Abstract
Objective
Premorbid cognitive deficits in schizophrenia are well documented and have been interpreted as supporting a neurodevelopmental etiological model. The authors investigated the following three unresolved questions about premorbid cognitive deficits: What is their developmental course? Do all premorbid cognitive deficits follow the same course? Are premorbid cognitive deficits specific to schizophrenia or shared by other psychiatric disorders?
Methods
Participants were members of a representative cohort of 1,037 males and females born between 1972 and 1973 in Dunedin, New Zealand. Cohort members underwent follow-up evaluations at specific intervals from age 3 to 32 years, with a 96% retention rate. Cognitive development was analyzed and compared in children who later developed schizophrenia or recurrent depression as well as in healthy comparison subjects.
Results
Children who developed adult schizophrenia exhibited developmental deficits (i.e., static cognitive impairments that emerge early and remain stable) on tests indexing verbal and visual knowledge acquisition, reasoning, and conceptualization. In addition, these children exhibited developmental lags (i.e., growth that is slower relative to healthy comparison subjects) on tests indexing processing speed, attention, visual-spatial problem solving ability, and working memory. These two premorbid cognitive patterns were not observed in children who later developed recurrent depression.
Conclusions
These findings suggest that the origins of schizophrenia include two interrelated developmental processes evident from childhood to early adolescence (ages 7–13 years). Children who will grow up to develop adult schizophrenia enter primary school struggling with verbal reasoning and lag further behind their peers in working memory, attention, and processing speed as they get older.
Cognitive impairment is ubiquitous in patients with schizophrenia (1) and is considered core to the pathophysiology of the illness (2). According to the neurodevelopmental model of schizophrenia, subtle behavioral, motor, and cognitive deviations are already apparent in childhood, years before overt clinical symptoms of adult schizophrenia manifest (3). Childhood cognitive deficits precede the appearance of adult schizophrenia (4–7). In a recent meta-analysis (8), it was estimated that on average, individuals who develop adult schizophrenia exhibit an 8-point deficit (0.5 standard deviations) in their childhood IQ. Although the premorbid cognitive deficit in schizophrenia is now well documented, three fundamental questions pertinent to the neurodevelopmental model remain unresolved.
The first question concerns the developmental course of cognitive functions prior to the onset of illness. Three hypotheses put forward to describe the developmental course of premorbid cognitive functioning in schizophrenia are illustrated in Figure 1. The developmental deterioration hypothesis (9–12) predicts premorbid decline in cognitive performance. The developmental deficit hypothesis (13) predicts static cognitive impairment, manifested as deficits that emerge early and remain stable. The developmental lag hypothesis (14–16) predicts growth in cognitive abilities, but growth that lags behind the more rapid growth in healthy individuals.
FIGURE 1. Schematic Representation of Three Hypotheses of the Developmental Course of Premorbid Cognitive Functioning in Schizophreniaa.
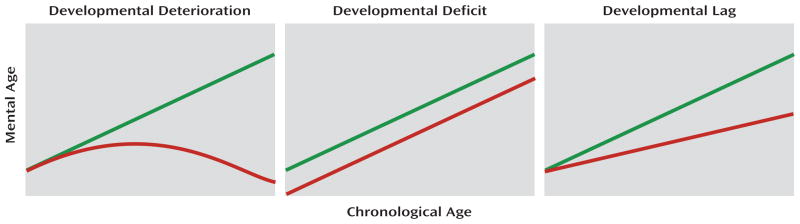
a The green line represents healthy comparison subjects and the red line represents children who grew up to meet diagnostic criteria for schizophrenia.
The second question is whether different cognitive functions follow similar or different developmental courses. Cross-sectional studies of premorbid cognitive functioning in schizophrenia conclude that most, if not all, cognitive functions are impaired. However, there is considerable variation in the magnitude of impairment across functions (4, 17–19). Some evidence suggests that the developmental course may differ for verbal versus nonverbal cognitive functions (4, 18, 19). This hypothesis should be tested for multiple specific cognitive abilities.
The third question is whether neurodevelopmental deficits are specific to schizophrenia or are common to other psychiatric disorders. Evidence from research in cognitive epidemiology (20) shows that low IQ elevates the risk of many psychiatric conditions, most notably depression (21–24). Such findings underscore the need to test the disorder specificity (versus disorder generality) of cognitive deficits.
The existing literature does not provide adequate answers to these three questions because of the following five reasons. First, many studies have used follow-back analyses of clinical samples and samples of convenience that may not represent the population of individuals with schizophrenia. Second, most previous studies have assumed, but not tested, the specificity of premorbid cognitive deficits in schizophrenia. Third, most epidemiological studies examine an omnibus index of cognitive functioning, namely IQ. Such indexes summarize the overall integrity of the brain, but they cannot be used to test hypotheses about specific cognitive functions. Fourth, many studies have used retrospective assessments that can only serve as a proxy for actual cognitive performance, have a narrow range of scores, usually index global cognitive functioning, and are not time specific. Fifth, most longitudinal studies have collected cognitive data at only one point in time. Studying developmental change requires measuring cognitive functioning on multiple occasions prior to illness. Some studies have measured cognitive functioning on multiple occasions but using different tests at each occasion, making it difficult to evaluate change in specific functions.
In the present study, we report data from a multidisciplinary health and development investigation that has followed more than 1,000 children in the general population from birth to age 32 years. We identified case subjects meeting diagnostic criteria for schizophrenia or depression through age 32 years, spanning the peak age period for these psychiatric disorders. Multiple cognitive functions were repeatedly assessed from childhood to early adolescence prior to the onset of schizophrenia or depression.
