 siRNA knockdown and EYFP-Stim2 overexpression on MeCh-induced Ca2+ oscillations.
A and B. Single experiments, 30–40 cells each, showing lack of effect of STIM2 knockdown on [Ca2+]i oscillations. C. Average rate of oscillations (spikes/5 min) for 3 such experiments, and including the effect of STIM1 knockdown. D. Average of 3 experiments showing that overexpression of STIM2, at 0.5 μg/well, had no effect of oscillation frequency, while at 2.0 μg/well there was a partial inhibition.
An EF-hand mutant of STIM2 is a poor activator of Orai1
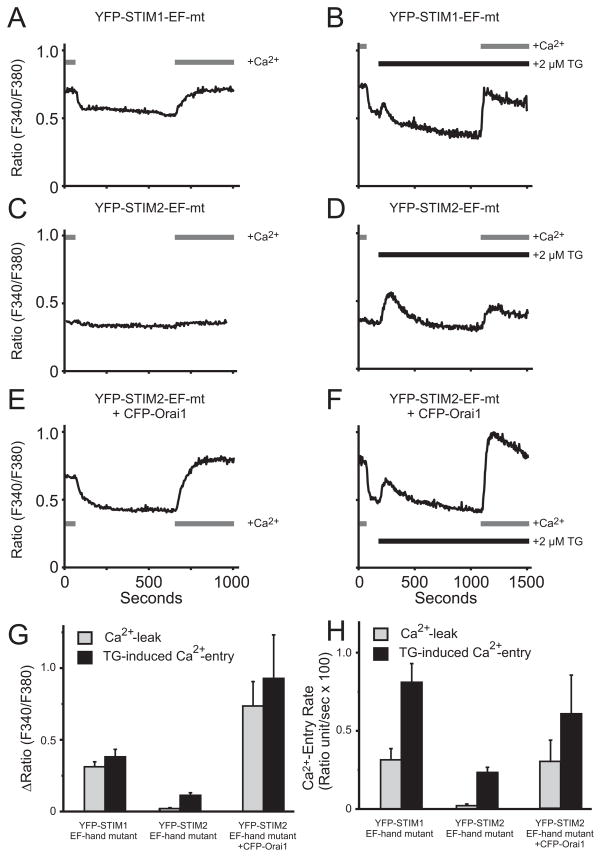
These results indicate that either overexpression or knockdown of STIM2 minimally affects Ca2+ oscillations. This could be because activation of Orai1 by STIM2 is significantly weaker than for STIM1. STIM2 has been shown to activate Orai1-dependent Ca2+ signals when both STIM2 and Orai1 are overexpressed [10], and we confirmed this observation (data not shown). However, when both proteins are overexpressed, their stoichiometry is unknown and the efficiency of interaction cannot be ascertained. When expressed on their own, neither STIM1 nor STIM2 substantially increases entry (Supplemental Fig. 1), probably because endogenous STIM proteins are sufficient for activation of endogenous Orai. One way to assess the ability of these proteins to activate endogenous Orai is to inhibit SOCE by knocking down STIM1, and then determine the ability of transfected STIMs to rescue entry. Previously, we demonstrated that with this protocol, only STIM1 and not STIM2 could restore SOCE [2]. A second strategy is to examine constitutive Ca2+ entry following overexpression of the respective EF-hand mutants of STIM1 and STIM2. Since Orai1 is not overexpressed, it is likely that in each case, all or most Orai1 channels are engaged by EF-hand mutant STIMs. Use of EF-hand mutants obviates the need for Ca2+ store depletion, and assures that only effects of the transfected STIMs will be seen. We utilized a D76N/D78N-EYFP-STIM1 which we described previously [11], and a mutant of EYFP-STIM2 (D80A) previously shown to have constitutive activity [4]. Overexpression of EF-hand mutant STIM1 caused substantial constitutive Ca2+ entry (Fig. 2A, B, G, F); however, EF-hand mutant STIM2 did not cause detectable Ca2+ entry (Fig. 2C, G, H). Thapsigargin-activated SOCE if anything was partially inhibited (Fig. 2D, G, H), as seen with wild-type STIM2 [5]. EF-hand mutant STIM2 was clearly present and constitutively active, because co-overexpression with Orai1 resulted in substantial constitutive Ca2+ entry (Fig. 2E, F, G, H). Co-overexpression of EF-hand mutant STIM1 and Orai1 resulted in cells that tended to detach and appeared apoptotic, presumably due to large constitutive Ca2+ influx. These findings suggest that the ability of STIM2 to activate Orai1 is severely limited in comparison to STIM1. Calcium influx through STIM2-activated channels that is sufficient to cause detectable elevations in cytoplasmic calcium can apparently only be demonstrated under conditions of Orai1 overexpression.
