Abstract
Background
Although a link between donor-specific antibodies against human leukocyte antigens type II (DSA II+) and transplant glomerulopathy has been clearly established, its role in cardiac allograft vasculopathy (CAV) is unclear.
Methods
DSA were evaluated using solid phase Single Antigen Bead assay before transplant in 51 heart transplant (HTX) recipients. Coronary angiography and three-dimensional intravascular ultrasound (3D IVUS) were performed at baseline and approximately one year after the baseline exam.
Results
There were 4 (7.8 %), 11 (21.5%), and 2 (3.9%) patients who had DSA against donor Class I (DSA I+), DSA II+, or both respectively. All patients had negative complement dependent cytotoxic cross match. There was accelerated progression of CAV in the DSA II+ group demonstrated by accelerated progression in plaque index (plaque volume/vessel volume) compared to patients with no DSA II+ antibodies (13.8±12% vs. −7.9±37%; p=0.01). The development of any angiographic CAV was also more common in DSA II+ patients as compared to the DSA− patients at 4 years (100±0% vs. 64.2±10%; p=0.05). All other traditional risk factors for CAV, or immunosuppression were similar between the groups (P>0.2 for all).
Conclusions
This is the first preliminary study demonstrating that HTX recipients with preformed Class II DSA may be at an increased risk for accelerated CAV as detected by consecutive volumetric 3D IVUS.
Keywords: Heart transplant, Cardiac allograft vasculopathy, Donor specific antibodies
Introduction
The clinical significance of Class II anti-Human Leucocyte Antigens (HLA) antibodies has been well established in renal transplantation (1), but their role in heart transplantation (HTX) is unclear. Previous studies have shown that HTX recipients who have anti-HLA donor-specific antibodies (DSA) are at increased risk for early acute rejection and have a lower graft survival (2, 3). Newly introduced solid-phase array techniques can distinguish between HLA type and Class I and II antibodies. These tests allow for simultaneous, sensitive and specific detection of multiple anti-HLA antibodies and the results are actively being used for recipient and donor selection (4). These improved techniques have been rapidly incorporated in clinical practice despite the significance of the different types and levels of HLA antibodies detected (Class I vs. Class II) being controversial in HTX. The role of DSA in cardiac allograft vasculopathy (CAV) which remains the leading cause of late morbidity and mortality in HTX recipients (5–7) is unclear. The aim of this study was therefore to evaluate the potential role of such alloantibodies on the progression of CAV as assessed by serial 3 dimensional (3-D) intravascular ultrasound (IVUS) examination and coronary angiography (8).
Results
Baseline patient characteristics
Table 1 shows the demographic, clinical and laboratory characteristics of the patients in the DSA I+, DSA II+, and DSA− groups. Because our aim was to evaluate the impact of each DSA type by itself we have excluded the 2 patients with both DSA I+ and DSA II+ from the analysis. DSA specificity and peak and total mean MFI values for each patient are shown in Table 1 in the supplemental digital content (see SDC 1, Table 1, http://links). There were no differences between the groups in recipient age, sex, donor age, conventional atherosclerosis risk factors, medical therapy and primary or secondary immunosuppression. Trough levels of CNI and SRL were within the therapeutic range in all the patients. History of ischemic cardiomyopathy was lower in the DSA I+ group compared to the other two groups. Flow cytometric cross matches were performed in 17 patients in the DSA− group, 3 patients in the DSA I+ group and 10 patients in the DSA II+ group. None of the patients in the DSA− and DSA I+ groups while 6 of 10 patients in the DSA II+ group had a positive flow cytometric cross match (p=0.0003). Three patients in the DSA II+ group, who had a positive flow cytometric crossmatch were treated with plasmapheresis immediately after transplant. Since only a small number of subjects had Class I DSA, the relationship between DSA I and CAV could not be investigated, thus analysis was performed only for the Class II DSA.
Table 1.
