Abstract
(xylyl-phanephos)Pt2+ in combination with XeF2 mediates the consecutive diastereoselective cation-olefin cyclization/fluorination of polyene substrates. Isolated yields were typically in the 60s while enantioselectivies reached as high as 87%. The data are consistent with a stereoretentive fluorination of a P2Pt-alkyl cation intermediate.
The fluorination of pharmaceutical drug candidates is an important strategy for masking metabolic hot spots.1 Despite recent progress with electrophilic fluorinating reagents,2 the synthesis of such compounds is still challenging and many deficiencies remain, especially in the asymmetric fluorination of non-enolate-based carbon nucleophiles.3,4,5 Fluorinated steroids (Scheme 1), in particular, are important bioactive compounds with a deficiency of methods for their synthesis.6,7,8
Scheme 1.

Common fluorinated steroids.
De novo syntheses of carbocycles with the flexibility for F-incorporation are rare, though such methods would considerably expand the accessibility of such privileged structures.1 Transition metal catalyzed cyclizations, if suitably coupled to M-C fluorination reactions,4 could provide a route to complex fluorinated carbo- and hetero-cycles with control of absolute and relative stereochemistry.
Electrophilic Pt(II) complexes are effective initiators of C-C bond forming cation-olefin cascades.9,10,11 The fate of the organometallic intermediate of these cascades can be controlled through ligand choice, and when the supporting ligand is a diphosphine, this intermediate is susceptible to β-H elimination and leads to net dehydrogenated products. If this complex could instead be intercepted by a Pt-C fluorination reaction, a catalytic cyclization/fluorination protocol would result with concomitant access to C3-fluorinated compounds.6d The rapidity and stereospecificity with which [(triphos)Pt-R][BF4] reacts with XeF2 to yield C-F products (Eq. 1) suggested that the desired interception might be capable of competing with β-H elimination.12
 |
(1) |
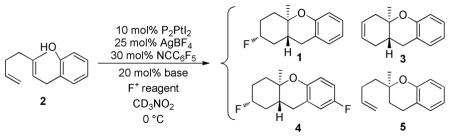
In the diphosphine catalyst series that we have examined, (S)-xylyl-phanephos ((S)-(–)-4,12-bis[di(3,5-xylyl)phosphine]-[2,2]-paracyclophane) has consistently provided the highest enantioselectivity for various cyclization chemistries. As a starting point for our catalytic cyclization/fluorination goal, we adapted conditions previously optimized for cyclization/β-H elimination reactions.10b The modified conditions included use of AgBF4 and NCC6F5 to generate the “active” [(S)-(xylyl-phanephos)Pt(NCC6F5)2][(BF4)2] catalyst. Subsequent addition of a base (to facilitate cyclization), substrate and an electrophilic fluorine source generated the desired product (1) as a single (stereoretentive) isomer, along with variable quantities of β-H eliminated product (3) and the Brønsted product (5) (Table 1); several additional phosphines are included for comparison.
Table 1.
Selected optimizing conditions.
| ||||||
|---|---|---|---|---|---|---|
| Entry | P2 | Base | % 1a (%ee)b | %3a | %4a | %5a |
| 1 | (R)-DTBM-SEGPHOS | None | 20 | 14 | 0 | 57 |
| 2 | (R)-DTBM-SEGPHOS | Ph2NH | 34 | 28 | trace | 14 |
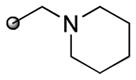
| 3 | (R)-DTBM-SEGPHOS |
|
63(13) | 8 | 11 | 0 |
| 4 | (R)-xylyl-MeO-BIPHEP |
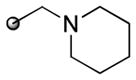
|
85(5) | 10 | 3 | 0 |
| 5 | (S)-xylyl-phanephos |
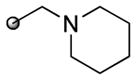
|
72(75) | 7 | 3 | trace |
Uncorrected GC percentages
Determined by chiral GC.
