Abstract
The Type B trichothecene deoxynivalenol (DON), a ribotoxic mycotoxin known to contaminate cereal-based foods, induces ribosomal RNA (rRNA) cleavage in the macrophage via p38-directed activation of caspases. Here we employed the RAW 264.7 murine macrophage model to test the hypothesis that this rRNA cleavage pathway is similarly induced by other ribotoxins. Capillary electrophoresis confirmed that the antibiotic anisomycin (≥25 ng/ml), the macrocylic trichothecene satratoxin G (SG) (≥10 ng/ml) and ribosome-inactivating protein ricin (≥300 ng/ml) induced 18s and 28s rRNA fragmentation patterns identical to that observed for DON. Also, as found for DON, inhibition of p38, double-stranded RNA-activated kinase (PKR) and hematopoietic cell kinase (Hck) suppressed MAPK anisomycin-induced rRNA cleavage, while, in contrast, their inhibition did not affect SG- and ricin-induced rRNA fragmentation. The p53 inhibitor pifithrin-μ and pan caspase inhibitor Z-VAD-FMK suppressed rRNA cleavage induced by anisomycin, SG and ricin, indicating that these ribotoxins shared with DON a conserved downstream pathway. Activation of caspase 8, 9 and 3 concurrently with apoptosis further suggested rRNA cleavage occurred in parallel with both extrinsic and intrinsic pathways of programmed cell death. When specific inhibitors cathepsin L and B (lysosomal cysteine cathepsins active at cytosolic neutral pH) were tested, only the former impaired anisomycin-, SG-, ricin- and DON-induced rRNA cleavage. Taken together, the data suggest that (1) all four ribotoxins induced p53-dependent rRNA cleavage via activation of cathepsin L and caspase 3, and (2) activation of p53 by DON and anisomycin involved p38 whereas SG and ricin activated p53 by an alternative mechanism.
INTRODUCTION
Several natural toxins are capable of inducing a “ribotoxic stress response” that has been linked to activation of mitogen-activated protein kinases (MAPKs), aberrant gene regulation, ribosomal RNA (rRNA) cleavage and apoptosis (Iordanov et al., 1997; Laskin et al., 2002; Bunyard et al., 2003; Xia et al., 2007; Zhou et al., 2005a; He et al., 2012). Such ribotoxins include both low-molecular-weight compounds (eg trichothecenes and anisomycin) that directly bind to ribosome and ribosome-inactivating proteins (RIPs) (eg. ricin and Shiga toxin). The mechanisms by which these toxins induce ribotoxic stress and its downstream sequelae remain incompletely understood.
The trichothecenes comprise a family of secondary sesquiterpenoid mycotoxin metabolites (>200), which are divided into four types (A, B, C, D) based on group differences on the conserved sesquiterpene backbone with a 9,10-double bond and a 12,13-epoxide (Kimura et al., 2007). The Type B trichothecene deoxynivalenol (DON), a frequent contaminant in cereal products worldwide that exerts adverse effects on human and animals, has been extensively studied with regard to mechanisms of its acute and chronic toxic effects (Pestka, 2010). The Type D (macrocyclic) trichothecene satratoxin G (SG) is produced by the black mold Stachybotrys and has been linked to damp building-related illnesses (DBRI) (Pestka et al., 2008). Anisomycin is an antibiotic produced by Streptomyces.
Ribosome-inactivating proteins are divided into two types based on the composition of peptide: type 1 RIP consists of a single peptide (A-chain) and type 2 is composed of two peptides (A- and B-chain). The A-chain of RIPs contains a RNA N-glycosidase domain that specifically cleaves adenine off the highly conserved sarcin/ricin (S/R) loop on eukaryotic 28s rRNA, while B-chain can bind to the cell surface and mediate the entrance of whole RIPs into the cell by endocytosis (Hartley and Lord, 2004). Ricin is a type 2 RIP found in castor beans. After entering the cells by endocytosis, ricin undergoes vesicular retrograde transport from early endosomes to the trans-Golgi network (TGN) and reaches the lumen of the ER, where A-chain is released and translocates into the cytosol to depurinate 28s rRNA (Olsnes, 2004), which has been proposed as the sensor for ribotoxic stress (Iordanov et al., 1997). Ricin additionally causes cleavage on 18s and 28s rRNA at A3560 and A4045 (Li and Pestka, 2008).
Mononuclear phagocytes of the innate immune system appear to be particularly susceptible to the ribotoxic stressors. We have previously observed that the DON induces cleavage of 18s and 28s RNA the RAW 264.7 murine macrophage model and that was closely linked to apoptosis onset (He et al., 2012). rRNA cleavage was linked to the sequential activation of two ribosome associated kinases (double-stranded RNA activated protein kinase [PKR] and hematopoietic cell kinase [Hck]), p38 MAPK, p53, caspase 8/9 and caspase 3. Interestingly, the small molecules anisomycin and SG as well the RIP ricin appear to evoke identical rRNA cleavage profiles in the macrophage but the mechanisms are not known. The purpose of this investigation was to test the hypothesis that ribotoxins share common mechanisms with DON for mediating rRNA cleavage in the well-studied RAW 264.7 model. Specifically, we compared signaling pathways for DON-, anisomycin-, SG- and ricin-induced rRNA cleavage in RAW 264.7. The results demonstrated that all of the ribotoxins induced rRNA cleavage involving mechanisms closely linked to both extrinsic and intrinsic apoptotic pathways. Critical common upstream elements included p53, caspases and cathepsin L. p53 activation by DON and anisomycin involved PKR, Hck and p38, while SG and ricin appeared to activated p53 via an alternative route.
MATERIALS AND METHODS
Chemicals
DON, anisomycin, PKR inhibitor C16 and Hck inhibitor PP1 were purchased from Sigma-Aldrich (St. Louis, MO). Ricin was obtained from Vector Labs Inc. (Burlingame, CA). Satratoxin G (SG) was purified as described previously (Islam et al., 2009). The p38 inhibitor SB203580, JNK inhibitor SP600125, ERK inhibitor PD98059, p53 inhibitor pifithrin-μ, pan cathepsin inhibitor and cathepsin L inhibitor I, and cathepsin B inhibitor II were purchased from EMD MilliporeChemicals Inc. (Gibbstown, NJ). Pan caspase inhibitor Z-VAD-FMK was supplied by BD Pharmingen (San Diego, CA). All other chemicals and medium components were obtained from Sigma-Aldrich, except where noted.
Macrophage cell culture
RAW 264.7 (ATCC, Rockville, MD), a mouse macrophage cell line, was cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), streptomycin (100 μg/ml) and penicillin (100 U/ml) at 37°C in a humidified atmosphere with 5% CO2. Macrophage cell number and viability were assessed by trypan blue dye exclusion using a hematocytometer. Cells (2.5 × 106) were seeded and cultured in 100-mm tissue culture plates for 24 h to achieve approximately 80% confluency prior to treatments with toxins (anisomycin, SG, ricin and DON) or inhibitors.
