Abstract
Parkinson’s disease (PD) is a neurodegenerative disease primarily characterized by cardinal motor symptoms and central nervous system pathology. As current drug therapies can often stabilize these cardinal motor symptoms attention has shifted to the other motor and non-motor symptoms of PD which are resistant to drug therapy. Dysphagia in PD is perhaps the most important drug resistant symptom as it leads to aspiration and pneumonia, the leading cause of death. Here, we present direct evidence for degeneration of the pharyngeal motor nerves in PD. In this study, we examined the cervical vagal (X) nerve, pharyngeal branch of the X nerve (Ph-X), and pharyngeal plexus innervating the pharyngeal muscles in 14 postmortem specimens, 10 subjects with PD and 4 age-matched control subjects. Synucleinopathy in the pharyngeal nerves was detected using an immunohistochemical method for phosphorylated α-synuclein. α-Synuclein aggregates were revealed in the X nerve and Ph-X and immunoreactive intramuscular nerve twigs and axon terminals within the neuromuscular junctions were identified in all the PD subjects and in none of the controls. These findings indicate that the motor nervous system of the pharynx is involved in the pathological process of PD. Notably, PD subjects with dysphagia had a higher density of α-synuclein aggregates in the pharyngeal nerves as compared with those without dysphagia. Motor involvement of the pharynx in PD appears to be one of the factors leading to oropharyngeal dysphagia commonly seen in PD patients.
Keywords: α-Synuclein aggregates, Dysphagia, Immunohistochemistry, Intramuscular nerve twigs, Lewy bodies, Lewy neurites, Motor nerve, Nerve degeneration, Parkinson’s disease, Peripheral nervous system, Pharyngeal constrictor muscles, Pharyngeal plexus, Swallowing, Upper esophageal sphincter, Vagus nerve
INTRODUCTION
Parkinson’s disease (PD) is a neurodegenerative disorder affecting the human central, peripheral, and enteric nervous systems. In PD, in addition to degeneration of the nigrostriatal dopaminergic pathway, a variety of neuronal systems are involved, causing multiple neuromediator dysfunctions that account for the complex patterns of functional deficits (1). In the upper aerodigestive tract, pharynx, larynx, and tongue play an important role in swallowing, airway protection, phonation and speech articulation. Bulbar dysfunction characterized by dysphagia, sialorrhoea, hypophonia, and dysarthria, frequently observed in patients with PD, can be equally or even more disabling than the cardinal features. Approximately 50% to 80% of subjects with PD develop dysphagia (2–8) and 60% to 90% of PD patients exhibit speech and voice disorders (9, 10). However, the mechanisms of the upper airway disorders in PD remain unclear.
The histopathological hallmark of PD is the presence of fibrillar aggregates of α-synuclein called Lewy bodies (LBs) and Lewy neurites (LNs). α-Synuclein, a 140 amino-acid and a 14 kDa protein, is a major component of the LB and LN in PD (11). It is expressed in the central and peripheral nervous systems (12, 13) and involved in neurodegenerative diseases, including PD (14, 15). At the peripheral level, Lewy pathology has been revealed in the autonomic nervous system in PD, including the cardiac plexus (16–20), enteric nervous system of the alimentary tract (21–23), and skin (24–28). Although α-synuclein histopathology has been found in peripheral autonomic and sensory nervous systems, little is known about its existence in the peripheral motor nerves in PD.
More recently, we demonstrated PD-induced morphohistological and histochemical alterations in the pharyngeal muscles. Specifically, pharyngeal muscles in PD exhibited pathological changes, including the presence of denervated and atrophied fibers, fiber type grouping, and fast-to-slow myosin heavy chain transformation (29). Additionally, pharyngeal muscle atrophy (29) and vocal fold atrophy (10, 30, 31) observed in subjects with PD indirectly indicate motor impairment in PD. Motor innervation of the pharyngeal and laryngeal muscles is provided by X nerve. The X nerve gives off several motor branches, including the Ph-X, recurrent (RLN) and superior (SLN) laryngeal nerves that innervate the pharyngeal, laryngeal, and some palatal muscles. The pharyngeal constrictor (PC) and cricopharyngeus (CP) muscles receive their motor innervation from the pharyngeal plexus formed by the Ph-X, glossopharyngeal (IX), and sympathetic nerves (32–36), whereas the laryngeal muscles are innervated by the RLN and the external branch of the SLN. The Ph-X and laryngeal nerves are derived from the cervical X nerve (37–41). Morphological and immunohistochemical alterations in the pharyngeal muscle fibers in PD (29) suggest that the motor nervous system in the pharynx may be affected by the neuropathological process of the disease.
Recent studies have demonstrated synucleinopathy in the cervical X nerve fibers in PD (23, 42–44). However, it remains unknown whether the α-synuclein-positive fibers identified in the X nerve are motor in nature as approximately 80–90% of its fibers are afferent (45). In addition, little is known about whether Lewy pathology exists in the intramuscular nerve twigs and axon terminals within neuromuscular junctions (NMJs) in PD.
The purpose of this study was to test the hypothesis that the myofiber atrophy oberved in PD pharyngeal muscles is caused by neurodegeneration of the pharyngeal motor nerves. To test this hypothesis, we examined pharyngeal motor nerves in PD, including the cervical X nerve trunk, the pharyngeal plexus formed mainly by the Ph-X, intramuscular nerve twigs, and axon terminals within NMJs to detect degenerated motor nerve fibers using an immunohistochemical method for phosphorylated α-synuclein.
MATERIALS AND METHODS
Human Subjects
Histochemical and immunohistochemical studies were performed on the nervous tissues obtained from 10 deceased human subjects with clinically diagnosed and neuropathologically confirmed PD and 4 age-similar controls. The PD pharynges were provided by the Brain and Body Donation Program (46) at Banner Sun Health Research Institute (BSHRI), which is part of Banner Health, a regional nonprofit health care provider centered in metropolitan Phoenix, Arizona. BSHRI and the Mayo Clinic Arizona are the principal institutional members of the Arizona Parkinson’s Disease Consortium, which conducts a longitudinal clinicopathological study of PD and normal aging subjects with annual examinations from entry until death and autopsy. The healthy autopsied pharynges were obtained from BSHRI (n = 2) and the Department of Pathology at Hackensack University Medical Center (n = 2) in New Jersey.
Clinical and Neuropathological Assessments
The clinical characteristics of the 10 subjects with PD are shown in Table 1. Detailed clinical and neuropathological data for each case were provided by the Arizona PD Consortium. Subjects received standardized neuropathological examinations. Clinical assessments were performed by experienced movement disorder specialists (CHA, JNC, HAS, JES), who rated the clinical severity of PD using Hoehn and Yahr (H&Y) scale (47) and disability and motor impairment using the Unified Parkinson’s Disease Rating Scale (UPDRS) (48). Specific clinicopathologic diagnostic criteria for PD (49) were used. Dysphagia was assessed subjectively using item 7 of the UPDRS part II scale [i.e., swallowing score (0–4): 0 (normal), 1 (rare choking), 2 (occasional choking), 3 (requires soft food), and 4 (requires NG tube or PEG feeding)]. Gross and microscopic neuropathologic assessments were made by a single neuropathologist (TGB), who provided a detailed report on the brain neuropathology for each subject with PD.
Table 1.