In screening a variety of electrophilic fluorine sources, it was discovered that only XeF2 effectively competed with β-H elimination. Other less reactive F+ sources showed predominant to exclusive β-H elimination to 3.13 In addition to the desired 1, over fluorination to 4 was also observed. Since controls showed that 1 does not react with XeF2 and acid is known to enhance the F+ potential of XeF2,14 we surmised that the HF byproduct of cyclization was activating the XeF2. This problem was easily solved by the addition of TMSOMe as an HF sponge, which additionally obviates the need for a base.15
Optimization studies included testing multiple bisphosphines, solvents, and other TMS-X derivatives.15 Once again (S)-xylyl-phanephos was uniquely enantioselective (~75%) for controlling the % ee of the cation-olefin cascades. Of the tested HF scavengers, TMSOMe was the most effective inhibitor of double fluorination and like previous ionic cascades, nitro-methane was the optimum solvent. A catalyst formulation comprised of 10 mol% (S)-(xylyl-phanephos)PtI2, 25 mol% AgBF4, 30 mol% NCC6F5 and stoichiometric quantities of XeF2 and TMSOMe at 0 °C provided 1 in 67% yield and with 75% enantiomeric excess.16
These optimized conditions were subsequently applied to a variety of alcohol and phenol terminated dienes and trienes (Table 2). In most cases, high conversion of substrate occurred within three hours, however, the reactions were allowed to proceed for 24 h at 0 °C to ensure complete consumption of the XeF2. For the substrate classes in Table 2, no Brønsted acid derived products like 5 were observed, and a single diastereomer consistent with stereoretentive fluorination of the intermediate P2Pt-alkyl cation, was observed.
Table 2.
Catalytic electrophilic fluorination.a
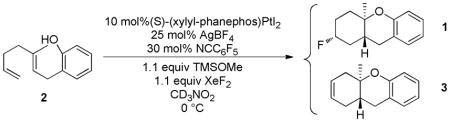
| ||||
|---|---|---|---|---|
| Entry | Polyene | Product | Yield 1 (%ee)b | Yield 3c |
| 1 |
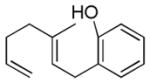
|

|
67% (75) | 14% |
| 2 |
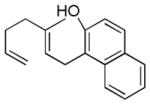
|

|
67% (73) | 15% |
| 3d |
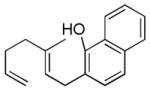
|
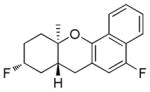
|
49% (86) | 15%e |
| 4 |
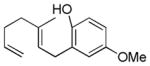
|
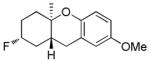
|
56% (81) | 16% |
| 5 |

|

|
60% (87) | 13% |
| 6 |

|
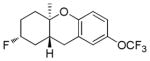
|
68% (83) | 24% |
| 7 |

|

|
80% (81) | trace |
| 8f |
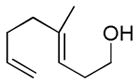
|
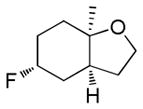
|
63%g | 22% |
| 9f |
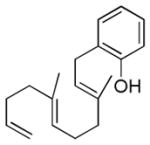
|
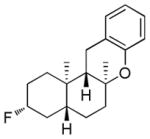
|
69% (10) | 10%e |
| 10h |
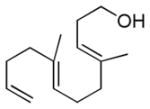
|
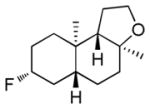
|
56% (78) | 0% |
Condtions: 10 mol% (S)-(xylyl-phanephos)Ptl2, 25 mol% AgBF4, 30 mol% NCC6F5, 1.1 equiv TMS-OMe, 1.1 equiv XeF2, 0.4 mL CD3NO2, 0 °C, 24 h. Starting material is mass balance of reaction.
Isolated yield, % ee determined by chiral GC.
GC yield.
Reaction run using 1.6 equiv XeF2.