RNA purification and cleavage analysis
RNAs were extracted by TRIZOL (Invitrogen, Carlsbad, CA) following manufacturer’s protocol and their concentrations measured using a Nanodrop reader (Thermo Fisher, Wilmington, DE). The cleavage of rRNA (300 ng/μl) was analyzed by capillary electrophoresis using an Agilent 2100 Bioanalyzer with a Nanochip (Agilent, Santa Clara, CA) following the manufacture’s instruction.
Immunoblotting
Western analyses were conducted using primary antibodies specific for murine forms of total/cleaved caspase-9, cleaved caspase 3 (Asp175), total caspase 8 and cleaved caspase 8 (Asp387), total and phosphorylated p38, JNK and ERK1/2 (Cell Signaling, Beverly, MA). Mouse β-actin antibody (Sigma) was to confirm loading. Cells were washed twice with ice-cold phosphate-buffered saline (PBS), lysed in boiling lysis buffer (1% [w/v] sodium dodecylsulfate, 1 mM sodium ortho-vanadate and 10 mM Tris, pH 7.4), boiled for 5 min and sonicated briefly; the lysate was centrifuged at 12,000 × g for 10 min at 4 °C. Protein in the resultant supernatant was measured with a BCA protein assay kit (Fisher, Pittsburgh, PA). Total cellular proteins (40 μg) were separated on BioRad precast 4–20% polyacrylamide gel (BioRad, Hercules, CA) and transferred to a polyvinylidene difluoride (PVDF) membrane (Amersham, Arlington Heights, IL). After incubating with blocking buffer (Li-Cor, Lincoln, NE) for 1 h at 25 °C, membranes were incubated with murine and/or rabbit primary antibodies (1:1000 dilution in Li-Cor blocking buffer) to immobilized proteins of interest overnight at 4 °C. After washing three times with Tris-Buffered Saline and Tween 20 (TBST) for 10 min each, blots were incubated with secondary IRDye 680 goat anti-rabbit and/or IRDye 800 goat anti-mouse IgG antibodies (Li-Cor) (1:2000 dilution in fresh Li-Cor blocking buffer) for 1 h at 25 °C. After washing three times, infrared fluorescence from these two antibody conjugates were simultaneously measured using a Li-Cor Odyssey Infrared Imaging System.
Morphometric measurement of apoptosis
Acridine orange/ethidium bromide (AO/EB) staining was performed using a previously described (Muppidi et al., 2004) with modifications. Briefly, microscope slides were sterilized by UV light, added to 100-mm tissue culture plates and then seeded with RAW 264.7 cells (2.5 × 106) for 24 h to achieve approximately 80% confluency. Cells were then treated with anisomycin (25 ng/ml), SG (10 ng/ml), or ricin (500 ng/ml) for 6 h and the slides with attached cells stained for 2 min in dye mixture consisting of 100 μg/ml acridine orange and 100 μg/ml ethidium bromide in PBS. After washing twice with cold PBS, slides were coverslipped and examined at 400x under Nikon fluorescence microscope equipped with a wide-band FITC filter. Cells (>200) were classified based on their nuclear morphology (bright chromatin, highly condensed or fragmented nuclei) in to four categories: viable normal (VN), viable apoptosis (VA), nonviable apoptosis (NVA), nonviable necrosis (NVN) and at least 200 cells were counted. The apoptotic index was calculated as follows:
Statistics
Data were analyzed by one-way ANOVA using Tukey’s test using Sigma Stat 3.11 (Jandel Scientific, San Rafael, CA). Data sets were considered significantly different when p < 0.05.
RESULTS
Anisomycin, SG, and ricin induce rRNA cleavage
Capillary electrophoresis has been previously used to demonstrate that DON induces cleavage of 18s and 28s RNA in RAW 264.7 macrophages (He et al., 2012). Similarly, exposure of these cells to anisomycin, SG and ricin for 6 h induced rRNA cleavage at concentrations as low as 10 ng/ml, 4 ng/ml and 50 ng/ml, respectively (Fig. 1 A, B, and C). In a follow-up kinetic study, anisomycin (25 ng/ml), SG (10 ng/ml) and ricin (300 ng/ml) were found to induce significant rRNA cleavage beginning at 3 h, 4 h, 5 h, respectively (Fig. 2 A, B, C). These latter concentrations were selected for subsequent mechanistic studies.
FIG. 1. Concentration dependence of anisomycin-, SG- and ricin-induced rRNA cleavage.
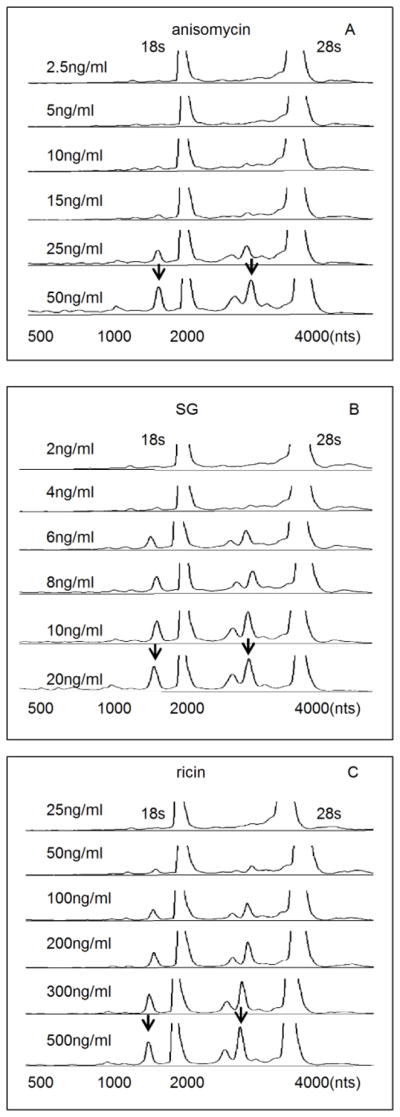
RAW 264.7 cells were treated with indicated concentrations of (A) anisomycin, (B) SG and (C) ricin, respectively, for 6 h and total RNA then analyzed by capillary electrophoresis. The 18s and 28s rRNAs peaks are labeled and only the regions between 500 to 4000 nts are shown. The Y-axis fluorescence unit (FU) cutoff is 50. Arrows indicate representative sites of cleavage. DON has been previously shown to similarly induce cleavage of 18s and 28s RNA in this model (He et al., 2012).
FIG. 2. Kinetics of anisomycin-, SG- and ricin-induced rRNA cleavage.