Demographic and Clinical Characteristics of Cases With Parkinson’s Disease (PD) and Control Subjects
| Case No. | Sex | Age at Death, y | Age at PD Onset, y | PD Duration, y | H&Y Stages | Motor UPDRS | Cause of Death | UPDRS Months Before Death | PMI (h) | Dysphagia |
|---|---|---|---|---|---|---|---|---|---|---|
| PD 1 | M | 75 | 55 | 20 | 2 | 17 | es-PD | 21 | 69 | Yes |
| PD 2 | M | 73 | 62 | 11 | 3 | 18 | es-PD | 26 | 26 | No |
| PD 3 | M | 78 | 59 | 19 | 4 | 51 | CAD | 8 | 76 | Yes |
| PD 4 | F | 84 | 64 | 20 | 3 | 29 | CPF | 10 | 48 | No |
| PD 5 | M | 80 | 69 | 11 | 4 | 53 | c-PD | 11 | 26 | No |
| PD 6 | M | 81 | 70 | 11 | 4 | 43 | es-PD | 19 | 58 | Yes |
| PD 7 | F | 79 | 68 | 11 | 4 | 47 | Pneu | 9 | 16 | No |
| PD 8 | M | 75 | 45 | 30 | 4 | 66 | es-PD | 1 | 36 | Yes |
| PD 9 | M | 80 | 63 | 17 | 5 | 40 | CPF | 5 | 23 | No |
| PD 10 | M | 79 | 56 | 23 | 2 | 28 | MNP | 9 | 34 | Yes |
| Mean (range) | 78 (73–84) | 61 (45–70) | 17 (11–30) | 3.5 (2–5) | 39 (17–66) | 12.0 (1–26) | 41 (16–76) | |||
| Control 1 | F | 74 | – | – | – | – | MSF | – | 24 | No |
| Control 2 | M | 80 | – | – | – | – | PC | – | 73 | No |
| Control 3 | M | 70 | – | – | – | – | stroke | – | 26 | No |
| Control 4 | F | 70 | – | – | – | – | CPF | – | 30 | No |
| Mean (range) | 73.5 (70–80) | 38 (24–73) |
CAD, coronary artery disease; c-PD, complications of PD; CPF, cardiopulmonary failure; es-PD, end stage of PD; F, female; H&Y, Hoehn-Yahr clinical rating scale (score range 1–5); M, male; MNP, malignant neoplasm of prostate; MSF, multisystem failure; PC, pancreatic cancer; PD, Parkinson’s disease; PMI, postmortem interval; Pneu, pneumonia; UPDRS, Unified Parkinson’s Disease Rating Scale.
Tissue Sampling and Preparation
The mean postmortem interval between expired time and tissue preparation was 41 hours (range, 16–76 hours) for PD specimens (Table 1) and 38 hours (range, 24–73 hours) for controls; this postmortem interval does not hamper reliable histochemical analysis of autopsied tissues (50, 51), provided the body has been stored in refrigerated area. The tissue samples were harvested as shown in Figure 1.
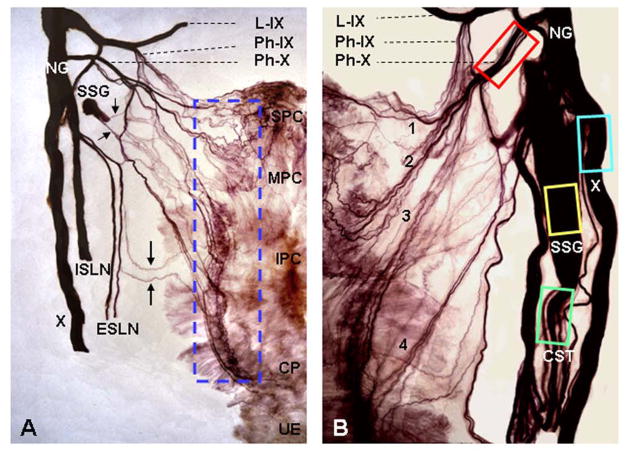
FIGURE 1.
Photographs of two human semipharynges processed with Sihler’s stain, wholemount nerve staining technique, showing the branching of cervical vagus (X) nerve, formation of the pharyngeal plexus, and tissue sampling sites for detecting neurodegenerative changes in the nerves and intramuscular nerve twigs. Pharyngeal plexus in humans provides motor innervation to pharyngeal constrictors (PCs) and cricopharyngeal (CP) sphincter. (A) Posterior view of a Sihler’s stained adult human semipharynx, showing the branching and distribution of the cervical X nerve and the pharyngeal plexus. Note that the X nerve gives off its pharyngeal branch (Ph-X) and superior laryngeal nerve (SLN). The SLN is then subdivided into internal (ISLN) and external (ESLN) branches which supply the laryngeal and pharyngeal mucosa (ISLN) and cricothyroid muscle (ESLN), respectively. The pharyngeal plexus is formed mainly by the Ph-X and less by the pharyngeal branch of the glossopharyngeal nerve (Ph-IX), ESLN (large arrows), and sympathetic nerve fibers (small arrows) from superior sympathetic ganglion (SSG). In this case, most of the SSG was removed in order to show nerve branching patterns. The enclosed region, which is the location of motor zone in the PCs and CP, indicates tissue sampling site of the PC and CP muscles for detecting neurodegenerative changes in the intramuscular nerve twigs and axon terminals. IPC, inferior pharyngeal constrictor; L-IX, lingual division of the IX nerve; MPC, middle pharyngeal constrictor; NG, nodose ganglion of X nerve; SPC, superior pharyngeal constrictor; UE, upper esophagus. x2. (B) Higher magnification image of a Sihler’s stained human semipharynx, showing anatomic relationship between different nerves and nerve branches and illustrating the nerve tissue sampling sites (enclosed regions) for histochemical and immunohistochemical studies. Note that the Ph-X gives off at least 4 primary branches (1, 2, 3, 4) to innervate the PC and CP muscles. x4. CST, cervical sympathetic trunk. Adapted and reproduced in part with permission from Mu and Sanders (35), Ann Otol Rhino Laryngol 2007;116:604–17.
The studied neural structures included cervical X nerve, Ph-X, cervical superior sympathetic ganglion (SSG), and cervical sympathetic trunk (ST). Firstly, a 3-cm-long segment of the cervical X nerve trunk on each side was sampled immediately after it gives off Ph-X and SLN. The removed X nerve trunk was then divided into 4 sub-segments (~7-mm in length/each). One nerve segment was prepared for α-synuclein immunohistochemistry and used in this study, whereas the remaining nerve segments were prepared for other purposes. Secondly, a 1-cm of the Ph-X was sampled immediately after it is divided from the X nerve. The removed Ph-X was then divided into 2 sub-segments (~5-mm in length/each), one of which was prepared for α-synuclein immunohistochemistry as described below and the other was prepared for a different purpose. Finally, entire SSG and a segment of ST (~7-mm in length/each) were also harvested to detect α-synuclein pathology as both components give off sympathetic nerve fibers to join pharyngeal plexus as shown in Figure 1. The removed nerve and SSG samples were fixed with 10% neutral buffered formalin overnight, embedded in paraffin, and sectioned (4 μm) longitudinally or transversely.
After nerve samples were removed, the motor zone in the PC and CP muscles, where the pharyngeal plexus is located, was outlined and sampled as shown in Figure 1. The muscle samples were frozen in isopentane cooled by dry ice, sectioned longitudinally (60-μm thick) on a cryostat (Reichert-Jung 1800; Mannheim, Germany) at −25°C, and stored at −80°C until staining procedures were performed. The muscle sections were stained immunohistochemically to identify α-synuclein immunoreactive intramuscular nerve twigs and axon terminals. The immunohistochemically stained muscle sections showing axon terminals were re-stained using conventional acetylcholinesterase and silver stain (AChE-Ag) to see if the axon terminals innervate NMJs.
Staining Methods
Immunohistochemistry and Staining
Nerve sections were deparaffinized in xylene and brought to distilled water through graded alcohols. The nerve and muscle sections were stained with an immunohistochemical method for phosphorylated α-synuclein as previously described (43, 44, 52–54). Briefly, the tissue sections were: (1) pretreated with 1:100 proteinase K (Enzo Life Sciences) diluted in in 0.1 M phosphate buffer (PBS) at 37°C for 30 min and washed 3 times in PBS; (2) immersed for 30 min in 1% hydrogen peroxide in 0.1 M PBS with 0.3% Triton X-100 (PBS-TX) at pH 7.4 and washed 3 times in PBS-TX; (3) incubated at room temperature (RT) overnight in anti-phosphorylated α-synucliein (psyn) monoclonal antibody (psyn#64; Wako) at 1:1000 dilution in PBS-TX and washed 3 times in PBS-TX; (4) incubated with a secondary biotinylated antibody (anti-mouse IgG diluted 1:1000 in PBS-TX; Vectastain, Vector Laboratories, Burlingame, CA) for 2 hr at RT and washed 3 times in PBS-TX; (5) treated for 30 min with avidin-biotin-complex (ABC, Vector), with A and B components of the kit both at 1:1000 dilution, and followed by 2 washes in PBS-TX and a last wash in 0.05 M Tris buffer at pH 7.6; (6) treated with 3,3′-diaminobenzidine (DAB, Sigma) (5 mg/100 ml) with added saturated nickel ammonium sulfate (2 ml/100 ml) and hydrogen peroxide (5 μl/100 ml of 1% hydrogen peroxide) for 30 min in the dark and washed 3 times in 0.05 M Tris buffer at PH 7.6; (7) cleared with xylene and coverslipped. Controls for staining specificity were omission of the primary antibody.