Method
Cohort
Participants were members of the Dunedin Multidisciplinary Health and Development Study, a longitudinal investigation of the health and behavior of a complete cohort of children born between 1972 and 1973 in Dunedin, New Zealand. A total of 1,037 children from the birth cohort (91% of eligible births; male subjects: 52%) participated in the follow-up assessment at 3 years of age, forming the base sample for the study. Cohort members represented the full range of socioeconomic status levels in the general population of New Zealand’s South Island and were primarily Caucasian. Follow-up evaluations were also conducted at ages 5, 7, 9, 11, 13, 15, 18, 21, 26, and, most recently, 32 years. At the 32-year-old follow-up evaluation, we assessed 972 (96%) of the 1,015 cohort members who were alive between 2004 and 2005. Participants attended our research unit for a full day of individual data collection.
Assessment of Cognitive Functions
The WISC–R (25) was administered to cohort members within 1 month of their birthdays at ages 7, 9, 11, and 13 years (26). Psychometrists were blind to the children’s performance on previous administrations of the WISC–R. The test was individually administered according to standard protocol. Participants were given eight subtests. Factor analytic studies have demonstrated that these subtests assess the following three major cognitive domains (27): verbal comprehension (information, vocabulary, and similarities), perceptual organization (block design, picture completion, and object assembly), and freedom from distractibility (arithmetic and digit symbol coding) (Table 1). Two subtests were omitted because they are supplementary and not part of the IQ (mazes, digit span), and two were omitted because of time constraints (comprehension, picture arrangement).
TABLE 1.
Characteristics of the Wechsler Intelligence Scale for Children Subtestsa
| Subtest | Description |
|---|---|
| Information | This is a test of general knowledge. It reflects the ability to acquire and store knowledge in long-term memory, to access it, and to express it verbally. Test items include questions about knowledge in history, geography, and art and are arranged in order of difficulty from the simplest to most difficult. |
| Similarities | This is a test of verbal concept formation and reasoning. It captures the ability to categorize and conceptualize information available in semantic memory (a form of long-term memory). The similarities subtest asks the child to explain what a pair of words has in common (e.g., that apples and oranges are both fruits), with word-pairs ranging in difficulty from concrete relations to abstract ones. |
| Vocabulary | This is a test of language skills and includes questions about the meaning of words (e.g., What does winter mean?). It captures language processes such as the ability to acquire word meaning, recall it, and effectively express it. |
| Block Design | This is a test of visual-spatial organization, executive planning, and problem solving skills. The child is required to put together two, four, or nine red and white blocks in a pattern according to specific designs being displayed. Test items are presented with increasing difficulty. Higher scores reflect both accuracy and speeded responses. |
| Object Assembly | This is a test of visual perception and construction ability. This test contains cut-up cardboard figures of familiar objects (puzzles), which are given in order of increasing difficulty. The child must analyze the object and construct the whole visual object from its parts within time constraints. Responses are scored for both accuracy and speed. |
| Picture Completion | This is a test of visual discrimination and reasoning. It requires knowledge of a variety of common objects and scenes. Children are shown incomplete pictures of human features, familiar objects, or scenes arranged in order of difficulty and are asked to identify the missing part by pointing to it and/or naming it. The child must look at the visual whole presented and analyze its parts to identify what is missing. |
| Arithmetic | This is a test that requires working memory processes to be applied to the manipulation of orally presented verbal information. It involves numerical knowledge, short-term memory, attention, and concentration. Children are presented with arithmetic problems in story format (e.g., Four men can finish a job in eight hours. How many men will be needed to finish it in one-half hour?). Performance requires holding information in short-term memory, accessing long-term memory to retrieve numerical rules of mathematical operation, and using the rules to manipulate the stored data. Items are arranged according to the level of difficulty and have time limits. |
| Digit Symbol | This is a test of psychomotor speed and coordination and attention/concentration. Better performance also depends on incidental learning. A key that pairs symbols and numbers is presented. Within a time constraint, the child is requested to fill in rows containing blank squares (each with a randomly assigned number above it) using the key. |
Data described previously (28).
Mental Age
A fundamental characteristic of cognitive abilities is their progressive development through childhood and adolescence. Thus, on each WISC–R subtest, a child is expected to complete more items and obtain better scores as he or she grows up. For each WISC subtest, raw subtest scores were transformed to mental age scores (using regression models derived from Table 21 in the WISC–R manual [25]). Mental age scores express the chronological age for which a given level of performance is normative and can be used to monitor each child’s intraindividual development over time. In contrast, the age-corrected subtest scaled score that is typically used in clinical practice is not appropriate for charting intraindividual developmental change. The IQ and subtest scaled scores were designed to make comparisons between a child and the population of children of the same chronological age: IQ=(mental age/chronological age)× 100. As such, the means are the same at every age and obscure growth of cognitive functions over time. To test hypotheses about the course of cognitive development, we focused on mental age scores (28).
Assessment of Schizophrenia Spectrum Disorder and Depression
Schizophrenia
In the Dunedin study, schizophrenia was assessed beginning at the 21-year-old follow-up evaluation. Previously, we referred to the diagnosed group as having “schizophreniform” or “schizophrenia syndrome” (29) because the young age of these individuals conferred some uncertainty about whether their symptoms constituted frank schizophrenia and because our analyses were based on research diagnosis and not clinical hospital discharge diagnosis. To enhance the validity of our research diagnosis, we have implemented special steps (29). First, we require the presence of hallucinations (which are not substance use-related) in addition to at least two other positive symptoms. This requirement is stricter than DSM-IV criteria (30), which do not require hallucinations. Second, because self-reports can be compromised by poor insight in schizophrenia, we require objective evidence that impairment is the result of psychosis, as reported by knowledgeable informants and as recorded in the Dunedin study’s life history calendars, which document continuous histories of life functioning in employment and relationships. Third, in our research, the Diagnostic Interview Schedule, Version IV (31), is administered by experienced mental health clinicians, not lay interviewers. These clinicians record detailed case notes in addition to interview responses. Our staff systematically rate observable symptoms manifested in grooming and speech during the full day participants spend at our research unit. Participants bring their medications, which are classified by a pharmacist. Informants report subjects’ positive and negative psychotic symptoms as well as their cognitive impairment via postal questionnaires. Finally, subjects’ parents were interviewed about their adult child’s psychotic symptoms and treatment as part of the Dunedin Family Health History Study, which was carried out from 2003 to 2005. These data were accumulated in the Dunedin study across repeated follow-up assessments at ages 21, 26, and 32 years and compiled into dossiers that were reviewed by four clinicians to achieve best-estimate diagnoses. By 32 years of age, 1% of the sample (N=11) met criteria for formal schizophrenia and had been hospitalized and prescribed antipsychotic medications. An additional 2.5% (N=24) met all criteria for schizophrenia, had hallucinations, and suffered significant life impairment but had not yet been registered with the New Zealand health service as schizophrenia patients.