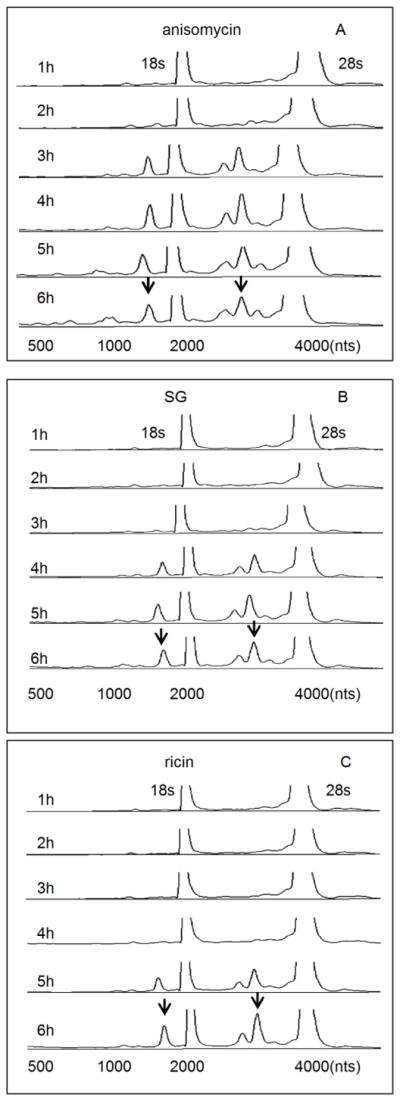
Cells were treated with (A) anisomycin (25 ng/ml), (B) SG (10 ng/ml) and (C) ricin (300 ng/ml), respectively, for indicated time intervals. RNA cleavage was analyzed by gcapillary electrophoresis as reported in Fig. 1 legend.
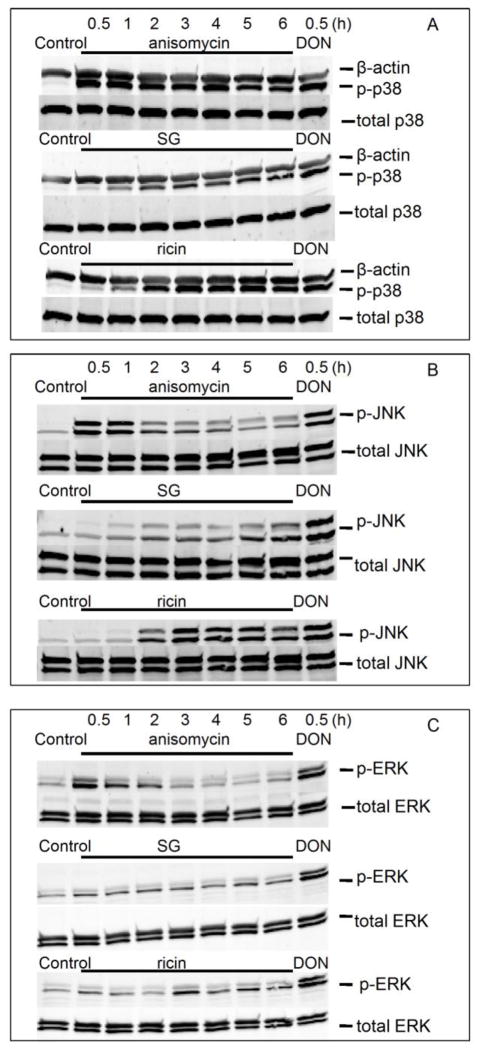
Anisomycin-induced MAPK activation differs temporally from that for SG and ricin
The capacities of ribotoxins to induce MAPK activation were compared to that of DON. Anisomycin induced robust phosphorylation of p38 at 30 min that lasted up to 6 h. SG and ricin activation of p38 was detected at 1 h, which was maximal at 2 h and lasted 6 h, (Fig. 3 A). Anisomycin also activated JNK and ERK1/2 at 30 min which were attenuated at ≥ 2 h. In contrast, SG and ricin activated JNK at ≥2 h (Fig. 3 B) as well as induced very modest phosphorylation of ERK2 (Fig. 3 C). When the activation of p38, JNK and ERK by anisomycin, SG and ricin at 30 min were compared to that of DON, only anisomycin showed a similar activation pattern (Fig. 3A, B, C).
FIG. 3. Anisomycin, SG and ricin differentially activate p38, JNK and ERK.
Cells were treated with anisomycin (25 ng/ml), SG (10 ng/ml) and ricin (300 ng/ml) for 0.5, 1, 2, 3, 4, 5 and 6 h, and DON (1000 ng/ml) for 0.5 h, respectively. Cells were then lysed and subjected to Western blotting analysis with total and phosphorylated (A) p38, (B) JNK and (C) ERK antibodies. β-actin was immunochemically stained as loading control (A).
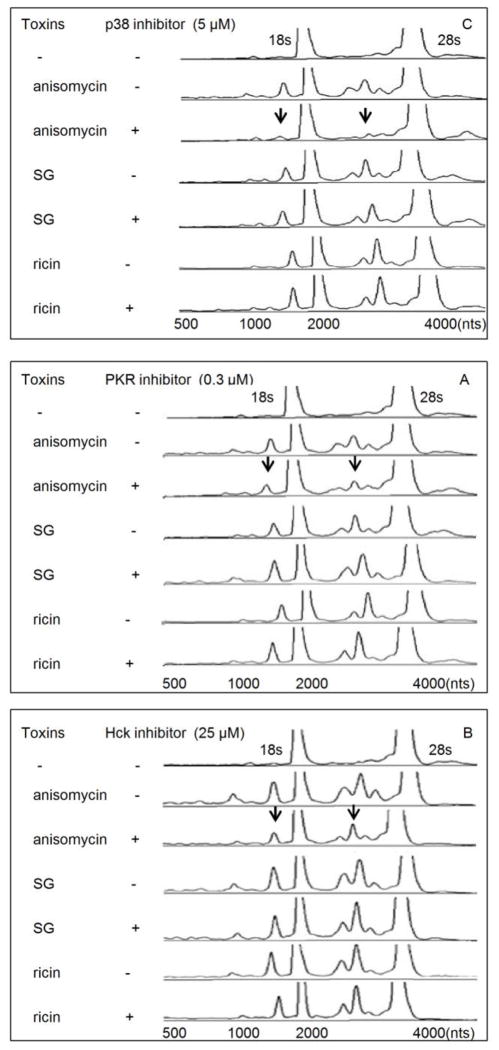
PKR, Hck and p38 inhibition suppresses induction of cleavage by anisomycin but not SG and ricin
As has previously been described for DON (He et al., 2012), pharmacological inhibition of p38 (5 μM, Fig. 4 A), suppressed anisomycin-induced rRNA cleavage. However, this inhibitor did not block SG- and ricin-induced rRNA cleavage. Also as observed for DON, anisomycin-, SG- and ricin-induced rRNA cleavage were not affected by JNK and ERK inhibition (data not shown). Both PKR and Hck have been shown in a prior studies to be upstream of DON-induced p38 activation (Zhou et al.,2003;2005b) and requisites for rRNA cleavage (He et al., 2012). Similar to DON, inhibition of these kinases blocked rRNA cleavage induction by anisomycin but not SG and ricin (Fig. 4 B, C). These data suggest that anisomycin shared a common upstream signaling pathway with DON, while SG and ricin apparently employ alternative upstream mechanisms.
FIG. 4. Anisomycin, but not SG and ricin, induce rRNA cleavage through p38, PKR and Hck.
