Abstract
The regulated response of endothelial cells (ECs) to signals in their environment is not only critical for the de novo formation of primordial vascular networks during early development (i.e. vasculogenesis), but is also required for the subsequent growth and remodelling of new blood vessels from pre-existing ones (i.e. angiogenesis). Vascular endothelial growth factors (Vegfs) and their endothelial-cell specific receptors play a crucial role in nearly all aspects of blood vessel growth. How the outputs from these pathways affect and coordinate endothelial behaviour is an area of intense research. Recently, numerous studies have highlighted roles for microRNAs in modulating Vegf signalling output in several different contexts. In this review we will provide an overview of how small RNAs regulate multiple aspects of the Vegf signalling pathway. In particular, we highlight areas where identification of microRNAs and their targets has provided new insight into the role of downstream effectors in modulating Vegf output during development. Since Vegf plays a broad role in multiple aspects of endothelial biology and has become a target for therapeutic manipulation of pathological blood vessel growth, microRNAs that affect Vegf signalling output will undoubtedly be major targets of clinical value.
Introduction
Formation of the vertebrate circulatory system requires coordination of diverse cellular behaviors during embryonic development 1. During vasculogenesis, endothelial cell (EC) progenitors must balance proliferation, differentiation into distinct lineages (e.g. artery and vein), and migration to the appropriate anatomical location to establish a primitive vascular network de novo 2. Subsequent angiogenesis entails the sprouting and growth of new blood vessels from this pre-existing network, which also requires coordination of proliferation and migration and dynamic regulation of ‘tip’ and ‘stalk’ cell identities within growing angiogenic sprouts 3. During development these growth processes must be carefully coordinated with lumen formation and the integration of hemodynamic forces provided by the initial onset of circulatory flow 4. Not surprisingly, perturbation of any of these steps has detrimental effects on circulatory function in the embryo. Likewise, many of these same processes are utilized in the context of pathological vascular growth 5. Thus, a better understanding of the signalling pathways that govern blood vessel growth and homeostasis will undoubtedly have clinical benefits.
Over the last two decades we have learned a great deal about the signals required for vascular growth in both embryonic and adult settings. These studies revealed an important role for many of the central developmental signalling pathways, such as Notch, BMP, and Wnt, in multiple aspects of vascular development and function 1, 5–7. In addition, several EC-specific pathways have been identified. Most notable in this regard are receptor tyrosine kinases for Vascular endothelial growth factor (Vegf) and angiopoietin ligands 8. In particular, ligands of the Vegf family play an integral role in nearly every aspect of vascular development and maintenance 9. Indeed, loss of even a single copy of Vegfa completely blocks vessel formation in mouse embryos leading to embryonic lethality 10, 11, while loss of this ligand in the endothelium of adult vasculature causes severe endothelial dysfunction 12, and inhibition of Vegfa signalling potently inhibits pathological angiogenesis 13. Vegf ligands can elicit a wide range of responses in ECs, including migration, proliferation, survival, differentiation and increased permeability 9. Given the importance of targeting this pathway in a variety of clinical settings 8, a detailed understanding of how Vegf ligands elicit a particular response in vivo is of great importance. Over the past several years, there has been an increasing amount of evidence implicating microRNAs in the control of Vegf signalling output at multiple levels. Indeed, microRNAs are now known to control the expression of Vegf ligands, receptors, and intracellular signalling components of the Vegf pathway, as well as proteins that cross-talk with Vegf receptors. Here, we will review the most recent findings regarding microRNA regulation of Vegf signalling output.
Regulation of angiogenesis by microRNAs
Originally identified in C. elegans in 1993 14, microRNAs are a group of highly conserved, non-coding single-strand RNA molecules (~21–23 nucleotides (nts)) that function to fine-tune protein expression through various mechanisms, including the degradation of target mRNA, the inhibition of translation elongation, or the sequestration of target mRNAs away from the translation machinery 15–17. This is accomplished by the incorporation of the microRNA into the RNA-Induced Silencing Complex (RISC) 18, which is then directed primarily to the 3′ untranslated region (UTR) of the target mRNA via the seed sequence of the microRNA. While microRNAs may affect the expression of hundreds of target genes 19, a small number of target mRNAs may adequately explain the biological effect of a particular microRNA in a given cellular context 20. Numerous studies have also demonstrated that microRNAs can target several genes within the same pathway to control biological responses 21.
MicroRNAs have been shown to play key roles in vascular development. For example, deletion of Dicer, an enzyme required for microRNA biogenesis, results in early embryonic lethality 22, while mice homozygous for a hypomorphic allele of Dicer survive to mid-gestation but have major defects in angiogenesis 23. Conditional deletion of Dicer in the endothelium results in reduced vascular growth in postnatal models of tumor angiogenesis and ischemia 24. While these mice did not display angiogenic defects during development, Dicer was not ablated from all ECs in this study 24, and it remains possible that full deletion of Dicer may well result in developmental vascular defects. In addition to these developmental studies, knockdown of Dicer in cultured ECs has been shown to result in significant defects in their responsiveness to angiogenic factors 25.
Control of VEGF ligand and receptor expression by microRNAs
The Vegf family is composed of several ligands (Vegfa, b, c, d and e and Placental growth factor (Plgf)), each of which has a particular preference for one of three endothelial expressed receptors (Vegfr-1/Flt1, Vegfr-2/Flk1/Kdr and Vegfr-3/Flt4) 9. Regarding vascular development, Vegfa is the most functionally significant and well-characterized member of the Vegf family. Upon binding to its receptor tyrosine kinase, Vegfr-2, Vegfa can elicit distinct responses, including proliferation, cell survival, permeability, differentiation, and migration 9. These effects are mediated through the activation of several downstream signalling effectors 9. Homozygous deletion of Vegfr-2 26, much like deletion of its ligand 10, 11, results in an absence of blood vessels and lethality in mice. Vegfr-3, which becomes almost exclusive to lymphatic ECs in late development 27, is also expressed on vascular endothelial tip cells during embryonic and post-natal angiogenesis and responds to Vegfc and Vegfd 28, 29. Vegfr-3 deletion also leads to embryonic lethality, which is due to a failure of vascular remodelling 30, and inhibition of Vegfr-3 activity can inhibit tumor angiogenesis 28. In contrast to Vegfr-2 and Vegfr-3, Vegfr-1 largely functions as a “decoy” receptor to negatively regulate Vegf signalling during development. 31, 32. The Vegfr-1 gene encodes an alternative soluble isoform that lacks the intracellular domain and has a much higher affinity for Vegfa than Vegfr-2 33. Accordingly, mice bearing a null allele of Vegfr-1 exhibit extensive vascular over-growth, while those bearing a deletion of only the tyrosine kinase domain display normal vascular development 34. Importantly, the levels of Vegfr-2/3 versus Vegfr-1 appear to be both spatially and temporally regulated during angiogenesis. Thus, the proper balance and regulation of Vegf receptor levels plays an important role in orchestrating angiogenic sprouting 31, 32.