Percentage is fluorinated elimination species only
Reaction with 20 mol% polystyrene-bound piperidine base run, no TMS-OMe, see SI for details.
Due to the volatility of this compound, a GC yield is reported.
Contains 23% unidenfied species, mass balance is unreacted starting material. Cannot separate the unidentified species from the product, therefore GC yield reported.
As shown in Table 2, variants on the phenol termini were well tolerated, except for α-naphthol (entry 3), wherein competitive fluorination of the aryl ether product occurs even with TMSOMe. In situ monitoring indicated that aryl fluorination occurred post cyclization / Pt-C fluorination. In this case, extra XeF2 was used to compensate for the difluorination stoichiometry. Unexpectedly, para-substituents improved the ee’s (entries 4–7).
Dienyl- and trienyl alcohols and phenols were also viable substrates though they behaved peculiarly. In the case of entries 8 and 9, the yields were poor under standard conditions, but could be recovered by exchanging TMSOMe for a polystyrene-bound piperidine base (see Table 1). In contrast, the triene alcohol in entry 10 performed better under the standard conditions. These base effects are not yet understood.
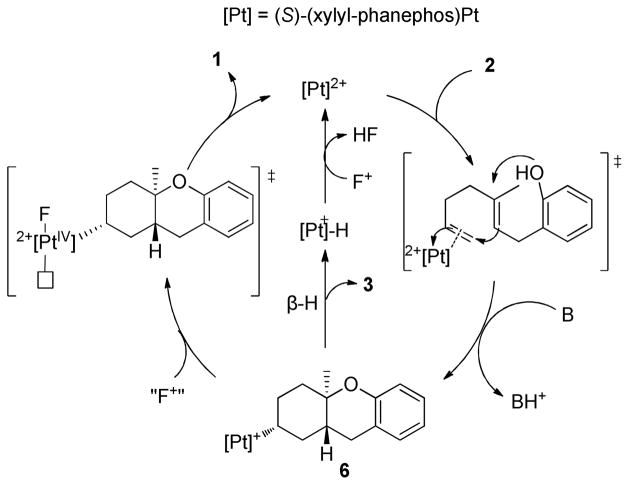
In situ monitoring of a cyclization/fluorination of 2 indicated that the alkyl cation (as the nitrile adduct, 6) serves as the catalyst resting state (31P NMR). These data support our current view of the mechanism (Scheme 2), that has 6 competitively undergoing β-H elimination or F+ attack to generate a [Pt]IVR(F) dication, which undergoes a stereoretentive reductive elimination to 1. Neither the [Pt]IV nor the [Pt]-H species are observable by NMR, however, literature precedence suggests that both routes are viable.17,5d,5f,5i,5k
Scheme 2.
Proposed catalytic cycle for electrophilic fluorination.
In summary, we illustrate that P2Pt-dicationic catalysts can mediate the enantioselective cation-olefin cyclization/fluorination reactions of polyprenoids to yield C3-fluorinated carbocycles. The key feature of the putative catalytic cycle is the selective reaction of XeF2 with P2Pt-alkyl cations over P2Pt-dications, which enables the sequential cyclization/fluorination.
Supplementary Material
Acknowledgments
Funding Sources
We thank the National Institute of General Medical Sciences for generous support (Grant GM-60578). H. N. also thanks the VEF for their support.