Baseline Demographic and Clinical Characteristics of the Patients
| Variable | DSA I+ (n=4) | DSA II+ (n=11) | DSA− (n=34) |
|---|---|---|---|
| Recipient age, years | 56.7 [44.8, 69.7] | 51.7 [47.9, 60.4] | 55.3 [46.9, 61.5] |
| Gender—male, n(%) | 3(75) | 9(82) | 26(76) |
| Time from transplant to 1st IVUS (years) | 0.99 [0.36, 1.15] | 0.20 [0.18, 1.05] | 0.62 [0.18, 1.22] |
| Time between IVUS exams | 1.00 [0.95, 1.05] | 0.84 [0.76, 0.92] | 0.96 [0.84, 1.06] |
| ICMP, n(%) | 0(0)* | 5(45) | 7(21) |
| Donor age (years) | 21.5 [20.0, 33.2] | 21 [17.0, 29.0] | 30.7 [22.1, 46.7] |
| Ischemic time (min) | 161.5 [158.0, 173.4] | 185 [160.0, 240.0] | 183.0 [131.2, 210.7] |
| BMI (kg/m2) | 22.8 [20.4, 24.0] | 27.3 [21.6, 31.9] | 26.0 [22.2, 29.5] |
| Uric acid (mg/dL) | 5.9 [5.2, 6.2] | 6.8 [5.7, 9.2] | 7.1 [5.6, 9.4] |
| GFR mL/min/1.73 m2 | 48.5 [37.8, 56.2] | 60.0 [48.0, 79] | 57.0 [43.0, 63.0] |
| Triglycerides (mg/dL) | 76.5 [62.7, 187] | 136.5 [87.5, 209.7] | 112.0 [73.0, 218.3] |
| Cholesterol (mg/dL) | 151.0 [111.0, 218.0] | 156.0 [116.0, 195.0] | 174.0 [135.0, 204.7] |
| Hypertension, n(%) | 1(25) | 2(18) | 8(23) |
| Diabetes, n(%) | 0(0) | 2(18) | 4(13) |
| Total rejection score | 0.34 [0.24, 0.47] | 0.23 [0.0, 0.5] | 0.20 [0.0, 0.4] |
| Any rejection score | 0.31 [0.18, 0.47] | 0.23 [0.0, 0.5] | 0.20 [0.0, 0.4] |
| Peak DSA (MFI) | 1278 [482, 2051] | 804 [530, 2221] | 0 [0, 0] |
| Positive flow cytometric crossmatch n(%)|| | 0(0)* | 6(60)*¶ | 0(0)¶ |
| CCB, n(%) | 1(25) | 3(27) | 9(27) |
| Statin n(%) | 2(50) | 8(72) | 24(71) |
| Aspirin, n(%) | 1(25) | 5(45) | 8(23) |
| SRL vs CNI n(%) | SRL 3(75); CNI 1(25) | SRL 8(72); CNI 3(28) | SRL 14(42); CNI 20(58) |
| MMF vs AZA, n(%) | MMF 3(75); AZA 1(25) | MMF 9(82); AZA 2(18) | MMF 24(71); AZA 10(29) |
| Prednisone, n(%) | 3(75) | 8(72) | 17(50) |
P<0.05, DSA I+ vs. DSA II+; + P<0.05, DSA I+ vs. DSA−.;
P<0.05, DSA II+ vs. DSA−
Assessed in 17 DSA−, 3 DSAI+ and 10 DSA II+ patients.
3-D IVUS findings
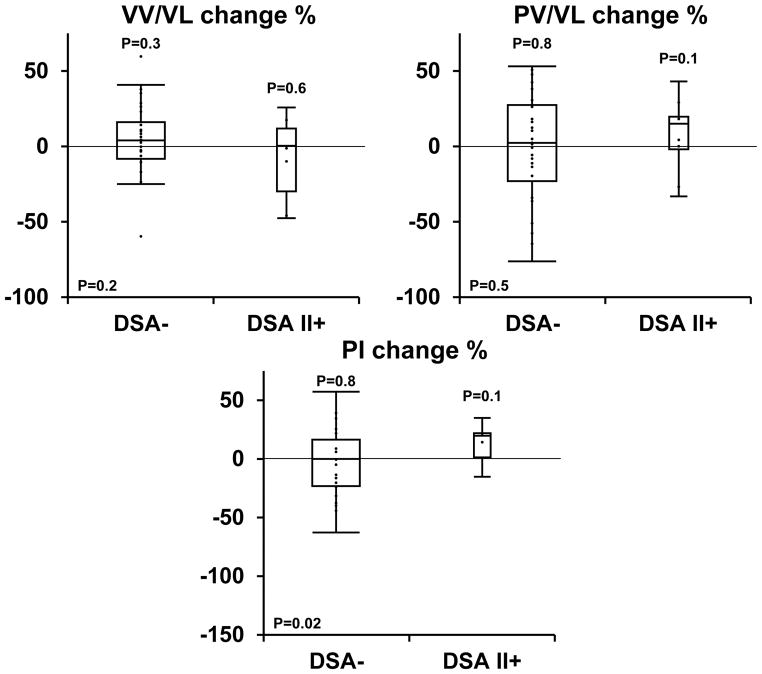
Table 2 outlines the 3-D IVUS findings in the DSA− and DSA II+ patients. There was significant progression of plaque index (PI) from baseline to the first follow up IVUS examination in the DSA II+ group (+0.04±0.03 mm3; p=0.01) but there was no progression of PI observed in the DSA− group (−0.003±0.07 mm3; p=0.8). The progression in PI in the DSA II+ group likely occurred due to a combination of a trend for accelerated progression in plaque volume and vessel constriction, a change measured from baseline to the first follow up IVUS. The rate of progression in PI was thus significantly greater in the DSA II+ group when compared to the DSA− group (+13.8±12% vs. −7.9±37% respectively; p=0.02) (Figure 2) despite the baseline PI being higher in the DSA− group. The same trend for increased rate of PI progression was also observed in those patients (n=6) who had low DSA II value (peak MFI less than 2000) when compared to the DSA− patients (+13.4±19% vs. −7.9±37% respectively). Similarly PI progression appeared to occur to the same extent in those DSA II+ HTX recipients who had a FCXM that was negative (n=5) as compared to the 19 DSA− recipients in which FCXM was performed and negative (+12.5±16% vs. −15.2±43% respectively). There were no significant differences in the baseline volumetric data or the change in the different parameters by 3D IVUS between the DSA I+ and the DSA− group (P>0.2 for all parameters).
Table 2.
