Abstract
Prostate cancer (PCa) contains small population of cancer stem cells (CSCs) that contribute to its initiation and progression. Development of specific markers for identification of the CSCs may lead to new diagnostic strategies of PCa. Increased aldehyde dehydrogenase 1A1 (ALDH1A1) activity has been found in stem cell populations of leukemia and some solid tumors. The goal of the study was to investigate the stem cell-related function and clinical significance of the ALDH1A1 in human PCa. Aldefluor assay was used to isolate ALDH1A1+ cells from PCa cell lines. Stem cell characteristics of the ALDH1A1+ cells were then investigated by in vitro and in vivo approaches. ALDH1A1 expression by immunohistochemestry in 18 normal prostate and 163 PCa tissues was also analyzed. The ALDH1A1+ PCa cells displayed high clonogenic and tumorigenic capacities, and serially reinitiated transplantable tumors that resembled histopathologic characteristics and heterogeneity of the parental PCa cells in mice. Immunohistochemical analysis of human prostate tissues showed that ALDH1A1+ cells were sparse and limited to the basal component in normal prostates. In tumor specimens, however, increased ALDH1A1 immunopositivity was found not only in secretory type cancer epithelial cells, but also in neuroendocrine tumor populations. Furthermore, the high ALDH1A1 expression in PCa was positively correlated with Gleason score (P=0.01) and pathologic stage (P=0.01), and inversely associated with overall survival and cancer-specific survival of the patients (P=0.00093, P=0.00017). ALDH1A1 could be a prostate CSC-related marker. Measuring its expression might provide a potential approach to study tumorigenesis of PCa and predict outcome of the disease.
Keywords: ALDH1A1, biomarkers, cancer stem cell, prostate cancer, prognosis
Prostate cancer (PCa) is the most frequently diagnosed tumor and the number two cancer killer in men. Its treatments depend on the stage of the disease, resulting in variable clinical outcome (1). Prognostic markers that can identify aggressive PCa and help select appropriate therapy to finally reduce the mortality are therefore urgently needed. However, current techniques are not accurate enough to predict outcome of PCa. New diagnostic modalities have to be developed (1).
The cancer stem cell (CSC) model proposes that tumorigenesis is driven by CSCs that might be derived from mutated adult stem cells (SCs). CSCs are functionally defined by the following criteria: high tumorigenicity, self-renewal capacity, recapitulating the heterogeneity of the original primary tumor (2). Although being present in small numbers in tumor tissues, CSCs are believed to be responsible for progression and relapse of cancer (3). Therefore, CSCs could provide a prognostic strategy for human malignancies. For example, CD133, a commonly used marker to characterize colon CSCs was proven to be an independent prognostic factor that correlated with low survival of colon cancer patients (4). Accumulating evidence posit that CSC concept is also relevant to PCa (5). The growth and metastasis of PCa might be promoted by CSCs that are responsible for its aggressiveness and frequently recur after therapy (6). Therefore, identification of specific markers for the CSCs would deep our understanding of the tumor biology of prostate. More importantly, these specific markers could be developed as a new diagnostic system for monitoring the progression of PCa and offering the best opportunity to prevent its recurrence, and hence probably cure the challenging malignancy.
Human mature prostatic glands consist of basal, secretory luminal, and neuroendocrine cells. Prostate CSs are suggested to localize in the basal cell layer (7). Several candidate populations of prostate stem/progenitors cells have been reported, including those expressing cell surface markers, CD44, α2ß1, or CD133 (7-8). For example, Collins et al. found that the α2ß1+ cells comprised approximately 15% of the CD44+ basal cells and possessed high colony-forming efficiency and an ability to generate prostate-like acini when engrafted in nude mice (7). Because CSCs share properties with normal SCs, CSCs of PCa have been isolated by using these cell surface markers (5). However, the PCa cells that highly express the markers do not always appear to tightly mark a cell population with the CSC characteristics (5). In particular, the ability of these cells to successively reinitiate transplantable tumors remains uncertain, while serial xenotransplantation is regarded as the gold standard for proving the existence of CSCs (9-10). Moreover, clinical significance of the surface markers, especially, relevance for PCa patients’ outcome is not clear.
Aldehyde dehydrogenase (ALDH) is an enzyme involved in intracellular retinoic acid production (11). ALDH1A is a major member of the ALDH family that includes 17 genes encoding different substrate specificities, of which ALDH1A1 catalyzes the oxidation of retinal to retinoic acid (11). Retinoic acid signaling is linked to cellular differentiation during development and has important function in stem cell self-protection throughout an organism’s lifespan (12-5). Activation of ALDH1A1 has been found in stem cell populations in multiple myeloma and acute myeloid leukemia (16). Ginestier et al. (17) showed that ALDH1A1 was a marker of normal and malignant human mammary SCs and a predictor of poor clinical outcome of breast cancer patients. We recently demonstrated that the ALDH1A1+ lung cancer cells could generate tumors that resembled the heterogeneity of the parental cancer cells (18). Furthermore, elevated ALDH1A1 expression was correlated with the stage and grade of lung tumors and associated to a poor prognosis for the lung cancer patients (18). In addition, high ALDH activity was recently proven to be a novel functional marker of murine prostate stem cells (19). However, no research has been reported concerning the role of ALDH1A1 in tumorigenesis of human prostate and its clinical importance in the disease.
In this study, we first used Aldefluor assay and fluorescence-activated cell sorting to isolate ALDH1A1+ cells from human PCa cell lines. The ALDH1A1+ cancer cells exhibited the important CSC properties: high in vitro tumorigenicity, in vivo tumor initiation and self-renewal capacities, and successively reinitiating transplantable tumors that gave rise to a heterogeneous population of cancer cells. Moreover, immunohistochemistry analysis of clinical specimens showed that ALDH1A1+ cells were spare and resided in the basal layers of normal prostate glands. In cancer specimens, however, ALDH1A1 immunopositivity were found not only within secretory type cancer epithelia, but also among neuroendocrine tumor cells. High ALDH1A1 expression correlated significantly with the patients’ poor survivals. ALDH1A1 could therefore be a prostate CSC-associated marker, and might provide a potential prognostic factor for the malignant progession of PCa.
Material and methods
Cell lines and cultures
PCa cell lines, PC3 cells and LNCaP cells, were obtained from the American Type Culture Collection (ATCC, Manassas, VA). The cells were maintained in the culture medium recommended by ATCC and harvested when they were in the logarithmic phase of growth for use in the following experiments.