Footnotes
Characterization data for all new compounds and synthetic procedures are included in the Supporting information. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Hiyama T. Organofluorine Compounds. Springer; Berlin: 2000. [Google Scholar]; (b) Hagmann WK. J Med Chem. 2008;51:4359. doi: 10.1021/jm800219f. [DOI] [PubMed] [Google Scholar]; (c) Furuya T, Kuttruff CA, Ritter T. Curr Opin Drug Discovery. 2008;11:803. and references therein. [PubMed] [Google Scholar]; (d) Kirk KL. Org Process Res Dev. 2008;12:305. [Google Scholar]; (e) Purser S, Moore PR, Swallow S, Gouverneur V. Chem Soc Rev. 2008;37:320. doi: 10.1039/b610213c. [DOI] [PubMed] [Google Scholar]; (f) Morgenthaler M, Schweizer E, Hoffmann-Röder A, Benini F, Martin RE, Jaeschke G, Wagner B, Fischer H, Bendels S, Zimmerli D, Schneider J, Diederich F, Kansy M, Müller K. ChemMedChem. 2007;2:1100. doi: 10.1002/cmdc.200700059. [DOI] [PubMed] [Google Scholar]; (g) Müller K, Faeh C, Diederich F. Science. 2007;317:1881. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- 2.For aromatic electrophilic fluorinations, see for example: Grushin VV. Acc Chem Res. 2010;43:160. doi: 10.1021/ar9001763.Gouverneur V. Nat Chem. 2012;4:152. doi: 10.1038/nchem.1280.Borodkin GI, Shubin VG. Russ Chem Rev. 2010;79:259.Fier PS, Hartwig JF. J Am Chem Soc. 2012;134:10795. doi: 10.1021/ja304410x.Chan KSL, Wasa M, Wang X, Yu JQ. Angew Chem Int Ed. 2011;50:9081. doi: 10.1002/anie.201102985.Tang P, Furuya T, Ritter T. J Am Chem Soc. 2010;132:12150. doi: 10.1021/ja105834t.Wang X, Mei TS, Yu JQ. J Am Chem Soc. 2009;131:7520. doi: 10.1021/ja901352k.Furuya T, Kaiser HM, Ritter T. Angew Chem Int Ed. 2008;47:5993. doi: 10.1002/anie.200802164.Hull KL, Anani WQ, Sanford MS. J Am Chem Soc. 2006;128:7134. doi: 10.1021/ja061943k.
- 3.For enantioselective fluorination reviews: Cahard D, Xu X, Couve-Bonnaire S, Pannecoucke X. Chem Soc Rev. 2010;39:558. doi: 10.1039/b909566g.Lectard S, Hamashima Y, Sodeoka M. Adv Synth Catal. 2010;352:2708.Ma JA, Cahard D. Chem Rev. 2008;108:PR1. doi: 10.1021/cr800221v.Pihko PM. Angew Chem Int Ed. 2006;45:544. doi: 10.1002/anie.200502425.Audouard C, Ma JA, Cahard D. Adv Org Synth. 2006;2:431.
- 4.For non-enolate organic enantioselective electrophilic fluorinations: Rauniyar V, Lackner AD, Hamilton GL, Toste FD. Science. 2011;334:1681. doi: 10.1126/science.1213918.Dinoiu V. Rev Roum Chim. 2007;52:219.Kim SM, Kang YK, Cho MJ, Mang JY, Kim DY. Bull Korean Chem Soc. 2007;28:2435.Togni A, Mezzetti A, Barthazy P, Becker C, Devillers I, Frantz R, Hintermann L, Perseghini M, Sanna M. Chimia. 2001;55:801.Qiu S, Xu T, Zhou J, Guo Y, Liu G. J Am Chem Soc. 2010;132:2856. doi: 10.1021/ja909716k.