Volumetric Assessment of Vascular Geometry and Progression of Allograft Vasculopathy During Follow-Up in patients with DSA II+ vs. DSA−
| DSA II+ (n = 11) | DSA− (n=34) | P Value | |
|---|---|---|---|
| Vessel Volume/Segment Length | |||
| Baseline (mm3/mm) | 14.7±4 | 14.9±4 | 0.75# |
| 2ND IVUS (mm3/mm) | 14.2±5 | 15.7±4 | 0.48# |
| Absolute difference (mm3/mm) | −0.51±3 | +0.81±4 | 0.3* |
| Change in % | −9.6±25 | +1.3±3 | 0.2* |
| P-value | 0.6¶ | 0.3¶ | |
| Plaque Volume/Segment Length | |||
| Baseline (mm3/mm) | 3.6±1 | 4.8±2 | 0.2# |
| 2 ND IVUS (mm3/mm) | 4.0±2 | 4.9±2 | 0.25# |
| Absolute difference (mm3/mm) | +0.51±1 | +0.06±1 | 0.42* |
| Change in % | +6.2±22 | −5.4±24 | 0.5* |
| P-value | 0.1¶ | 0.8¶ | |
| Plaque index | |||
| Baseline (mm3) | 0.24±0.06 | 0.31±0.1 | 0.02# |
| 2 ND IVUS (mm3) | 0.27±0.07 | 0.31±0.11 | 0.40# |
| Absolute difference (mm3) | +0.04±0.03 | −0.003±0.07 | 0.05* |
| Change in % | +13.8±12 | −7.9±18 | 0.02* |
| P-value | 0.01¶ | 0.8¶ | |
SL: segment length; VV: vessel volume; LV: lumen volume; PV: plaque volume; PI: plaque index.
ANCOVA test; baseline value is a covariate.
Paired t test
t test
Figure 2. Relative changes in vessel volume, plaque volume and plaque index progression between the baseline and second IVUS examination in patients with and without DSA II+.
a) The graph presents relative changes (in percent) in vessel volume (a), plaque volume (b) and plaque index (c) (plaque volume/vessel volume) between the baseline IVUS examination performed during the first year after transplant and the second IVUS exam performed a year afterwards in patients with DSA II+ compared to patients with no DSA pre transplant. There is a trend for accelerated vessel constriction and plaque progression, and a significant acceleration in plaque index progression with pre transplant donor specific antibodies against HLA class II antigens
Results are represented by box plots (middle hash of the box indicates the median; 25th to 75th percentiles are represented by end caps of the box; whiskers extend to the last observed value still within 1.5 times the interquartile range [difference between the 25th and 75th percentiles] above or below the 25th and 75th percentiles). Results of the paired t test comparing baseline and the second IVUS exam performed a year afterwards are represented by P above each box plot. Results of the t test comparing between both groups are represented by p in the right lower corner.
The primary aim of our study was to evaluate the potential role of DSA on the progression of CAV. A cutoff value of anti-HLA antibodies of >300 MFI was used as this value has been validated in our laboratory to be above the “background noise” level, to define positivity for DSA. However, the significance of the various DSA MFI values is unknown. Many transplant programs use a cutoff of MFI>1000 for recipient and donor selection, we have therefore also performed a separate analysis using a cutoff of MFI>1000 and present it in Table 3.
Table 3.
Volumetric Assessment of Vascular Geometry and Progression of Allograft Vasculopathy During Follow-Up in patients with DSA II+ and MFI>1000 vs. DSA−
| DSA II+ (n = 6) | DSA− (n=34) | P Value | |
|---|---|---|---|
| Vessel Volume/Segment Length | |||
| Baseline (mm3/mm) | 12.9±5 | 14.9±4 | 0.4# |
| 2ND IVUS (mm3/mm) | 11.5±7 | 15.7±4 | 0.2# |
| Absolute difference (mm3/mm) | −1.4±3 | +0.81±4 | 0.2* |
| Change in % | −21.1±12 | +1.3±3 | 0.1* |
| P-value | 0.3¶ | 0.3¶ | |
| Plaque Volume/Segment Length | |||
| Baseline (mm3/mm) | 2.9±1 | 4.8±2 | 0.04# |
| 2ND IVUS (mm3/mm) | 2.8±1 | 4.9±2 | 0.03# |
| Absolute difference (mm3/mm) | −0.1±0.7 | +0.06±1 | 0.4* |
| Change in % | −5.5±11 | +5.4±24 | 0.8* |
| P-value | 0.7¶ | 0.8¶ | |
| Plaque index | |||
| Baseline (mm3) | 0.2±0.04 | 0.3±0.1 | 0.003# |
| 2ND IVUS (mm3) | 0.25±0.03 | 0.3±0.1 | 0.02# |
| Absolute difference (mm3) | +0.03±0.04 | −0.003±0.07 | 0.1* |
| Change in % | +12.3±7 | −7.9±18 | 0.05* |
| P-value | 0.08¶ | 0.82¶ | |
SL: segment length; VV: vessel volume; LV: lumen volume; PV: plaque volume; PI: plaque index.
ANCOVA test; baseline value is a covariate.
Paired t test
t test
Using the higher DSA values, the results were similar. The rate of progression in PI was significantly greater in the DSA II+ group when compared to the DSA− group (+12.3±7% vs. −7.9±18% respectively; p=0.05). There was a strong trend for progression of plaque index (PI) from baseline to the first follow up IVUS examination in the DSA II+ group (+0.03±0.04mm3; p=0.08) but there was no progression of PI observed in the DSA− group (−0.003±0.07mm3; p=0.8).