AChE-Ag Staining
Conventional AChE-Ag staining as described (55) was also used in this study. AChE-Ag stains both the axons and their innervating NMJs. Some longitudinal muscle sections stained with α-synuclein immunohistochemistry were re-stained using AChE-Ag staining to confirm if some α-synuclein immunoreactive axon terminals in the pharyngeal muscles were motor to innervate NMJs.
Photographing
The stained sections of the nerves and muscles were examined under a Zeiss photomicroscope (Axioplan; Carl Zeiss, Germany) and photographed with a Sony digital camera (model DXC-5500, Japan) attached to the photomicroscope and connected to a personal computer with image analysis software.
Semiquantitative Assessment of α-Synuclein Positive Inclusions
Stained sections from both PD subjects and controls were assessed by a single investigator (JC) without knowledge of subject identity or diagnosis. For a given tissue sample from either the X nerve or Ph-X, 3 nerve sections at different spatial levels stained for α-synuclein immunohistochemistry were chosen to count LNs, each of which was counted separately. For each section, the microscopic field with the highest density of LNs was identified and the number of LNs was counted at x200 magnification (field diameter 1 mm) with a computerized image analysis system (SigmaScan; Jandel Scientific, San Rafael, California). The density of the LNs in the 3 selected sections for each sample was averaged and the mean density was used for evaluating lesion severity. The severity of α-synuclein positive lesions in each of the nerve samples was rated according to the mean density of the LNs and assessed semiquantitatively following an arbitrary grading system: −, no lesions; +, 1–20 lesions/field (mild); ++, 21–50 lesions/field (moderate); +++, >50 lesions/field (severe). The data on the density of lesions (and similarly Semiquantitative Assessment rates) from the nerve samples collected from the same subject and experimental condition were averaged.
Data Analysis
The data on the frequency of α-synuclein aggregates were compared between X nerve and Ph-X in PD subjects with and without dysphagia. The influence of dysphagia on the density of α-synuclein lesions was evaluated in the PD group with 2-way repeated measure ANOVA. The main factors were presence or absence of dysphagia (yes or no) and nerve type (X nerve and Ph-X). In addition, statistical analysis of the semi-quantitative rates on α-synuclein lesions were compared between 4 experimental conditions (combination of nerve type and presence or absence of dysphagia) with the Kruskal-Wallis test, followed by post-hoc analysis with Benferroni corrected multiple comparisons. Statistical computations were performed using the Statistical Analysis System 9.2 (SAS, Inc., Cary, NC). Statistical significance was set at p < 0.05.
RESULTS
Demographic Features
The demographic and clinical data are summarized in Table 1. There were 10 subjects with PD (8 men and 2 women) with a mean age of 78 years (range, 73–84 years). All PD subjects were white. The mean age at onset of PD was 61 years (range, 45–70 years) and the mean duration of PD at time of death was 17 years (range, 11–30 years). In this group, 7 PD subjects had developed dementia. The mean Hoehn and Yahr stage was 3.5; 2 subjects were stage 2; 2 subjects were stage 3; 5 subjects were stage 4; and 1 subject was stage 5. The mean score for the motor UPDRS (UPDRS: Part III) was 39 points (range, 17–66). The mean interval between last neuro/movement examination and autopsy (UPDRS months before death) was 12 moths (range, 1–26 months) for PD cases (Table 1). The 4 age-similar control subjects consisted of 2 men and 2 women with a mean age of 73.5 years (range, 70–80 years). The cause of death for each of PD cases and normal control subjects was also give in Table 1.
Based on the UPDRS part II scale, dysphagia occurred in 5 out of the 10 PD subjects (Table 1). In the 5 dysphagic subjects, the swallowing score was rated as 1 in 2 cases (PD 1, 6) and 2 in 3 cases (PD 3, 8, 10). It looks like the PD duration in the dysphagia group was longer than that in the non-dysphagia group. However, a comparison of the demographic features showed that there were no significant differences between dysphagia (PD 1, 3, 6, 8, 10) and non-dysphagia (PD 2, 4, 5, 7, 9) groups in the mean age (77.6 vs 79.2 years), mean PD duration (20.6 vs 14.0 years), mean Hoehn and Yahr stage (3.2 vs 3.8), and mean score for the motor UPDRS (41.0 vs 37.4 points) (p >0.05).
Neuropathological Findings in Brains with PD
All autopsied brains of the PD subjects included in this study met neuropathological criteria for PD based on examination by T.G.B. Microscopic examinations revealed that the substantia nigra and locus ceruleus showed moderate to marked depletion or loss of pigmented neurons and there were several LBs in each region (data not shown). Immunohistochemical staining for phosphorylated α-synuclein showed frequent immunoreactive LBs and LNs in the olfactory bulb, brainstem, amygdala, transentorhinal area and cingulate gyrus, with variable densities in the 3 neocortical regions examined (temporal, frontal, and parietal). The major spinal cord subdivisions were examined in 7 cases; 6 of these had positive Lewy-type synucleinopathy in the spinal cord. Using the Unified Staging System for Lewy Body Disorders (54), 6 cases were classified as “neocortical stage” and 2 were “brainstem and limbic stage”. Seven of the PD cases also had dementia, and of these, 5 cases also met consensus neuropathological criteria for Alzheimer’s disease (AD) (56). One other case had dementia on the basis of progression of PD without concurrent AD.
Overview of Branching of the Cervical X Nerve and Formation of the Pharyngeal Plexus
The X nerve originates in the medulla oblongata and extends through the jugular foramen below the head to the neck, chest and abdomen. It carries both afferent and efferent fibers. The somatic motor fibers of the X nerve, which arise from the cells of the nucleus ambiguus in the medulla, form the Ph-X and laryngeal nerves which innervate pharyngeal, laryngeal, and some palatal muscles.
Figure 1 illustrates the branching and distribution patterns of the cervical X nerve and the nerve branches forming the pharyngeal plexus. Sihler’s stain, a wholemount nerve staining technique, shows that upon leaving the jugular foramen, the X nerve gives off its Ph-X and then the internal and external branches of the SLN. The Ph-X arises from the upper part of the nodose ganglion of the X nerve and passes across the internal carotid artery to the upper border of the middle pharyngeal constrictor, where it divides into numerous intramuscular twigs. The PCs and CP sphincter receive their motor innervation from the pharyngeal plexus which is formed mainly by the Ph-X and less by the pharyngeal branch of glossopharyngeal nerve (IX), external branch of the SLN, and sympathetic nerve fibers derived from the SSG. These findings indicate that the somatic motor nerve fibers innervating the PC and CP muscles are carried by X nerve, concentrated in the Ph-X, run in the nerve fascicles forming the pharyngeal plexus, and distributed to the motor zone in the middle portion of the muscles, where they give off axon terminals to innervate the NMJs. Therefore, identification of α-synuclein aggregates and degenerated axons in these neural structures would provide direct evidence of involvement of the peripheral motor nervous system controlling the pharynx in PD.
α-Synuclein Pathology in the X Nerve, Ph-X, Intramuscular Nerve Twigs (INT) and Axon Terminals within Neuromuscular Junctions (NMJ) in PD
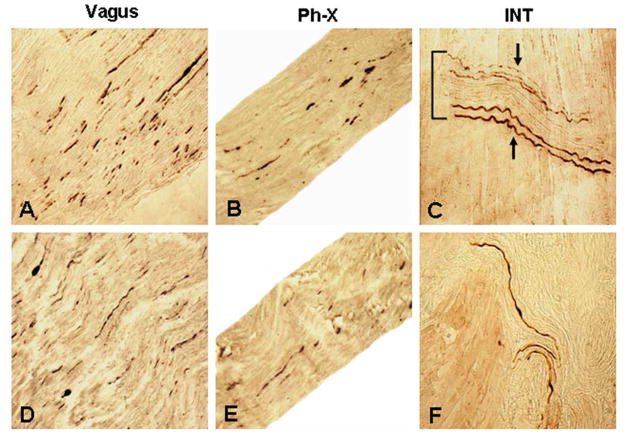
Figure 2 shows photomicrographs of immunostained longitudinal sections of the cervical X nerve, Ph-X, and INTs in PD. Aggregated axonal α-synuclein inclusions were identified in both the cervical X nerve trunk (Fig. 2A, D) and Ph-X (Fig. 2B, E). Immunoreactive INTs (Figs. 2C, F; 3A–D) and axon terminals within NMJs (Fig. 3E–H) were revealed in the PD IPC and CP muscles.