- 5.For electrophilic fluorinations that proceed through M-C bonds, see for examples: Furuya T, Kamlet AS, Ritter T. Nature. 2011;473:470. doi: 10.1038/nature10108.Vigalok A. Organometallics. 2011;30:4802.Engle KM, Mei TS, Wang X, Yu JQ. Angew Chem Int Ed. 2011;50:1478. doi: 10.1002/anie.201005142.Vigalok A, Kaspi AW. Top Organomet Chem. 2010;31:19.Mankad NP, Toste FD. Chem Sci. 2012;3:72. doi: 10.1039/C1SC00515D.Racowski JM, Gary JB, Sanford MS. Angew Chem Int Ed. 2012;51:3414. doi: 10.1002/anie.201107816.Dubinsky-Davidchik IS, Potash S, Goldberg I, Vigalok A, Vedernikov AN. J Am Chem Soc. 2012;134:14027. doi: 10.1021/ja3039272.Bloom S, Pitts CR, Miller DC, Haselton N, Holl MG, Urheim E, Lectka T. Angew Chem Int Ed. 2012;51:10580. doi: 10.1002/anie.201203642.Furuya T, Benitez D, Tkatchouk E, Strom AE, Tang P, Goddard WA, Ritter T. J Am Chem Soc. 2010;132:3793. doi: 10.1021/ja909371t.Furuya T, Klein JEMN, Ritter T. Synthesis. 2010;11:1804. doi: 10.1055/s-0029-1218742.Ball ND, Sanford MS. J Am Chem Soc. 2009;131:3796. doi: 10.1021/ja8054595.Kaspi AW, Yahav-Levi A, Goldberg I, Vigalok A. Inorg Chem. 2008;47:5. doi: 10.1021/ic701722f.Hull KL, Anani WQ, Sanford MS. J Am Chem Soc. 2006;128:7134. doi: 10.1021/ja061943k.Dick AR, Kampf JW, Sanford MS. J Am Chem Soc. 2005;127:12790. doi: 10.1021/ja0541940.
- 6.For steroid fluorination see for example: Poss AJ, Shia GA. Tet Lett. 1995;36:4721.Rozen S, Ben-Shushan G. J Org Chem. 1986;51:3522.Bowers A, Ringold HJ. Tetrahedron. 1958;3:14.For C3 fluorinated steroids: Liu W, Huang X, Cheng M-J, Nielsen RJ, Goddard WA, Groves JT. Science. 2012;337:1322. doi: 10.1126/science.1222327.
- 7.For a recent enantioselective I+ initiated cascade to yield C3-halopolyprenoids, see: Chen G, Ma S. Angew Chem Int Ed. 2010;49:8306. doi: 10.1002/anie.201003114.Sakakura A, Ukai A, Ishihara K. Nature. 2007;445:900. doi: 10.1038/nature05553.Sakakura A, Ishihara K. ChimicaOggi-Chemistry Today. 2007;25:9.
- 8.For asymmetric polyolefin cyclization methodologies, see: Zhu S, MacMillan DWC. J Am Chem Soc. 2012;134:10815. doi: 10.1021/ja305100g.Schafroth MA, Sarlah D, Krautwald S, Carreira EM. J Am Chem Soc. 2012;134:20276. doi: 10.1021/ja310386m.Jones SB, Simmons B, Mastracchio A, MacMillan DWC. Nature. 2011;475:183. doi: 10.1038/nature10232.Rendler S, MacMillan DWC. J Am Chem Soc. 2010;132:5027. doi: 10.1021/ja100185p.Knowles RR, Lin S, Jacobsen EN. J Am Chem Soc. 2010;132:5030. doi: 10.1021/ja101256v.Knowles RR, Jacobsen EN. Proc Natl Acad Sci. 2010;107:20678. doi: 10.1073/pnas.1006402107.Surendra K, Corey EJ. J Am Chem Soc. 2008;130:8865. doi: 10.1021/ja802730a.Zhao YJ, Loh TP. J Am Chem Soc. 2008;130:10024. doi: 10.1021/ja802896n.Uyanik M, Ishihara K, Yamamoto H. Org Lett. 2006;8:5649. doi: 10.1021/ol062378t.Uyanik M, Ishibashi H, Ishihara K, Yamamoto H. Org Lett. 2005;7:1601. doi: 10.1021/ol050295r.Taylor MS, Jacobsen EN. Proc Natl Acad Sci USA. 2004;101:5368. doi: 10.1073/pnas.0307893101.Kang SH, Lee SB, Park CM. J Am Chem Soc. 2003;125:15748. doi: 10.1021/ja0369921.