ECs are highly sensitive and responsive to Vegf gradients. These gradients are established through the alternative splicing of Vegfa transcripts, which results in an array of protein variants with diverse functional properties, such as differential diffusion rates, as well as variable association with the extra-cellular matrix (ECM), leading to distinct abilities to activate cell signalling pathways and to induce vascular morphogenesis 35. In humans, Vegfa isoforms contain 121, 165, 189 or 206 amino acids; all except Vegfa121 contain a basic stretch near the carboxyl terminus with variable affinity for heparan sulfate proteoglycans (HSPGs) and Neuropilin-1 (co-receptors for Vegfa) 35. As a result Vegfa121, which is unable to bind HSPGs, is freely diffusible and can influence EC proliferation, though contributing little to EC migration 36. On the contrary, Vegfa165 and Vegfa189, which have strong affinities for HSPGs, are tightly associated with the ECM, forming a gradient to allow for the directional migration of ECs by promoting filopodia extensions 36. One possible explanation for the vast array of cellular responses elicited by different Vegfa isoforms is the preferential activation of one downstream signalling effector over another. For example, unlike its soluble counterpart, matrix-bound Vegfa can induce prolonged tyrosine kinase activity of Vegfr-2 leading to increased phosphorylation of p38/Mitogen Activated Protein Kinase (MAPK), thereby up-regulating angiogenic sprouting. This is mediated by interaction between Vegfr-2 and β1-integrins 37.
Given the considerable dynamic control of Vegf ligand and receptor expression during vascular development, it is not surprising that microRNAs can control this pathway by targeting transcripts encoding these proteins (Fig. 1). Since enhanced expression of Vegfa occurs in diseases such as cancer and diabetic retinopathy and drives pathological vascular growth 38, alterations in microRNA expression may contribute to these diseases. Indeed, microRNA (miR)-93 39 and miR-200b 40 were recently found to be down-regulated by hyperglycemic conditions. These microRNAs both target the Vegfra 3′ UTR, and knock-down of these microRNAs enhances Vegfa expression in vivo 39, 40. MiR-15a also directly represses Vegf and Fibroblast growth factor 2 (Fgf2) in ECs to control angiogenesis 41, and miR-20b represses Vegf expression in tumor cells to affect their cell survival in hypoxic conditions 42. MicroRNAs are also induced down-stream of Vegf signalling itself 24. For example, Vegf induces the expression of miR-16 and miR-424, which share a common seed sequence 43. These microRNAs act in a negative feedback loop to control angiogenesis through combined targeting of Vegfa, Vegfr-2 and the FGF receptor, FGFR1 43. Other microRNAs, such as miR-296, indirectly regulate Vegfr-2 expression 44. Vegfr-2 turnover is regulated by hepatocyte growth factor-regulated tyrosine kinase substrate (HGS), which mediates sorting of ligand/receptor complexes to lysosomes for degradation 45. MiR-296 expression is enhanced in tumor vasculature and is induced by Vegf signalling, and through its repression of HGS expression in ECs, miR-296 facilitates enhanced expression of Vegfr-2 on the cell surface and potentiates Vegf signalling 44.
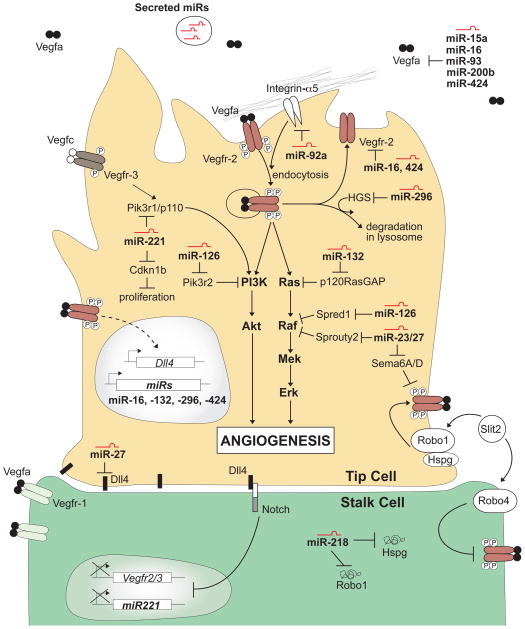
Fig 1. MicroRNAs impinge on Vegf signalling to regulate angiogenesis.
Binding to their receptor tyrosine kinases (Vegfr-2 and Vegfr-3), Vegfa and Vegfc can drive angiogenesis through the activation of several downstream signaling effectors (e.g. PI3K and MAPK/ERK). The output of these effectors is modulated by microRNAs. In particular, recent evidence highlight the important roles of microRNAs in controlling Vegf signalling by titrating the levels of Vegf ligand (miR-15a, 16, 93, 200b, and 424), Vegf receptors (miR-16, and 424), as well as positive and negative regulators of the Vegf signal transduction cascade (miR-23, -27, -126, -132, -218 and -221). The identification of these regulatory microRNAs has emphasized the importance of fine-tuning both the PI3K and MAPK signaling pathways downstream of Vegf signaling and has provided new insights into how different outputs can be modulated through post-transcriptional control.
Control of intracellular signalling downstream of Vegf by microRNAs
In addition to regulation at the level of ligand and receptor, several studies reveal that intracellular signalling effectors utilized downstream of Vegf are targeted by microRNAs (Fig. 1). In particular, this work has underscored the importance of both the phosphoinositide-3-kinase (PI3K) and MAPK/ERK signalling pathways in modulating Vegf signalling outputs during vascular development.