Cells were pre-treated with (A) SB-203580 (5 μM), (B) C-16 (0.3 μM) or (C) PP1 (25 μM) for 1 h before anisomycin (25 ng/ml), SG (10 ng/ml) and ricin (300 ng/ml) treatment for 6 h, respectively. RNAs were purified and analyzed by capillary electrophoresis as described in Fig. 1 legend. Results are representative of three separate experiments. Arrows indicate that fragmentation to major cleavage peaks were suppressed by inhibitors.
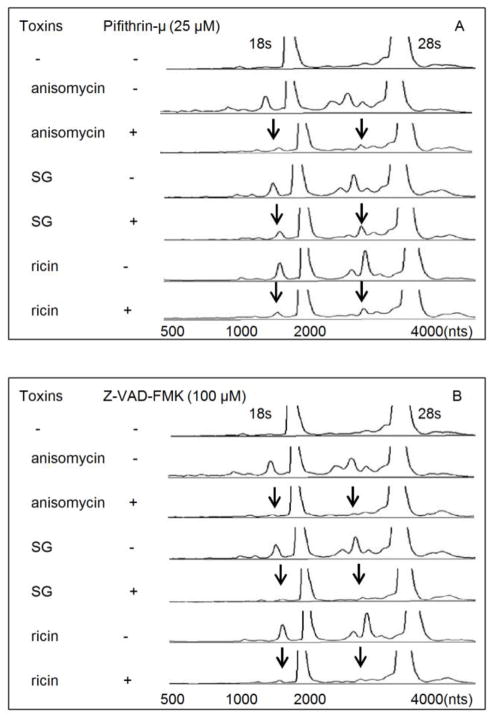
p53 and caspase inhibitors suppress SG-, anisomycin- and ricin-induced rRNA cleavage
As demonstrated earlier for DON (He et al., 2012), anisomycin-, SG- and ricin-induced rRNA cleavage were markedly suppressed by the p53 inhibitor pifithrin-μ and broad spectrum caspase inhibitor Z-VAD-FMK (Fig. 5 A, B). Thus, all four share a conserved downstream pathway involving p53 and caspases to induce rRNA cleavage.
FIG. 5. Anisomycin-, SG- and ricin-induced rRNA cleavage involves p53 and caspase.
Cells were pre-treated with (A) pifithrin-μ (25 μM) or (B) Z-VAD-FMK (100 μM) for 1 h before SG (10 ng/ml), anisomycin (25 ng/ml) and ricin (300 ng/ml) exposure for 6 h, respectively. RNAs were analyzed by capillary electrophoresis as described in Fig. 1 legend. Results are representative of three separate experiments. Arrows indicate that fragmentation to major cleavage peaks were suppressed by inhibitors.
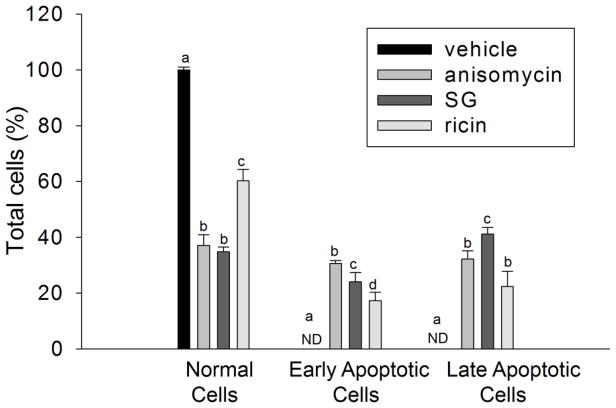
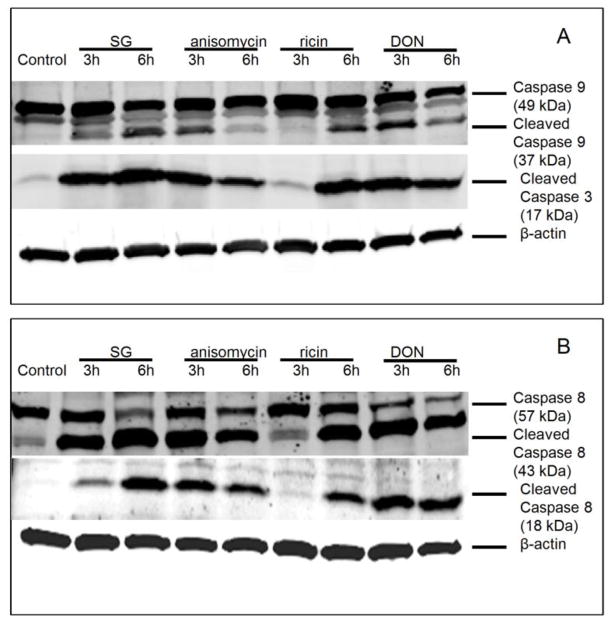
Anisomycin, SG and ricin induce apoptosis through extrinsic and intrinsic pathways
DON-induced rRNA cleavage was previously associated with apoptosis (He et al., 2012), AO/EB staining revealed that, likewise, anisomycin, SG and ricin markedly induced apoptosis concurrently with rRNA cleavage at 6 h (Fig. 6). Apoptosis can be via extrinsic and intrinsic pathways, which involve caspase 3 activation through caspase 8 and caspase 9, respectively. DON and anisomycin strongly activated caspase 9/3/8 at 3 h, which were attenuated at 6 h (7 A, B). On the contrary, SG and ricin evoked more caspase 9/3/8 cleavage at 6 h than 3 h (7 A, B). These data were consistent with time course studies of four toxins, in which DON and anisomycin caused rRNA cleavage at 2 to 3 h, whereas SG and ricin induced cleavage at 4 and 5 h, respectively.
FIG. 6. Anisomycin, SG and ricin induce apoptosis in RAW 264.7 cells.
Cells were treated with vehicle, anisomycin (25 ng/ml), SG (10 ng/ml) and ricin (300 ng/ml) for 6 h, respectively. Acridine orange/ethidium bromide staining (AO/EB) was used to calculate percentage of normal, early and late apoptotic adherent cells. Data are mean ± SE of triplicate wells. ND indicates non-detectable. Bars without the same letter differ (p<0.5).