Figure 2. EF-hand mutant of STIM2 weakly interacts with Orai1.
A and B. Expression of EYFP-STIM1-EF hand mutant (D76N, D78N) induces a constitutive Ca2+ entry (A) that is slightly enhanced following thapsigargin treatment (B). C and D. In contrast, expression of EYFP-STIM2-EF hand mutant (D80A) has no effect on basal Ca2+ leak (C) and appears to exert an inhibitory effect on thapsigargin-induced Ca2+ entry (D). E and F. Co-expressing CFP-Orai1 with EYFP-STIM2-EF hand mutant induces a constitutive Ca2+ entry (E) that is slightly enhanced following thapsigargin treatment (F). All plasmid concentrations were 0.5μg per transfection. Data summarized in G (peak Ca2+ rise) and H (rate of Ca2+ rise) are mean ± SEM for n=4 experiments (A–D) and n=3 (E–F).
Intracellular localization of STIM1 and STIM2 during Ca2+ oscillations
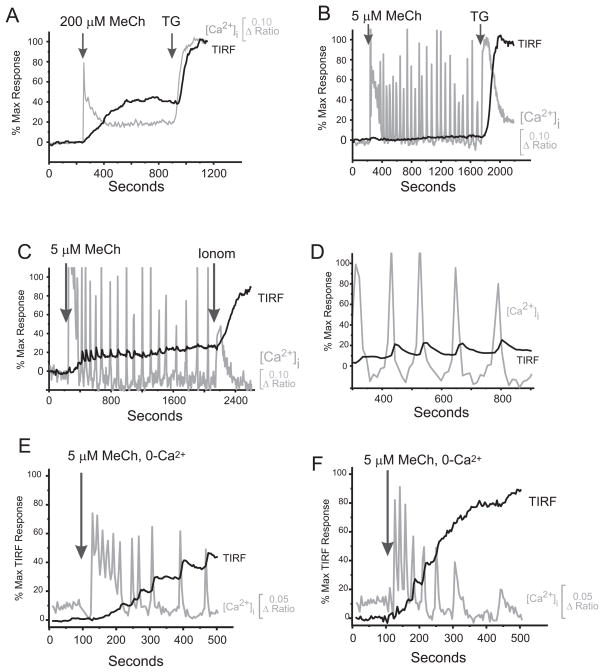
A critical step for SOCE activation is redistribution of STIM into distinct punctae close to the plasma membrane. Substantial depletion of intracellular stores appears necessary for STIM1 translocation (Supplemental Fig. 1), yet the RNAi data clearly indicate an obligatory role for STIM1 during oscillations when global Ca2+ depletion is minimal [2]. Thus, we examined movements of STIM1 and STIM2 under conditions of submaximal, physiological receptor activation that gives rise to Ca2+ oscillations. We employed a technique combining total internal reflection fluorescence microscopy (TIRFM) and [Ca2+]i monitoring. Maximal depletion of stores with 200 μM MeCh (Fig. 3A), 2 μM thapsigargin or 10 μM ionomycin activated robust translocation of STIM1 to near membrane regions without affecting other endoplasmic reticulum markers (not shown). With a low concentration of MeCh, inducing oscillations [9], we observed a variety of responses. In a minority of cells there was no detectable TIRFM response despite obvious [Ca2+]i oscillations (39/91 cells, Fig. 3B). However, in 52/91 cells, a TIRFM response developed, always after some delay (Fig. 3C; ~100s see Supplemental Figure 3C). In some of these responding cells (10/52 cells), agonist-induced [Ca2+]i-transients were closely followed by transient movements of EYFP-STIM1 to and from the plasma membrane (Fig. 3C, expanded in Fig. 3D). Coordination of EYFP-STIM1 movements with [Ca2+]i-transients implies that intracellular Ca2+-stores sense their fill status and relay this information in a proportional and synchronized fashion. The reason that some cells did not show a TIRF response while oscillating is not known, but there are a number of possibilities. First, the time constant for activation of Icrac is tens of seconds [12] which may be too slow in some cells to resolve as discrete events. Second, overexpression of EYFP-STIM1 may occlude subtle movements of STIM1 in small cellular compartments. Third, STIM1 movements may occur in membrane areas other than those near the coverslip, which is the only surface observable by TIRFM.