The 3.5% prevalence rate in the Dunedin study cohort seems high at first. However, unlike prior epidemiological surveys, the study is able to count disordered individuals in the community who are overlooked in most studies. Perala et al. (32) showed empirically that undercounting occurs because individuals with psychotic disorders often refuse to participate in surveys and die prematurely, and surveys often exclude homeless or institutionalized individuals with psychosis. Our birth cohort, with a 96% participation rate, lets us count these psychotic individuals overlooked by prior surveys, some of whom are not yet registered patients because of their youth. Our prevalence rate matches that of a recent study using multisource methods to ascertain the prevalence of psychosis in the population, which was more than 3% (32, 33).
Recurrent depression
At ages 18, 21, 26, and 32 years, depression was assessed using the Diagnostic Interview Schedule (31). Criteria did not change from DSM-III-R (34) to DSM-IV (30) because we required impairment at all ages. Details about depression diagnoses are reported elsewhere (35). In the present study, we compared individuals with schizophrenia versus individuals with recurrent episodes of depression in an effort to make the comparison group more comparable regarding the chronic course and severity of illness. Recurrence was defined as meeting criteria for depression at two or more follow-up assessment periods (ages 18, 21, 26, and 32 years), resulting in 13.4% of cohort members demonstrating evidence for recurrent depression.
Statistical Analysis
Our analyses compared the following three mutually exclusive groups: cohort members diagnosed with schizophrenia (N=35) or recurrent depression (N=145) and healthy comparison subjects (N=556) (who had no other disorders such as substance abuse or anxiety disorders). For the purposes of the present analyses, subjects who were comorbid for schizophrenia and depression were assigned to the schizophrenia group.
Growth-curve models were fitted to examine developmental change in specific cognitive functions from age 7 to 13 years (36). The growth-curve models used represent the following three parameters of interest: intercept (representing the starting value of the growth curve), linear slope (representing the rate of change across time), and quadratic slope (representing curvature in the developmental pattern over time). Positive or negative slope values indicate the direction of developmental change. Growth curves were used to test predictions derived from the deterioration, deficit, and lag hypotheses.
The developmental deterioration hypothesis predicts premorbid decline in cognitive performance. In growth-curve models, this decline is characterized by negative quadratic slopes, indicating an inverted U shape in development (Figure 1). To test this hypothesis, we examined the statistical significance and direction of the quadratic slopes for each cognitive function in the two diagnostic groups.
The developmental deficit hypothesis predicts static cognitive impairment, which is manifested in early emerging cognitive deficits that remain stable. In growth-curve models, this course is characterized by a significantly lower value of the intercept in future case subjects relative to comparison subjects but no significant between-group differences in linear slope. To test this hypothesis, we examined contrasts between both diagnostic groups and healthy comparison subjects in the intercepts and linear slopes for each cognitive function.
The developmental lag hypothesis predicts slower premorbid cognitive development relative to more rapid growth in healthy individuals. In growth-curve models, this course is characterized by a lower value of the slope in future case subjects relative to comparison subjects. To test this hypothesis, we examined contrasts between both diagnostic groups and healthy comparison subjects in the linear slopes for each cognitive function.
All models were fit with the SAS PROC MIXED procedure, version 9.1 (SAS Institute, Cary, N.C.) using restricted maximum-likelihood estimation. For all outcomes, intercepts and linear slopes were treated as random effects (i.e., individual-level variation was allowed), while quadratic effects were fixed within group (e.g., subjects with schizophrenia had the same estimated curvature).
Results
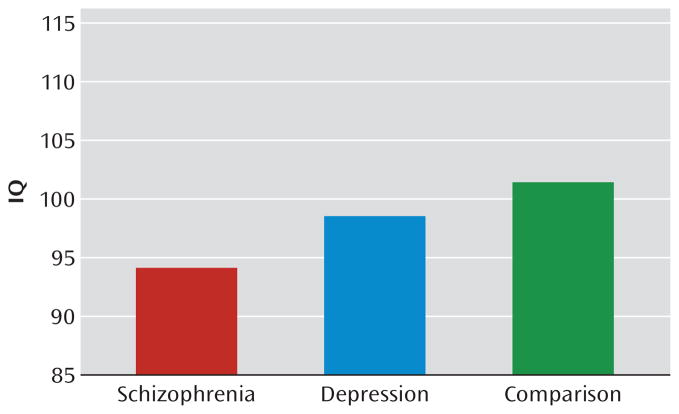
As we have previously shown (23), in the Dunedin study cohort, lower childhood IQ predicted significantly increased risk of being diagnosed with schizophrenia or depression as an adult, even after controls for social class. The average childhood IQ was 94 for cohort members who met diagnostic criteria for schizophrenia as an adult, 98 for those who met criteria for recurrent depression as an adult, and 101 for healthy comparison subjects (Figure 2). In the present analyses, we disaggregated IQ into specific cognitive functions and tested the decline, deficit, and lag hypotheses from age 7 to 13 years.
FIGURE 2.
Childhood IQ Scores for Subjects Who Grew Up to Meet Diagnostic Criteria for Schizophrenia or Recurrent Depression and for Healthy Comparison Subjects
The following figures present the premorbid cognitive test scores, between ages 7 to 13 years, for children who grew up to meet diagnostic criteria for schizophrenia or recurrent depression and healthy comparison subjects. The cognitive tests are organized according to the cognitive ability areas they assess: verbal comprehension (Figure 3), perceptual organization (Figure 4), and freedom from distractibility (Figure 5) (also see the table in the data supplement accompanying the online version of this article.).