Since the majority of Class II DSA were against DQ, it was possible that the association was caused by DQ rather than Class II antibodies. We repeated the analysis with only patients with DQ antibodies (see SDC 1, Table 2, http://links). The results demonstrate a similar trend, but the difference in rate of progression in PI was not significant
DSA and angiographic CAV
There were 36 (71%) of 51 patients who had grade I CAV or greater during a median follow up period of 3.7 [2.2, 5.2] years. Kaplan-Meier curves show considerable difference in the presence of angiographic CAV significantly affecting the DSA II+ group as compared to DSA− patients at 1 year (42.5±18% vs. 19.6±7%), 2 years (57.2±18% vs. 36.2±9%) and 4 years (100.0±0 vs. 64.2±9%, p=0.05). There were 7 patients (14%) who had grade II or greater CAV during the median follow up period of 3.7 years. There was a clear trend for increased rate of developing grade II or greater CAV in DSA II+ patients as compared to DSA− patients at 1 year (16.6±15% vs. 3.3±3%), and 4 years (58.3±30% vs. 18.2±8%).
Discussion
This is the first, preliminary study that demonstrates a significant association of Class II DSA with progression of CAV as detected by 3-D IVUS and coronary angiography early after HTX. Therefore, preformed Class II DSA may be a risk factor for accelerated CAV after HTX. Since only a few patients had Class I DSA, the relationship between DSA I and CAV could not be investigated.
MHC class II antigens are constitutively expressed in human capillary and venous endothelium including that of coronary arteries and could be a target for allo-immune responses (9, 10). DSA may cause direct endothelial cell damage via complement activation or by targeting Natural Killer cells and macrophages. DSA have also been shown to activate endothelial exocytosis of granules that contain prothrombotic mediators, resulting in exaggerated mitosis and inflammation of the vessel (11). Whether such responses as a result of class II DSA play a role in a “smoldering” inflammatory response, contributing to accelerated plaque progression remains speculative and will have to be assessed in further studies.
This study could have important implications for clinical practice in HTX. First, we suggest that Class II DSA be considered as an important component of the overall assessment of immunologic risk in HTX recipients. Second, HTX in the presence of demonstrable DSA against Class II (even in lower MFIs) may necessitate close post-transplant monitoring for CAV. Third, the role of a virtual cross-match by excluding donors with unacceptable antigens to which recipients have DSA especially of Class II type in minimizing the development of CAV needs to be further explored. Newer interventions that may attenuate the formation or levels of DSA such as rituximab may also affect the development of subsequent CAV. (12)
Prior to the advent of sensitive techniques for detecting anti-HLA antibodies, risk factors of CAV were considered to be more traditional risk factors of atherosclerosis such as hyperlipidemia, diabetes, donor age, ischemic time, etc. (6, 13) The important role of immune factors in CAV emerges from more recent studies (13, 14) suggesting that both adaptive (cellular and humoral) and innate immune responses result in the progression of CAV.
The introduction of solid phase based techniques for HLA antibody analysis has enabled specific and sensitive determination of the presence of donor specific HLA class I and class II (IgG) antibodies in transplant recipients. There are an increasing number of transplant centers making determinations of transplant eligibility and donor suitability based on the results obtained by these solid phase arrays. However, the impact of DSA detected by these sensitive techniques on long-term post transplant outcomes such as graft vasculopathy is unclear. Most published studies in this field have focused on renal transplant recipients (1, 15, 16) and have shown that DSA against HLA class II antigens are associated with an increased risk of developing transplant glomerulopathy and AMR. There is paucity of data on the role of HLA class II antibodies on HTX outcomes (17, 18). Although previous animal and clinical trials have implicated the role of AMR on the progression of CAV (19–23) DSA may not necessarily be associated with AMR and this finding may have been related to the sensitivity of the tests used to detect DSA (24).
Flow cytometry crossmatch methods are more sensitive than the cytotoxic assays and a positive flow crossmatch has been shown to be associated with AMR and graft survival (18). However prospective flow crossmatches are not readily available to make decisions regarding HTX feasibility. In this study, no significant difference in mortality or in the incidence of AMR was observed between patients with or without DSA. The lack of difference may be due to the small number of patients assessed and the limited duration of follow up, emphasizing the need for larger observational studies. Furthermore, most of our patients had low DSA values and perhaps higher DSA values are required for the development of AMR.
The principal aim of the present study was to determine if the presence of DSA before HTX is associated with accelerated progression of CAV as detected by 3D IVUS. 3D IVUS is presently considered the gold standard for the evaluation of CAV (25–27) and quantifies both intimal thickening and changes in external elastic membrane area, a process of arterial remodeling. This is especially important considering that several studies have emphasized that lumen loss in CAV may be caused not only by intimal thickening and plaque progression but also by changes in external elastic membrane area (27–29). Our study showed a trend towards accelerated vessel volume constriction despite a trend towards higher plaque progression in patients with class II DSA in the first year post transplant culminating in significant plaque index progression in the DSA II+ group. This suggests that the mechanism of early lumen loss in these patients may be due to a combination of accelerated rate of intimal hyperplasia and adverse vessel remodeling. (28) The presence of CAV assessed by subsequent routine coronary angiography validates our IVUS findings. Interestingly CAV occurs irrespective whether patients had low or high Class II DSA values and thus may question the strategy being used in transplant centers of higher anti-HLA antibody values as a cut off to list unacceptable antigens for HTX recipients if the goal is to prevent CAV.