Figure 3. Temporal effects of Methacholine (MeCh) on Ca2+ mobilization and the EYFP-STIM1 TIRF signal.
A. Time course of TIRF and Ca2+ rise in HEK293 cells activated with a high concentration of MeCh (200 μM in HBSS plus 1.8mM Ca2+). B and C. Time course of TIRF and Ca2+ rise in HEK293 cells activated with either a low, oscillatory concentration of MeCh (5 μM). Intracellular Ca2+ and TIRF signal from EYFP-STIM1 were monitored simultaneously as described in Methods. In 39/91 cells no detectable TIRF response was observed (eg. Panel B). In 52/91 cells a TIRF response was initiated after a delay and in 10/52 cells oscillations in EYFP movement were synchronized with, and just following each Ca2+ oscillation (eg. Panel C). D. An expanded segment from Panel C. E and F. TIRF responses to 5 μM MeCh in the absence of extracellular Ca2+. Under these conditions, a greater proportion of EYFP-STIM1cells (63/69) gave a TIRFM response and the response was initiated after a shorter delay compared to responses in the presence of extracellular Ca2+. See Supplemental Fig. 3 for summary of low [MeCh] and extracellular Ca2+ effects on TIRFM response parameters.
The subtle movements of STIM1 during [Ca2+]i oscillations were amplified in the absence of extracellular Ca2+ (Fig. 3E, F). Again, in some cells the EYFP-STIM1 TIRFM signal increased incrementally in synchrony with, and just behind individual [Ca2+]i oscillations. While the proportion of cells demonstrating coordinated TIRF and [Ca2+]i oscillations was not substantially increased in the absence of extracellular Ca2+ (16/63 cells), the percentage of cells giving a TIRFM response (~91%, 63/69 cells) and the magnitude were increased (65.5%, Supplemental Fig. 3A) and the delay to the TIRFM response was reduced (78 sec, Supplemental Fig. 3C). Presumably these changes reflect enhanced depletion of a critical Ca2+ pool when there is no entry to replenish it. Interestingly, the global loss of Ca2+ from intracellular stores is increased by only a miniscule amount by removal of extracellular Ca2+ [9].
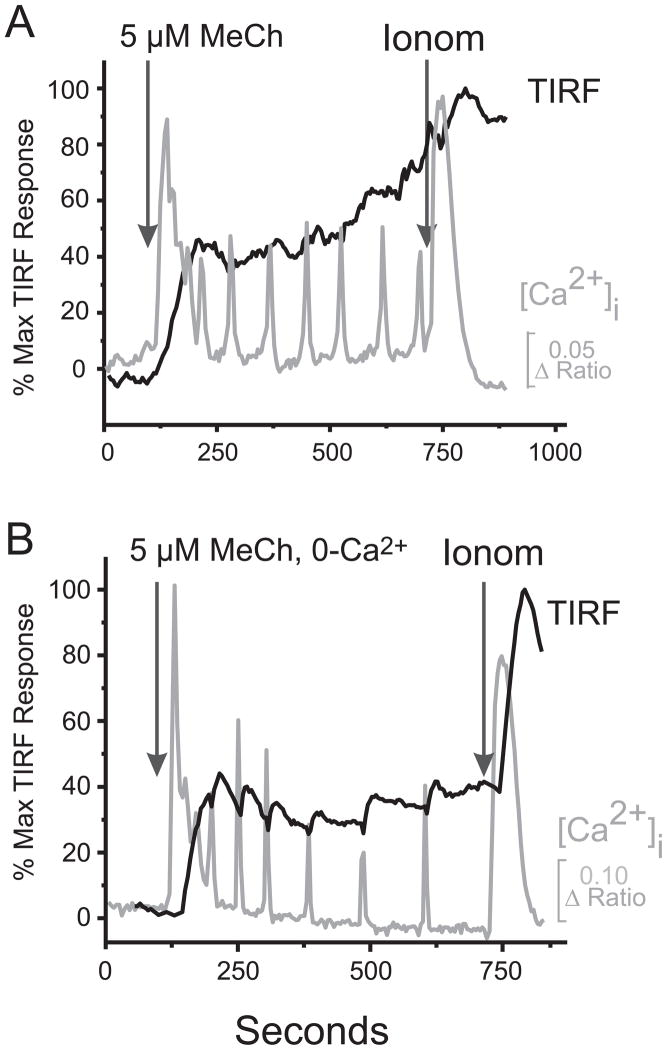
We also examined movements of EYFP-STIM2 in oscillating cells (Fig. 4). Consistent with the observed greater sensitivity of STIM2 to small changes in ER Ca2+, all cells responding with calcium oscillations (23/23) showed significant STIM2 TIRFM signals. The magnitude of the STIM2 TIRFM response was substantially greater (88.6%, see Supplemental Fig. 3B), and the delay to onset of the response was substantially shorter (28.1 sec, see Supplemental Fig. 3D) than for EYFP-STIM1 (16.7% and 102.9 sec, respectively, see Supplemental Fig. 3). Like EYFP-STIM1, in some cells transient EYFP-STIM2 TIRFM responses were coordinated with agonist-induced [Ca2+]i-transients (Fig. 4). This was observed mainly in the absence of extracellular calcium (5/32 cells, see below), and only once (1/23 cells) in the presence of extracellular calcium. In contrast to EYFP-STIM1, the EYFP-STIM2 TIRFM response was not enhanced in the absence of extracellular Ca2+ (Supplemental Figs. 4B, 3B, D) probably because the response was nearly maximal in the presence of Ca2+. Thus, STIM2 responds strongly to the limited Ca2+ discharges associated with [Ca2+]i oscillations, but apparently cannot produce sufficient activation of Orai channels to support the necessary underlying Ca2+ entry (consistent with data in Fig. 1 and 2).