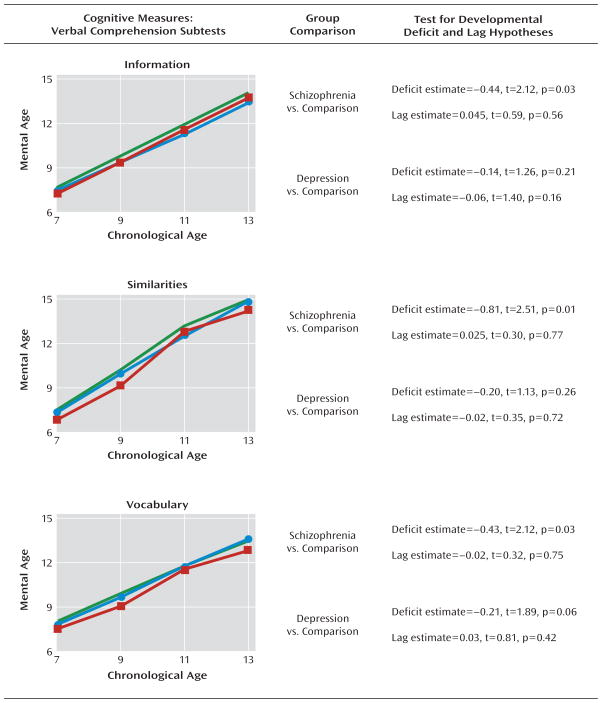
FIGURE 3. Mental Age Verbal Comprehension Scores by Assessment Age Among Children Who Later Developed Adult Schizophrenia or Recurrent Depression and Healthy Comparison Subjectsa.
a Data illustrate mean scores (green line represents healthy comparison subjects [N=556]; red line represents children who grew up to meet diagnostic criteria for schizophrenia [N=35]; blue line represents children who grew up to meet diagnostic criteria for recurrent depression [N=145]). The results show nonstandardized estimates of the differences between groups in the intercept and linear slope along with corresponding t tests and significance values from the growth-curve models.
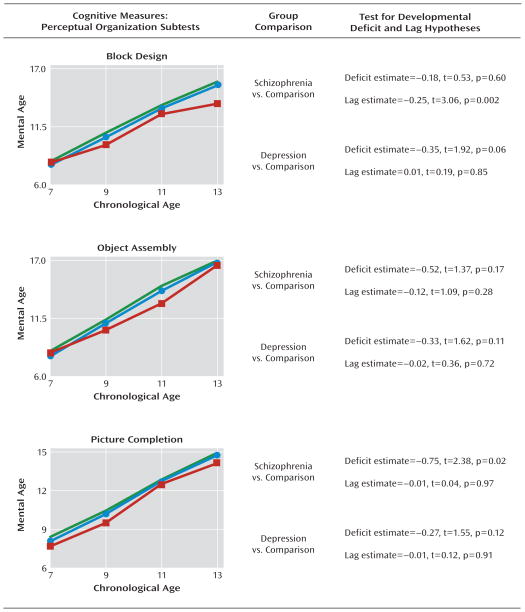
FIGURE 4. Mental Age Perceptual Organization Scores by Assessment Age Among Children Who Later Developed Adult Schizophrenia or Recurrent Depression and Healthy Comparison Subjectsa.
a Data illustrate mean scores (green line represents healthy comparison subjects [N=556]; red line represents children who grew up to meet diagnostic criteria for schizophrenia [N=35]; blue line represents children who grew up to meet diagnostic criteria for recurrent depression [N=145]). The results show nonstandardized estimates of the differences between groups in the intercept and linear slope along with corresponding t tests and significance values from the growth-curve models.
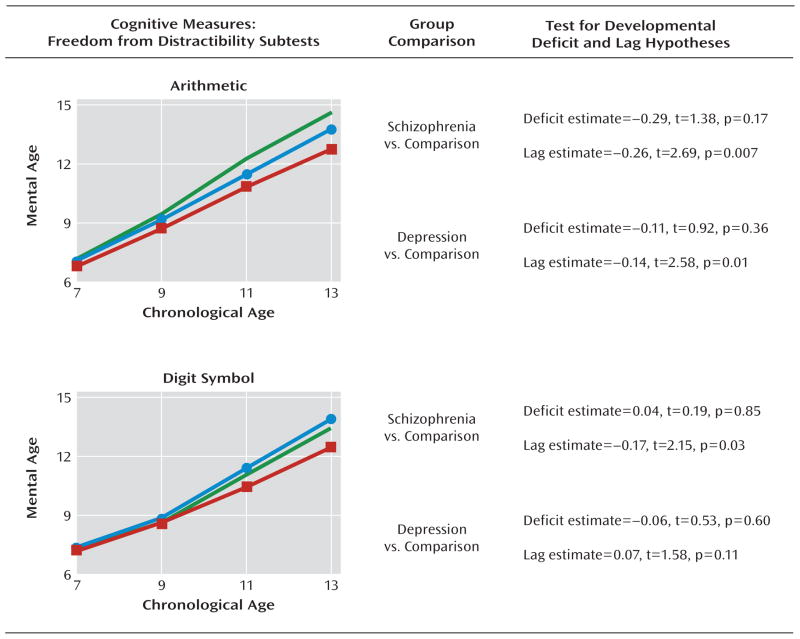
FIGURE 5. Mental Age Freedom From Distractibility Scores by Assessment Age Among Children Who Later Developed Adult Schizophrenia or Recurrent Depression and Healthy Comparison Subjectsa.
a Data illustrate mean scores (green line represents healthy comparison subjects [N=556]; red line represents children who grew up to meet diagnostic criteria for schizophrenia [N=35]; blue line represents children who grew up to meet diagnostic criteria for recurrent depression [N=145]). The results show nonstandardized estimates of the differences between groups in the intercept and linear slope along with corresponding t tests and significance values from the growth-curve models.