PI3K activity is an essential downstream effector of Vegf signalling during vascular development 46. Class I PI3Ks are heterodimers composed of a catalytic subunit, which can be encoded by 4 different genes (p110α, β, δ, and γ: referred to hereafter as pik3ca, b, d, and g) and a regulatory subunit, for which 5 different genes have been identified (pik3r1-5) 47. Notably, Pik3r1, 2, and 3 possess SH2 domains that mediate direct interactions with upstream receptor tyrosine kinases. Pik3ca, b, and g, as well as most regulatory subunits are expressed in ECs 48, 49 and appear to have distinct roles in modulating Vegf output. For example, pik3ca is essential for Vegfa-mediated endothelial migration during developmental angiogenesis 46, while pik3cg plays an important role in Vegfa-stimulated vascular permeability in adult vasculature 49. PI3Ks phosphorylate membrane bound phosphoinositol-4,5-bisphosphate to generate phosphoinositol-1,4,5-trisphosphate, which serves as a docking and activation site for downstream signalling molecules, such as Akt1, a serine/threonine kinase. Given the importance of PI3K signalling in cell survival and growth, constitutive activation of this pathway is typically associated with cellular transformation in non-endothelial cell types. In many of these cases, activation is due to the loss of PI3K regulatory subunits, which are thought to exist in a 1:1 ratio with the catalytic subunits and usually inhibit kinase activity in the absence of upstream activation 50. Several recent findings suggest that these regulatory subunits are important targets of microRNAs to regulate Vegf signalling output in ECs. In particular, miR-126 and miR-221 have been found to target Pik3r2 51, 52 and Pik3r1 53, respectively.
MiR-126 control of Vegfr-2 signalling
MiR-126 was the first EC-specific microRNA identified and is located within intron 7 of the Egfl7 gene 51, 54. Deletion of miR-126 in mice (without altering the expression of the host gene), results in severe defects in blood vessel development, including delayed angiogenic sprouting, haemorrhage and partial early embryonic lethality 52, 54. These defects are similar to phenotypes associated with loss of Vegf signalling, in both the mouse 55 as well as the zebrafish 51, suggesting that miR-126 acts to modulate Vegf signalling output. Indeed, knockdown of miR-126 in ECs results in decreased phosphorylation of Akt, as well as ERK1/2 in response to Vegf treatment 51, 52, 54. Furthermore, miR-126 targets transcripts encoding Pik3r2 51, 52 and Spred1 51, 52, 54, 56, an inhibitor of MAPK signalling, providing a direct link between this microRNA and the Vegf signalling pathway.
Interestingly, results from zebrafish suggest that miR-126-mediated repression of Spred1 and Pik3r2 may be context dependent. Unlike mammalian genomes, which encode one copy of miR-126 that does not appear to be regulated by blood flow 57, the zebrafish genome encodes two copies of miR-126, one of which is induced by blood flow in the aortic arches where it is required for Vegfa-dependent angiogenic remodelling 58. In this case, Spred1 over-expression mimics the loss of miR-126, while reduction of Spred1 levels in miR-126 deficient embryos rescues aortic arch remodelling. Thus, Spred1, but not Pik3r2, is required for flow-mediated angiogenic remodelling of the aortic arches in the zebrafish. Further investigation using compound mouse knockouts deficient for miR-126 and its targets will be insightful to determine the context-dependent requirements for Spred1 and Pik3r2 in mediating ERK and PI3K signalling downstream of Vegfa during embryonic and postnatal angiogenesis.
MiR-221 control of Vegfr-3 signalling
Similar to miR-126, recent data suggest that miR-221 also acts to modulate Vegf receptor signalling through regulation of a PI3K regulatory subunit. Although previously characterized in other systems 59, miR-221 was identified through deep sequencing efforts as an endothelial-enriched microRNA in zebrafish embryos 53. Knockdown of miR-221 does not affect initial blood vessel development but causes angiogenesis and lymphatic defects 53 that are remarkably similar to loss of Vegfr-3, a receptor for the Vegfc ligand 29. Furthermore, epistasis studies suggest that miR-221 acts within the Vegfc/Vegfr-3 signalling pathway and in parallel to Vegfa/Vegfr-2. Mosaic analysis revealed that miR-221 deficient cells do not contribute well to the tip cell position in developing angiogenic sprouts. By contrast, overexpression of miR-221 induces tip cell behaviors, such as enhanced proliferation and migration. The effect of miR-221 on cell proliferation appears to be largely due to repression of cyclin-dependent kinase inhibitor 1B (Cdkn1b), and miR-221 also targets Pik3r1, which was previously shown to interact with Vegfr-3 60. Both reduction and over-expression of Pik3r1 result in inhibition of angiogenesis 53, suggesting that precisely tuned levels of this regulatory subunit are required for optimal PI3K output.
These studies suggest that a common mechanism by which microRNAs regulate intracellular growth factor signalling is by precisely tuning the level of PI3K regulatory subunits. Studies showing that Pik3r1 and Pik3r2 are targeted by several different microRNAs during growth factor signalling in non-endothelial cell types further suggest that this may be a general theme 61–63. Interestingly, in these contexts, microRNA-mediated repression of the regulatory subunit is associated with reduced proliferation and apoptosis. By contrast, both miR-126 and miR-221 promote endothelial growth and angiogenesis suggesting that they fine-tune, rather than simply inhibit, PI3K activity to elicit context-dependent Vegf signalling outputs. Moreover, these microRNAs appear to control output through two distinct receptors: miR-126 regulates Vegfr-2, while microRNA-221 regulates Vegfr-3 signalling. These studies would further imply that these receptors each utilize different PI3K signalling complexes. In general, these microRNAs could provide a mechanism by which a cell may respond differentially to, or appropriately integrate, signalling in response to Vegfa and/or Vegfc.
MiR-132 control of Ras activity
Ras is a key regulator that acts downstream of the Vegf receptor to mediate activation of the MAPK/ERK pathway, and recent findings have suggested that Ras activity in ECs is controlled by miR-132 during tumor angiogenesis. Activity of Ras is controlled by opposing GTPase activating proteins (GAPs) and GTP exchange factors (GEFs) that dictate whether Ras is an a GTP-associated active form, or a GDP-associated inactive form. MiR-132 was found to be highly expressed in tumor ECs, but not in normal ECs, and its induction is driven by angiogenic factors such as Vegf and Fgf2 64. Silencing of miR-132 expression decreases the miR-132 target, p120RasGAP 65, which negatively regulates Ras, and contributes to vascular development and remodelling 66. Induction of miR-132 expression in ECs may act as an angiogenic switch during tumor neovascularization, and antagonism of miR-132 markedly decreases tumor angiogenesis in mouse models 64. Furthermore, the role of miR-132 appears to be EC specific, as its inhibition has little effect on the expression of p120RasGAP in other cell types 64.
