Abstract
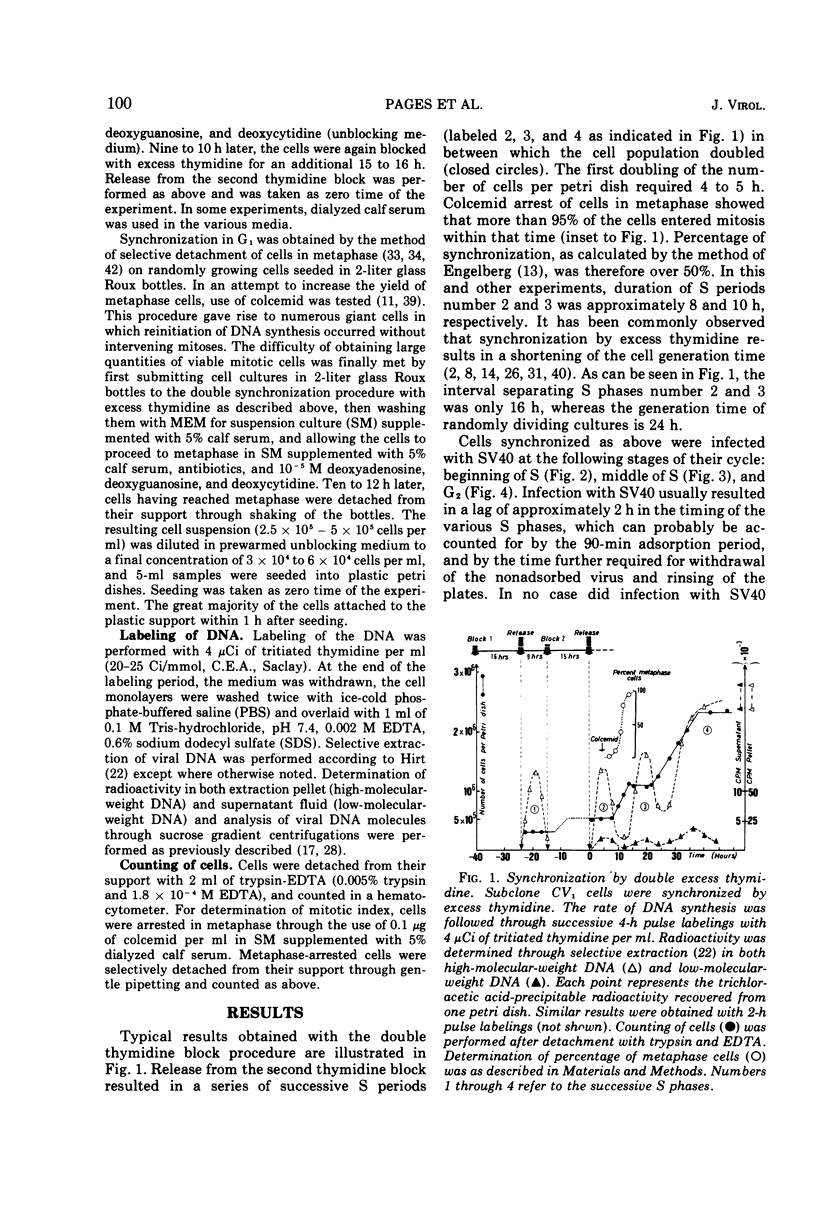