Figure 4. Temporal effects of Methacholine (MeCh) on Ca2+ mobilization and the EYFP-STIM2 TIRF signal.
A. Intracellular Ca2+ and TIRF signal from EYFP-STIM2 were monitored simultaneously in HEK293 cells as described in Methods. In the presence of extracellular calcium, low concentration of MeCh (5 μM) induced a TIRFM response in all cells (23/23 cells). The delay to the onset of the TIRF response was much shorter for EYFP-STIM2 than observed with EYFP-STIM1 (~28 sec and ~103 sec respectively). B. The absence of extracellular Ca2+ did not augment the EYFP-STIM2 TIRFM response, but rather modestly attenuated it. (see Supplemental Figure 3 for summary).
The limited ability of STIM2 to activate Orai channels is understandable if the function of STIM2 is primarily for long-term maintenance of intracellular Ca2+ homeostasis [4]. The more efficient STIM1 requires substantial Ca2+ loss from intracellular stores for its activation, and this may be important in protecting cells from excessive cytoplasmic Ca2+ fluctuations from minimal perturbations of Ca2+ stores. This may also explain why overexpression of STIM2 has an inhibitory effect [5]; when present in excess it may behave like a partial agonist. The inability of STIM2 to strongly activate Orai1 may be due to the presence of intracellular inhibitor [10]. If so, STIM2 could possibly function in a signaling capacity in other cell types where the inhibitor is present at lower concentration. Recently reported findings with a STIM2 knockout mouse have shown a clear albeit subtle function of STIM2 in long-term T-cell activation [13]. We believe that the major function of STIM2 is maintenance of steady-state cellular Ca2+ homeostasis as originally suggested by Brandman et al [4]. Since STIM2 appears to be partially active in resting cells, it would seem problematic if it were fully capable of activating Orai channels. With low concentrations of agonist, producing intracellular Ca2+ oscillations, STIM2 responds more robustly than STIM1 to this minimal degree of intracellular store depletion. However, the inability of STIM2 to strongly activate Orai apparently prevents significant contribution to the underlying Ca2+ entry. Rather, it appears that during [Ca2+]i oscillations, ER Ca2+ must transiently drop into the range sensed by STIM1 to adequately activate Orai channels and Icrac. This substantial localized drop in Ca2+ while global Ca2+ stores change very little [9] supports previous suggestions that a small critical pool of stored Ca2+ plays the major role in controlling store-operated channels [14,15]. Avoiding the need for global ER Ca2+ depletion to activate SOCE would minimize deleterious effects on other important ER functions such as protein synthesis and packaging.