MicroRNAs regulate signalling pathways that cross-talk with Vegfr-2
Cross-talk with several signalling pathways also contributes to the precise modulation of Vegf signal output to govern appropriate EC responses 1. Several recent studies have revealed that these pathways are also targeted by microRNAs in ECs (Fig. 1). These include components of the Slit/Robo pathway, which are widely known for their roles as repulsive cues during neuronal guidance 67, 68, the Notch pathway, which plays a central role in switching off angiogenic behaviors induced by Vegf signalling 29, as well as integrins, which can modulate Vegfr-2 signalling 69.
MiR-218 control of Slit/Robo signalling
The Slit family of ligands (Slit1-3) bind to their cognate Roundabout (Robo) receptors (Robo1-4) to control cell migratory behaviors. While this pathway is best known for its regulation of neuronal guidance 67, 68, this ligand/receptor system also impinges on the Vegf signalling pathway in ECs. For example, Slit2 can activate Robo1 and Robo4 receptors, which are expressed on ECs 70, to elicit divergent EC responses. Slit2 activates a Robo4-dependent signalling pathway in ECs that represses Vegf signalling by inhibiting the small GTPase, Arf6 71, 72. In contrast, Robo1 potentiates Vegf signalling by enhancing phosphorylation of Vegfr-273. Recently, a microRNA family (miR-218) was found to be intronically encoded in the Slit2 and Slit3 genes 73, 74. Intriguingly, Slit-encoded miR-218 targets the Slit receptor, Robo1, as well as components of the HSPG biosynthetic pathway and negatively regulates angiogenic responses in ECs 73, 74. HSPGs have previously been shown to enhance Slit binding to Robo receptors 75, and they also influence Vegf ligand/receptor interactions 35. Since miR-218 does not target Robo4, this microRNA may alter the activation of Robo1- vs Robo4-dependent pathways, which have opposing effects on Vegf signalling. Knockdown of miR-218 in the mouse retina results in defective angiogenesis 74, and knockdown of miR-218 in zebrafish results in defects in the Vegf-dependent migration of the endocardium to the midline during the early stages of heart development 73, illustrating the requirement of the Slit/miR-218/Robo1 pathway for normal cardiovascular development 73. The contribution of this pathway to pathological vascular growth remains to be determined.
Notch, microRNAs and Vegf
In sprouting blood vessels, Notch signalling plays a vital role in reducing the angiogenic response normally induced by Vegf 29, 76, 77. In this case, Vegfa stimulates migration of leading tip cells from a pre-existing blood vessel. During this process, Vegfa induces expression of the Notch ligand, Dll4 in the tip cell, which activates Notch signalling in adjacent cells, reducing their migration and proliferation. This provides an elegant mechanism to license a restricted number of cells to sprout from a pre-existing vessel, and allows the growing sprout to maintain its connection to the patent blood vessel. Notch activation is thought to inhibit Vegfa-stimulated migration and proliferation by downregulating Vegfr-3 signalling 29, while also inducing the expression of soluble Vegfr-1 78, which presumably acts as a sponge to bind surrounding Vegfa to prevent activation of Vegfr-2 31.
Several studies suggest that cross-talk between Notch and Vegf signalling is further modulated by microRNAs. As mentioned above, miR-221 promotes tip cell behavior, in part through regulation of Pik3r1 and modulation of signalling downstream of Vegfr-3 53. Interestingly, Notch activation represses the expression of miR-221 53. Also, excessive tip cell behaviors normally associated with loss of Notch signalling are blocked by loss of miR-221. Thus, Notch appears to affect the output of Vegf-3 signalling, in part through the regulation of miR-221 levels. Notch signalling components themselves have also been identified as microRNA targets during angiogenesis. In zebrafish embryos, miR-27b was found to repress the transcript encoding Dll4. Accordingly, miR-27b-deficient zebrafish embryos have reduced filopodia formation and impaired sprouting 79, similar to embryos with activated Notch 29. This is associated with increased expression of Dll4 and upregulation of Vegfr-1 79. Interestingly, recent studies in cultured human cells and mice have shown that the miR-23/27/24 cluster is highly expressed in endothelial cells and that miR-23 and miR-27 are also capable of coordinately repressing Sprouty2 80, a negative regulator of Raf1 81, and Semaphorin 6A and D 80, 82, which inhibit Vegfr-2 signalling 83. Thus, similar to miR-126 and miR-221, miR-27 can control the expression of multiple targets to modulate Vegf signalling output.
MiR-92a control of integrin expression
Endothelial cells interact with several ECM proteins, including fibronectin, which can affect angiogenesis. For example, fibronectin can facilitate the migration of tip cells by enhancing Vegfr-2 signalling 84. Fibronectins signal through several integrin proteins that are expressed on ECs. The importance of integrins for vascular growth is illustrated by the embryonic lethality and major vascular defects that are observed in Integrin-α5 (ITGA5) knock-out mice 85. Recently, miR-92a was shown to control angiogenesis through the targeting of ITGA5 in the endothelium 86. Over-expression of miR-92a inhibits sprout formation and vascular network formation in vitro, and also represses vascular invasion of matrigel plugs loaded with angiogenic factors in vivo. Of particular interest, inhibition of miR-92a in mouse models of hind limb ischemia or myocardial infarction results in enhanced angiogenesis and tissue regeneration, strongly suggesting that miR-92a normally suppresses angiogenic signalling through its targeting of ITGA5 86.
Emerging areas of microRNA biology with possible implications for Vegf signalling and angiogenesis
In general, microRNAs are thought to act cell-autonomously to repress their target transcripts. The aforementioned examples of microRNA regulation have focused on this well-known role for microRNAs. However, recent findings suggest that microRNAs may act as paracrine or even endocrine factors. Furthermore, microRNA target transcripts in a given cell may also serve as sponges to titrate microRNA levels and function rather than being functional targets themselves. While little is known concerning the importance of these two new concepts in the context of Vegf signalling and angiogenesis, there are hints from recent studies that they will be relevant in this context. Therefore, we provide a brief overview of these new aspects of microRNA biology.