Patients were not typed for HLA-DPB1 although DP antibodies can be important, thus we may have underestimated the influence of Class II DSA.
Conclusion
In our preliminary study, HTX recipients with pre transplant Class II DSA compared to DSA− recipients have an accelerated rate of coronary plaque progression as assessed by 3-D IVUS and increased development of angiographic CAV. Larger, multi-center observational studies will be required to validate these observations.
Material and Methods
Study Design
The study was a nonrandomized, retrospective, single-center study approved by the Mayo Clinic institutional review board. From January 2006 to December 2009, 104 patients were transplanted in our institution from which we identified 40 HTX recipients in whom anti-HLA antibodies were determined before transplant by Single Antigen Bead assay (SAB) (One Lambda, Inc., Canoga Park, CA; Luminex platform) and had baseline and a one-year follow-up IVUS study as part of a standard surveillance protocol. An additional 11 randomly chosen patients, transplanted between January 2004 to January 2006, who had sera available for SAB assay and had baseline and one-year follow-up IVUS study performed, were included in the study. Primary immunosuppressive agents (calcineurin inhibitors [CNI] or sirolimus [SRL]), and secondary immunosuppressive agents mycophenolate mofetil (MMF) or azathioprine (AZA), and dose of prednisone was not modified based on the presence or absence of DSA. All HTX recipients received induction therapy with antithymocyte globulin (ATG), as part of a standard induction protocol. Immunosuppression was managed and dosed as previously described. (26) Three patients in the DSA II+ group, who had a positive flow cytometric crossmatch were treated with plasmapheresis immediately after transplant. Routine endomyocardial biopsies were performed weekly for 6 weeks after transplantation, beginning a week after completing induction therapy, every 2 weeks from 6 weeks to 3 months, monthly from 3 to 6 months, then at 3-month intervals until the end of the second year as well as 10 to 15 days after any biopsy specimens that showed a rejection of grade 2R or higher. Total rejection score (TRS) was calculated for each patient. Each biopsy result was graded and assigned based on ISHLT R grading as 0R = 0, 1R = 1, 2R = 2, and 3R = 3. Total rejection score (TRS) was calculated by dividing the cumulative scores for all the biopsies by the total number of biopsy specimens taken until the time of the last IVUS evaluation. Any rejection score (ARS) was calculated as 0R = 0, 1R = 1, 2R = 1, and 3R = 1, and represented the total number of rejections, regardless of severity, experienced by that patient during the same time period normalized for the total number of biopsy specimens.
Coronary angiography and IVUS Examination and Analysis
CAV was categorized using recent ISHLT guidelines (8).
IVUS was performed at baseline (0.30 [0.17, 1.15] years after transplant), and 0.93 [0.83, 1.05] years after the baseline examination.
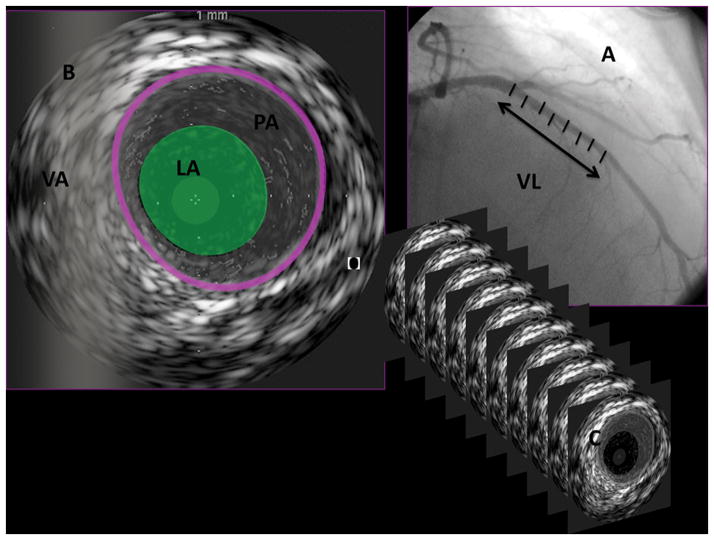
The method for performing IVUS has been described elsewhere.(26, 30)Briefly, IVUS was performed during routine coronary angiography after intracoronary administration of 100 to 200 μg nitroglycerin. Mechanical pullback (0.5 mm/s) was performed from the mid to distal left anterior descending coronary artery to the left main coronary artery with a 20-MHz, 2.9F, monorail, electronic Eagle Eye Gold IVUS imaging catheter (Volcano Therapeutics Inc, Rancho Cordova, Calif) and a dedicated IVUS scanner (Volcano Therapeutics). The IVUS images were stored on a CD-ROM for later offline 3D volumetric IVUS analysis. Offline volumetric analysis of IVUS data was performed (echo Plaque 2, version 2.5, INDECSystems Inc, Santa Clara, Calif) by operators who were unaware of group assignment. The Simpson rule for volumetric measurement was used. Proximal and mid left anterior descending coronary artery regions were defined for the interrogated artery. Starting with the first complete vascular ring distal to the bifurcation with the left circumflex artery lumen, plaque and vessel volume were analyzed. Each measured volume was normalized to the examined segment length (mm3/mm) to compensate for differences in examined vessel segment length. A plaque index (PI) was calculated as follows: (PV/vessel volume), where PV is plaque volume. Changes in PV, lumen volume, and vessel volume or PI were defined as last follow-up minus baseline volume measures value and as percent change. The semi automated contour detection of both the lumen and the media-adventitia interface was performed at intervals of either 16 or 32 frames, depending on the heterogeneity of the image. All other measurements were carried out automatically. Border detection was corrected manually in all frames after automatic border detection. An example of an IVUS study defining each of the measurements is shown in Figure 1.