Isolation of ALDH1A1+ cell population by Aldefluor assay and fluorescence-activated cell sorting (FACS)
An Aldefluor kit (StemCell Technologies, Durham, NC) optimized for interaction with human ALDH1A1 was used to identify ALDH1A1+ cells as previously described (18). Briefly, the brightly fluorescent ALDH1A1 expressing cells were detected by using an Aria cell sorter (BD Biosciences, San Jose, CA). Side-scatter and forward-scatter profiles were used to reduce cell doublets. Nonviable cells were eliminated using the viability dye 4′,6-diamidino-2-phenylindole (DAPI) (Sigma, St. Louis, MO). Specific ALDH1A1 activity was based on the difference between the presence/absence of the Aldefluor inhibitor diethylaminobenzaldehyde (DEAB) (Sigma). Data were analyzed by using Cell Quest software (B-D Biosciences). Each experiment was repeated three times.
Clonal analysis and clonogenic assays
Cells were plated at density of 200 per well in a six-well tissue culture dish, as described in our previous report (18). Clones with >50 cells were scored at the end of week two. The percentage of cells that initiated a clone was presented as cloning efficiency. For clonogenic assays, cells were plated at 1,000 per well in six-well culture dishes coated with a thin layer of 1% solidified agar (9, 18). Spheres that arose within three weeks were considered as clonogenicity. For each cell type, triplicate samples were performed and the clones or spheres were counted by two individuals in a blind fashion.
Xenografting
Cells were resuspended in serum-replacement medium at 1,000 cells per 50 ml and mixed with an equal volume of matrigel (BD Biosciences). The cells were then implanted into the dorsal prostates of ten NOD/SCID mice, which has been widely used as an ‘‘orthotopic’’ implantation site for human PCa (9). Tumorigenicity was measured mainly by tumor incidence (i.e., the number of tumors/number of injections), size of tumor, and latency (i.e., time from injection to detection of palpable tumors). The mice were observed for six weeks to allow tumor growth and then euthanized under deep anesthesia with pentobarbital (Sigma). Tumor volume (mm3) was calculated as (W2 × L) / 2. A part of each engrafted tumor tissue was dissociated with collagenase IV (Sigma) and incubated with mechanical disruption as previously described (18). Serial transplantations of the PCa xenografts were performed by regrafting the freshly disassociated cells into male mice. The disassociated cells were also reanalyzed by using Aldefluor assay for FACS analysis as described above. Immunofluorescence study was undertaken on the cells from the engrafted tumors to evaluate expressions of ALDH1A1, CD44, and androgen receptor (AR) as described below. Moreover, a portion of each tumor tissue was fixed in 10% formaldehyde and embedded in paraffin for histopathologic study.
Immunohistochemical (IHC) and immunofluorescence analyses
To explore expression patterns of ALDH1A1 in normal prostate and PCa tissues and their relationship with those of CD44, AR, and chromogranin A, we obtained tissue microarrays (TMAs) (US Biomax, Frederick, MD) constructed from 18 normal prostate and 64 PCa tissues (Table 1). IHC staining was performed on the consecutive TMA sections by using antibodies against ALDH1A1 (Santa Cruz Biotechnology, Santa Cruz, CA), CD44 (eBioscience, San Diego, CA), AR (Santa Cruz Biotechnology), and chromogranin A (Vector Laboratories, Inc., Burlingame, CA) as previously described (20-21). Immunoreactive staining intensity for each antibody was rated according to the following scale: no visible staining = 0, faint staining = 1, moderate staining = 2, and strong staining = 3. The total number of cells with positive staining for the antibodies was quantized on each tissue spot (0.6 mm2) of the TMAs. Percentage of cells with positive staining was graded as 0%, <10%, 10–25%, 25–50%, 50–75% or higher.
Table 1.
Immunohistochemical analysis of prostate cancer specimens for ALDH1A1 expression on tissue microarrays constructed from18 normal prostate and 64 PCa tissues a
| Characteristics | No. of tissues |
High ALDH1A1 expression b |
P c |
|---|---|---|---|
| All tissues | 82 | ||
| Age (years) | 0.62 | ||
| ≤ 65 | 22 | 4 | |
| > 65 | 60 | 9 | |
| Diagnosis | |||
| Normal | 18 | 0 | |
| Prostate cancer | 64 | 13 | |
| Pathologic | |||
| stage | |||
| pT2 | 39 | 5 | 0.01 |
| ≥pT3 | 25 | 8 | |
| Gleason | |||
| grade | 0.01 | ||
| ≤7 | 44 | 7 | |
| 8-10 | 20 | 6 |
The tissue microarrays (TMAs) constructed from 18 normal prostate and 64 PCa tissues were obtained from US Biomax, (Biomax, Frederick, MD). The clinical information, including prostatectomy, radiation, androgen deprivation, follow-up date, was not available.
A specimen with more than 10% overall score of ALDH1A1 was defined as one with high ALDH1A1 expression.
All P - values were determined by two-sided tests. P -values ≤ 0.05 were considered statistically significant.
Because the clinical follow-up information for the specimens on the TMAs was not available, to evaluate the associations between ALDH1A1 expression and PCa patients’ outcomes, we obtained formalin-fixed and paraffin-embedded PCa tissue sections with follow-up outcomes from the School of Clinical Medicine of Southeast University in China. The specimens were collected from ninety-nine PCa patients who underwent radical retropubic prostatectomy between 1991 and 2003. The patients also received androgen-deprivation therapy. Rdiation therapy was unavailable for many patients. Therefore, analysis influence of radiation on the patients was not included in the study. The minimum available follow-up on all patients was 6.72 (0.6-16.4) years. Overall survival was measured using the dates of death from any cause. Cancer-specific survival was calculated using the dates of death from PCa. The study was conducted under a protocol approved by the institutional review board for human subjects’ research. Geographic characteristics of the patients, including age, histopathologic diagnosis, and the survivals are shown in Table 2. IHC with ALDH1A1 antibody was performed and staining intensity was rated as described above. The total number of cells with positive staining for the antibodies was quantized in 20 fields on each paraffin tissue section.
Table 2.
Immunohistochemical staining of ALDH1A1 on prostate cancer specimens from ninetynine PCa patients with clinicopathological characteristics and follow-up date
| Characteristics | No. of Patients |
High ALDH1A1 expression a | Pb |
|---|---|---|---|
| All cases | 99 | 19 | |
| Age (years) | 0.83 | ||
| ≤ 65 | 34 | 6 | |
| > 65 | 65 | 13 | |
| Follow-up time (years) | 0.6-16.4 | ||
| Median Follow-up (years) |
6.72 | ||
| Pathologic stage | |||
| pT2 | 68 | 11 | 0.02 |
| ≥pT3 | 31 | 8 | |
| Gleason grade | 0.02 | ||
| ≤7 | 70 | 10 | |
| 8-10 | 29 | 9 |
A specimen with more than 10% overall score of ALDH1A1 was defined as one with high ALDH1A1 expression.