Developmental Deterioration Hypothesis
There was no evidence of cognitive deterioration among future schizophrenia case subjects. For all eight cognitive tests, the linear slopes of the growth curves were positive and significant (all p values <0.001), indicating that on average, future case subjects, similar to healthy comparison subjects, showed developmental increases in their cognitive functions between ages 7 and 13 years. None of the negative quadratic slopes in the schizophrenia group were significant (range of all p values: 0.15–0.94). Because the test of quadratic slope was potentially underpowered in our sample, we also examined the magnitude of the effect sizes (r values) for the negative quadratic effects, where r=0.10 represents a small effect. All effect sizes were uniformly smaller than small (range of all r values: 0.002–0.039). To complement the group-level analysis, we also examined individual growth curves in the schizophrenia group. No future schizophrenia case subject’s last score at age 13 was lower than his or her first score at age 7 on any of the cognitive functions measured, except for one child’s score on the block design subtest. No cognitive deterioration was evident in future recurrent depression case subjects.
Developmental Deficit Hypothesis
Future schizophrenia case subjects exhibited early and static cognitive deficits on the following four cognitive tests: information, similarities, vocabulary, and picture completion (Figure 3, Figure 4). On these tests, growth-curve analyses showed that future schizophrenia subjects had significantly lower intercept values than healthy comparison subjects but did not differ significantly in their slope values, indicating that their cognitive impairment on tests indexing verbal and visual knowledge acquisition, reasoning, and conceptualization had already emerged by age 7 and remained steady throughout puberty. Calculated in terms of mental age, the extent of impairment on these tests ranged from 0.4 to 0.8 years.
Developmental deficits were also apparent, but less striking, among case subjects who met criteria for recurrent depression as adults. These subjects had lower intercept values than healthy comparison subjects on all eight subtests, but these differences reached marginal significance on only two subtests: vocabulary (Figure 3) and block design (Figure 4).
Developmental Lag Hypothesis
On three cognitive tests (block design, arithmetic, and digit symbol [Figure 4, Figure 5]), growth-curve analyses showed that future schizophrenia case subjects had lower linear slope values than healthy comparison subjects, indicating that their growth on tests measuring freedom from distractibility and visual-spatial problem solving skills was developmentally slower. Examining the linear slope values for these three tests revealed that on average, for every year between the ages of 7 and 13, future schizophrenia case subjects fell behind healthy comparison subjects by an additional 0.17 to 0.26 mental age years. Developmental lags were less apparent among subjects who later developed recurrent depression. These subjects exhibited slower cognitive growth on only the arithmetic test (Figure 5).
An important question is whether the early deficits and the later developmental lags reflect two processes that occur in the same individuals. To address this, we tested whether lower intercept values (set at age 7 years) on the four “deficit” subtests predicted lower slope values on the three “developmental lag” subtests among the 35 future schizophrenia case subjects (Table 2). The correlation coefficients were strong and positive, suggesting that those children with early verbal deficits also exhibited developmental lags in processing speed and working memory. The correlations were marginally stronger among subjects who later met criteria for schizophrenia (average r=0.61) relative to healthy comparison subjects (average r=0.43), suggesting that this developmental process may be exaggerated in schizophrenia.
TABLE 2.
Correlations Between Intercept Values on the Four “Deficit” Subtests and Slope Values on the Three “Developmental Lag” Subtests of the Wechsler Intelligence Scale for Children–Revised Among Future Schizophrenia Case Subjects and Healthy Comparison Subjects
| Intercept on the Wechsler Intelligence Scale for Children–Revised | Slope on the Wechsler Intelligence Scale for Children–Revised | Correlation
|
Analysis
|
||
|---|---|---|---|---|---|
| Future Schizophrenia Case Subjects (N=35) | Healthy Comparison Subjects (N=556) | Difference Between Two Independent Correlations (z)a | p | ||
| Vocabulary | Block design | 0.78 | 0.42 | 3.33 | 0.00 |
| Arithmetic | 0.80 | 0.59 | 2.28 | 0.01 | |
| Digit symbol | 0.55 | 0.27 | 1.83 | 0.03 | |
| Picture completion | Block design | 0.80 | 0.58 | 2.44 | 0.01 |
| Arithmetic | 0.57 | 0.41 | 1.17 | 0.12 | |
| Digit symbol | 0.52 | 0.21 | 2.03 | 0.02 | |
| Similarities | Block design | 0.57 | 0.43 | 0.97 | 0.17 |
| Arithmetic | 0.72 | 0.52 | 1.80 | 0.04 | |
| Digit symbol | 0.57 | 0.26 | 2.09 | 0.02 | |
| Information | Block design | 0.53 | 0.51 | 0.12 | 0.45 |
| Arithmetic | 0.67 | 0.65 | 0.27 | 0.39 | |
| Digit symbol | 0.28 | 0.28 | −0.01 | 0.50 | |
| Average correlation | 0.61 | 0.43 | 1.53 | 0.06 | |
Analyses performed for differences between correlations after Fisher’s r-to-z transformation of correlations (one-tailed test).
Discussion
The neurodevelopmental model of schizophrenia posits the existence of deviations in cognitive development many years prior to the emergence of overt clinical symptoms of adult schizophrenia (3). Findings from this study add to what is known about the neurodevelopmental model in three ways. First, our findings point to both cognitive developmental deficits and cognitive developmental lags during childhood in individuals who will go on to develop schizophrenia as an adult. Second, different cognitive functions appear to follow different developmental courses from childhood to early adolescence. The developmental deficit model appears to apply to verbal and visual knowledge acquisition, reasoning, and conceptualization abilities. The developmental lag model appears to apply to freedom from distractibility and visual-spatial problem solving abilities. Third, these patterns of cognitive deviations from childhood to early adolescence in schizophrenia are not shared in recurrent depression. Research (24), including prior analyses of the present cohort (23), has identified lower IQ scores among subjects who were later diagnosed with recurrent depression relative to healthy comparison subjects. The results from the present study—which focused on multiple cognitive functions measured by 8 subtests of the WISC–R—suggest that the small premorbid IQ difference between future depressed case and comparison subjects most likely reflects the pooled effect of their slightly lower scores across multiple, diverse cognitive functions.