FIGURE 2.
Photomicrographs of the longitudinal sections of cervical X nerve trunk (A, D), pharyngeal branch of the X nerve (Ph-X) (B, E), and pharyngeal muscles (C, F) immunostained for phosphorylated α-synuclein (psyn) in PD subjects with dysphagia (A–C) and without dysphagia (D–F), showing Lewy neurites (LNs) in the nerves examined and immunoreactive intramuscular nerve twigs (INTs) in the muscles. (A) A stained section of a cervical X nerve trunk from a PD subject with dysphagia (PD 3, 78-year-old man with disease duration of 19 years, Hoehn & Yahr 4, and motor UPDRS 51; lesion severity: severe, +++). Note that there were numerous LNs (darkly stained threads and dots) in the X nerve. x200. (B) A stained section of the Ph-X from the same PD subject as in A. Note that the Ph-X in this case also contained frequent LNs (lesion severity: moderate, ++). x200. (C) A stained section of the inferior pharyngeal constrictor (IPC) from the same PD subject as in B. Note that an INT running across the muscle fibers was horizontally sectioned. Several axons on the superior and inferior margins of the INT were positively immunostained with anti-psyn immunohistochemistry (arrows), whereas the normal axons in the center of the INT remained unstained. x200. (D) A stained section of cervical X nerve trunk from a PD subject without dysphagia (PD 9, 80-year-old man with disease duration of 17 years, Hoehn & Yahr 5, and motor UPDRS 40; lesion severity: moderate, ++), showing frequent LNs in the X nerve. x200. (E) A stained section of the Ph-X from the same PD subject as in D. Note that there were several LNs in the Ph-X (lesion severity: mild, +). x200. (F) A stained section of cricopharyngeal (CP) sphincter from the same PD subject as in E, showing three immunoreactive axons in the muscle. x200.
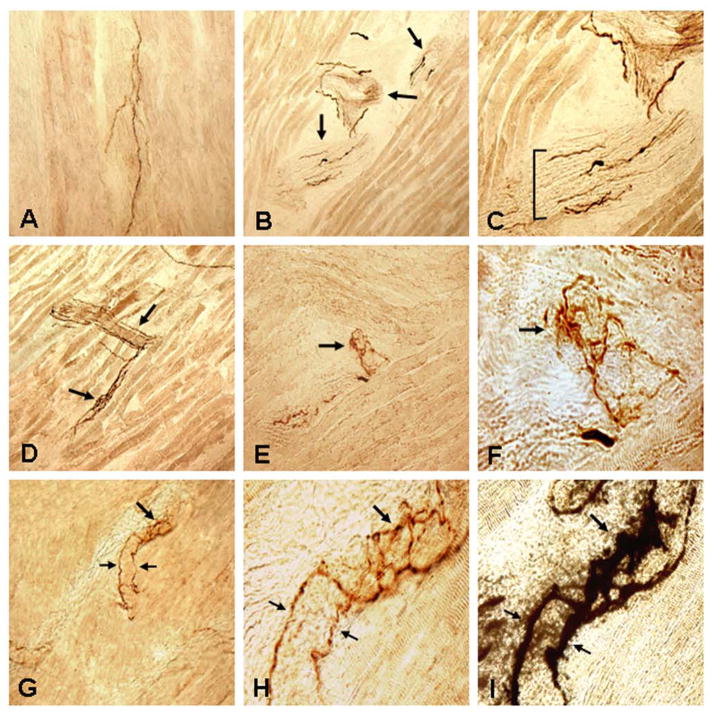
FIGURE 3.
Photomicrographs of the longitudinal sections of inferior pharyngeal constrictor (IPC) and cricopharyngeal (CP) sphincter immunostained with anti-phosphorylated α-synuclein immunohistochemistry in PD subjects, showing immunoreactive intramuscular nerve twigs (INTs) and axon terminals within neuromuscular junctions (NMJs). (A–C) Images of a stained section of IPC muscle from a PD subject with dysphagia (PD 8, 75-year-old man with disease duration of 30 years, Hoehn & Yahr 4, and motor UPDRS 66). (A) A stained muscle section showing immunoreactive axons (darkly stained threads). x100. (B) A stained muscle section containing three obliquely cut intramuscular nerve branches (arrows). Note that there were several α-synuclein immunoreactive axons (darkly stained threads) in each of the nerve branches. x100. (C) Magnification of B, showing the profiles of the nerve branches and α-synuclein positive and negative (unstained) axons within the intramuscular nerve branches. x200. (D–F) Images of stained sections of IPC muscle from a PD subject without dysphagia (PD 9, 80-year-old man with disease duration of 17 years, Hoehn & Yahr 5, and motor UPDRS 40), showing α-synuclein immunoreactive INTs and axon terminals within NMJs. (D) Low-power view of a stained muscle section, showing immunoreactive INTs (arrows) containing α-synuclein positive axons. x100. (E) Higher-power view of a stained muscle section, showing α-synuclein positive axon terminals within an NMJ (arrow). x200. (F) Closeup photograph of E, showing the profiles of the immunoreactive axon terminals within the NMJ (arrow). x640. (G–I) Photomicrographs of stained sections of CP muscle from a PD subject with dysphagia (PD 10, 79-year-old man with disease duration of 23 years, Hoehn & Yahr 2, and motor UPDRS 28), showing immunoreactive axon terminals. (G) Higher-power view of a stained PD CP, showing two α-synuclein positive preterminal axons (small arrows) and their terminals within the NMJ (large arrow). x200. (H) Closeup photograph of G, showing the organization of the axon terminals within the NMJ. Note that two immunoreactive preterminal axons (small arrows) innervating an NMJ give off terminals within the NMJ (large arrow). x640. (I) The muscle section used in H was restained with AChE-Ag. Note that the preterminal axons (small arrows) innervated an NMJ (large arrow) and that the immunoreactive axon terminals as shown in H were located within the AChE-Ag stained NMJ. x640.
A marked difference in occurrence of α-synuclein pathology in the studied nerves and INTs was observed between PD and control groups. For example, in PD group, the most frequently affected structure was the X nerve (10/10), followed by the Ph-X (8/9) and the INTs (7/10). In controls, no LNs were observed in both the X nerve and the Ph-X and no immunoreactive INTs were found in the muscles studied (data not shown).
On average, the cervical X nerve contained more α-synuclein aggregates than the Ph-X in PD. Variable lesion severity in PD X nerve and Ph-X was summarized in Table 2. The severity of α-synuclein lesions in each of the nerve samples for each subject with PD was rated according to the mean density of α-synuclein aggregates. In PD group, the severity of α-synuclein aggregates in X nerve was rated as mild (+) in 3 cases, moderate (++) in 4 cases, and severe (+++) in 3 cases. Ph-X was obtained from 9 PD subjects. The severity of α-synuclein lesions was rated as negative (no lesions) in 1 case, mild in 3 cases, and moderate in 5 cases. In this series, the X nerve was moderately to severely affected in 7 cases (7/10), whereas the Ph-X was moderately affected in 5 cases (5/9). In PD, the density or severity of α-synuclein lesions in the X nerve was larger as compared with that in the Ph-X (Table 2).
Table 2.