All P - values were determined by two-sided tests. P -values ≤ 0.05 were considered statistically significant.
To elucidate whether ALDH1A1+ PCa cells could generate engrafted tumors with heterogeneity in mice, we also examined expressions of ALDH1A1, CD44, and AR by using immunofluorescence assay with the above primary antibodies on the disassociated cells from the xenografts. The cells were then stained with a fluorescently conjugated IgG (Abcam Inc. Cambridge, MA) and examined under a Leica microscope (Leica Microsystems, Inc. Buffalo, NY), as previously described (18). Each batch of slides contained a positive control and a negative control.
Statistical analysis
The statistical software SPSS12.0 (SPSS Inc., Chicago, IL) was used to analyze the differences between ALDH1A1+ and ALDH1A1− cells with unpaired or paired t tests for statistical significance. Kaplan-Meier models were applied to generate Life tables. Differences between groups were examined using the log-rank test. Univariate and multivariate Cox regression analysis was performed using stepwise methods for analysis of ALDH1A1 staining result, age, histopathologic parameters, and the survivals. Due to the long follow-up period for this patient set, PSA value was unavailable for many patients. Therefore, analysis of correlation of ALDH1A1 expression with PSA level was not included in the study.
Results
Identification of a small ALDH1A1+ subpopulation in PCa cell lines with increased properties of clonogenicity
We utilized the Aldefluor assay followed by FACS analysis to assess the presence and size of the cell population with ALDH1A1 positive expression in two human PCa cell lines. The PC3 and LNCaP cell lines had a small size of ALDH1A1+ population, respectively. The PC3 showed a population of 2.1% (2.06 ± 1.27) ALDH1A1+ cells (Fig. 1A), and the LNCaP had 2.0% (1.99 ± 1.18) ALDH1A1+ cells. However, with DEAB, an Aldefluor inhibitor, the cells exhibited less than 0.03% ALDH1A1+ population.
Fig. 1.
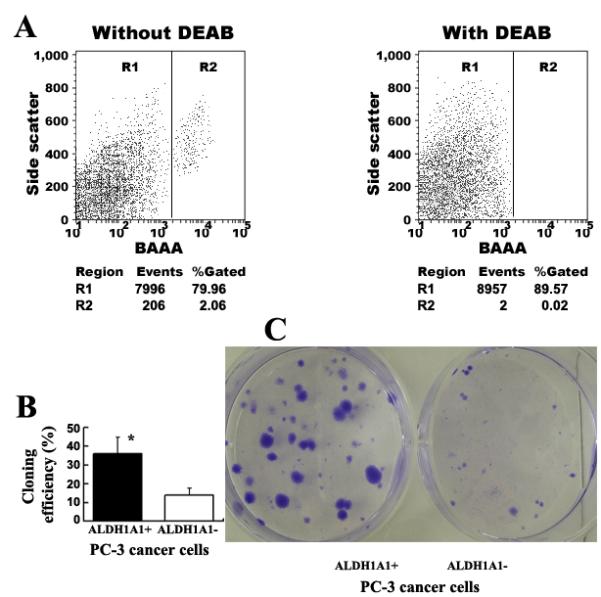
ALDH1A1+ PCa cells had tumor stem cell properties in vitro. A. FACS analysis of cancer cells using the Aldefluor assay. The brightly fluorescent ALDH1A1-expressing cells (ALDH1A1+ cells) were detected in the green fluorescence channel. The cells incubated with DEAB were used to establish the baseline fluorescence of these cells (R1) and define the ALDEFLUOR (ALDH1A1)-positive region (R2). The Fig.1A showed the result from PC3 cell line. B. ALDH1A1+ PCa cells possessed significantly higher colony-forming efficiency compared with ALDH1A1− PCa populations. ALDH1A1+ and ALDH1A1− cells were plated in a six-well dish. Two weeks after plating, clones were counted and results were presented as percent cloning efficiency (Y axis). Column indicates mean from three independent experiments; bars, SE. *, P < 0.0001. C. ALDH1A1+ PCa populations formatted larger and more colonies as compared with the ALDH1A1− cells by clonogenicity assays. Spheres were counted three weeks after plating in triplicate at 1,000 per well in a six-well plate coated with soft agar. The experiments were undertaken on all PCa cell lines and repeated three times. The Fig.1 only showed the result from PC3 cell line.
The ALDH1A1+ PC3 and ALDH1A1+ LNCaP cells displayed significantly higher colony-forming efficiency (Fig. 1B) by forming larger and more clones compared with the ALDH1A1− PC3 and ALDH1A1− LNCaP cells at the end of the clonal assays. The data indicated that ALDH1A1+ PCa cells could possess high proliferative capacity. Because anchorage-independent growth is an approximation of tumorigenesis, and CSCs are thought to be the tumor-initiating cells (2), we further tested the ability of the ALDH1A1+ and ALDH1A1− PCa cells to produce colonies by using soft agar assay. The ALDH1A1+ PC3 cells generated at least three times as many colonies as the ALDH1A1− PC3 cells (Fig. 1C). The ALDH1A1+ LNCaP cells also resulted in approximate four times as many colonies as did the ALDH1A1− LNCaP cells. Furthermore, the colonies from the ALDH1A1+ cells formed faster and were larger in size (approximately four times) compared with ones from the ALDH1A1− cells (all p<0.01). Although ALDH1A1+ and ALDH1A1− PCa cells generated similar size and number of clones in soft agar, time from plating the cells to forming clones from ALDH1A1+ PC3 cancer cells was one week earlier (7±2 days) compared with ALDH1A1+ LNCaP cells. These observations indicate that ALDH1A1+ PCa cells present increased in vitro clonogenicity and tumorigenicity. Moreover, ALDH1A1+ PC3 cancer cells might have higher clonogenicity compared with ALDH1A1+ LNCaP cells.