Several studies have now shown that microRNAs are abundant and relatively stable in circulation, suggesting that they may play a paracrine role in controlling gene expression 87, 88. MicroRNAs have been associated with a variety of carriers, including lipid-encapsulated microvesicles (MV), such as exosomes (50–100 nm), microparticles (0.1–1 μm) and apoptotic bodies (0.5–2 μm), as well as HDL and LDL complexes, and these carriers can deliver microRNAs to recipient cells 89–91. Additionally, circulating Ago2-containing protein complexes that contain microRNAs may be the main carriers of circulating microRNAs 92, but it is unclear whether these non-lipid-encapsulated complexes can be delivered to cells. Tumor cells can secrete microRNA-containing MVs that are taken up by ECs to alter EC phenotype, including their angiogenic properties 93–95. It is currently not known whether secreted microRNAs contribute to vascular development, but this is an exciting prospect. A recent study has implied that levels of EC-enriched microRNAs, such as miR-126 and miR-92a may be reduced in vascular diseases such as coronary artery disease 96, but whether this is a cause or consequence of disease is not currently known. Further analyses in larger cohorts of age-matched patients will be necessary to clarify these initial findings.
While the functional effects of microRNAs are typically thought to be manifested through the regulation of protein levels from a target transcript, recent observations suggest that many target transcripts may serve a different purpose. In this case, mRNAs containing microRNA binding sites can affect the expression of other mRNAs in a protein coding-independent manner via competition for the binding of microRNAs to their 3′ UTRs 97, 98. These RNAs have been named competing endogenous RNAs (ceRNAs). Their function was first illustrated by the finding that a pseudogene for PTEN, PTENP1, can affect the expression of the PTEN gene by acting as a microRNA decoy 99. Considering the central role that PTEN plays in controlling PI3K activity, an important output for Vegf signalling 100, this finding will likely be relevant to vascular growth. It has subsequently been shown that multiple coding transcripts can control PTEN expression by titrating away microRNAs from the PTEN 3′ UTR 101, 102. A long non-coding RNA has also been shown to regulate muscle differentiation by acting as a ceRNA for miR-133 103. It will be interesting to determine whether ceRNAs affect angiogenic signalling by competition for important pro- or anti-angiogenic microRNAs. CeRNAs represent another layer of regulation that may impact on the dynamic control of angiogenic signalling pathways.
Conclusion and clinical implications
It is evident that the Vegf signalling pathway is highly regulated at multiple levels. Recent studies demonstrate that microRNAs provide an additional layer of regulation by titrating the levels of proteins that are involved in the transduction of angiogenic signals. The general theme from these studies is that microRNAs are important nodes for controlling particular endothelial behaviors downstream of Vegf. Based on these observations, targeting microRNAs to manipulate subtle aspects of vascular growth in a clinical setting will be highly desirable. This is of particular importance since many early angiogenesis inhibitors that bluntly target Vegf ligand/receptor interactions or Vegfr kinase activity result in side effects, such as hypertension and proteinuria 104. MicroRNAs can be targeted therapeutically 105, and several findings from mouse models indicate that targeting microRNAs, including those implicated in Vegf signalling, may be useful in clinical settings where precise control of vascular growth is desired. For example, silencing miR-126 has been shown to impair neoangiogenesis following myocardial infarction 54 or hind limb ischemia 106. Interestingly, the host transcript for miR-126, Egfl7, is highly upregulated in tumor endothelium 107, but the role of miR-126 in tumor angiogenesis has not yet been addressed. Antagonism of miR-132 64 or miR-296 44, which are upregulated in tumor endothelium, has promising inhibitory effects on tumor angiogeneis. In contrast, inhibiting miR-92a enhances vascular growth in the setting of myocardial infarction and hind limb ischemia 86. These exciting results provide an impetus to further understand the role of microRNAs in modulating the signalling output of Vegf.
Acknowledgments
Research in the Fish lab is supported by grants from the Canadian Institutes of Health Research (MOP-119506) and the Heart and Stroke Foundation of Ontario (NA-7282). J.F. is the recipient of a Heart and Stroke Foundation New Investigator Award and an Early Researcher Award from the Ontario Ministry of Economic Development and Innovation. Research in the Lawson lab is supported by grants from the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
References
- 1.Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011;12:551–564. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poole TJ, Coffin JD. Vasculogenesis and angiogenesis: Two distinct morphogenetic mechanisms establish embryonic vascular pattern. Journal of Experimental Zoology. 1989;251:224–231. doi: 10.1002/jez.1402510210. [DOI] [PubMed] [Google Scholar]
- 3.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. The Journal of Cell Biology. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.le Noble F, Klein C, Tintu A, Pries A, Buschmann I. Neural guidance molecules, tip cells, and mechanical factors in vascular development. Cardiovasc Res. 2008;78:232–241. doi: 10.1093/cvr/cvn058. [DOI] [PubMed] [Google Scholar]
- 5.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 6.Chappell JC, Bautch VL. Vascular development: genetic mechanisms and links to vascular disease. Curr Top Dev Biol. 2010;90:43–72. doi: 10.1016/S0070-2153(10)90002-1. [DOI] [PubMed] [Google Scholar]
- 7.Dejana E. The role of wnt signaling in physiological and pathological angiogenesis. Circ Res. 2010;107:943–952. doi: 10.1161/CIRCRESAHA.110.223750. [DOI] [PubMed] [Google Scholar]