Figure 1. The methods for conducting 3-dimensional intravascular ultrasound (IVUS) examinations and definition of IVUS measurements.
IVUS was performed during routine coronary angiography with mechanical pullback (0.5 mm/s) from the mid to distal left anterior descending coronary artery to the left main coronary artery. The vessel length (VL), which was interrogated for each examination, was measured (A). The semiautomated contour software detected both the blood-media interface defined as lumen area (LA) and the media-adventitia interface defined as vessel area (VA). Plaque area (PA) was defined as the difference between VA and LA for each 2-dimensional image (B). Border detection was corrected manually in all frames after automatic border detection. Two-dimensional interrogations were performed at intervals of either 16 or 32 frames, depending on the heterogeneity of the image (C). Next, the vessel volume (VV), lumen volume (LV), and plaque volume (PV; mm3) were calculated with the Simpson rule for volumetric measurement and corrected for the segment length (mm3/mm). Plaque index (PI) was calculated as follows: PI=(PV/VV)×100%.
Donor HLA Class I and Class II typing
Low resolution Class I HLA-A, B, Cw and Class II HLA-DRB1, DQB1 was performed using the LABType® SSO (One Lambda) which uses sequence-specific oligonucleotide probes (SSO) bound to fluorescently coded microspheres using the LABScan™ 100 flow analyzer (Luminex). The assignment of the HLA typing is based on the reaction pattern compared to patterns associated with published HLA gene sequences using the HLA-Visual software (One Lambda).
Single-antigen flow-bead analysis of alloantibody (SAB)
Pre-transplant sera were screened for HLA-Abs using a panel of up to 100 different color-coded beads each coated with purified single HLA Class I and Class II antigens (LABScreen™ Single Antigen Beads, One Lambda) using Luminex based technology (Luminex Corp., Austin TX). Donor typing performed was at low-to-medium resolution and serologic equivalents were reported. In cases with more than one allele, if only one bead was positive, while the other was negative, based on the low to medium resolution typing and considering the common well defined allele, the bead that would correspond to the donor type was considered as DSA. For DQ, as the beads have different DQA1 and DQB1 combinations, in cases where DQA1 donor typing was available, bead representing the donor DQA1-DQB1 combination were considered to define the donor specific antibody. In cases where DQA1 typing was not available, the common DRB1-DQA1-DQB1 haplotypes were considered to define a positive DSA. For example if the donor typed as DR17-DQ2, DQA1*05-DQB1*02:01 bead was considered as the DSA. However, in this study we did not find any DSA with only DQA1 specificity. One to eight dilutions were performed on highly sensitized individuals to rule out interfering substances. In addition commercial products were utilized when the negative control bead had a very high MFI value. The results were expressed as mean fluorescence intensity (MFI) value for each HLA-Abs, which corresponds with the strength of the antibody: the higher the MFI, the stronger the reaction. DSA were defined as HLA-Abs to the HLA antigens shared by the donor and were defined at serological equivalent levels. MFI>300 was defined as a positive result for the presence of DSA. The highest level of MFI for HLA class I and II DSA was then used to classify patients by SAB DSA level.
Flow cytometric cross-match
Three-color FCXM was performed for 60% (31/51) of patients before transplant (FACSCalibur, BD Biosciences, San Jose, CA, USA). Alloantibody binding was assessed using flourescein isothiocyanante (FITC) conjugated F(ab)′2 goat anti-human IgG serum by way of indirect immunofluoresence and three fluorescence parameters (CD3,CD19) were used to identify T and B cells. The interpretation of the FCXM was compared by directly comparing the fluorescence intensity of the donor T and B lymphocytes after treatment with patient serum to the intensity of donor cells after treatment with a negative control serum using a 1024 scale. A median channel fluorescence shift greater than 52 was interpreted as positive for T cells and greater than 106 for B cells
Statistical Analysis
Continuous parameters were presented as means ± SD and compared using the Student’s t-test. Ordinal data were presented by median, 1st and 3rd quartiles and compared using the exact nonparametric Wilcoxon rank sum test. Differences from baseline to the follow-up IVUS exams were compared by use of a paired t test. IVUS values at end of follow up between groups were compared by ANCOVA, with the baseline value of the term included in the analysis as a covariate. Categorical data were compared between groups using the χ2 or the Fisher’s exact test whenever the expected value in one of the cells was less than five. The time to onset of CAV distributions were calculated from the time of transplant according to the Kaplan–Meier method and compared by means of the log-rank test. Time of onset of CAV was defined as the time of diagnosis of ISHLT CAV grade 1 (Mild) categorized using the recent ISHLT guidelines. All P values were two-sided, and values of less than 0.05 were considered to indicate statistical significance. All data were analyzed with the JMP System software version 8.0 (SAS Institute, Inc, Cary, NC).