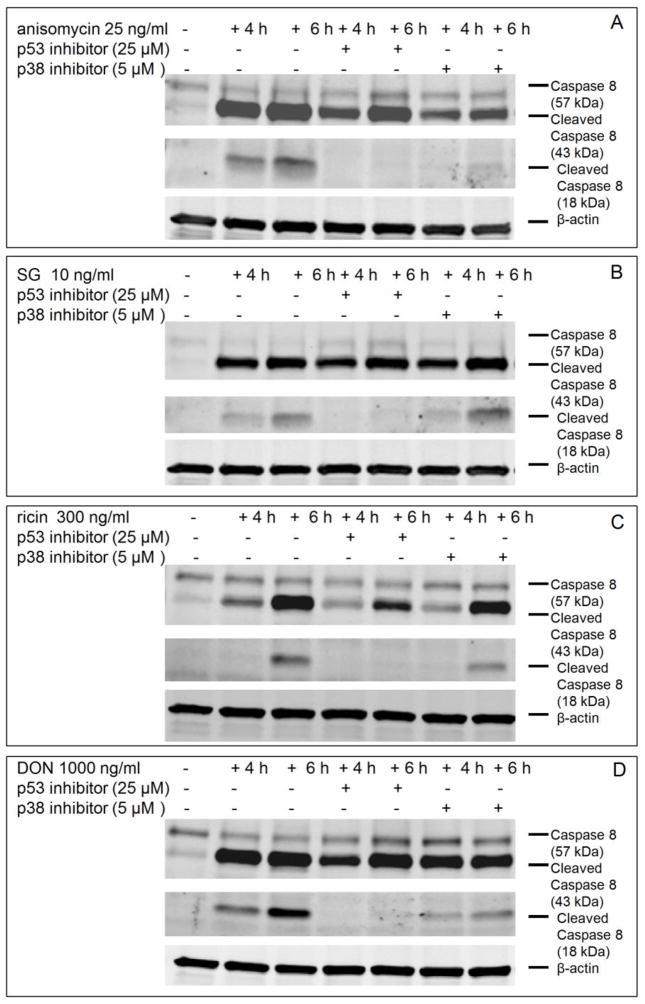
p53 inhibition suppresses caspase 8 activation by all four toxins but p38 inhibition suppresses only DON- and anisomycin-induced caspase 8 activation
Inhibition of p53 markedly suppressed anisomycin-, SG-, ricin- and DON-induced the cleavage of caspase 8 to its 18 KDa subunit (p18) (Fig. 8, A, B, C, D). p38 inhibition only suppressed anisomycin- and DON-induced caspase 8 activation (Fig. 8 A, D), which was again consistent with the prior observation that p38 mediates DON- and anisomycin-induced rRNA cleavage but not that of SG and ricin(Fig. 4 A).
Fig. 8. p38 inhibition suppresses only DON- and anisomycin-induced caspase 8 activation but p53 inhibition inhibits caspase 8 activation by all four toxins.
Cells were pretreated with vehicle, p53 inhibitor pifithrin-μ (25 μM) or p38 inhibitor SB-203580 (5 μM) for 1 h prior to treatment of (A) anisomycin (25 ng/ml), (B) SG (10 ng/ml), (C) ricin (300 ng/ml) and (D) DON (1000 ng/ml) for 4 and 6 h, respectively. Cells were lysed and subjected to Western blotting analysis with total and cleaved caspase 8. β-actin was also stained as loading control and shown at the bottom (A, B, C, D).
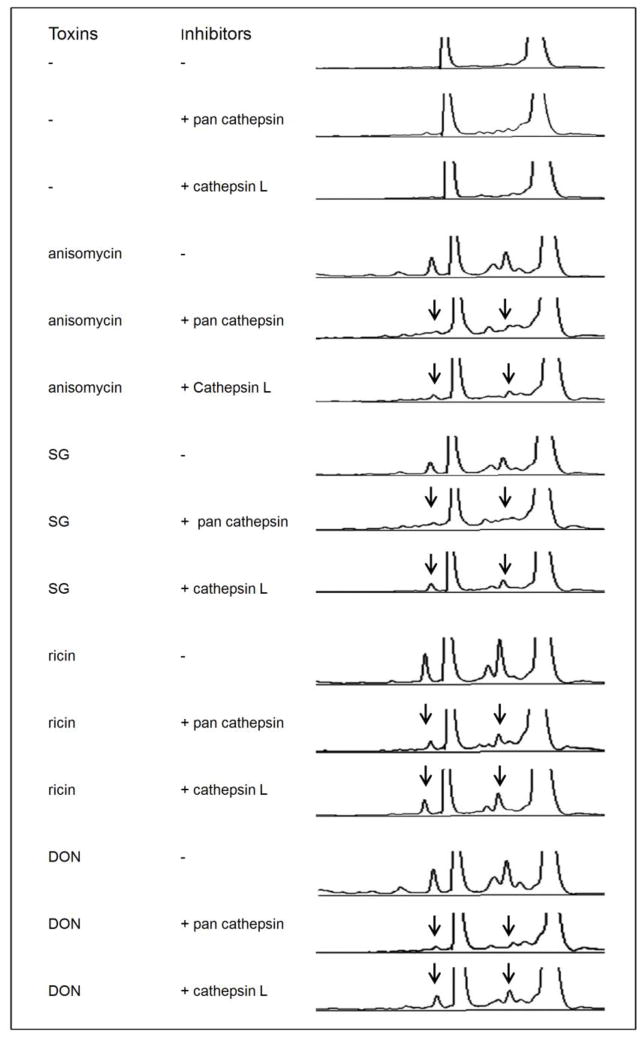
Cathepsin L is involved in anisomycin-, SG-, ricin- and DON-induced apoptosis-associated rRNA cleavage
Lysosome membrane permeabilization (LMP) and subsequent cathepsin release are believed to contribute to caspase-dependent apoptosis through the cleavage of BID and proapoptotic Bcl-2 homologues (Droga-Mazovec et al., 2008). The pan cathepsin inhibitor suppressed anisomycin-, SG-, ricin- and DON-induced rRNA cleavage (Fig. 9). Since cathepsin B and L derived from lysosomes are the most active cysteine cathepsins under neutral cytosolic pH (Boya and Kroemer, 2008), specific inhibitors for them were used to identify the executing cathespin. Cathepsin L inhibitor I, which specifically inhibits cathepsin L and B (Esser et al., 1994), significantly suppressed anisomycin-, SG-, ricin- and DON-induced rRNA cleavage (Fig. 9). In contrast, cathepsin B inhibitor II, which specifically inhibits cathepsin B (McConnell et al., 1993), was without effect up to concentrations of 20 μM (data not shown). Thus, in addition to cathepsin L’s known executive action during apoptosis, its activation also appears to be critical for ribotoxin-induced rRNA cleavage.
FIG. 9. Cathepsin L is involved in anisomycin-, SG-, ricin- and DON-induced rRNA cleavage.
Cells were pretreated with vehicle, pan cathepsin inhibitor (40 μM) or cathepsin L inhibitor (20 μM) for 1 h followed by anisomycin (25 ng/ml), SG (10 ng/ml), ricin (300 ng/ml) and DON (1000 ng/ml) exposure for 6 h, respectively. RNAs were analyzed by capillary electrophoresis as described in Fig. 1 legend. The results are representative of three separate experiments. Arrows indicate that fragmentation to major cleavage peaks is suppressed by inhibitors.
DISCUSSION
The results described herein demonstrate that four ribotoxins, namely, DON, anisomycin, SG and ricin, although different in structure and activity, induce apoptosis-associated rRNA cleavage. Both DON and anisomycin triggered rRNA cleavage through PKR/Hck-mediated p38 activation and subsequent p53-dependent induction of the extrinsic and intrinsic apoptotic pathways. While SG and ricin also could evoke rRNA cleavage via activation of p53 and associated apoptotic pathways, p38 or its upstream kinases were not involved. These findings are quite consistent with the rapid, robust p38 activation by DON and anisomycin compared to relatively slower and weaker activation of p38 by SG and ricin. Taken together, ribotoxins appear to induce apoptosis and rRNA cleavage through conserved activation of p53 and caspases involving cathepsins, especially cathepsin L, but differed with respect to the requirement for upstream p38 activation (Fig. 10).