Tumor formation of ALDH1A1+ PCa cells in vivo
The gold standard in determining CSCs is whether the testing cells can preferentially initiate tumor development in animal models (9, 22). We therefore implanted ALDH1A1+ PC3, ALDH1A1− PC3, ALDH1A1+ LNCaP, and ALDH1A1− LNCaP cells into ten NOD/SCID mice, respectively. After four weeks, 5 × 102 ALDH1A1+ PC3 cells yielded tumors with an average of 17±1.2 mm3 in all ten mice, while the same number of ALDH1A1− cells did not produce tumors in any mouse (Fig. 2A). Furthermore, 5 × 104 ALDH1A1+ PC3 cells generated much larger tumors in all mice with an average of 28±1.5 mm3, whereas the same amount of ALDH1A1− PC3 cell produced a small tumor mass (8 mm3) in only one of the ten mice (Fig. 2B). Likewise, 5 × 102 ALDH1A1+ LNCaP cells yielded tumors with an average of 13±1.1 mm3 in all ten mice, while the same dose of ALDH1A1− cells did not generate tumors. 5 × 104 ALDH1A1+ LNCaP cells created tumors in all mice with an average of 25±1.3 mm3. However, the same numbers of ALDH1A1− LNCaP cell resulted in a small tumor mass (7 mm3) in only one of the ten mice. Therefore, the ALDH1A1+ PCa cells had at least 100 times more in vivo tumorigenicity compared with the ALDH1A1− PCa cells, implying that the ALDH1A1+ cancer cells might be more tumorigenic than their isogenic ALDH1A1− cancer cells.
Fig. 2.
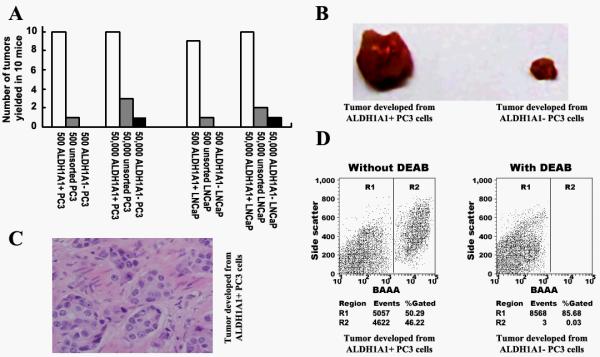
ALDH1A1+ PCa cells had tumor stem cell properties in vivo. A. Tumor formation ability of ALDH1A1+ cells was greater than that of ALDH1A1− cancer cells. 5 × 102 and 5 × 104 ALDH1A1+ PC cells, ALDH1A1− PC cells, or unsorted corresponding PC cells were injected into the dorsal prostate of ten NOD/SCID mice, respectively, as indicted in X axis. Tumor incidence refers to the number of tumors developed in the ten mice, as shown in the Y axis. After four weeks, the 5 × 102 ALDH1A1+ PC3 cells yielded tumors in all mice, whereas the same amount of ALDH1A1− PC3 cell didn’t produce tumor mass in any mouse.The 5 × 104 ALDH1A1+ PC3 cells yielded tumors in all mice, whereas the same amount of ALDH1A1− PC3 cell produced a small tumor mass in only one mouse. B. ALDH1A1+ PCa cells generated larger tumors compared with ALDH1A1− cancer cells. 5 × 104 ALDH1A1+ PC3 cells yielded tumors with an average of 28±1.5 mm3, whereas the same dose of ALDH1A1− PC3 cell produced a small tumor mass (8 mm3) in only one of the mice. C. Histopathologic examination of the engrafted tumors formed by the ALDH1A1+ PC3 cells revealed a highly cellular mass with characteristics of adenocarcinoma of prostate. D. ALDH1A1+ PCa cells could generate PCa tumors with heterogeneity in vivo. Reanalyzing cells of the engrafts generated from the ALDH1A1+ and ALDH1A1− cells by using the Aldefluor assay showed that the cells from the tumor produced by ALDH1A1+ PC3 cancer cells (left panel) gave rise to 50% ALDH1A1+ population and 46% ALDH1A1− cells. However, the cells from the tumor formed by ALDH1A1− PC3 cells (right panel) only produced ALDH1A1− PC3 population.
Although at the dose of 5 × 102 cells, ALDH1A1+ PC3 and ALDH1A1+ LNCaP generated similar tumor incidence and size in mice. However, latency of generating the xenograft tumors from ALDH1A1+ PC3 cancer cells was statistically shorter (8±2 day) than that (15±3 day) of ALDH1A1+ LNCaP cells (P<0.05). The results indicate that ALDH1A1+ PC3 cells could have more tumorigenic property compared with ALDH1A1+ LNCaP cells.
In addition, serial transplantation experiments showed that PCa tumors developed from ALDH1A1+ cells could be regenerated for 6 cycles until we prepared the paper. Histopathologic examination revealed a highly cellular mass with characteristics of PCa in the engrafts from the ALDH1A1+ PCa cancer cells (Fig. 2B). The observation provides functional evidence that the ALDH1A1+ PCa cells have the property of in vivo self-renewal.
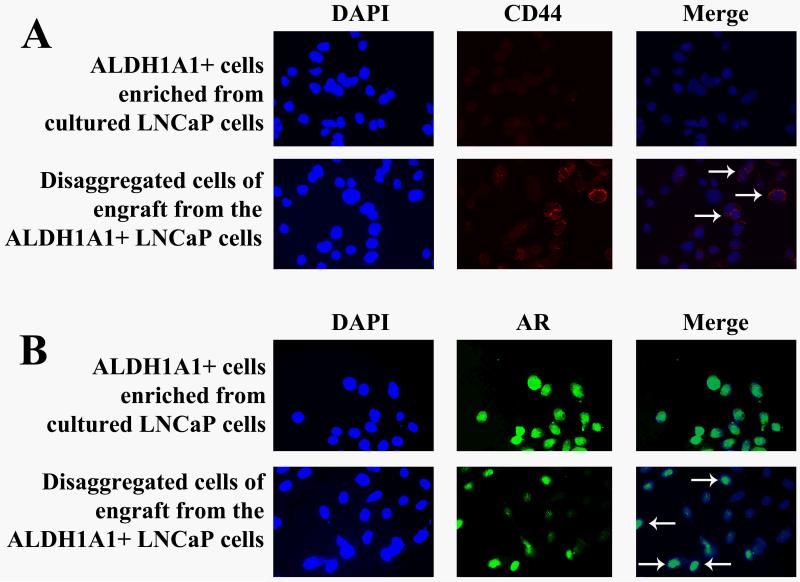
To elucidate whether ALDH1A1+ PCa cells could create PCa tumors with heterogeneity in vivo, Aldefluor analysis was performed on disassociated cells of the tumor engrafts of the first generation. The engrafted tumors from ALDH1A1+ PC3 cells gave rise to 50% (50±3.4%) ALDH1A1+ cells and 46% (46±2.5%) ALDH1A1− cells (Fig. 2C). Furthermore, immunofluorescence analysis showed that the disaggregated cells of the engrafted tumors from the ALDH1A1+ LNCaP cells that were initially negative for CD44 and positive for AR displayed 37% (36±1.3%) CD44+ cells and 56% (56±2.2%) AR− cells, respectively (Fig. 3). In addition, the engraftments from the ALDH1A1+ PC3 population that was originally positive for CD44 and negative for AR exhibited 29% (28±1.2%) CD44− cells and 32% (31±1.8%) AR+ populations. The results imply that ALDH1A1+ PCa cells could produce heterogeneous populations of tumorigenic phenotypes.