- 8.Saharinen P, Eklund L, Pulkki K, Bono P, Alitalo K. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol Med. 2011;17:347–362. doi: 10.1016/j.molmed.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 10.Carmeliet P. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara N. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 12.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 14.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 15.Eulalio A, Behm-Ansmant I, Izaurralde E. P bodies: at the crossroads of post-transcriptional pathways. Nat Rev Mol Cell Biol. 2007;8:9–22. doi: 10.1038/nrm2080. [DOI] [PubMed] [Google Scholar]
- 16.Giraldez AJ, Mishima Y, Rihel J, Grocock RJ, Van Dongen S, Inoue K, Enright AJ, Schier AF. Zebrafish MiR-430 Promotes Deadenylation and Clearance of Maternal mRNAs. Science. 2006;312:75–79. doi: 10.1126/science.1122689. [DOI] [PubMed] [Google Scholar]
- 17.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell. 2005;123:631–640. doi: 10.1016/j.cell.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 19.Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valastyan S, Benaich N, Chang A, Reinhardt F, Weinberg RA. Concomitant suppression of three target genes can explain the impact of a microRNA on metastasis. Genes Dev. 2009;23:2592–2597. doi: 10.1101/gad.1832709. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature. 2011;469:336–342. doi: 10.1038/nature09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 23.Yang WJ, Yang DD, Na S, Sandusky GE, Zhang Q, Zhao G. Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem. 2005;280:9330–9335. doi: 10.1074/jbc.M413394200. [DOI] [PubMed] [Google Scholar]
- 24.Suarez Y, Fernandez-Hernando C, Yu J, Gerber SA, Harrison KD, Pober JS, Iruela-Arispe ML, Merkenschlager M, Sessa WC. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc Natl Acad Sci U S A. 2008;105:14082–14087. doi: 10.1073/pnas.0804597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007;101:59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- 26.Shalaby F. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 27.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tammela T, Zarkada G, Wallgard E, Murtomaki A, Suchting S, Wirzenius M, Waltari M, Hellstrom M, Schomber T, Peltonen R, Freitas C, Duarte A, Isoniemi H, Laakkonen P, Christofori G, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–660. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- 29.Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781–784. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- 30.Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, Breitman M, Alitalo K. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282:946–949. doi: 10.1126/science.282.5390.946. [DOI] [PubMed] [Google Scholar]
- 31.Chappell JC, Taylor SM, Ferrara N, Bautch VL. Local guidance of emerging vessel sprouts requires soluble Flt-1. Dev Cell. 2009;17:377–386. doi: 10.1016/j.devcel.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zygmunt T, Gay CM, Blondelle J, Singh MK, Flaherty KM, Means PC, Herwig L, Krudewig A, Belting HG, Affolter M, Epstein JA, Torres-Vazquez J. Semaphorin-PlexinD1 signaling limits angiogenic potential via the VEGF decoy receptor sFlt1. Dev Cell. 2011;21:301–314. doi: 10.1016/j.devcel.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kappas NC, Zeng G, Chappell JC, Kearney JB, Hazarika S, Kallianos KG, Patterson C, Annex BH, Bautch VL. The VEGF receptor Flt-1 spatially modulates Flk-1 signaling and blood vessel branching. J Cell Biol. 2008;181:847–858. doi: 10.1083/jcb.200709114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci U S A. 1998;95:9349–9354. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shibuya M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis. Genes & Cancer. 2011;2:1097–1105. doi: 10.1177/1947601911423031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruhrberg C. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16:2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen TT. Anchorage of VEGF to the extracellular matrix conveys differential signaling responses to endothelial cells. J Cell Biol. 2010;188:595–609. doi: 10.1083/jcb.200906044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 39.Long J, Wang Y, Wang W, Chang BH, Danesh FR. Identification of microRNA-93 as a novel regulator of vascular endothelial growth factor in hyperglycemic conditions. J Biol Chem. 2010;285:23457–23465. doi: 10.1074/jbc.M110.136168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McArthur K, Feng B, Wu Y, Chen S, Chakrabarti S. MicroRNA-200b regulates vascular endothelial growth factor-mediated alterations in diabetic retinopathy. Diabetes. 2011;60:1314–1323. doi: 10.2337/db10-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yin KJ, Olsen K, Hamblin M, Zhang J, Schwendeman SP, Chen YE. Vascular Endothelial Cell-specific MicroRNA-15a Inhibits Angiogenesis in Hindlimb Ischemia. J Biol Chem. 2012;287:27055–27064. doi: 10.1074/jbc.M112.364414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lei Z, Li B, Yang Z, Fang H, Zhang GM, Feng ZH, Huang B. Regulation of HIF-1alpha and VEGF by miR-20b tunes tumor cells to adapt to the alteration of oxygen concentration. PLoS ONE. 2009;4:e7629. doi: 10.1371/journal.pone.0007629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chamorro-Jorganes A, Araldi E, Penalva LO, Sandhu D, Fernandez-Hernando C, Suarez Y. MicroRNA-16 and microRNA-424 regulate cell-autonomous angiogenic functions in endothelial cells via targeting vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1. Arterioscler Thromb Vasc Biol. 2011;31:2595–2606. doi: 10.1161/ATVBAHA.111.236521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wurdinger T, Tannous BA, Saydam O, Skog J, Grau S, Soutschek J, Weissleder R, Breakefield XO, Krichevsky AM. miR-296 regulates growth factor receptor overexpression in angiogenic endothelial cells. Cancer Cell. 2008;14:382–393. doi: 10.1016/j.ccr.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ewan LC, Jopling HM, Jia H, Mittar S, Bagherzadeh A, Howell GJ, Walker JH, Zachary IC, Ponnambalam S. Intrinsic tyrosine kinase activity is required for vascular endothelial growth factor receptor 2 ubiquitination, sorting and degradation in endothelial cells. Traffic. 2006;7:1270–1282. doi: 10.1111/j.1600-0854.2006.00462.x. [DOI] [PubMed] [Google Scholar]
- 46.Graupera M, Guillermet-Guibert J, Foukas LC, Phng LK, Cain RJ, Salpekar A, Pearce W, Meek S, Millan J, Cutillas PR, Smith AJ, Ridley AJ, Ruhrberg C, Gerhardt H, Vanhaesebroeck B. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature. 2008;453:662–666. doi: 10.1038/nature06892. [DOI] [PubMed] [Google Scholar]