Frequency and Severity of α-Synuclein Positive lesions in Cervical X Nerve and Ph-X in Subjects With Parkinson’s Disease
| Case No. | X nerve
|
Ph-X
|
||||||
|---|---|---|---|---|---|---|---|---|
| LNs (range) | Severity of Lesions | LNs (range) | Severity of Lesions | |||||
| 1 | 26 (22–30) | ++ | NA | |||||
| 2 | 13 (10–14) | + | 6 (3–10) | + | ||||
| 3 | 60 (42–69) | +++ | 24 (18–30) | ++ | ||||
| 4 | 45 (33–56) | ++ | 22 (14–27) | ++ | ||||
| 5 | 20 (14–26) | + | 7 (3–11) | + | ||||
| 6 | 48 (35–57) | ++ | 21 (12–35) | ++ | ||||
| 7 | 17 (12–24) | + | – | − | ||||
| 8 | 54 (39–62) | +++ | 23 (12–31) | ++ | ||||
| 9 | 40 (21–55) | ++ | 10 (8–12) | + | ||||
| 10 | 55 (46–61) | +++ | 21 (13–26) | ++ | ||||
|
| ||||||||
| Mean (range) | 38 (13–60) | 17 (6–24) | ||||||
The severity of α-synuclein positive lesions in the X nerve and Ph-X was rated according to the mean density of the LNs and assessed semiquantitatively following an arbitrary grading system: −, no lesions; +, 1–20 lesions/field (mild); ++, 21–50 lesions/field (moderate); +++, >50 lesions/field (severe). LNs, Lewy neurites; NA, not available.
PD subjects with dysphagia had a higher density of α-synuclein lesions in either the X nerve or the Ph-X as compared with those without dysphagia (Table 2). For example, in the 5 PD subjects with dysphagia (PD 1, 3, 6, 8, 10) the X nerve exhibited moderate lesions in 2 cases (PD 1, 6) and severe lesions in 3 cases (PD 3, 8, 10). In contrast, in the 5 PD subjects without dysphagia (PD 2, 4, 5, 7, 9), the X nerve disclosed mild lesions in 3 cases (PD 2, 5, 7) and moderate lesions in 2 cases (PD 4, 9).
Two-way repeated ANOVA (factors: presence dysphagia and nerve type) showed a statistically significant effect of both factors and the interaction between them. The density of α-synuclein lesions in the dysphagia group was larger than that in the non-dysphagia group (F=10.69, df=1/7, p=0.014). The density of α-synuclein lesions in X nerve was also larger than that in Ph-X (F=94.04, df=1/7, p=0.0001). The significant interaction between presence of dysphagia and nerve type (F=5.63, df=1/7, p=0.049) showed that the difference between the subjects with dysphagia and without dysphagia was larger in X group (52.3 vs 27.2) than in Ph-X group (23.3 vs 9.6). The semi-quantitative rates on α-synuclein aggregates were compared with the Kruscal-Wallis test. The test showed presence of statistically significant differences between experimental conditions (p <0.01, Chi-Square 11.5, df=3). Post-hoc analysis with Bonferroni corrected multiple comparisons showed significantly larger severity of lesions in X nerve between subjects with dysphagia and without dysphagia (rank difference 8.1, p=0.012). In Ph-X nerve there was a trend in similar direction, but the difference did not reach the level of statistical significance (rank difference 6.7, p=0.064).
Longitudinal sections of the PC and CP muscles stained with anti-psyn immunohistochemistry showed immunoreactive axons and INTs (Figs. 2C, F; 3A–D), preterminal axons and axon terminals within NMJs (Fig. 3E–H). Some muscle sections stained with α-synuclein immunohistochemistry (Fig. 3H) were also restained with AChE-Ag (Fig. 3I). AChE-Ag staining showed that the preterminal axons innervated NMJs and the immunoreactive axon terminals were located within the NMJs (Fig. 3I), indicating that they were motor in nature. In control muscles, no immunoreactive INTs and axon terminals were observed (data not shown).
α-Synuclein Aggregates in the Cervical Superior Sympathetic Ganglion (SSG) and Sympathetic Nerve (SN) in PD
As shown in Figure 1, cervical SSG and SN furnish nerve fibers to join the pharyngeal plexus which supplies the PC and CP muscles. In this study, SSG and SN obtained from 7 PD (PD 4–10) and 3 control subjects were examined using α-synuclein immunohistochemistry. All of the 7 PD SSG (Fig. 4A–C) and SN (Fig. 4D–F) samples exhibited α-synuclein aggregates. The SSG and SN were affected slightly in 2 cases (PD 4, 7), moderately in 2 cases (PD 5, 6), and severely in 3 cases (PD 8, 9, 10). In controls, no α-synuclein aggregates were identified in both the SSG and SN (data not shown).
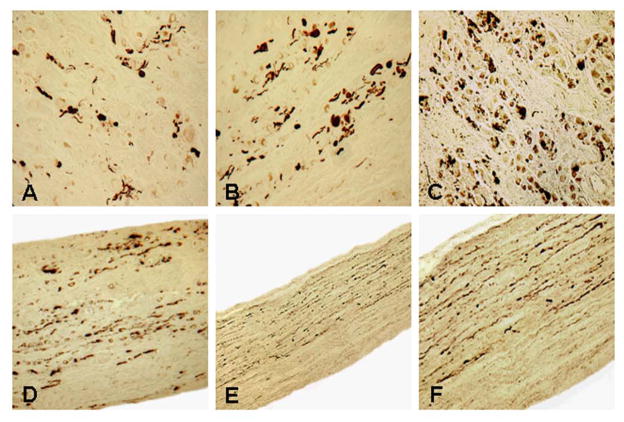
FIGURE 4.
Photomicrographs of cross sections of the cervical superior sympathetic ganglion (SSG) (A–C) and longitudinal sections of the cervical sympathetic nerve (D–F) from subjects with PD immunostained with anti-phosphorylated α-synuclein immunohistochemistry. (A) A stained section of SSG from a PD subject without dysphagia (PD 7, 79-year-old woman with disease duration of 11 years, Hoehn & Yahr 4, and motor UPDRS 47). The SSG was affected slightly as it contained some α-synuclein aggregates. x200. (B) A stained section of SSG from a PD subject without dysphagia (PD 5, 80-year-old man with disease duration of 11 years, Hoehn & Yahr 4, and motor UPDRS 53). The SSG was affected moderately as it exhibited abundant α-synuclein aggregates. x200. (C) A stained section of SSG from a PD subject with dysphagia (PD 10, 79-year-old man with disease duration of 23 years, Hoehn & Yahr 2, and motor UPDRS 28). The SSG was affected severely as it contained numerous α-synuclein aggregates. x200. (D) A stained longitudinal section of a cervical sympathetic trunk from the same PD subject as in B (PD 5). Note that there were abundant α-synuclein aggregates in the sympathetic nerve. x200. (E) Low-power view of a stained longitudinal section of a cervical sympathetic trunk from the same PD subject as in C (PD 10). Note that this sympathetic nerve contained numerous α-synuclein aggregates. Also note that α-synuclein immunoreactive axons were not randomly distributed throughout the nerve, instead they were concentrated in two-thirds of the nerve. x100. (F) Higher-power view of E. x200.
DISCUSSION
To our knowledge, this study is the first to demonstrate α-synuclein pathology in the peripheral motor nerves innervating the pharynx in PD. Our studies showed that some nerve fibers of the pharyngeal plexus innervating the pharyngeal constrictors and cricopharyngeal sphincter undergo degeneration in PD. Degenerative changes in the motor nerve fibers were documented with anti-psyn immunohistochemistry. There are several notable findings. Firstly, using α-synuclein immonohistochemistry we detected Lewy pathology in the nerves forming the pharyngeal plexus. Specifically, α-synuclein aggregates were identified in cervical X nerve, Ph-X, cervical superior sympathetic ganglion and sympathetic trunk in PD. Secondly, immunoreactive intramuscular nerve twigs and axon terminals within the NMJs in the PD PC and CP muscles were identified using α-synuclein immunostaining. Finally, some α-synuclein immunoreactive axon terminals in the pharyngeal muscles were motor in nature since they were located within the NMJs and positively stained with AChE-Ag method. Notably, PD subjects with dysphagia had a higher density of α-synuclein aggregates in the nerves studied as compared with those without dysphagia. Axonal degeneration of the pharyngeal motor nerves could be responsible for denervation atrophy of the pharyngeal muscle fibers as described (29). Involvement of the peripheral motor nervous system controlling the pharynx in PD is most likely a major factor leading to the oropharyngeal dysphagia that is commonly seen in patients with PD.