Fig. 3.
ALDH1A1+ PCa cells produced heterogeneous populations of tumorigenic phenotypes. A. Immunofluorescence analysis of ALDH1A1+ cells freshly enriched from cultured LNCaP cells showed that most of the cells were negative for CD44 (weak red fluorescent staining). B. Part of the disassociated cells of engraft developed from the ALDH1A1+ LNCaP cells were positively stained for CD44, as illustrated by red fluorescence staining of the cell membrane (arrows). C. ALDH1A1+ cells newly isolated from the same cultured LNCaP cells demonstrated that the majority of the cells were positive for androgen receptor (AR) (green fluorescent staining of the nuclei). D. Only fraction of the disaggregated cells of engraft produced from the ALDH1A1+ LNCaP cells were positively stained for AR, as demonstrated by green fluorescent staining of the nucleus (arrowheads). 4′,6-diamidino-2-phenylindole (DAPI) was used to stain nuclei.
Altogether, our in vivo data suggest that ALDH1A1+ PCa cells possess unique features of cancer stem-like cells, including initiation of tumorigenesis, self renewal, and reinitiating serially transplantable tumors with multipotent differentiation potential.
ALDH1A1 expression was sparse and limited to the basal component in normal prostates
As shown in Fig.4A, only a few ALDH1A1+ cells, which displayed positive cytoplasmic staining, resided in the basal cell layers of normal prostate tissues. In the adjacent sections from the same normal prostate tissues, CD44+ cells also existed in the basal component, indicating that ALDH1A1+ and CD44+ cells were at the same locations in prostate glands (Fig. 4A). However, comparison of ALDH1A1 and CD44 staining on the consecutive tissue sections revealed that ALDH1A1+ cells accounted for a portion of ALDH1A1+ population, and percentage of ALDH1A1+ cells was statistically lower compared with CD44+ cells in the same basal cell layers (Fig.4B) (P<0.0001). Together with previous reports that the basal cell fraction includes prostate SCs (5) and CD44+ cells represent normal SCs (5, 10), our findings of ALDH1A1+ cells comprising a subset of CD44+ cells in normal basal layer component might imply that the ALDH1A1+ cells could be enriched in SCs of prostate.
Fig. 4.
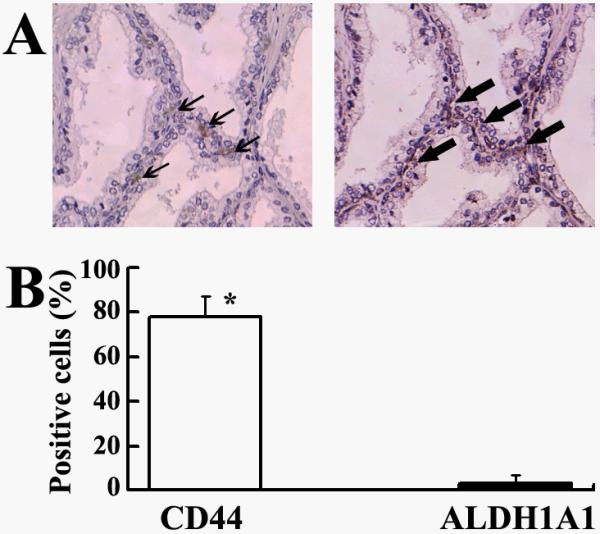
ALDH1A1 expression was sparse and limited to the CD44+ basal component in normal prostates. A. Immunohistochemical study of adjacent sections of a normal prostate tissue specimen for the expression of ALDH1A1 and CD44 showed that only a few ALDH1A1+ cells (arrows in left panel), which displayed positive cytoplasmic staining, resided in the CD44 positive basal cells (arrowheads in right panel). B. Comparison of expression of ALDH1A1 and CD44 in 18 normal prostate tissues. Bars reflect the mean percentage of positive staining cells ± SE for each antibody. *, P < 0.0001.
ALDH1A1 expression existed in PCa tumors with heterogeneous phenotypes
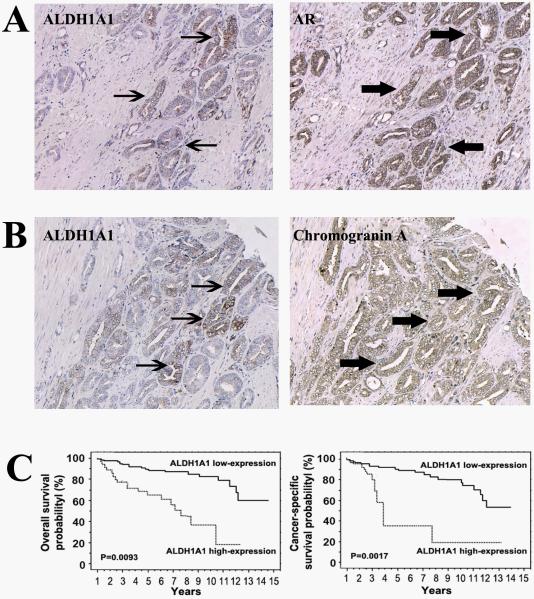
ALDH1A1 expression was found in tumor tissues as illustrated by strong cytoplasm staining. Furthermore, in contrast to rare ALDH1A1+ cells in normal basal component, a higher percentage of ALDH1A1+ population was found in tumor tissues (P<0.0001). In addition, IHC analysis of serial tumor sections displayed that ALDH1A1+ cells existed not only within secretory type cancer epithelial cells that showed strong AR immunopositivity (Fig. 5A), but also among neuroendocrine tumor cells that had positive chromogranin A (Fig. 5B). Although the ALDH1A1 positive cells only account for a subset of the AR+ or chromogranin A+ cancer cell populations, the observation suggests that ALDH1A1 highly expresses in a form of heterogeneity in tumor tissues, being consistent with CSC concept, in which, CSCs have multipotent differentiation feature.
Fig. 5.
ALDH1A1 expression existed in PCa tumors with heterogeneous phenotypes and was associated with a poor prognosis for the patients. A. Immunohistochemical study of adjacent sections of a PCa tumor for the expression of ALDH1A1 and AR showed that ALDH1A1+ cells (arrows in left panel) exist in portion of secretory type cancer epithelial cells that had strong AR immunopositivity (arrowheads in right panel). B. ALDH1A1+ cells (arrows in left panel) were present in part of neuroendocrine tumor cells that had positive chromogranin A (arrowheads in right panel). C. Probability of overall survival (left panel) and cancer-specific survival (right panel) by levels of ALDH1A1 expression in the 99 PCa patients.