Previous studies of [Ca2+]i oscillations have suggested that their all-or-none digital nature provides an unambiguous signal for activation of downstream effectors ([16] and references therein). However, a number of recent reports have highlighted the essential role of store-operated channels in providing the Ca2+ that activates downstream effectors [17–21], including those channels activated during Ca2+ oscillations [22]. The current and previous [2] studies indicate that [Ca2+]i oscillations can readily mobilize STIM1 and activate Icrac signaling. While we cannot measure directly the small current underlying Ca2+ oscillations, given the interspike interval of the order of several minutes, and the time constant of Icrac of the order of tens of seconds, it is possible that influx may sometimes be similarly digitized in synchrony with Ca2+ release and STIM1 movement. But regardless of the temporal nature of the resulting Ca2+ influx, what is more important is its obligatory link to the digital release event. Each Ca2+ oscillation will transiently drop ER Ca2+ content into the STIM1 sensitivity range, assuring some degree of STIM1 activation even at very low agonist concentration. This conclusion fits nicely with the report by Di Capite et al. [22] who demonstrated that in oscillating mast cells, Ca2+ entering through CRAC channels preferentially initiates signaling to increase gene expression. Thus we may have to rethink the idea that store-operated entry functions simply to maintain Ca2+ stores for a digitally encoded Ca2+ release signal; rather, in at least some instances the function of the regenerative Ca2+ release spikes may be to activate STIM1-dependent store-operated Ca2+ entry that constitutes the primary signal to downstream effectors.
Methods and Materials
Cell Culture
HEK293 cells (ATCC) were cultured in Dulbecco’s minimum essential medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum and 2 mM glutamine and maintained in a humidified 95% air, 5% CO2 incubator at 37°C. In preparation for cDNA transfection, cells were transferred to 6-well plates and allowed to grow to ~90% confluence. In preparation for Ca2+ measurements, cells were cultured to about 70% confluence and then transferred onto 30 mm round glass coverslips (#1. 5 thickness). The cells were allowed to attach for a period of 6 h, after which additional DMEM was added to the coverslip, and the cells were maintained in culture for an additional 24–36 h before use in Ca2+ measurements.
cDNA Transfection
HEK293 cells were transfected using either Lipofectamine 2000 (Invitrogen; 2 μl per well) or electroporation (Amaxa, Nuclefector Kit V) with cDNA for either EYFP, EYFP-STIM1, or EYFP-STIM2. After a 6 h incubation period, the medium bathing the cells was replaced with complete DMEM and maintained in culture. After 24 h, cDNA treated cells were transferred to 30 mm glass coverslips in preparation for Ca2+ measurements as described above, which were carried out on the following day. The concentrations of plasmids used were 0.5 μg/well for EYFP-STIM1 and 0.5 or 2.0 μg/well for EYFP-STIM2.
siRNA Knockdown
HEK293 cells were plated in a 6-well plate on day 1. On day 2, cells were transfected with siRNA (100 nM) against STIM1 (IDT), STIM2 (IDT), Orai1 (Invitrogen) or siCONTROL (Dharmacon) using Metafectene (Biontex Laboratories GmbH, Martinsried/Planegg, Germany, 7 μl per well), and including siGLO (Dharmacon) as a marker. The sequences of the siRNA used were: STIM1 agaaggagcuagaaucucac [23]; STIM2 aactgagaagcagttggtctg [6], Orai1 cccuucggccugaucuuuaucgucu [23]. After an 8-h incubation period, the medium bathing the cells was replaced with complete DMEM and maintained in culture. On day 3, cells were transferred to 30-mm glass coverslips in preparation for Ca2+ measurements as described above, which were carried out on day 4 or 5. The effectiveness of these siRNA in knocking down protein levels was determined by Western blot (Supplemental Fig. 2).
Combined Calcium Measurements and TIRF Microscopy on Single HEK293 Cells
Fluorescence measurements were made in HEK293 cells transfected with EYFP-STIM1 or EYFP-STIM2 and loaded with the ratiometric calcium sensitive dye, fura-5F. Briefly, coverslips with transfected cells were mounted in a Teflon chamber and incubated in DMEM with 1 μM fura-5F/AM at 37° C in the dark for 25 min. Before [Ca2+]i measurements were made, cells were washed 3 times and incubated for 15–30 min at room temperature (25° C) in a HEPES-buffered salt solution (HBSS, in mM: NaCl 120; KCl 5.4; MgCl2 0.8; HEPES 20; CaCl2 1.8 and glucose 10 mM, with pH 7.4 adjusted by NaOH). In these experiments, nominally Ca2+-free solutions were HBSS with no added CaCl2.