In this study, Lewy pathology in the peripheral motor nerves innervating the pharyngeal muscles was detected using immunohistochemistry for α-synuclein which is involved in neurodegenerative diseases, including PD (14, 15, 44, 52). AChE-Ag staining stains both the axons and NMJs. The AChE-Ag stained intramuscular nerve fascicles and axons are motor in nature if they innervate NMJs. We found that the longitudinal muscle sections stained with α-synuclein immunohistochemistry can be re-stained with AChE-Ag. This gave us a good opportunity to determine if the α-synuclein positive axons and terminals are motor. The same muscle section re-stained with AChE-Ag showed that some α-synuclein positive axons innervated NMJs, thus confirming that the α-synuclein immunostained preterminal axons and axon terminals were motor.
Disability in PD is mostly attributed to motor impairment (49, 57). The peripheral motor system has been demonstrated to be involved in PD. Electrophysiological abnormalities include decreased muscle activity (58), abnormal motor unit morphology (59), prolonged latencies and smaller motor nerve action potentials (60), and chronic partial denervation in the PD limb muscles (59, 61–66). Motor unit number estimation is a unique electrophysiologic means that can provide a numeric estimate of the number of axons innervating a muscle. Using this method, Caviness et al., (65) demonstrated that mild motor neuron drop-out with reinnervation occurs in PD. The detectable electrophysiological changes suggest that motor degeneration is part of the pathologic picture of PD. These observations gain support from immunihistochemical studies by Beach et al., (44), who reported that occasional large anterior horn neurons as well as some fibers within the vagus and sciatic nerves in PD subjects were immunoreactive for phosphorylated α-synuclein.
Motor disturbances of upper aerodigestive tract such as oropharyngeal dysphagia, speech and voice disorders in PD are not uncommon. There are probably 8 million or more individuals in the world each year that have or will have swallowing and speech disorders during the course of their PD (7). Swallowing and speech problems in PD were thought to be associated with orofacial–laryngeal bradykinesia and rigidity (67). However, studies using simultaneous videoradiography and pharyngeal manometry found no correlation between overall muscle rigidity score and abnormal pharyngeal wall motion in PD patients (68). Importantly, pharmacological and surgical interventions for PD-related swallowing, speech and voice disorders are minimally effective. While neuropharmacologic and brain stimulation have marked therapeutic effects on limb motor functions, their effects on speech and swallowing in PD are less impressive, and in some cases, adverse (7, 8, 69–72). These findings suggest that oropharyngeal dysphagia in PD may not be caused solely by a reduction in basal ganglia dopamine activity. Therefore, other neurotransmitter systems (6, 70, 73) or nondopaminergic mechanisms (6) may also be involved. Various neuronal systems are likely involved in PD (1), thus resulting in multiple neuromediator dysfunctions that account for the complex patterns of functional deficits (74).
More recently, we reported that pharyngeal muscles in PD exhibit pronounced pathological changes, including the presence of considerable numbers of atrophied fibers, fiber type grouping, and fast-to-slow myosin heavy chain transformation, all suggestive of muscle denervation (29, 35). Decreased motor activity has been found in PD patients with dysphagia. By using pharyngeal manometry, some investigators (68) found that PD patients with dysphagia had a higher hypopharyngeal intrabolus pressure and lower pharyngeal contraction pressures, suggesting decreased muscle function. Clinical and radiological assessments also revealed that dysphagic PD patients had decreased tongue movements (75, 76) and pharyngeal peristalsis (70, 75), indicating impaired motor function. Here, we provide direct neural histochemical and immunohistochemical evidence for involvement of the peripheral motor nervous system controlling the pharyngeal muscles. Evidently, structural and functional changes in pharyngeal muscles are caused at least in part by axonal degeneration of the pharyngeal motor plexus.
Motor dysfunction of the pharynx and larynx could be due to degenerative alterations in the peripheral nervous system and/or neuropathological lesions in motoneurons. In the present study, we identified Lewy neurites in the X nerve and Ph-X which provide motor innervation to the pharyngeal constrictors and upper esophageal sphincter. Degenerated peripheral motor nerve fibers in both nerves suggest that Lewy pathology could occur in their motoneurons. It is well known that the nucleus ambiguus gives origin to the somatic motor fibers of the X nerve which innervate the striated muscles of the pharynx, larynx, and upper esophagus (77). Therefore, the lesions at both the peripheral and central levels could lead to swallowing and voice disorders in PD. However, Lewy pathology in the nucleus ambiguus has not been observed. Studies showed that the motoneurons in the nucleus ambiguus with their myelinated axons remained free of Lewy bodies and Lewy neurites during the course of PD (78–80). The negative findings may be due to the fact that the nucleus ambiguus in PD has not been systemically investigated. It is also possible that the pathological process of PD may affect the pharyngeal plexus before the nucleus ambiguus becomes involved. Therefore, it remains unknown about whether axonal degeneration of the pharyngeal motor nerves progresses from distal (peripheral) to proximal (central) versus from proximal to distal. We hypothesized that the nucleus ambiggus and hypoglossal nucleus may be affected by the pathological process of PD. Several reports have specifically noted that these nuclei do not appear to be affected. However, as we clearly see involvement in the axons arising from these nuclei, we suspect that subtle involvement may have been missed by prior investigators. A variety of alternative explanations are possible. It has been demonstrated that Lewy pathology occurs at early stage of the disease in the peripheral autonomic nervous system, including the cardiac plexus (16–20) and enteric nervous system of the alimentary tract (21–23). Braak and colleagues (22, 78, 79) hypothesized that neuroactive substances or pathogens may pass through the mucosa of the gastrointestinal tract to enter the CNS via peripheral autonomic nerve fibers. A similar neuropathologic pathway might be appliable to the upper aerodigestive tract, where pathogens may affect pharyngeal plexus first and then progress gradually from peripheral nerves to the nucleus ambiguus. However, more work is needed to determine α-synuclein-expression pathway in the upper aerodigestive tract.
At present, compared with the brain lesions in PD, much less is known about the disease-induced alterations in the peripheral motor and sensory nervous systems controlling upper airway structures, including pharynx, larynx, and tongue. Despite the high incidence of swallowing, speech and voice impairment, the neural basis for these upper airway disorders in PD remains unclear (2–10). The data from this study point to the need for further systematic investigation of the peripheral nervous system elements that control the upper aerodigestive tract which plays important roles in swallowing, speech articulation and voice production. Determination of the neural alterations in the oral, pharyngeal and laryngeal structures will shed light on the elucidation of the neural mechanisms of swallowing, voice and speech disorders in PD.
Acknowledgments
This research is supported by National Institutes of Health Grant 5 R01 DC004728 from the National Institute on Deafness and Other Communication Disorders (to Dr. Mu). The Brain and Body Donation Program is supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research.
The authors thank the Banner Sun Health Research Institute Brain and Body Donation Program and the Arizona Parkinson’s Disease Consortium for the provision of whole-mount tongue-pharynx-larnyx specimens and associated clinical and neuropathologic data from PD subjects.
We thank the anonymous reviewers for their constructive comments on the manuscript.