Elevated ALDH1A1 expression is associated with a poor prognosis for patients with PCa
To assess the potential use of ALDH1A1 as a prognostic marker in PCa patients, an overall score of ALDH1A1 expression in the tissue specimens was assigned by multiplying the intensity score by the mean percentage of cells staining. ALDH1A1 expression was then stratified at three levels: absent, present and high level of expression of ALDH1A1, that was, a specimen without any expression of ALDH1A1 was considered as one with absent ALDH1A1 expression, a specimen had <10% of cells for ALDH1A1 expression with faint staining was considered as one with present ALDH1A1 expression. A specimen with more than 10% overall score was defined as one with high ALDH1A1 expression. All normal prostate tissues had ≤10% of cells for ALDH1A1 expression with faint staining. Therefore, >10% overall score was used as a cut-off, that was, a tumor specimen with more than 10% overall score was defined as one with high ALDH1A1 expression.
Applying this criterion, ALDH1A1 high-expression was found in 13 of the 64 (20%) PCa tumors on the TMAs (Table 1). High ALDH1A1 expression was predominately found in high Gleason cancers (P=0.01) and high pathologic stage (P=0.01). Similarly, of the 99 PCa tumors with clinical follow-up information, there were 19 (19%) tissues showing high ALDH1A1 expression (Table 2). Among the 99 cancers, high ALDH1A1 expression was associated with high Gleason score (P=0.02), high pathologic stage (P=0.02) of the tumors. However, there was no significant association between ALDH1A1 expression and patient’s age (Tables 1 and 2). The data from two independent PCa patient populations demonstrate that ALDH1A1 high-expression is correlated to aggressive behavior of prostate tumorigenesis.
Kaplan—Meier survival curve analyses of the 99 PCa tumors with clinical follow-up information showed that patients with high ALDH1A1 expression in their tumors had a significantly reduced overall survival rate compared with patients who had low ALDH1A1 expression (P=0.0093) (Fig. 5C). The difference was more prominent in the prostate cancer-specific survival data (P=0.0017). Furthermore, univariate analysis demonstrated that high ALDH1A1 expression in PCa tumors was significantly related to overall and cancer-specific survivals with hazard ratios of 1.79 (95% CI 1.226−2.632, P=0.0027) and 1.07 (95% CI 1.032−1.154, P=0.0019), respectively.
Parameters, including age, high ALDH1A1 expression, Gleason score (≤7 versus >8), pathologic stage (≥pT3 versus pT2), were included for Cox regression multivariate analysis. Overall and cancer-specific survivals were used as endpoints. ALDH1A1 high-expression consistently showed a strong and independent prognostic effect on overall and cancer-specific survivals with hazard ratios of 1.73 (95% CI 1.163−2.527, P=0.0066) and 1.05 (95% CI 1.028−1.107, P=0.0062), respectively. Furthermore, Gleason score of the tumors was significantly associated with the overall and cancer-specific survivals with hazard ratios of 1.34 (95% CI 1.010−2.193, P=0.0016) and 1.11 (95% CI 1.021−2.215, P=0.0012). Pathologic stage was also significantly related to the overall and cancer-specific survivals with hazard ratios of 2.23 (95% CI 1.713−2.899, P=0.0029) and 2.10 (95% CI 1.771−2.736, P=0.0032). However, age only was shown as an independent prognostic factor for overall survival of the patients with hazard ratios of 1.62 (95% CI 1.052−2.632, P=0.0458).
Discussion
Development of specific markers to isolate and characterize CSCs of prostate tumors would greatly advance our understanding of tumor biology of prostate. A Hoechst 33342 efflux assay for collecting side population (SP) that preferentially expresses drug-efflux pumps has been used to enrich CSCs of leukemia and some solid tumors (2-3). However, the approach failed to detect a reliable SP in several commonly cultured human PCa cell lines (9-10). Recently, cell surface markers have been utilized to isolate CSCs from human PCa cells. For instance, CD44+/α2ß1+ PCa cells demonstrated high colony-forming efficiency and capacity to differentiate into several cell types of prostate carcinoma (9-10). However, the ability of these cells to serially reinitiate transplantable tumors has not been well documented (10). Furthermore, the reliability of using the markers to identify CSCs remains controversial (5, 10). For example, Collins et al demonstrated that a subpopulation of CD44+/a2b1high/CD133+ cells in clinical PCa specimens had stem cell characteristics in vitro (5). However, Pfeiffer et al (23) recently used CD133 to isolate cancer stem cells from PCa cell lines. Only one (DU145) of 6 PCa cell lines (DU145, DuCaP, LAPC-4, 22Rv1, LNCaP, and PC-3) had detectable CD133+ population (0.01%). There was no difference in the ability of the CD133+, CD133−, and unselected DU145 cells to form colonies. In addition, although α2ß1+ PCa cells showed higher proliferative and clonogenic potentials in vitro compared with the isogenic α2ß1− PCa cells, α2ß1+ PCa cells were no more tumorigenic than the corresponding α2ß1− cells in vivo (9). Moreover, several other cell populations have been identified that share the same marker phenotype, including circulating fibrocytes (22-6), thus limiting their application in enriching CSCs from clinical specimens (24).
In this present study, we successfully isolated ALDH1A1+ cells from PCa cell lines by uisng Aldefluor assay. Several pieces of evidence support that ALDH1A1+ PCa cells could be enriched in CSCs or stem-like cancer cells. First, the in vitro assays revealed that ALDH1A1+ PCa cells had higher clone formation efficiency than did ALDH1A1− PCa cells. Moreover, ALDH1A1+ PCa cells grew in an anchorage-independent manner. Therefore, ALDH1A1+ PCa cells were highly in vitro colongenic and tumorigenic cells. Second, our in vivo experiments showed that the ALDH1A1+ PCa cells were at least 100 times more tumorigenic than ALDH1A1− PCa cells. Furthermore, the engrafted tumors illustrated histopathologic patterns similar to those of the primary PCa cells, implying that the ALDH1A1+ population could resemble the characteristics of the tumor subtype and possess the capacity to self-renew. Third, the dissociated cells of the engraftments created from ALDH1A1+ PCa cells presented an average of 46% ALDH1A1− PCa cells. Furthermore, the engrafted tumors from the ALDH1A1+ PC3 cells that were initially negative for AR staining displayed an average of 32% AR+ cells. Vice versa, the engrafts from the ALDH1A1+ LNCaP cancer cells that were primarily negative for CD44 staining exhibited a population of 37% CD44+ cells. The data demonstrated that ALDH1A1+ cells might give rise to a heterogeneous property of tumors of PCa. Fourth, importantly, ALDH1A1+ PCa cells could be serially passaged at least 6 times in vivo. Considering that the most crucial standard for CSCs is their ability to re-initiate successively transplantable xenografts that resemble the original tumor histology and heterogeneity, the ALDH1A1+ PCa cells could represent CSCs of prostate tumors.