- 47.Morello F, Perino A, Hirsch E. Phosphoinositide 3-kinase signalling in the vascular system. Cardiovasc Res. 2009;82:261–271. doi: 10.1093/cvr/cvn325. [DOI] [PubMed] [Google Scholar]
- 48.Go YM, Park H, Maland MC, Darley-Usmar VM, Stoyanov B, Wetzker R, Jo H. Phosphatidylinositol 3-kinase gamma mediates shear stress-dependent activation of JNK in endothelial cells. Am J Physiol. 1998;275:H1898–1904. doi: 10.1152/ajpheart.1998.275.5.H1898. [DOI] [PubMed] [Google Scholar]
- 49.Serban D, Leng J, Cheresh D. H-ras regulates angiogenesis and vascular permeability by activation of distinct downstream effectors. Circ Res. 2008;102:1350–1358. doi: 10.1161/CIRCRESAHA.107.169664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jaiswal BS, Janakiraman V, Kljavin NM, Chaudhuri S, Stern HM, Wang W, Kan Z, Dbouk HA, Peters BA, Waring P, Dela Vega T, Kenski DM, Bowman KK, Lorenzo M, Li H, Wu J, et al. Somatic mutations in p85alpha promote tumorigenesis through class IA PI3K activation. Cancer Cell. 2009;16:463–474. doi: 10.1016/j.ccr.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuhnert F, Mancuso MR, Hampton J, Stankunas K, Asano T, Chen CZ, Kuo CJ. Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development. 2008;135:3989–3993. doi: 10.1242/dev.029736. [DOI] [PubMed] [Google Scholar]
- 53.Nicoli S, Knyphausen C-P, Zhu Lihua J, Lakshmanan A, Lawson Nathan D. miR-221 Is Required for Endothelial Tip Cell Behaviors during Vascular Development. Developmental cell. 2012;22:418–429. doi: 10.1016/j.devcel.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee S. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nicoli S, Standley C, Walker P, Hurlstone A, Fogarty KE, Lawson ND. MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature. 2010;464:1196–1200. doi: 10.1038/nature08889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 58.Nicoli S. MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature. 2010;464:1196–1200. doi: 10.1038/nature08889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Felli N, Fontana L, Pelosi E, Botta R, Bonci D, Facchiano F, Liuzzi F, Lulli V, Morsilli O, Santoro S, Valtieri M, Calin GA, Liu CG, Sorrentino A, Croce CM, Peschle C. MicroRNAs 221 and 222 inhibit normal erythropoiesis and erythroleukemic cell growth via kit receptor down-modulation. Proc Natl Acad Sci U S A. 2005;102:18081–18086. doi: 10.1073/pnas.0506216102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang JF, Zhang X, Groopman JE. Activation of vascular endothelial growth factor receptor-3 and its downstream signaling promote cell survival under oxidative stress. J Biol Chem. 2004;279:27088–27097. doi: 10.1074/jbc.M314015200. [DOI] [PubMed] [Google Scholar]
- 61.Zheng Y, Yin L, Chen H, Yang S, Pan C, Lu S, Miao M, Jiao B. miR-376a suppresses proliferation and induces apoptosis in hepatocellular carcinoma. FEBS Lett. 2012;586:2396–2403. doi: 10.1016/j.febslet.2012.05.054. [DOI] [PubMed] [Google Scholar]
- 62.Lal A, Thomas MP, Altschuler G, Navarro F, O’Day E, Li XL, Concepcion C, Han YC, Thiery J, Rajani DK, Deutsch A, Hofmann O, Ventura A, Hide W, Lieberman J. Capture of microRNA-bound mRNAs identifies the tumor suppressor miR-34a as a regulator of growth factor signaling. PLoS Genet. 2011;7:e1002363. doi: 10.1371/journal.pgen.1002363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park SY, Lee JH, Ha M, Nam JW, Kim VN. miR-29 miRNAs activate p53 by targeting p85 alpha and CDC42. Nat Struct Mol Biol. 2009;16:23–29. doi: 10.1038/nsmb.1533. [DOI] [PubMed] [Google Scholar]
- 64.Anand S. MicroRNA-132-mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nature Med. 2010;16:909–914. doi: 10.1038/nm.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anand S, Majeti BK, Acevedo LM, Murphy EA, Mukthavaram R, Scheppke L, Huang M, Shields DJ, Lindquist JN, Lapinski PE, King PD, Weis SM, Cheresh DA. MicroRNA-132-mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nat Med. 2010;16:909–914. doi: 10.1038/nm.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boon LM, Revencu N, Vikkula M. eLS. John Wiley & Sons, Ltd; 2001. Capillary Malformation–Arteriovenous Malformation and RASA1 Mutations. [Google Scholar]
- 67.Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96:795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- 68.Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 69.Somanath PR, Malinin NL, Byzova TV. Cooperation between integrin alphavbeta3 and VEGFR2 in angiogenesis. Angiogenesis. 2009;12:177–185. doi: 10.1007/s10456-009-9141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sheldon H, Andre M, Legg JA, Heal P, Herbert JM, Sainson R, Sharma AS, Kitajewski JK, Heath VL, Bicknell R. Active involvement of Robo1 and Robo4 in filopodia formation and endothelial cell motility mediated via WASP and other actin nucleation-promoting factors. FASEB J. 2009;23:513–522. doi: 10.1096/fj.07-098269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jones CA, London NR, Chen H, Park KW, Sauvaget D, Stockton RA, Wythe JD, Suh W, Larrieu-Lahargue F, Mukouyama YS, Lindblom P, Seth P, Frias A, Nishiya N, Ginsberg MH, et al. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nature Med. 2008;14:448–453. doi: 10.1038/nm1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jones CA, Nishiya N, London NR, Zhu W, Sorensen LK, Chan AC, Lim CJ, Chen H, Zhang Q, Schultz PG, Hayallah AM, Thomas KR, Famulok M, Zhang K, Ginsberg MH, et al. Slit2–Robo4 signalling promotes vascular stability by blocking Arf6 activity. Nature Cell Biol. 2009;11:1325–1331. doi: 10.1038/ncb1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fish JE, Wythe JD, Xiao T, Bruneau BG, Stainier DY, Srivastava D, Woo S. A Slit/miR-218/Robo regulatory loop is required during heart tube formation in zebrafish. Development. 2011;138:1409–1419. doi: 10.1242/dev.060046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Small EM, Sutherland LB, Rajagopalan KN, Wang S, Olson EN. MicroRNA-218 regulates vascular patterning by modulation of Slit-Robo signaling. Circ Res. 2010;107:1336–1344. doi: 10.1161/CIRCRESAHA.110.227926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hu H. Cell-surface heparan sulfate is involved in the repulsive guidance activities of Slit2 protein. Nat Neurosci. 2001;4:695–701. doi: 10.1038/89482. [DOI] [PubMed] [Google Scholar]