FIG. 10. Model for ribotoxin-induced rRNA cleavage model in RAW 264.7 cells.
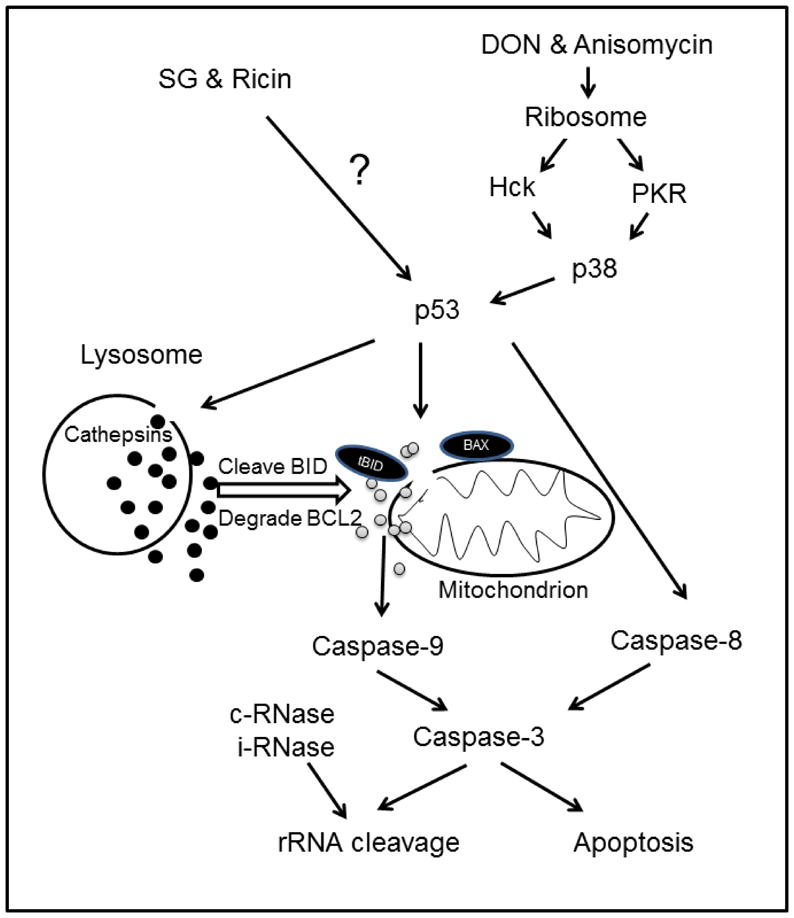
Picture depicts putative signaling pathways for induction of rRNA cleavage by DON, anisomycin, SG and ricin. DON and anisomycin sequentially activate PKR/Hck, p38, p53 and caspases leading to apoptosis-associate rRNA cleavage, while SG and ricin activate p53 and caspases by an alternative mechanism. Cathepsin L released from lysosome promotes DON-, anisomycin-, SG- and ricin-induced apoptosis and rRNA cleavage. Both constitutive (c) and inducible (i) RNases might contribute to the ribotoxin-induced cleavage of rRNA exposed by caspase action. Black and gray circles represent cathepsins and cytochrome c, respectively.
Apoptosis is mediated by intrinsic and extrinsic apoptotic pathways. The intrinsic pathway is characterized by mitochondrial dysfunction, release of apoptotic activators and sequential activation of caspase 9 and 3 (Chandra et al., 2004) both of which were found to be activated by anisomycin, SG and ricin. The canonical extrinsic pathway is mediated by death receptors, the binding of ligands to which will recruit the procaspase 8 to the death-inducing signaling complex (DISC) via the interaction with FADD (von Roretz and Gallouzi, 2010). Inactive procaspase 8 is activated by sequential cleavage at Asp374/384 and Asp216, releasing small and large subunits (p10 and p18), which assemble into active heterotetramer caspase 8 (Zhao et al., 2010). We demonstrate here that DON, anisomycin, SG and ricin caused cleavage of procaspase 8 and generated the p18 subunit
As determined previously for DON, p53 plays a pivotal role in caspase activation and induction of rRNA cleavage (He et al., 2012). Our data showed that inhibitors for p53 and caspases similarly abrogate apoptosis-associated rRNA cleavage by anisomycin, SG and ricin, suggesting they are the conserved mediators for ribotoxin-induced rRNA cleavage. This result is consistent with previous studies demonstrating that p53 also induces cell apoptosis through cytochrome c release and caspase 9 activation (Soengas et al., 1999; Schuler and Green, 2001; Zhou et al., 2005a). Our data are further consistent with previous observations that p53 upregulates caspase 8 expression (Ehrhardt et al., 2008) and mediates caspase 8 activation in both transcription-dependent and -independent manners (Ding et al., 2000; Yao et al., 2007).
It has been suggested that ribotoxins that share common binding sites on rRNA might initiate identical signal transduction (Iordanov et al., 1997). Anisomycin and DON are small molecules that freely diffuse through cell membrane and bind to the peptidyl transferase center (PTC) on 28s rRNA (Iordanov et al., 1997; Shifrin and Anderson, 1999), possibly activating same signaling pathways. DON induces posphorylation of PKR and Hck possibly by perturbation of rRNA tertiary structure which, in turn, activate the MAPKs p38, JNK and ERK (Pestka, 2010). These processes apparently drive competing apoptotic (p38/p53/Bax/mitochondria/caspase-3) and survival (ERK/AKT/p90Rsk/Bad) pathways in macrophages (Zhou et al., 2005a). Anisomycin rapidly activated MAPKs as observed for DON, and furthermore, PKR, Hck and p38 inhibition suppressed anisomycin-induced rRNA cleavage, suggesting DON and anisomycin activate the identical signaling pathway upstream to rRNA cleavage.
Ricin possesses N-glycosidase activity and inhibits translation by depurinating the 28S rRNA to induce apoptosis. Although the potential linkage between depurination and apoptosis is not fully understood, it is generally accepted that inhibition of protein synthesis by RIPs triggers a mitochondrial stress response followed by loss of mitochondrial membrane potential (MMP), rapid release of cytochrome c and activation of caspase 9 (Narayanan et al., 2005). Consistent with that concept, our data showed ricin-induced apoptosis-associated rRNA cleavage was mediated by p53 and caspase 8/9/3.
Lysosomes are acidic (pH ≤5), highly dynamic single-membrane bound organelles that contain hydrolytic enzymes that degrade intracellular macromolecules. Cathepsins are the best-characterized proteases in lysosome and include cysteine cathepsins with a few serine (A and G) and aspartic cathespins (D and E) (Johansson et al., 2010). In response to a variety of stress stimuli, lysosomal membrane permeabilization (LMP) occurs leading to release of cathepsins into cytosol. Cathepsin B, L and D are active at neutral cytosolic pH, and promote apoptosis (Boya and Kroemer, 2008). Active cathepsins are released upon LMP and cleave various protein substrates including pro-apoptotic BID and anti-apoptotic BCL-2 molecules (BCL-2, BCL-XL and MCL-1) leading to MMP, the central event in the intrinsic apoptotic pathway regulating the release of cytochrome c (Repnik and Turk, 2010). Our findings suggest that lysosome-derived cathepsin L was a key protease in rRNA cleavage induction by all four ribotoxins.