Previous studies strongly suggested that the basal cell layer of adult human prostate contained SCs (5, 10). For example, more than 70% proliferating cells and molecules important in maintaining fundamental properties of SC localized in this basal compartment (10, 27). Furthermore, less than 5% dissociated basal layer cells from adult human prostate possessed extensive proliferative capacity (27-8). In addition, under suitable culture conditions, a small population of prostatic basal cells maintained some developmental plasticity, in which, the cells transdifferentiated into neuronal/glial cells (29). Finally, CD44 was expressed on most basal cells and purified CD44+ prostate epithelial cells, when co-cultured with stromal cells, produced increased amounts of PSA, probably due to the differentiation of CD44+ cells into luminal cells (10, 30). CD44+ cells were therefore believed to contain normal prostate SCs and CD44 was considered as a marker for SCs in prostate epithelia (5, 10). In this study, we demonstrate that a small ALDH1A1+ cell population is restricted to the CD44 positive basal layer cells of normal prostates, suggesting that the ALDH1A1+ cells in normal prostate glands might be SCs. Investigation of whether the ALDH1A1+ normal cells can engage a multilineage repopulation of a tissue or organ, one of the most important characteristic of SC, is undertaken at our laboratory.
Although higher percentage ALDH1A1 positive cells with stronger staining signals were observed in PCa tissues compared with normal prostate tissues, only 19% and 20% tumors showed high ALDH1A1 expression in the two sets of cancer specimens. The finding is consistent with the notion that CSCs constituted a minority of the tumor population. Interestingly, ALDH1A1 positive staining was present not only in secretory type cancer epithelia, but also in neuroendocrine differentiated tumor cells. The data provide the evidence that ALDH1A1 expressed in a form of hertergentity in human PCa tissues. The observation is in agreement with findings in our animal experiments, in which, the disaggregated cells of the tumors generated from the ALDH1A1+ PCa cells give rise to 46% ALDH1A1− cells. The observation is also compatible with that the engrafted tumors from the ALDH1A1+ PC3 cells originally being negative for AR show 32% AR+ cells, while the xenografts from the ALDH1A1+ LNCaP cells initially being negative for CD44 display 37% CD44+ cells. The results imply that ALDH1A1+ cancer cells might be able to differentiate into secretory and neuroendocrine epithelial cancer cells within tumor tissues. Furthermore, our observations also confirm concept of a hierarchical organization of cancer cells existing in PCa proposed by Tang et al and others (9, 10, 31). Based on the concept, CSCs could derive from mutated CSs, and PCa cells are organized as a hierarchy with CSCs sitting at the apex and having the ability to develop (or differentiate) into a spectrum of more mature progeny. The potential of ALDH1A1+ PCa cells to “transdifferentiate” into other cell types could help explain biological conundrum of prostate carcinogenesis, in which, although PCa, clonal by origin, is rather heterogeneous in its cellular composition. Nevertheless, further investigation of whether and how normal ALDH1A1+ cells can transform into ALDH1A1+ cancer populations is required.
PC3 cancer cells are androgen-independent and characterized by a marked tumorigenic phenotype. Whereas LNCaP is an androgen-dependent cell line endowed with low tumorigenic and clonogenic abilities. Here we find that although ALDH1A1+ and ALDH1A1− PCa cells generated similar size and number of clones in soft agar, time from plating the cells to forming clone from ALDH1A1+ PC3 cancer cells was one week earlier compared with ALDH1A1+ LNCaP cells. Furthermore, ALDH1A1+ PC3 cancer cells and ALDH1A1+ LNCaP cancer cells demonstrate similar tumor incidence and endpoint tumor size in mice. However, latency of generating the xenograft tumors from ALDH1A1+ PC3 cancer cells is much shorter compared with that of ALDH1A1+ LNCaP cells at the same dose. The results indicate that although PC3 and LNCaP cells have similar size of ALDH1A1+ population or cancer stem cell population, ALDH1A1+ PC3 cells or stem cell population of PC3 cells could have more clonogenic and tumorigenic property compared with those of ALDH1A1+ LNCaP cells. Our observation may support that PC3 cancer cells are more tumorigenic than LNCaP prostate cancer cells on the basis of CSC theory. Whether ALDH1A1 is an androgen-regulated gene is currently investigated at our laboratory.
There are few markers clinically available for accurate prediction of prognosis of PCa patients besides PSA and Gleason sum (32). Because of the heterogeneous nature of PCa, it has become clear that a combination of accurate prognostic indicators is needed to improve the ability for identifying aggressive disease, so that more individualized treatments could be offered. We here show that high ALDH1A1 expression occurs in the PCa samples, and is significantly associated with the Gleason score and pathologic stage. More importantly, ALDH1 expression inversely correlates with survivals of the patients with PCa. A limitation of the study is that a small sample size. If the ALDH1A1 expression could be proven as a prognostic biomarker in a future large PCa population with comprehensive clinical information, the assay for analysis of ALDH1A1 might be added to the currently available prognostic factors for improving the accuracy of outcome predictions of PCa.
In summary, utilizing in vitro and in vivo experimental systems, we show that ALDH1A1+ cancer cells are endowed with extensive proliferation and self-renewal potential, being able to generate tumors that might resemble the histopathologic characteristics and heterogeneity of the parental PCa cells, and therefore might have the properties of CSCs. However, further molecular biologic analysis of ALDH1A1 in carcinogenesis of prostate and validating its prognostic value in a large population of PCa will be warranted.
Acknowledgments
Grant support: This work was supported in part by National Cancer Institute grants CA-113707 and CA-133956, American Cancer Society-Research Scholar Grant in Basic, Preclinical, Clinical and Epidemiology Research, a Scholar Award from NIH-K12- Multidisciplinary Clinical Research Career Development Program-University of Maryland, and a clinical innovator award from Flight Attendant Medical Research Institute (all to F. J.).