Both TIRFM and calcium measurements were performed on an Olympus IX71 inverted microscope equipped with a 60x 1. 45 NA objective and an Olympus (Melville, NY) IX2-RFAEVA-2 illumination system. Illumination for TIRFM was provided by a 488-nm argon ion laser (Melles Griot, Carlsbad, CA) directed through a fiber optic cable. At the same time, excitation wavelengths for fura-5F (360nm and 380nm) were provided by a Xenon arc lamp and a motorized filter wheel. The emitted fluorescence passed through D525/50m filter. All images were captured by a Photometrics Cascade 512F cooled CCD camera (Roper Scientific, Tucson, AZ). Control of image acquisition and analysis of was performed with MetaFluor software (Molecular Devices Corp., Downington, PA). Changes in intracellular calcium are represented as the ratio of fura-5F fluorescence due to excitation at 360 nm to that due to excitation at 380 nm (Ratio= F360/F380). Changes in the TIRFM signal as the ratio measured fluorescence, F, divided by the TIRFM signal at the beginning of the experiment, Fo (Ratio= F/Fo). The effects of agonist stimulation on the TIRFM signal were also expressed as a percentage of the maximal TIRFM signal elicited by thapsigargin or ionomycin treatment in the same cell (see Supplemental Fig. 2D).
When only monitoring intracellular calcium, cells were mounted onto a Nikon TS-100 inverted microscope equipped with a 20x fluor objective (0.75 NA) and fluorescence images recorded and analyzed with a digital fluorescence imaging system (InCyt Im2, Intracellular Imaging Inc., Cincinnati, OH) equipped with a light sensitive CCD camera (Cooke PixelFly, ASI, Eugene, OR). Fura-5F fluorescence was monitored by alternately exciting the dye at 340 and 380 nm, and collecting the emission wavelength at 510 nm. Changes in intracellular calcium are represented as the ratio of fura-5F fluorescence due to excitation at 340 nm to that due to excitation at 380 nm (Ratio= F340/F380). We utilized the lower affinity indicator fura-5F (Kd = 400 nM) to assure that the data are well below saturation; the ratios we report are for the most part <1.0, well below the average observed Rmax of 5.6. The ratio changes in fields of fura-5F-loaded cells were collected from a multiple regions of interest (ROI), with each ROI representing an individual cell. Typically, 25 to 35 ROIs were monitored per experiment. In all cases, ratio values have been corrected for contributions by autofluorescence, which is measured after treating cells with 10 μM ionomycin and 20 mM MnCl2.
Information on the sources of plasmids and other materials, Western blots and statistical analyses can be found with Supplemental Materials.
Supplementary Material
Acknowledgments
We thank Drs. Jerrel Yakel, Steve Shears and Wayne DeHaven who read the manuscript and provided helpful comments. This work was supported by funds from the Intramural Program of the NIEHS and NIH.