LPM can be induced by a range of distinct agents and molecules, such as ROS, p53, lysosomotropic agents (Boya and Kroemer, 2008). Anisomycin-, SG-, ricin- and DON-induced apoptosis-associated rRNA cleavage was suppressed by inhibitors of p53 and cathepsins, indicating that p53 mediates both ribotoxin-induced LMP and cathepsin release. p53 has been found to induce LMP in transcription-independent fashions (Johansson et al., 2010). After being phosphorylated at Ser15, p53 translocates to lysosome membrane and directly trigger LMP (Li et al., 2007). Association of phospho-p53 (ser15) with the lysosome not only destabilizes its membrane but also concurrently increases the cytosolic cathepsin L activity (Fogarty et al., 2010). Indeed, as observed here, cathepsin and cathepsin L inhibitors suppressed anisomycin-, SG-, ricin- and DON-induced rRNA cleavage. Thus it might be speculated that p53 coordinates LMP- and MMP-dependent apoptotic pathways in conjunction with rRNA cleavage. However, the exact mechanisms for p53-mediated LMP and the crosstalk between LMP and MMP remain to be determined.
To summarize, the results presented here demonstrate that anisomycin, SG, ricin and DON caused apoptosis-associated rRNA cleavage via a common conserved downstream signaling pathway involving both caspases and cathepsin L (Fig. 10). DON- and anisomycin-induced rRNA cleavage requires prior sequential activation of PKR/Hck, p38, p53 and caspases, while SG and ricin activated p53 and caspases did not. Future work should focus on clarifying the mechanisms by which lysosome membrane integrity is disrupted leading to release of cathepsins as well as the exact roles of cathepsin L in induction of apoptosis and rRNA cleavage. It will be of further interest to understand crosstalk that exists between lysosome-dependent and caspase-dependent apoptotic pathway and to identify the executing RNases specifically cleaving rRNA. Additionally, it will be necessary to determine how SG and ricin activate p53. Ultimately, in vivo studies of the ribotoxin-induced apoptosis-associated rRNA cleavage will be essential to establish the systemic biological significance of rRNA cleavage to immune dysfunction.
FIG. 7. Anisomycin, SG, ricin and DON activate caspases 8, 9 and 3.
Cells were treated with SG (10 ng/ml), anisomycin (25 ng/ml), ricin (300 ng/ml) and DON (1000 ng/ml) for 3 and 6 h, respectively. Cells were lysed and subjected to Western blotting analysis with (A) total and cleaved caspase 9 and cleaved caspase 3 antibodies or (B) total and cleaved caspase 8 antibodies. β-actin was also stained as loading control and shown at the bottom (A, B).
Highlights.
Deoxynivalenol (DON) anisomycin, satratoxin G (SG) and ricin are ribotoxins.
Ribotoxins induce 18s and 28s rRNA cleavage in the RAW 264.7 macrophage model.
Ribotoxins induce rRNA cleavage via activation of p53, caspases and cathepsins.
DON- and anisomycin-triggered rRNA cleavage is p38-dependent
SG- and ricin-induced rRNA cleavage is p38-independent
Acknowledgments
This study was supported by ES003358 from the National Institutes of Health grant as well as USDA Wheat and Barley SCAB Initiative Award 59-0206-9-058 and USDA NIFA Award 2011-D635. We thank Mary Rosner for technical assistance as well as Laura Vines for advice and suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boya P, Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene. 2008;27:6434–6451. doi: 10.1038/onc.2008.310. [DOI] [PubMed] [Google Scholar]
- Bunyard P, Handley M, Pollara G, Rutault K, Wood I, Chaudry M, Alderman C, Foreman J, Katz DR, Chain BM. Ribotoxic stress activates p38 and JNK kinases and modulates the antigen-presenting activity of dendritic cells. Mol Immunol. 2003;39:815–827. doi: 10.1016/s0161-5890(02)00262-6. [DOI] [PubMed] [Google Scholar]
- Chandra D, Choy G, Deng X, Bhatia B, Daniel P, Tang DG. Association of active caspase 8 with the mitochondrial membrane during apoptosis: potential roles in cleaving BAP31 and caspase 3 and mediating mitochondrion-endoplasmic reticulum cross talk in etoposide-induced cell death. Mol Cell Biol. 2004;24:6592–6607. doi: 10.1128/MCB.24.15.6592-6607.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding HF, Lin YL, McGill G, Juo P, Zhu H, Blenis J, Yuan J, Fisher DE. Essential role for caspase-8 in transcription-independent apoptosis triggered by p53. J Biol Chem. 2000;275:38905–38911. doi: 10.1074/jbc.M004714200. [DOI] [PubMed] [Google Scholar]
- Droga-Mazovec G, Bojic L, Petelin A, Ivanova S, Romih R, Repnik U, Salvesen GS, Stoka V, Turk V, Turk B. Cysteine cathepsins trigger caspase-dependent cell death through cleavage of bid and antiapoptotic Bcl-2 homologues. J Biol Chem. 2008;283:19140–19150. doi: 10.1074/jbc.M802513200. [DOI] [PubMed] [Google Scholar]
- Ehrhardt H, Hacker S, Wittmann S, Maurer M, Borkhardt A, Toloczko A, Debatin KM, Fulda S, Jeremias I. Cytotoxic drug-induced, p53-mediated upregulation of caspase-8 in tumor cells. Oncogene. 2008;27:783–793. doi: 10.1038/sj.onc.1210666. [DOI] [PubMed] [Google Scholar]
- Esser RE, Angelo RA, Murphey MD, Watts LM, Thornburg LP, Palmer JT, Talhouk JW, Smith RE. Cysteine proteinase inhibitors decrease articular cartilage and bone destruction in chronic inflammatory arthritis. Arthritis & Rheumatism. 1994;37:236–247. doi: 10.1002/art.1780370213. [DOI] [PubMed] [Google Scholar]
- Fogarty MP, McCormack RM, Noonan J, Murphy D, Gowran A, Campbell VA. A role for p53 in the beta-amyloid-mediated regulation of the lysosomal system. Neurobiol Aging. 2010;31:1774–1786. doi: 10.1016/j.neurobiolaging.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Grollman AP. Inhibitors of protein biosynthesis. II Mode of action of anisomycin. J Biol Chem. 1967;242:3226–3233. [PubMed] [Google Scholar]
- Hartley MR, Lord JM. Cytotoxic ribosome-inactivating lectins from plants. Biochim Biophys Acta. 2004;1701:1–14. doi: 10.1016/j.bbapap.2004.06.004. [DOI] [PubMed] [Google Scholar]