Supplementary Material
Acknowledgments
Source of funding:
Supported in part by HL 84904 (Heart Failure Clinical Research Network) (NLP), KL2RR024151 (NLP), UL1RR24150 (NLP)
Footnotes
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
Disclosures
The authors of this manuscript have no conflicts of interest to disclose.
Authorship Contributions:
Yan Topilsky, MD (Y.T): 1,2,3,5
Manish J. Gandhi, MD (M.J.G): 1,2,3,4
Eugenia Raichlin, MD (E.R): 1,2
Barry A. Boilson, MD (B.A.B): 1,2
John A. Schirger, MD (J.A.S): 1,2
Brooks S. Edwards, MD (B.S.E): 1,2
Alfredo L. Clavell, MD (A.L.C): 1,2
Richard J. Rodeheffer, MD (R.J.R): 1,2
Robert P. Frantz, MD (R.P.F): 1,2
Participated in research design
Participated in the writing of the paper
Participated in the performance of the research
Contributed new reagents or analytic tools
Participated in data analysis
References
- 1.Gebel HM, Bray RA, Nickerson P. Pre-transplant assessment of donor-reactive, HLA-specific antibodies in renal transplantation: contraindication vs. risk. Am J Transplant. 2003 Dec;3(12):1488–500. doi: 10.1046/j.1600-6135.2003.00273.x. [DOI] [PubMed] [Google Scholar]
- 2.Tambur AR, Bray RA, Takemoto SK, Mancini M, Costanzo MR, Kobashigawa JA, et al. Flow cytometric detection of HLA-specific antibodies as a predictor of heart allograft rejection. Transplantation. 2000 Oct 15;70(7):1055–9. doi: 10.1097/00007890-200010150-00011. [DOI] [PubMed] [Google Scholar]
- 3.Przybylowski P, Balogna M, Radovancevic B, Frazier OH, Susskind B, Van Buren C, et al. The role of flow cytometry-detected IgG and IgM anti-donor antibodies in cardiac allograft recipients. Transplantation. 1999 Jan 27;67(2):258–62. doi: 10.1097/00007890-199901270-00012. [DOI] [PubMed] [Google Scholar]
- 4.Smith JD, Hamour IM, Banner NR, Rose ML. C4d fixing, luminex binding antibodies - a new tool for prediction of graft failure after heart transplantation. Am J Transplant. 2007 Dec;7(12):2809–15. doi: 10.1111/j.1600-6143.2007.01991.x. [DOI] [PubMed] [Google Scholar]
- 5.Costanzo MR, Naftel DC, Pritzker MR, Heilman JK, 3rd, Boehmer JP, Brozena SC, et al. Heart transplant coronary artery disease detected by coronary angiography: a multiinstitutional study of preoperative donor and recipient risk factors. Cardiac Transplant Research Database. J Heart Lung Transplant. 1998 Aug;17(8):744–53. [PubMed] [Google Scholar]
- 6.Taylor DO, Edwards LB, Boucek MM, Trulock EP, Aurora P, Christie J, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult heart transplant report--2007. J Heart Lung Transplant. 2007 Aug;26(8):769–81. doi: 10.1016/j.healun.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Miller LW, Schlant RC, Kobashigawa J, Kubo S, Renlund DG. 24th Bethesda conference: Cardiac transplantation. Task Force 5: Complications. J Am Coll Cardiol. 1993 Jul;22(1):41–54. doi: 10.1016/0735-1097(93)90814-h. [DOI] [PubMed] [Google Scholar]
- 8.Mehra MR, Crespo-Leiro MG, Dipchand A, Ensminger SM, Hiemann NE, Kobashigawa JA, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant. Jul;29(7):717–27. doi: 10.1016/j.healun.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Taflin C, Charron D, Glotz D, Mooney N. Immunological function of the endothelial cell within the setting of organ transplantation. Immunol Lett. May 27; doi: 10.1016/j.imlet.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Al-Lamki RS, Bradley JR, Pober JS. Endothelial cells in allograft rejection. Transplantation. 2008 Nov 27;86(10):1340–8. doi: 10.1097/TP.0b013e3181891d8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamakuchi M, Kirkiles-Smith NC, Ferlito M, Cameron SJ, Bao C, Fox-Talbot K, et al. Antibody to human leukocyte antigen triggers endothelial exocytosis. Proc Natl Acad Sci U S A. 2007 Jan 23;104(4):1301–6. doi: 10.1073/pnas.0602035104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelishadi SS, Azimzadeh AM, Zhang T, Stoddard T, Welty E, Avon C, et al. Preemptive CD20+ B cell depletion attenuates cardiac allograft vasculopathy in cyclosporine-treated monkeys. J Clin Invest. Apr 1;120(4):1275–84. doi: 10.1172/JCI41861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmauss D, Weis M. Cardiac allograft vasculopathy: recent developments. Circulation. 2008 Apr 22;117(16):2131–41. doi: 10.1161/CIRCULATIONAHA.107.711911. [DOI] [PubMed] [Google Scholar]
- 14.Weis M, von Scheidt W. Coronary artery disease in the transplanted heart. Annu Rev Med. 2000;51:81–100. doi: 10.1146/annurev.med.51.1.81. [DOI] [PubMed] [Google Scholar]