- 9.(a) Fürstner A. Chem Soc Rev. 2009;38:3208. doi: 10.1039/b816696j. [DOI] [PubMed] [Google Scholar]; (b) Fürstner A, Davies PW. Angew Chem Int Ed. 2007;46:3410. doi: 10.1002/anie.200604335. [DOI] [PubMed] [Google Scholar]; (c) Chianese AR, Lee SJ, Gagné MR. Angew Chem Int Ed. 2007;46:4042. doi: 10.1002/anie.200603954. [DOI] [PubMed] [Google Scholar]
- 10.(a) Sokol JG, Korapala CS, White PS, Becker JJ, Gagné MR. Angew Chem Int Ed. 2011;50:5658. doi: 10.1002/anie.201100463. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mullen CA, Campbell AN, Gagné MR. Angew Chem Int Ed. 2008;47:6011. doi: 10.1002/anie.200801423. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Mullen CA, Gagné MR. J Am Chem Soc. 2007;129:11880. doi: 10.1021/ja073573l. [DOI] [PubMed] [Google Scholar]; (d) Kerber WD, Gagné MR. Org Lett. 2005;7:3379. doi: 10.1021/ol051277c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Koh JH, Gagné MR. Angew Chem Int Ed. 2004;43:3459. doi: 10.1002/anie.200453913. [DOI] [PubMed] [Google Scholar]; (f) Kerber WD, Koh JH, Gagné MR. Org Lett. 2004;6:3013. doi: 10.1021/ol048780u. [DOI] [PubMed] [Google Scholar]; (g) Koh JH, Larsen AO, Gagné MR. Org Lett. 2001;3:1233. doi: 10.1021/ol015702n. [DOI] [PubMed] [Google Scholar]
- 11.For related electrophilic polyene cyclizations: Pradal A, Chen Q, Faudot dit Bel P, Toullec PY, Michelet V. Synlett. 2012;23:74.Peng H, Liu G. Org Lett. 2011;13:772. doi: 10.1021/ol103039x.Chen CC, Yang SC, Wu MJ. J Org Chem. 2011;76:10269. doi: 10.1021/jo201795t.Toullec P, Michelet V, Soriano E, Marco-Contelles J. Topics in Current Chemistry. Vol. 302. Springer; Berlin, Heidelberg: 2011. p. 31.Sethofer SG, Mayer T, Toste FD. J Am Chem Soc. 2010;132:8276. doi: 10.1021/ja103544p.Toullec PY, Blarre T, Michelet V. Org Lett. 2009;11:2888. doi: 10.1021/ol900864n.
- 12.Zhao SB, Becker JJ, Gagné MR. Organometallics. 2011;30:3926. doi: 10.1021/om200515f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.A comprehensive list of F+ reagents and TMS-X sources tested during optimization is available in the supporting information.
- 14.(a) Tius MA. Tetrahedron. 1995;51:6605. [Google Scholar]; (b) Tramšek M, Žemva B. J Fluorine Chem. 2006;127:1275. [Google Scholar]
- 15.NMR data and a comprehensive list of tested silyl ethers are available in the supporting information.
- 16.Note that in the absence of [Pt] no conversion of starting material was observed.
- 17.(a) Zhao SB, Wang RY, Nguyen H, Becker JJ, Gagné MR. Chem Commun. 2012;48:443. doi: 10.1039/c1cc15006e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Grice K, Scheuermann M, Goldberg K, Canty AJ. Top Organomet Chem. Vol. 503. Springer; Berlin, Heidelberg: 2011. p. 1. [Google Scholar]; (c) Yahav-Levi A, Goldberg I, Vigalok A. J Fluorine Chem. 2010;131:1100. [Google Scholar]; (d) Furuya T, Ritter T. J Am Chem Soc. 2008;130:10060. doi: 10.1021/ja803187x. [DOI] [PubMed] [Google Scholar]; (e) Wang T, Love JA. Organometallics. 2008;27:3290. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.