- He K, Zhou HR, Pestka JJ. Targets and Intracellular Signaling Mechanisms for Deoxynivalenol-Induced Ribosomal RNA Cleavage. Toxicol Sci. 2012;127:382–390. doi: 10.1093/toxsci/kfs134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanov MS, Pribnow D, Magun JL, Dinh TH, Pearson JA, Chen SL, Magun BE. Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the alpha-sarcin/ricin loop in the 28S rRNA. Mol Cell Biol. 1997;17:3373–3381. doi: 10.1128/mcb.17.6.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam Z, Shinozuka J, Harkema JR, Pestka JJ. Purification and comparative neurotoxicity of the trichothecenes satratoxin G and roridin L2 from Stachybotrys chartarum. J Toxicol Environ Health A. 2009;72:1242–1251. doi: 10.1080/15287390903129234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson AC, Appelqvist H, Nilsson C, Kagedal K, Roberg K, Ollinger K. Regulation of apoptosis-associated lysosomal membrane permeabilization. Apoptosis. 2010;15:527–540. doi: 10.1007/s10495-009-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Tokai T, Takahashi-Ando N, Ohsato S, Fujimura M. Molecular and genetic studies of fusarium trichothecene biosynthesis: pathways, genes, and evolution. Biosci Biotechnol Biochem. 2007;71:2105–2123. doi: 10.1271/bbb.70183. [DOI] [PubMed] [Google Scholar]
- Laskin JD, Heck DE, Laskin DL. The ribotoxic stress response as a potential mechanism for MAP kinase activation in xenobiotic toxicity. Toxicol Sci. 2002;69:289–291. doi: 10.1093/toxsci/69.2.289. [DOI] [PubMed] [Google Scholar]
- Li M, Pestka JJ. Comparative induction of 28S ribosomal RNA cleavage by ricin and the trichothecenes deoxynivalenol and T-2 toxin in the macrophage. Toxicol Sci. 2008;105:67–78. doi: 10.1093/toxsci/kfn111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Zheng Y, Chen W, Wang C, Liu X, He W, Xu H, Cao X. Adaptor protein LAPF recruits phosphorylated p53 to lysosomes and triggers lysosomal destabilization in apoptosis. Cancer Res. 2007;67:11176–11185. doi: 10.1158/0008-5472.CAN-07-2333. [DOI] [PubMed] [Google Scholar]
- McConnell RM, York JL, Frizzell D, Ezell C. Inhibition studies of some serine and thiol proteinases by new leupeptin analogs. J Med Chem. 1993;36:1084–1089. doi: 10.1021/jm00060a016. [DOI] [PubMed] [Google Scholar]
- Muppidi J, Porter M, Siegel RM. Measurement of apoptosis and other forms of cell death. Curr Protoc Immunol. 2004;Chapter 3(Unit 3 17) doi: 10.1002/0471142735.im0317s59. [DOI] [PubMed] [Google Scholar]
- Narayanan S, Surendranath K, Bora N, Surolia A, Karande AA. Ribosome inactivating proteins and apoptosis. FEBS Lett. 2005;579:1324–1331. doi: 10.1016/j.febslet.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Olsnes S. The history of ricin, abrin and related toxins. Toxicon. 2004;44:361–370. doi: 10.1016/j.toxicon.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Pestka JJ. Mechanisms of deoxynivalenol-induced gene expression and apoptosis. Food Addit Contam. 2008;24:1–13. doi: 10.1080/02652030802056626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka JJ, Yike I, Dearborn DG, Ward MD, Harkema JR. Stachybotrys chartarum, trichothecene mycotoxins, and damp building-related illness: new insights into a public health enigma. Toxicol Sci. 2008;104:4–26. doi: 10.1093/toxsci/kfm284. [DOI] [PubMed] [Google Scholar]
- Pestka JJ. Deoxynivalenol-induced proinflammatory gene expression: mechanisms and pathological sequelae. Toxins (Basel) 2010;2:1300–1317. doi: 10.3390/toxins2061300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repnik U, Turk B. Lysosomal-mitochondrial cross-talk during cell death. Mitochondrion. 2010;10:662–669. doi: 10.1016/j.mito.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Schuler M, Green DR. Mechanisms of p53-dependent apoptosis. Biochem Soc Trans. 2001;29:684–688. doi: 10.1042/0300-5127:0290684. [DOI] [PubMed] [Google Scholar]
- Shifrin VI, Anderson P. Trichothecene mycotoxins trigger a ribotoxic stress response that activates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase and induces apoptosis. J Biol Chem. 1999;274:13985–13992. doi: 10.1074/jbc.274.20.13985. [DOI] [PubMed] [Google Scholar]
- Soengas MS, Alarcon RM, Yoshida H, Giaccia AJ, Hakem R, Mak TW, Lowe SW. Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science. 1999;284:156–159. doi: 10.1126/science.284.5411.156. [DOI] [PubMed] [Google Scholar]
- von Roretz C, Gallouzi IE. Protein kinase RNA/FADD/caspase-8 pathway mediates the proapoptotic activity of the RNA-binding protein human antigen R (HuR) J Biol Chem. 2010;285:16806–16813. doi: 10.1074/jbc.M109.087320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Li Y, Rosen EM, Laterra J. Ribotoxic stress sensitizes glioblastoma cells to death receptor induced apoptosis: requirements for c-Jun NH2-terminal kinase and Bim. Mol Cancer Res. 2007;5:783–792. doi: 10.1158/1541-7786.MCR-06-0433. [DOI] [PubMed] [Google Scholar]
- Yao Z, Duan S, Hou D, Heese K, Wu M. Death effector domain DEDa, a self-cleaved product of caspase-8/Mch5, translocates to the nucleus by binding to ERK1/2 and upregulates procaspase-8 expression via a p53-dependent mechanism. EMBO J. 2007;26:1068–1080. doi: 10.1038/sj.emboj.7601571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Sui X, Ren H. From procaspase-8 to caspase-8: revisiting structural functions of caspase-8. J Cell Physiol. 2010;225:316–320. doi: 10.1002/jcp.22276. [DOI] [PubMed] [Google Scholar]
- Zhou HR, Lau AS, Pestka JJ. Role of double-stranded RNA-activated protein kinase R (PKR) in deoxynivalenol-induced ribotoxic stress response. Toxicol Sci. 2003;74:335–344. doi: 10.1093/toxsci/kfg148. [DOI] [PubMed] [Google Scholar]
- Zhou HR, Islam Z, Pestka JJ. Induction of competing apoptotic and survival signaling pathways in the macrophage by the ribotoxic trichothecene deoxynivalenol. Toxicol Sci. 2005a;87:113–122. doi: 10.1093/toxsci/kfi234. [DOI] [PubMed] [Google Scholar]
- Zhou HR, Jia Q, Pestka JJ. Ribotoxic stress response to the trichothecene deoxynivalenol in the macrophage involves the SRC family kinase Hck. Toxicol Sci. 2005b;85:916–926. doi: 10.1093/toxsci/kfi146. [DOI] [PubMed] [Google Scholar]