References
- 1.Cookson MS, Aus G, Burnett AL, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177:540–5. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 2.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 3.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–61. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 4.Horst D, Kriegl L, Engel J, et al. CD133 expression is an independent prognostic marker for low survival in colorectal cancer. Br J Cancer. 2008;99:1285–9. doi: 10.1038/sj.bjc.6604664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang SH, Frame FM, Collins AT. Prostate cancer stem cells. J Pathol. 2009;217:299–306. doi: 10.1002/path.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly K, Yin JJ. Prostate cancer and metastasis initiating stem cells. Cell Res. 2008;18:528–37. doi: 10.1038/cr.2008.50. [DOI] [PubMed] [Google Scholar]
- 7.Collins AT, Habib FK, Maitland NJ, et al. Identification and isolation of human prostate epithelial stem cells based on alpha(2)beta(1)-integrin expression. J Cell Sci. 2001;114:3865–72. doi: 10.1242/jcs.114.21.3865. [DOI] [PubMed] [Google Scholar]
- 8.Richardson GD, Robson CN, Lang SH, et al. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci. 2004;117:3539–45. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- 9.Patrawala L, Calhoun-Davis T, Schneider-Broussard R, et al. Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+alpha2beta1+ cell population is enriched in tumor-initiating cells. Cancer Res. 2007;67:6796–805. doi: 10.1158/0008-5472.CAN-07-0490. [DOI] [PubMed] [Google Scholar]
- 10.Tang DG, Patrawala L, Calhoun T, et al. Prostate cancer stem/progenitor cells: identification, characterization, and implications. Mol Carcinog. 2007;46:1–14. doi: 10.1002/mc.20255. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida A, Hsu LC, Davé V. Retinal oxidation activity and biological role of human cytosolic aldehyde dehydrogenase. Enzyme. 1992;46:239–44. doi: 10.1159/000468794. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong L, Stojkovic M, Dimmick I, et al. Phenotypic characterization of murine primitive hematopoietic progenitor cells isolated on basis of aldehyde dehydrogenase activity. Stem Cells. 2004;22:1142–51. doi: 10.1634/stemcells.2004-0170. [DOI] [PubMed] [Google Scholar]
- 13.Chute JP, Muramoto GG, Whitesides J, et al. Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc Natl Acad Sci USA. 2006;103:11707–12. doi: 10.1073/pnas.0603806103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hess DA, Craft TP, Wirthlin L, et al. Widespread Non-Hematopoietic Tissue Distribution by Transplanted Human Progenitor Cells with High Aldehyde Dehydrogenase Activity. Stem Cells. 2006;26:611–20. doi: 10.1634/stemcells.2007-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hess DA, Wirthlin L, Craft TP, et al. Selection based on CD133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells. Blood. 2006;107:2162–9. doi: 10.1182/blood-2005-06-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearce DJ, Taussig D, Simpson C, et al. Characterization of cells with a high aldehyde dehydrogenase activity from cord blood and acute myeloid leukemia samples. Stem Cells. 2005;6:752–60. doi: 10.1634/stemcells.2004-0292. [DOI] [PubMed] [Google Scholar]
- 17.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell. 2007;15:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang F, Qiu Q, Khanna A, et al. Aldehyde dehydrogenase 1 is a tumor stem cellassociated marker in lung cancer. Mol Cancer Res. 2009;7:330–8. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burger PE, Gupta R, Xiong X, et al. High ALDH Activity: A Novel Functional Marker of Murine Prostate Stem/Progenitor Cells. Stem Cells. 2009;28 doi: 10.1002/stem.135. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palapattu GS, Wu C, Silvers CR, et al. Selective expression of CD44, a putative prostate cancer stem cell marker, in neuroendocrine tumor cells of human prostate cancer. Prostate. 2009;69:787–98. doi: 10.1002/pros.20928. [DOI] [PubMed] [Google Scholar]
- 21.Sotomayor P, Godoy A, Smith GJ, et al. Oct4A is expressed by a subpopulation of prostate neuroendocrine cells. Prostate. 2009;69:401–10. doi: 10.1002/pros.20895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–44. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 23.Pfeiffer MJ, Schalken JA. Stem Cell Characteristics in Prostate Cancer Cell Lines. Eur Urol. 2009;19 doi: 10.1016/j.eururo.2009.01.015. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Croker AK, Allan AL. Cancer stem cells: implications for the progression and treatment of metastatic disease. J Cell Mol Med. 2008;12:374–90. doi: 10.1111/j.1582-4934.2007.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balicki D. Moving Forward in Human Mammary Stem Cell Biology and Breast Cancer Prognostication Using ALDH1. Cell Stem Cell. 2007;15:485–7. doi: 10.1016/j.stem.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Sreerama L, Sladek NE. Class 1 and class 3 aldehyde dehydrogenase levels in the human tumor cell lines currently used by the National Cancer Institute to screen for potentially useful antitumor agents. Adv Exp Med Biol. 1997;414:81–94. doi: 10.1007/978-1-4615-5871-2_11. [DOI] [PubMed] [Google Scholar]
- 27.Bhatia B, Tang S, Yang P, et al. Cell-autonomous induction of functional tumor suppressor 15-lipoxygenase 2 (15-LOX2) contributes to replicative senescence of human prostate progenitor cells. Oncogene. 2005;24:3583–3595. doi: 10.1038/sj.onc.1208406. [DOI] [PubMed] [Google Scholar]
- 28.Hudson DL, O’Hare M, Watt FM, et al. Proliferative heterogeneity in the human prostate: Evidence for epithelial stem cells. Lab Invest. 2000;80:1243–1250. doi: 10.1038/labinvest.3780132. [DOI] [PubMed] [Google Scholar]
- 29.Raff M. Adult stem cell plasticity: Fact or artifact? Annu Rev Cell Dev Biol. 2003;19:1–22. doi: 10.1146/annurev.cellbio.19.111301.143037. [DOI] [PubMed] [Google Scholar]
- 30.Liu AY, True LD, LaTray L, et al. Cell-cell interaction in prostate gene regulation and cytodifferentiation. Proc Natl Acad Sci USA. 1997;94:10705–10710. doi: 10.1073/pnas.94.20.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu G, Yuan J, Wills M, et al. Prostate cancer cells with stem cell characteristics reconstitute the original human tumor in vivo. Cancer Res. 2007;67:4807–15. doi: 10.1158/0008-5472.CAN-06-4608. [DOI] [PubMed] [Google Scholar]
- 32.Cohen BL, Gomez P, Omori Y, et al. Cyclooxygenase-2 (COX-2) expression is an independent predictor of prostate cancer recurrence. Int J Cancer. 2006;119:1082–7. doi: 10.1002/ijc.21749. [DOI] [PubMed] [Google Scholar]