Our findings should also be interpreted in light of some limitations. First, premorbid cognitive assessments were conducted only through age 13 years, which constrains interpretation of our findings in two ways. 1) In regard to the testing of developmental deterioration, it is possible that cognitive deterioration may occur after puberty and closer to the onset of illness. For example, one study (11) reported decreases in scholastic achievement between ages 13 and 16 years in subjects who later developed schizophrenia. Our data only demonstrate that premorbid cognitive deterioration does not occur up to puberty. 2) In regard to the testing of the developmental lag hypothesis, our data can only identify lags during the period from childhood to early adolescence. Nevertheless, the period from age 7 to 13 years parallels the early school years, a period characterized by extensive knowledge acquisition and rapid cognitive development, making it an informative time for detecting abnormal development. Second, the number of schizophrenia case subjects was small, which had the practical effect of reducing the power to detect potentially meaningful group differences. Moreover, we reported findings for schizophrenia subjects who had been identified and received treatment as well as for those who had not. The premorbid functioning of the 11 case subjects who had been treated for schizophrenia was indistinguishable from that of the 24 subjects who had not received treatment. Thus, our findings represent the population of affected individuals. Third, the results of this study are limited to one birth cohort in one part of the world. Future research will need to establish whether these findings generalize to other cultures. In this regard, it is pertinent to note that the 8-point premorbid IQ deficit (0.5 standard deviations) observed in this cohort matches the deficits observed in the cohorts of other studies conducted around the world (8).
What Do These Findings Suggest Regarding the Etiology of Schizophrenia?
The developmental deficit model for the etiology of schizophrenia is supported by our data. This model posits that there is insult to the brain acquired or inherited in early development. The resulting developmental deficits are hypothesized to be manifested as early emerging cognitive impairments that remain stable up until the onset of illness. The present longitudinal findings point to early emerging, static developmental deficits in knowledge acquisition, concept formation, and verbal reasoning processes. These deficits are apparent by the time children begin primary school. However, our findings suggest that the developmental deficit model alone is insufficient to describe the course of cognitive impairments in schizophrenia.
Our findings contribute new evidence consistent with the developmental lag hypothesis, which posits that there is slower premorbid cognitive development among individuals who will meet criteria for a diagnosis of schizophrenia as adults. The findings point to developmental lag from childhood to early adolescence in cognitive functions that involve the ability to focus attention, process information quickly, and maintain and manipulate this information via “online” storage. The latter has been suggested as the core function of working memory (37). Taken together, these findings suggest that schizophrenia has origins in two interrelated developmental processes: an early static neuropathology (13) and a later developmental lag. In practical terms, children who will grow up to develop schizophrenia enter primary school struggling with verbal reasoning and fall further behind their peers in attention and working memory as they get older.
What Do These Findings Suggest Regarding Early Detection and Intervention in Schizophrenia?
Whereas the majority of prodromal research focuses on postpubertal functioning, the present study adds to a growing body of research suggesting that cognitive features of the so-called “prodrome” may start before puberty and continue progressively (38, 39). However, our findings do not recommend IQ testing for early identification. There is no single cognitive function that can be assessed at a single point in time prior to puberty to identify individuals who will develop schizophrenia later. Nevertheless, to inform etiological theories, multiple mental functions need to be assessed at multiple time points to detect the unique and subtle premorbid cognitive patterns that characterize individuals with schizophrenia at the group level.
Our findings provide support to etiologic (40, 41), endophenotypic (42–44), and intervention (45–47) research that focuses on processing speed and online storage as well as manipulation of information in schizophrenia. Recent research has demonstrated that processing speed, as measured by the digit symbol subtest, is the most severely impaired function in chronic schizophrenia patients (48). Our developmental data from the digit symbol test suggest that children who will develop adult schizophrenia already begin to lag behind normally developing children in the primary school years. If we extrapolate the average annual delay observed in our data (from age 7 to 13 years [Figure 5]) forward over a 10-year period to age 23 years—the peak age of onset in schizophrenia—our results would then predict a standard deviation unit difference ≥1 between future schizophrenia case subjects and healthy comparison subjects on the digit symbol test, which is indeed comparable to the difference observed in studies of adult schizophrenia patients (1).
Conclusions
Developmental studies in psychiatry are shifting from examining risk factors (e.g., from asking whether low IQ predicts later illness) to testing models that can document age-related changes in brain function. Although individuals with schizophrenia and individuals with depression share premorbid low IQ, our results show a clear distinction between schizophrenia and recurrent depression in the developmental processes that lead to low IQ. Future research should explore the pathogenic mechanisms underlying the different developmental processes.
Supplementary Material
Acknowledgments
Supported by the United Kingdom Medical Research Council grant G0100527; by NIMH grants MH-45070, MH-49414, and MH-077874; by the National Institute on Aging grant AG032282; by funding for the Dunedin Multidisciplinary Health and Development Research Unit from the New Zealand Health Research Council; by partial support of Dr. Reichenberg by NIMH grant MH-066105; and by the Royal Society-Wolfson Merit Award to Dr. Caspi.
The Otago Ethics Committee approved each phase of the study.
The authors thank the cohort members of the Dunedin study as well as their families and friends and the study’s founder Dr. Phil Silva.
Footnotes
Dr. Reichenberg has received speaker’s honoraria from AstraZeneca. Dr. Keefe has served as a consultant to Cortex, Shering-Plough Abbott, Acadia, AstraZeneca, BiolineRx, Bristol-Myers Squibb, Cephalon, Dainippon Sumitomo Pharma, Eli Lilly, Johnson and Johnson, Lundbeck, Memory Pharmaceuticls, Merck, NeuroSearch, Orexigen, Orion, Otsuka, Pfizer, Roche, Sanofi/Avenits, Targacept, Wyeth, and Xenoport; he has received royalties from the Brief Assessment of Cognition (BACS) and MATRICS Battery (BACS Symbol Coding); he has also received funding from Pfizer and Organon Pharmaceuticals. Dr. Murray has received speaker’s honoraria from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen, and Novartis. Drs. Caspi, Houts, Poulton, and Moffitt and Ms. Harrington report no financial relationships with commercial interests.