Reference List
- 1.Berridge MJ, Galione A. Cytosolic calcium oscillators. FASEB J. 1988;2:3074–3082. doi: 10.1096/fasebj.2.15.2847949. [DOI] [PubMed] [Google Scholar]
- 2.Wedel B, Boyles RR, Putney JW, Bird GS. Role of the Store-operated Calcium Entry Proteins, Stim1 and Orai1, in Muscarinic-Cholinergic Receptor Stimulated Calcium Oscillations in Human Embryonic Kidney Cells. J Physiol. 2007;579:679–689. doi: 10.1113/jphysiol.2006.125641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cahalan MD, Zhang SL, Yeromin AV, Ohlsen K, Roos J, Stauderman KA. Molecular basis of the CRAC channel. Cell Calcium. 2007;42:133–144. doi: 10.1016/j.ceca.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandman O, Liou J, Park WS, Meyer T. STIM2 Is a Feedback Regulator that Stabilizes Basal Cytosolic and Endoplasmic Reticulum Ca(2+) Levels. Cell. 2007;131:1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soboloff J, Spassova MA, Hewavitharana T, He LP, Xu W, Johnstone LS, Dziadek MA, Gill DL. STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ Entry. Curr Biol. 2006;16:1465–1470. doi: 10.1016/j.cub.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 6.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shuttleworth TJ. What drives calcium entry during [Ca2+]i oscillations?- challenging the capacitative model. Cell Calcium. 1999;25:237–246. doi: 10.1054/ceca.1999.0022. [DOI] [PubMed] [Google Scholar]
- 9.Bird GS, Putney JW. Capacitative calcium entry supports calcium oscillations in human embryonic kidney cells. J Physiol. 2005;562:697–706. doi: 10.1113/jphysiol.2004.077289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parvez S, Beck A, Peinelt C, Soboloff J, Lis A, Monteilh-Zoller M, Gill DL, Fleig A, Penner R. STIM2 protein mediates distinct store-dependent and store-independent modes of CRAC channel activation. FASEB J. 2007;22:752–761. doi: 10.1096/fj.07-9449com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeHaven WI, Jones BF, Petranka JG, Smyth JT, Tomita T, Bird GS, Putney JW. TRPC channels function independently of STIM1 and Orai1. The Journal of Physiology. 2009;587:2275–2298. doi: 10.1113/jphysiol.2009.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol (Lond) 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribeiro CMP, Putney JW. Differential effects of protein kinase C activation on calcium storage and capacitative calcium entry in NIH 3T3 cells. J Biol Chem. 1996;271:21522–21528. doi: 10.1074/jbc.271.35.21522. [DOI] [PubMed] [Google Scholar]
- 15.Parekh AB, Fleig A, Penner R. The store-operated calcium current ICRAC: nonlinear activation by InsP3 and dissociation from calcium release. Cell. 1997;89:973–980. doi: 10.1016/s0092-8674(00)80282-2. [DOI] [PubMed] [Google Scholar]
- 16.Thomas AP, Bird GStJ, Hajnóczky G, Robb-Gaspers LD, Putney JW. Spatial and temporal aspects of cellular calcium signalling. FASEB J. 1996;10:1505–1517. [PubMed] [Google Scholar]
- 17.Cooper DMF, Yoshimura M, Zhang Y, Chiono M, Mahey R. Capacitative Ca2+ entry regulates Ca2+-sensitive adenylyl cyclases. Biochem J. 1994;297:437–440. doi: 10.1042/bj2970437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willoughby D, Cooper DM. Ca2+ stimulation of adenylyl cyclase generates dynamic oscillations in cyclic AMP. J Cell Sci. 2006;119:828–836. doi: 10.1242/jcs.02812. [DOI] [PubMed] [Google Scholar]
- 19.Chang WC, Di Capite J, Singaravelu K, Nelson C, Halse V, Parekh AB. Local Ca2+ Influx through Ca2+ Release-activated Ca2+ (CRAC) Channels Stimulates Production of an Intracellular Messenger and an Intercellular Pro-inflammatory Signal. J Biol Chem. 2008;283:4622–4631. doi: 10.1074/jbc.M705002200. [DOI] [PubMed] [Google Scholar]
- 20.Ng SW, Di Capite J, Singaravelu K, Parekh AB. Sustained Activation of the Tyrosine Kinase Syk by Antigen in Mast Cells Requires Local Ca2+ Influx through Ca2+ Release-activated Ca2+ Channels. J Biol Chem. 2008;283:31348–31355. doi: 10.1074/jbc.M804942200. [DOI] [PubMed] [Google Scholar]
- 21.Chang WC, Nelson C, Parekh AB. Ca2+ influx through CRAC channels activates cytosolic phospholipase A2, leukotriene C4 secretion, and expression of c-fos through ERK-dependent and -independent pathways in mast cells. FASEB J. 2006;20:2381–2383. doi: 10.1096/fj.06-6016fje. [DOI] [PubMed] [Google Scholar]
- 22.Di Capite J, Ng SW, Parekh AB. Decoding of cytoplasmic Ca(2+) oscillations through the spatial signature drives gene expression. Curr Biol. 2009;19:853–858. doi: 10.1016/j.cub.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 23.Mercer JC, DeHaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, Putney JW. Large store-operated calcium-selected currents due to co-expression of orai1 or orai2 with the intracellular calcium sensor, stim1. J Biol Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.