References
- 1.Jellinger KA. Pathology of Parkinson’s disease. Changes other than the nigrostriatal pathway. Mol Chem Neuropathol. 1991;14:153–97. doi: 10.1007/BF03159935. [DOI] [PubMed] [Google Scholar]
- 2.Johnston BT, Li Q, Castell JA, et al. Swallowing and esophageal function in Parkinson’s disease. Am J Gastroenterol. 1995;90:1741–46. [PubMed] [Google Scholar]
- 3.Leopold NA, Kagel MC. Prepharyngeal dysphagia in Parkinson’s disease. Dysphagia. 1996;11:14–22. doi: 10.1007/BF00385794. [DOI] [PubMed] [Google Scholar]
- 4.Coates C, Bakheit AM. Dysphagia in Parkinson’s disease. Eur Neurol. 1997;38:49–52. doi: 10.1159/000112902. [DOI] [PubMed] [Google Scholar]
- 5.Fuh JL, Lee RC, Wang SJ, et al. Swallowing difficulty in Parkinson’s disease. Clin Neurol Neurosurg. 1997;99:106–12. doi: 10.1016/s0303-8467(97)00606-9. [DOI] [PubMed] [Google Scholar]
- 6.Ertekin C, Tarlaci S, Aydogdu I, et al. Electrophysiological evaluation of pharyngeal phase of swallowing in patients with Parkinson’s disease. Mov Disord. 2002;17:942–49. doi: 10.1002/mds.10240. [DOI] [PubMed] [Google Scholar]
- 7.Sapir S, Ramig L, Fox C. Speech and swallowing disorders in Parkinson disease. Curr Opin Otolaryngol Head Neck Surg. 2008;16:205–10. doi: 10.1097/MOO.0b013e3282febd3a. [DOI] [PubMed] [Google Scholar]
- 8.Sapir S, Ramig L, Fox C. Voice, speech and swallowing disorders. In: Factor S, Weiner W, editors. Parkinson disease: diagnosis and clinical management. New York City, New York: Demos Medical Publishing; 2008. pp. 77–97. [Google Scholar]
- 9.Logemann JA, Fisher HB, Boshes B, et al. Frequency and cooccurrence of vocal tract dysfunctions in the speech of a large sample of Parkinson patients. J Speech Hear Disord. 1978;42:47–57. doi: 10.1044/jshd.4301.47. [DOI] [PubMed] [Google Scholar]
- 10.Hartelius L, Svensson P. Speech and swallowing symptoms associated with Parkinson’s disease and multiple sclerosis: a survey. Folia Phoniatr Logop. 1994;46:9–17. doi: 10.1159/000266286. [DOI] [PubMed] [Google Scholar]
- 11.Spillantini MG, Schmidt ML, Lee VM, et al. α-Synuclein in Lewy bodies. Nature. 1997;388:839–40. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 12.Marrachelli VG, Miranda FJ, Alabadi JA, et al. Perivascular nerve fiber alpha-synuclein regulates contractility of mouse aorta: a link to autonomic dysfunction in Parkinson’s disease. Neurochem Int. 2010;56:991–98. doi: 10.1016/j.neuint.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Papachroni K, Ninkina N, Wanless J, et al. Peripheral sensory neurons survive in the absence of alpha- and gamma-synuclein. J Mol Neurosci. 2005;25:157–64. doi: 10.1385/JMN:25:2:157. [DOI] [PubMed] [Google Scholar]
- 14.Clayton DF, George JM. The synucleins: A family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 1998;21:249–54. doi: 10.1016/s0166-2236(97)01213-7. [DOI] [PubMed] [Google Scholar]
- 15.Bennett MC. The role of alpha-synuclein in neurodegenerative diseases. Pharmacol Ther. 2005;105:311–31. doi: 10.1016/j.pharmthera.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Amino T, Orimo S, Itho Y, et al. Profound cardiac sympathetic denervation occurs in Parkinson disease. Brain Pathol. 2005;15:29–34. doi: 10.1111/j.1750-3639.2005.tb00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orimo S, Amino T, Itoh Y, et al. Cardiac sympathetic denervation precedes neuronal loss in the sympathetic ganglia in Lewy body disease. Acta Neuropathol. 2005;109:583–88. doi: 10.1007/s00401-005-0995-7. [DOI] [PubMed] [Google Scholar]
- 18.Orimo S, Takahashi A, Uchihara T, et al. Degeneration of cardiac sympathetic nerve begins in the early disease process of Parkinson’s disease. Brain Pathol. 2007;17:24–30. doi: 10.1111/j.1750-3639.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orimo S, Uchihara T, Nakamura A, et al. Axonal α-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain. 2008;131:642–50. doi: 10.1093/brain/awm302. [DOI] [PubMed] [Google Scholar]
- 20.Fujishiro H, Frigerio R, Burnett M, et al. Cardiac sympathetic denervation correlates with clinical and pathologic stages of Parkinson’s disease. Mov Disord. 2008;23:1085–92. doi: 10.1002/mds.21989. [DOI] [PubMed] [Google Scholar]
- 21.Wakabayashi K, Takahashi H, Ohama E, et al. Parkinson’s disease: An immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol (Berl) 1990;79:581–83. doi: 10.1007/BF00294234. [DOI] [PubMed] [Google Scholar]
- 22.Braak H, de Vos RAI, Bohl J, et al. Gastric α-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Bloch A, Probst A, Bissig H, et al. α-Synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol. 2006;32:284–95. doi: 10.1111/j.1365-2990.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 24.Michell AW, Luheshi LM, Barker RA. Skin and platelet a-synuclein as peripheral biomarkers of Parkinson’s disease. Neurosci Lett. 2005;381:294–98. doi: 10.1016/j.neulet.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 25.Dabby R, Djaldetti R, Shahmurov M, et al. Skin biopsy for assessment of autonomic denervation in Parkinson’s disease. J Neural Transm. 2006;113:1169–76. doi: 10.1007/s00702-005-0431-0. [DOI] [PubMed] [Google Scholar]
- 26.Ikemura M, Saito Y, Sengoku R, et al. Lewy body pathology involves cutaneous nerves. J Neuropathol Exp Neurol. 2008;67:945–53. doi: 10.1097/NEN.0b013e318186de48. [DOI] [PubMed] [Google Scholar]
- 27.Miki Y, Tomiyama M, Ueno T, et al. Clinical availability of skin biopsy in the diagnosis of Parkinson’s disease. Neurosci Lett. 2010;469:357–59. doi: 10.1016/j.neulet.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 28.Shishido T, Ikemura M, Obi T, et al. α-synuclein accumulation in skin nerve fibers revealed by skin biopsy in pure autonomic failure. Neurology. 2010;74:608–10. doi: 10.1212/WNL.0b013e3181cff6d5. [DOI] [PubMed] [Google Scholar]
- 29.Mu L, Sobotka S, Chen J, et al. Altered pharyngeal muscles in Parkinson’s disease. J Neuropathol Exp Neurol. 2012;71:520–30. doi: 10.1097/NEN.0b013e318258381b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stelzig Y, Hochhaus W, Gall V, et al. Kehlkopfbefunde bei Patienten mit Morbus Parkinson. Laryngeal findings of patients with Parkinson’s disease [with English abstract] Laryngo-Rhino-Otol. 1999;78:544–51. doi: 10.1055/s-1999-8758. [DOI] [PubMed] [Google Scholar]
- 31.Meyer TK. The Larynx for neurologists. The Neurologist. 2009;15:313–18. doi: 10.1097/NRL.0b013e3181b1cde5. [DOI] [PubMed] [Google Scholar]
- 32.Mu L, Sanders I. The innervation of the human upper esophageal sphincter. Dysphagia. 1996;11:234–38. doi: 10.1007/BF00265207. [DOI] [PubMed] [Google Scholar]
- 33.Mu L, Sanders I. Neuromuscular organization of the human upper esophageal sphincter. Ann Otol Rhinol Laryngol. 1998;107:370–77. doi: 10.1177/000348949810700502. [DOI] [PubMed] [Google Scholar]
- 34.Mu L, Sanders I. Neuromuscular compartments and fiber-type regionalization in the human inferior pharyngeal constrictor muscle. Anat Rec. 2001;264:367–77. doi: 10.1002/ar.10020. [DOI] [PubMed] [Google Scholar]
- 35.Mu L, Sanders I. Neuromuscular specializations within human pharyngeal constrictor muscles. Ann Otol Rhinol Laryngol. 2007;116:604–17. doi: 10.1177/000348940711600809. [DOI] [PubMed] [Google Scholar]
- 36.Mu L, Sanders I. Newly revealed cricothyropharyngeus muscle in the human laryngopharynx. Anat Rec. 2008;291:927–38. doi: 10.1002/ar.20727. [DOI] [PubMed] [Google Scholar]
- 37.Sanders I, Wu BL, Mu L, et al. The innervation of the human larynx. Arch Otolaryngol Head Neck Surg. 1993;119:934–39. doi: 10.1001/archotol.1993.01880210022003. [DOI] [PubMed] [Google Scholar]
- 38.Sanders I, Mu L, Wu BL, et al. The intramuscular nerve supply of the human lateral cricoarytenoid muscle. Acta Otolaryngol (Stock) 1993;113:679–82. doi: 10.3109/00016489309135884. [DOI] [PubMed] [Google Scholar]