References
- 1.Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: integration of performance-based and brain imaging findings. Psychol Bull. 2007;133:833–858. doi: 10.1037/0033-2909.133.5.833. [DOI] [PubMed] [Google Scholar]
- 2.Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14:1–21. [PubMed] [Google Scholar]
- 3.Rapoport JL, Addington AM, Frangou S. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry. 2005;10:434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- 4.Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 Birth Cohort. Lancet. 1994;344:1398–1402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- 5.David AS, Malmberg A, Brandt L, Allebeck P, Lewis G. IQ and risk for schizophrenia: a population-based cohort study. Psychol Med. 1997;27:1311–1323. doi: 10.1017/s0033291797005680. [DOI] [PubMed] [Google Scholar]
- 6.Davidson M, Reichenberg A, Rabinowitz J, Weiser M, Kaplan Z, Mark M. Behavioral and intellectual markers for schizophrenia in apparently healthy male adolescents. Am J Psychiatry. 1999;156:1328–1335. doi: 10.1176/ajp.156.9.1328. [DOI] [PubMed] [Google Scholar]
- 7.Cannon TD, Bearden CE, Hollister JM, Rosso IM, Sanchez LE, Hadley T. Childhood cognitive functioning in schizophrenia patients and their unaffected siblings: a prospective cohort study. Schizophr Bull. 2000;26:379–393. doi: 10.1093/oxfordjournals.schbul.a033460. [DOI] [PubMed] [Google Scholar]
- 8.Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. Am J Psychiatry. 2008;165:579–587. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]
- 9.Jones PB, Done D. From birth to onset: a developmental perspective of schizophrenia in two national birth cohorts. In: Keshavan MS, Murray RM, editors. Neurodevelopment and Adult Psychopathology. Cambridge, United Kingdom: Cambridge University Press; 1997. pp. 119–136. [Google Scholar]
- 10.Reichenberg A, Weiser M, Rapp MA, Rabinowitz J, Caspi A, Schmeidler J, Knobler HY, Lubin G, Nahon D, Harvey PD, Davidson M. Elaboration on premorbid intellectual performance in schizophrenia: premorbid intellectual decline and risk for schizophrenia. Arch Gen Psychiatry. 2005;62:1297–1304. doi: 10.1001/archpsyc.62.12.1297. [DOI] [PubMed] [Google Scholar]
- 11.Fuller R, Nopoulos P, Arndt S, O’Leary D, Ho BC, Andreasen NC. Longitudinal assessment of premorbid cognitive functioning in patients with schizophrenia through examination of standardized scholastic test performance. Am J Psychiatry. 2002;159:1183–1189. doi: 10.1176/appi.ajp.159.7.1183. [DOI] [PubMed] [Google Scholar]
- 12.Bilder RM, Reiter G, Bates J, Lencz T, Szeszko P, Goldman RS, Robinson D, Lieberman JA, Kane JM. Cognitive development in schizophrenia: follow-back from the first episode. J Clin Exp Neuropsychol. 2006;28:270–282. doi: 10.1080/13803390500360554. [DOI] [PubMed] [Google Scholar]
- 13.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 14.Fish B, Marcus J, Hans SL, Auerbach JG, Perdue S. Infants at risk for schizophrenia: sequelae of a genetic neurointegrative defect: a review and replication analysis of pandysmaturation in the Jerusalem Infant Development Study. Arch Gen Psychiatry. 1992;49:221–235. doi: 10.1001/archpsyc.1992.01820030053007. [DOI] [PubMed] [Google Scholar]
- 15.Bedwell JS, Keller B, Smith AK, Hamburger S, Kumra S, Rapoport JL. Why does postpsychotic IQ decline in childhood-onset schizophrenia? Am J Psychiatry. 1999;156:1996–1997. doi: 10.1176/ajp.156.12.1996. [DOI] [PubMed] [Google Scholar]
- 16.Wood SJ, De Luca CR, Anderson V, Pantelis C. Cognitive development in adolescence: cerebral underpinnings, neural trajectories and the impact of aberrations. In: Keshavan M, Kennedy J, Murray RM, editors. Neurodevelopment and Schizophrenia. Cambridge, United Kingdom: Cambridge University Press; 2004. pp. 81–82. [Google Scholar]
- 17.Niendam TA, Bearden CE, Rosso IM, Sanchez LE, Hadley T, Nuechterlein KH, Cannon TD. A prospective study of childhood neurocognitive functioning in schizophrenic patients and their siblings. Am J Psychiatry. 2003;160:2060–2062. doi: 10.1176/appi.ajp.160.11.2060. [DOI] [PubMed] [Google Scholar]
- 18.Caspi A, Reichenberg A, Weiser M, Rabinowitz J, Kaplan Z, Knobler H, Avidson-Sagi N, Davidson M. Cognitive performance in schizophrenia patients assessed before and following the first psychotic episode. Schizophr Res. 2003;65:87–94. doi: 10.1016/s0920-9964(03)00056-2. [DOI] [PubMed] [Google Scholar]
- 19.Seidman LJ, Buka SL, Goldstein JM, Tsuang MT. Intellectual decline in schizophrenia: evidence from a prospective birth cohort 28 year follow-up study. J Clin Exp Neuropsychol. 2006;28:225–242. doi: 10.1080/13803390500360471. [DOI] [PubMed] [Google Scholar]
- 20.Deary IJ, Batty GD. Cognitive epidemiology. J Epidemiol Community Health. 2007;61:378–384. doi: 10.1136/jech.2005.039206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Os J, Jones P, Lewis G, Wadsworth M, Murray RM. Developmental precursors of affective illness in a general population birth cohort. Arch Gen Psychiatry. 1997;54:625–631. doi: 10.1001/archpsyc.1997.01830190049005. [DOI] [PubMed] [Google Scholar]