- 15.Issa N, Cosio FG, Gloor JM, Sethi S, Dean PG, Moore SB, et al. Transplant glomerulopathy: risk and prognosis related to anti-human leukocyte antigen class II antibody levels. Transplantation. 2008 Sep 15;86(5):681–5. doi: 10.1097/TP.0b013e3181837626. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 16.Pollinger HS, Stegall MD, Gloor JM, Moore SB, Degoey SR, Ploeger NA, et al. Kidney transplantation in patients with antibodies against donor HLA class II. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007 Apr;7(4):857–63. doi: 10.1111/j.1600-6143.2006.01699.x. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 17.McCarthy JF, Cook DJ, Smedira NG, O’Malley KJ, Massad MG, Sano Y, et al. Vascular rejection in cardiac transplantation. Transplant Proc. 1999 Feb-Mar;31(1–2):160. doi: 10.1016/s0041-1345(98)02106-x. [DOI] [PubMed] [Google Scholar]
- 18.McCarthy JF, Cook DJ, Massad MG, Sano Y, O’Malley KJ, Ratliff NR, et al. Vascular rejection post heart transplantation is associated with positive flow cytometric cross-matching. Eur J Cardiothorac Surg. 1998 Aug;14(2):197–200. doi: 10.1016/s1010-7940(98)00159-6. [DOI] [PubMed] [Google Scholar]
- 19.Russell PS, Chase CM, Colvin RB. Alloantibody- and T cell-mediated immunity in the pathogenesis of transplant arteriosclerosis: lack of progression to sclerotic lesions in B cell-deficient mice. Transplantation. 1997 Dec 15;64(11):1531–6. doi: 10.1097/00007890-199712150-00005. [DOI] [PubMed] [Google Scholar]
- 20.Russell PS, Chase CM, Winn HJ, Colvin RB. Coronary atherosclerosis in transplanted mouse hearts. II. Importance of humoral immunity. J Immunol. 1994 May 15;152(10):5135–41. [PubMed] [Google Scholar]
- 21.Wehner J, Morrell CN, Reynolds T, Rodriguez ER, Baldwin WM., 3rd Antibody and complement in transplant vasculopathy. Circ Res. 2007 Feb 2;100(2):191–203. doi: 10.1161/01.RES.0000255032.33661.88. [DOI] [PubMed] [Google Scholar]
- 22.Kaczmarek I, Deutsch MA, Kauke T, Beiras-Fernandez A, Schmoeckel M, Vicol C, et al. Donor-specific HLA alloantibodies: long-term impact on cardiac allograft vasculopathy and mortality after heart transplant. Exp Clin Transplant. 2008 Sep;6(3):229–35. [PubMed] [Google Scholar]
- 23.Michaels PJ, Espejo ML, Kobashigawa J, Alejos JC, Burch C, Takemoto S, et al. Humoral rejection in cardiac transplantation: risk factors, hemodynamic consequences and relationship to transplant coronary artery disease. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2003 Jan;22(1):58–69. doi: 10.1016/s1053-2498(02)00472-2. [DOI] [PubMed] [Google Scholar]
- 24.Uber WE, Self SE, Van Bakel AB, Pereira NL. Acute antibody-mediated rejection following heart transplantation. Am J Transplant. 2007 Sep;7(9):2064–74. doi: 10.1111/j.1600-6143.2007.01900.x. [DOI] [PubMed] [Google Scholar]
- 25.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987 May 28;316(22):1371–5. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 26.Raichlin E, Bae JH, Khalpey Z, Edwards BS, Kremers WK, Clavell AL, et al. Conversion to sirolimus as primary immunosuppression attenuates the progression of allograft vasculopathy after cardiac transplantation. Circulation. 2007 Dec 4;116(23):2726–33. doi: 10.1161/CIRCULATIONAHA.107.692996. [DOI] [PubMed] [Google Scholar]
- 27.Tsutsui H, Ziada KM, Schoenhagen P, Iyisoy A, Magyar WA, Crowe TD, et al. Lumen loss in transplant coronary artery disease is a biphasic process involving early intimal thickening and late constrictive remodeling: results from a 5-year serial intravascular ultrasound study. Circulation. 2001 Aug 7;104(6):653–7. doi: 10.1161/hc3101.093867. [DOI] [PubMed] [Google Scholar]
- 28.Lim TT, Liang DH, Botas J, Schroeder JS, Oesterle SN, Yeung AC. Role of compensatory enlargement and shrinkage in transplant coronary artery disease. Serial intravascular ultrasound study. Circulation. 1997 Feb 18;95(4):855–9. doi: 10.1161/01.cir.95.4.855. [DOI] [PubMed] [Google Scholar]
- 29.Pethig K, Heublein B, Meliss RR, Haverich A. Volumetric remodeling of the proximal left coronary artery: early versus late after heart transplantation. J Am Coll Cardiol. 1999 Jul;34(1):197–203. doi: 10.1016/s0735-1097(99)00159-x. [DOI] [PubMed] [Google Scholar]
- 30.Bae JH, Rihal CS, Edwards BS, Kushwaha SS, Mathew V, Prasad A, et al. Association of angiotensin-converting enzyme inhibitors and serum lipids with plaque regression in cardiac allograft vasculopathy. Transplantation. 2006 Oct 27;82(8):1108–11. doi: 10.1097/01.tp.0000230378.61437.a5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.