- 39.Sanders I, Wu BL, Mu L, et al. The innervation of the human posterior cricoarytenoid muscle. Laryngoscope. 1994;104:880–84. doi: 10.1288/00005537-199407000-00019. [DOI] [PubMed] [Google Scholar]
- 40.Mu L, Sanders I, Wu BL, et al. The intramuscular innervation of the human interarytenoid muscle. Laryngoscope. 1994;104:33–39. doi: 10.1288/00005537-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Mu L, Sanders I. The human cricothyroid muscle: Three muscle bellies and their innervation patterns. J Voice. 2009;23:21–28. doi: 10.1016/j.jvoice.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Del Tredici K, Braak H. A not entirely benign procedure: progression of Parkinson’s disease. Acta Neuropathol. 2008;115:379–84. doi: 10.1007/s00401-008-0355-5. [DOI] [PubMed] [Google Scholar]
- 43.Del Tredici K, Hawkes CH, Ghebremedhin E, et al. Lewy pathology in the submandibular gland of individuals with incidental Lewy body disease and sporadic Parkinson’s disease. Acta Neuropathol. 2010;119:703–13. doi: 10.1007/s00401-010-0665-2. [DOI] [PubMed] [Google Scholar]
- 44.Beach TG, Adler CH, Sue LI, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119:689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 46.Beach TG, Sue LI, Walker DG, et al. The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007. Cell Tissue Bank. 2008;9:229–45. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 48.Fahn S, Elton R, Committee DU. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent Developments in Parkinson’s Disease. New York: MacMillan; 1987. pp. 153–63. [Google Scholar]
- 49.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 50.Eriksson PO, Eriksson A, Ringqvist M, et al. The reliability of histochemical fiber typing of human necropsy muscles. Histochemistry. 1980;65:193–205. doi: 10.1007/BF00493169. [DOI] [PubMed] [Google Scholar]
- 51.Eriksson PO, Thornell LE. Histochemical and morphological muscle-fiber characteristics of the human masseter, the medial pterygoid and the temporal muscles. Arch Oral Biol. 1983;28:781–95. doi: 10.1016/0003-9969(83)90034-1. [DOI] [PubMed] [Google Scholar]
- 52.Beach TG, White CL, Hamilton RL, et al. Evaluation of alpha-synuclein immunohistochemical methods used by invited experts. Acta Neuropathol. 2008;116:277–88. doi: 10.1007/s00401-008-0409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fujiwara H, Hasegawa M, Dohmae N, et al. α-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–64. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 54.Beach TG, Adler CH, Lue L, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117:613–34. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tam SL, Archibald V, Tyreman N, et al. Increased neuromuscular activity reduces sprouting in partially denervated muscles. J Neurosci. 2001;21:654–67. doi: 10.1523/JNEUROSCI.21-02-00654.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.The National Institute on Aging and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 57.Litvan I, Bhatia KP, Burn DJ, et al. Movement Disorders Society Scientific Issues Committee. Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord. 2003;18:467–86. doi: 10.1002/mds.10459. [DOI] [PubMed] [Google Scholar]
- 58.Berardelli A, Rothwell JC, Thompson PD, et al. Pathophysiology of bradykinesia in Parkinson’s disease. Brain. 2001;124:2131–46. doi: 10.1093/brain/124.11.2131. [DOI] [PubMed] [Google Scholar]
- 59.Caviness JN, Smith BE, Stevens JC, et al. Motor unit changes in sporadic idiopathic Parkinson’s disease. Mov Disord. 2000;15:238–43. doi: 10.1002/1531-8257(200003)15:2<238::aid-mds1006>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 60.Toth C, Breithaupt K, Ge S, et al. Levodopa, methylmalonic acid, and neuropathy in idiopathic Parkinson disease. Ann Neurol. 2010;67:28–36. doi: 10.1002/ana.22021. [DOI] [PubMed] [Google Scholar]
- 61.Brait K, Fahn S, Schwarz GA. Sporadic and familial parkinsonism and motor neuron disease. Neurology. 1973;23:990–1002. doi: 10.1212/wnl.23.9.990. [DOI] [PubMed] [Google Scholar]
- 62.Sica RE, Herskovits E, Aguilera N, et al. An electrophysiological investigation of skeletal muscle in Parkinson’s disease. J Neurol Sci. 1973;18:411–20. doi: 10.1016/0022-510x(73)90135-4. [DOI] [PubMed] [Google Scholar]
- 63.Sica RE, Sanz OP. An electrophysiological study of the functional changes in the spinal motoneurones in Parkinson’s disease. Electromyogr Clin Neurophysiol. 1976;16:409–17. [PubMed] [Google Scholar]
- 64.Taly AB, Muthane UB. Involvement of peripheral nervous system in juvenile Parkinson’s disease. Acta Neurol Scand. 1992;85:272–75. doi: 10.1111/j.1600-0404.1992.tb04043.x. [DOI] [PubMed] [Google Scholar]
- 65.Caviness JN, Smith BE, Clarke SJ, et al. Motor unit number estimates in idiopathic Parkinson’s disease. Parkinsonism Relat Disord. 2002;8:161–64. doi: 10.1016/s1353-8020(01)00007-4. [DOI] [PubMed] [Google Scholar]
- 66.Abbruzzese G, Pigullo S, Schenone A, et al. Does parkin play a role in the peripheral nervous system? A family report. Mov Disord. 2004;19:978–81. doi: 10.1002/mds.20113. [DOI] [PubMed] [Google Scholar]
- 67.Hunker CJ, Abbs JH, Barlow SM. The relationship between parkinsonian rigidity and hypokinesia in the orofacial system: a quantitative analysis. Neurology. 1982;32:749–54. doi: 10.1212/wnl.32.7.749. [DOI] [PubMed] [Google Scholar]
- 68.Ali GN, Wallace KL, Schwartz R, et al. Mechanisms of oral-pharyngeal dysphagia in patients with Parkinson’s disease. Gastroenterology. 1996;110:383–92. doi: 10.1053/gast.1996.v110.pm8566584. [DOI] [PubMed] [Google Scholar]
- 69.Hunter PC, Crameri J, Austin S, et al. Response of parkinsonian swallowing dysfunction to dopaminergic stimulation. J Neurol Neurosurg Psychiatry. 1997;63:579–83. doi: 10.1136/jnnp.63.5.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leopold NA, Kagel MC. Pharyngo-esophageal dysphagia in Parkinson’s disease. Dysphagia. 1997;12:11–18. doi: 10.1007/pl00009512. [DOI] [PubMed] [Google Scholar]
- 71.Sapir S, Ramig L, Fox C. The Lee Silverman Voice Treatment® for voice, speech and other orofacial disorders in patients with Parkinson’s disease. Future Neurology. 2006;1:563–70. [Google Scholar]
- 72.Ramig L, Fox C, Sapir S. Speech Treatment for Parkinson disease. Expert Rev Neurotherapeutics. 2008;8:299–311. doi: 10.1586/14737175.8.2.297. [DOI] [PubMed] [Google Scholar]
- 73.Nilsson H, Ekberg O, Olsson R, et al. Quantitative assessment of oral and pharyngeal function in Parkinson’s disease. Dysphagia. 1996;11:144–50. doi: 10.1007/BF00417905. [DOI] [PubMed] [Google Scholar]
- 74.Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–76. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 75.Bushmann M, Dobmeyer SM, Leeker L, et al. Swallowing abnormalities and their response to treatment in Parkinson’s disease. Neurology. 1989;39:1309–14. doi: 10.1212/wnl.39.10.1309. [DOI] [PubMed] [Google Scholar]
- 76.Kurihara K, Kita K, Hirayama K, et al. Dysphagia in Parkinson’s disease. Clin Neruol. 1993;33:150–54. [PubMed] [Google Scholar]
- 77.Standring S, Ellis H, Healy JC, et al. Gray’s Anatomy. The Anatomical Basis of Clinical Practice. 39. New York: Churchill Livingstone; 2005. [Google Scholar]
- 78.Braak H, Rub U, Gai WP, et al. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. 2003;110:517–36. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- 79.Del Tredici K, Rub U, De Vos RAI, Bohl JRE, Braak H. Where does Parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol. 2002;61:413–26. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- 80.Hawkes CH, Del Tredici K, Braak H. Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]