- 22.Zammit S, Allebeck P, David AS, Dalman C, Hemmingsson T, Lundberg I, Lewis G. A longitudinal study of premorbid IQ score and risk of developing schizophrenia, bipolar disorder, severe depression, and other nonaffective psychoses. Arch Gen Psychiatry. 2004;61:354–360. doi: 10.1001/archpsyc.61.4.354. [DOI] [PubMed] [Google Scholar]
- 23.Koenen KC, Moffitt TE, Roberts AL, Martin LT, Kubzansky L, Harrington H, Poulton R, Caspi A. Childhood IQ and adult mental disorders: a test of the cognitive reserve hypothesis. Am J Psychiatry. 2009;166:50–57. doi: 10.1176/appi.ajp.2008.08030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gale CR, Deary IJ, Boyle SH, Barefoot J, Mortensen LH, Batty GD. Cognitive ability in early adulthood and risk of 5 specific psychiatric disorders in middle age: the Vietnam Experience Study. Arch Gen Psychiatry. 2008;65:1410–1418. doi: 10.1001/archpsyc.65.12.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wechsler D. Wechsler Intelligence Scale for Children–Revised. New York: Psychological Corp; 1974. [Google Scholar]
- 26.Moffitt TE, Caspi A, Harkness AR, Silva PA. The natural history of change in intellectual performance: Who changes? How much? Is it meaningful? J Child Psychol Psychiatry. 1993;34:455–506. doi: 10.1111/j.1469-7610.1993.tb01031.x. [DOI] [PubMed] [Google Scholar]
- 27.Kaufman AS. Factor analysis of the WISC–R at 11 age levels between 6 1/2 and 16 1/2 years. J Consult Clin Psychol. 1975;43:135–147. doi: 10.1037//0022-006x.43.5.661. [DOI] [PubMed] [Google Scholar]
- 28.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4. New York: Oxford University Press; 2004. [Google Scholar]
- 29.Poulton R, Caspi A, Moffitt TE, Cannon M, Murray R, Harrington H. Children’s self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Arch Gen Psychiatry. 2000;57:1053–1058. doi: 10.1001/archpsyc.57.11.1053. [DOI] [PubMed] [Google Scholar]
- 30.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Publishing; Washington, DC: 1994. [Google Scholar]
- 31.Robins LN, Cottler L, Bucholz K, Compton W. The Diagnostic Interview Schedule, Version IV. St Louis, Mo: Washington University School of Medicine; 1995. [Google Scholar]
- 32.Perala J, Suvisaari J, Saarni SI, Kuoppasalmi K, Isometsa E, Pirkola S, Partonen T, Tuulio-Henriksson A, Hintikka J, Kieseppa T, Harkanen T, Koskinen S, Lonnqvist J. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry. 2007;64:19–28. doi: 10.1001/archpsyc.64.1.19. [DOI] [PubMed] [Google Scholar]
- 33.McGrath JJ. The surprisingly rich contours of schizophrenia epidemiology. Arch Gen Psychiatry. 2007;64:14–16. doi: 10.1001/archpsyc.64.1.14. [DOI] [PubMed] [Google Scholar]
- 34.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3. Washington, DC: American Psychiatric Publishing; 1987. [Google Scholar]
- 35.Moffitt TE, Harrington H, Caspi A, Kim-Cohen J, Goldberg D, Gregory AM, Poulton R. Depression and generalized anxiety disorder: cumulative and sequential comorbidity in a birth cohort followed prospectively to age 32 years. Arch Gen Psychiatry. 2007;64:651–660. doi: 10.1001/archpsyc.64.6.651. [DOI] [PubMed] [Google Scholar]
- 36.Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 37.Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- 38.White T, Anjum A, Schulz SC. The schizophrenia prodrome. Am J Psychiatry. 2006;163:376–380. doi: 10.1176/appi.ajp.163.3.376. [DOI] [PubMed] [Google Scholar]
- 39.Rabinowitz J, De Smedt G, Harvey PD, Davidson M. Relationship between premorbid functioning and symptom severity as assessed at first episode of psychosis. Am J Psychiatry. 2002;159:2021–2026. doi: 10.1176/appi.ajp.159.12.2021. [DOI] [PubMed] [Google Scholar]
- 40.Erlenmeyer-Kimling L. Neurobehavioral deficits in offspring of schizophrenic parents: liability indicators and predictors of illness. Am J Med Genet. 2000;97:65–71. doi: 10.1002/(sici)1096-8628(200021)97:1<65::aid-ajmg9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 41.Nuechterlein KH, Dawson ME. Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophr Bull. 1984;10:160–203. doi: 10.1093/schbul/10.2.160. [DOI] [PubMed] [Google Scholar]
- 42.Egan MF, Goldberg TE, Gscheidle T, Weirich M, Rawlings R, Hyde TM, Bigelow L, Weinberger DR. Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biol Psychiatry. 2001;50:98–107. doi: 10.1016/s0006-3223(01)01133-7. [DOI] [PubMed] [Google Scholar]
- 43.Egan MF, Goldberg TE, Gscheidle T, Weirich M, Bigelow LB, Weinberger DR. Relative risk of attention deficits in siblings of patients with schizophrenia. Am J Psychiatry. 2000;157:1309–1316. doi: 10.1176/appi.ajp.157.8.1309. [DOI] [PubMed] [Google Scholar]
- 44.Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kern RS, Nuechterlein KH, Green MF, Baade LE, Fenton WS, Gold JM, Keefe RS, Mesholam-Gately R, Mintz J, Seidman LJ, Stover E, Marder SR. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. Am J Psychiatry. 2008;165:214–220. doi: 10.1176/appi.ajp.2007.07010043. [DOI] [PubMed] [Google Scholar]
- 46.Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, Gold JM, Goldberg TE, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 47.Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 48.Dickinson D, Ramsey MB, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64:532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.