- 76.Williams CK, Li JL, Murga M, Harris AL, Tosato G. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood. 2006;107:931–939. doi: 10.1182/blood-2005-03-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 78.Krueger J. Flt1 acts as a negative regulator of tip cell formation and branching morphogenesis in the zebrafish embryo. Development. 2011;138:2111–2120. doi: 10.1242/dev.063933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Biyashev D, Veliceasa D, Topczewski J, Topczewska JM, Mizgirev I, Vinokour E, Reddi AL, Licht JD, Revskoy SY, Volpert OV. miR-27b controls venous specification and tip cell fate. Blood. 2012;119:2679–2687. doi: 10.1182/blood-2011-07-370635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou Q, Gallagher R, Ufret-Vincenty R, Li X, Olson EN, Wang S. Regulation of angiogenesis and choroidal neovascularization by members of microRNA-23~27~24 clusters. Proc Natl Acad Sci U S A. 2011;108:8287–8292. doi: 10.1073/pnas.1105254108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yusoff P, Lao DH, Ong SH, Wong ES, Lim J, Lo TL, Leong HF, Fong CW, Guy GR. Sprouty2 inhibits the Ras/MAP kinase pathway by inhibiting the activation of Raf. J Biol Chem. 2002;277:3195–3201. doi: 10.1074/jbc.M108368200. [DOI] [PubMed] [Google Scholar]
- 82.Urbich C, Kaluza D, Frömel T, Knau A, Bennewitz K, Boon RA, Bonauer A, Doebele C, Boeckel J-N, Hergenreider E, Zeiher AM, Kroll J, Fleming I, Dimmeler S. MicroRNA-27a/b controls endothelial cell repulsion and angiogenesis by targeting semaphorin 6A. Blood. 2012;119:1607–1616. doi: 10.1182/blood-2011-08-373886. [DOI] [PubMed] [Google Scholar]
- 83.Dhanabal M, Wu F, Alvarez E, McQueeney KD, Jeffers M, MacDougall J, Boldog FL, Hackett C, Shenoy S, Khramtsov N, Weiner J, Lichenstein HS, LaRochelle WJ. Recombinant semaphorin 6A-1 ectodomain inhibits in vivo growth factor and tumor cell line-induced angiogenesis. Cancer Biol Ther. 2005;4:659–668. doi: 10.4161/cbt.4.6.1733. [DOI] [PubMed] [Google Scholar]
- 84.Stenzel D, Lundkvist A, Sauvaget D, Busse M, Graupera M, van der Flier A, Wijelath ES, Murray J, Sobel M, Costell M, Takahashi S, Fassler R, Yamaguchi Y, Gutmann DH, Hynes RO, et al. Integrin-dependent and -independent functions of astrocytic fibronectin in retinal angiogenesis. Development. 2011;138:4451–4463. doi: 10.1242/dev.071381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Francis SE, Goh KL, Hodivala-Dilke K, Bader BL, Stark M, Davidson D, Hynes RO. Central roles of alpha5beta1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler Thromb Vasc Biol. 2002;22:927–933. doi: 10.1161/01.atv.0000016045.93313.f2. [DOI] [PubMed] [Google Scholar]
- 86.Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, Chavakis E, Potente M, Tjwa M, Urbich C, Zeiher AM, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 87.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, Li Q, Li X, Wang W, Wang J, Jiang X, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 89.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 90.Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, Hristov M, Koppel T, Jahantigh MN, Lutgens E, Wang S, Olson EN, Schober A, Weber C. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2:ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 91.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, Tetta C, Bussolati B, Camussi G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71:5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 94.Umezu T, Ohyashiki K, Kuroda M, Ohyashiki JH. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene. 2012 doi: 10.1038/onc.2012.295. E-Pub, Jul 16. [DOI] [PubMed] [Google Scholar]
- 95.Zhuang G, Wu X, Jiang Z, Kasman I, Yao J, Guan Y, Oeh J, Modrusan Z, Bais C, Sampath D, Ferrara N. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 2012;31:3513–3523. doi: 10.1038/emboj.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Roxe T, Muller-Ardogan M, Bonauer A, Zeiher AM, Dimmeler S. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 97.Seitz H. Redefining microRNA targets. Curr Biol. 2009;19:870–873. doi: 10.1016/j.cub.2009.03.059. [DOI] [PubMed] [Google Scholar]
- 98.Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandolfi PP. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465:1033–1038. doi: 10.1038/nature09144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hamada K, Sasaki T, Koni PA, Natsui M, Kishimoto H, Sasaki J, Yajima N, Horie Y, Hasegawa G, Naito M, Miyazaki J, Suda T, Itoh H, Nakao K, et al. The PTEN/PI3K pathway governs normal vascular development and tumor angiogenesis. Genes Dev. 2005;19:2054–2065. doi: 10.1101/gad.1308805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tay Y, Kats L, Salmena L, Weiss D, Tan SM, Ala U, Karreth F, Poliseno L, Provero P, Di Cunto F, Lieberman J, Rigoutsos I, Pandolfi PP. Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs. Cell. 2011;147:344–357. doi: 10.1016/j.cell.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Karreth FA, Tay Y, Perna D, Ala U, Tan SM, Rust AG, DeNicola G, Webster KA, Weiss D, Perez-Mancera PA, Krauthammer M, Halaban R, Provero P, Adams DJ, Tuveson DA, et al. In vivo identification of tumor- suppressive PTEN ceRNAs in an oncogenic BRAF-induced mouse model of melanoma. Cell. 2011;147:382–395. doi: 10.1016/j.cell.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, Tramontano A, Bozzoni I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358–369. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Izzedine H, Rixe O, Billemont B, Baumelou A, Deray G. Angiogenesis inhibitor therapies: focus on kidney toxicity and hypertension. Am J Kidney Dis. 2007;50:203–218. doi: 10.1053/j.ajkd.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 105.van Rooij E, Purcell AL, Levin AA. Developing microRNA therapeutics. Circ Res. 2012;110:496–507. doi: 10.1161/CIRCRESAHA.111.247916. [DOI] [PubMed] [Google Scholar]
- 106.van Solingen C, Seghers L, Bijkerk R, Duijs JM, Roeten MK, van Oeveren-Rietdijk AM, Baelde HJ, Monge M, Vos JB, de Boer HC, Quax PH, Rabelink TJ, van Zonneveld AJ. Antagomir-mediated silencing of endothelial cell specific microRNA-126 impairs ischemia-induced angiogenesis. J Cell Mol Med. 2009;13:1577–1585. doi: 10.1111/j.1582-4934.2008.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Parker LH, Schmidt M, Jin SW, Gray AM, Beis D, Pham T, Frantz G, Palmieri S, Hillan K, Stainier DY, De Sauvage FJ, Ye W. The endothelial-cell-derived secreted factor Egfl7 regulates vascular tube formation. Nature. 2004;428:754–758. doi: 10.1038/nature02416. [DOI] [PubMed] [Google Scholar]