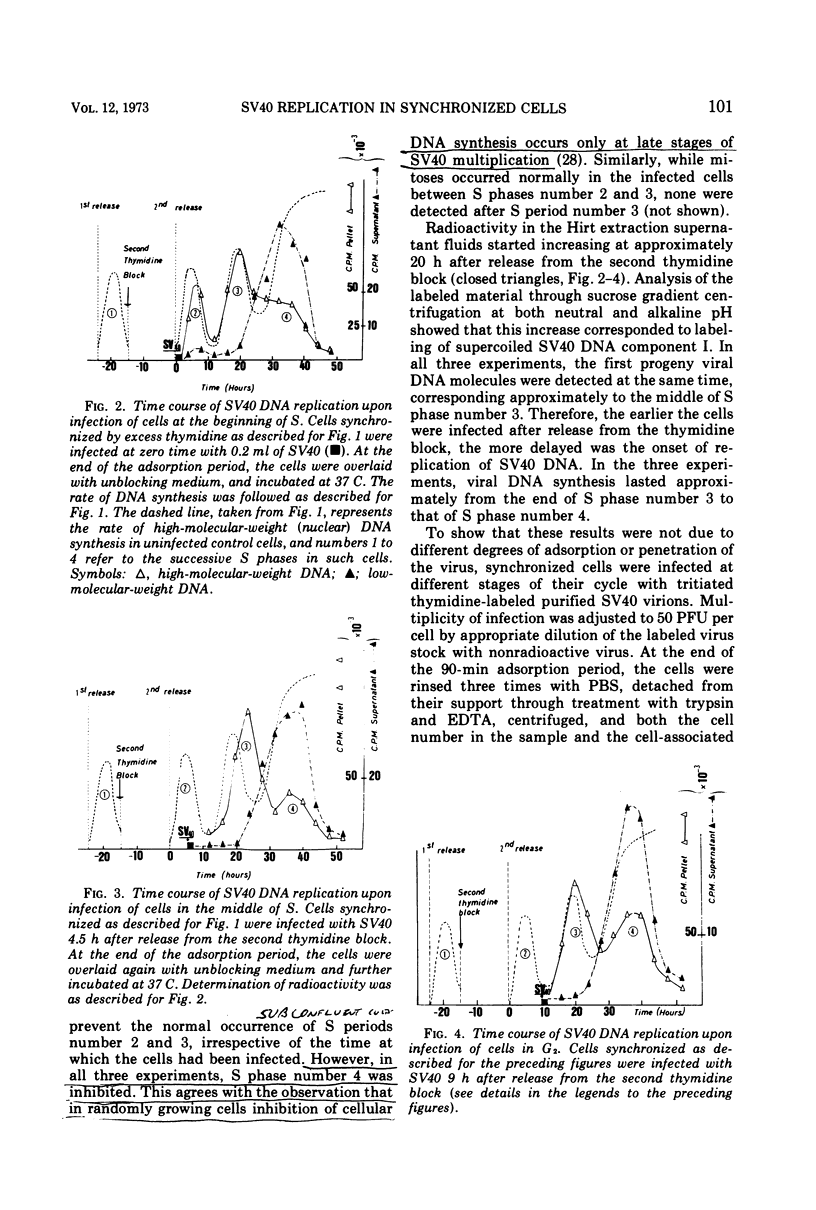
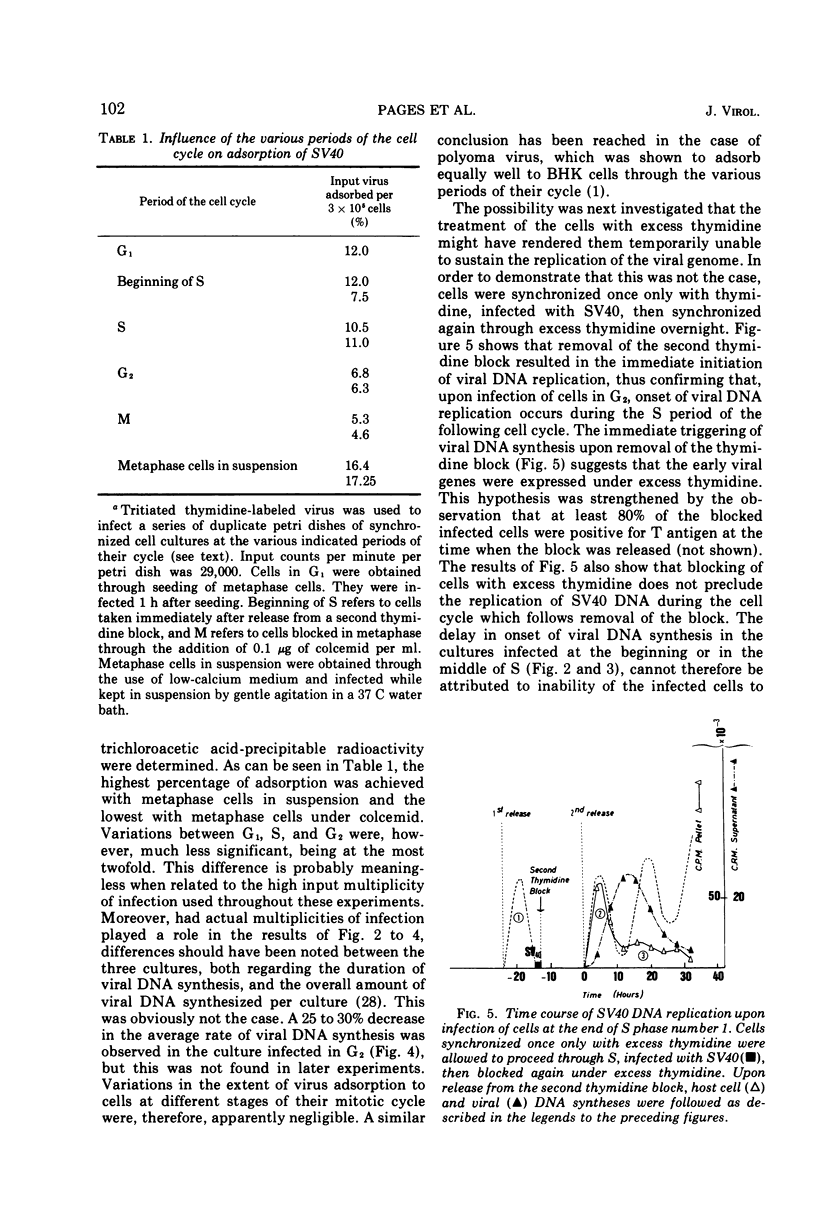
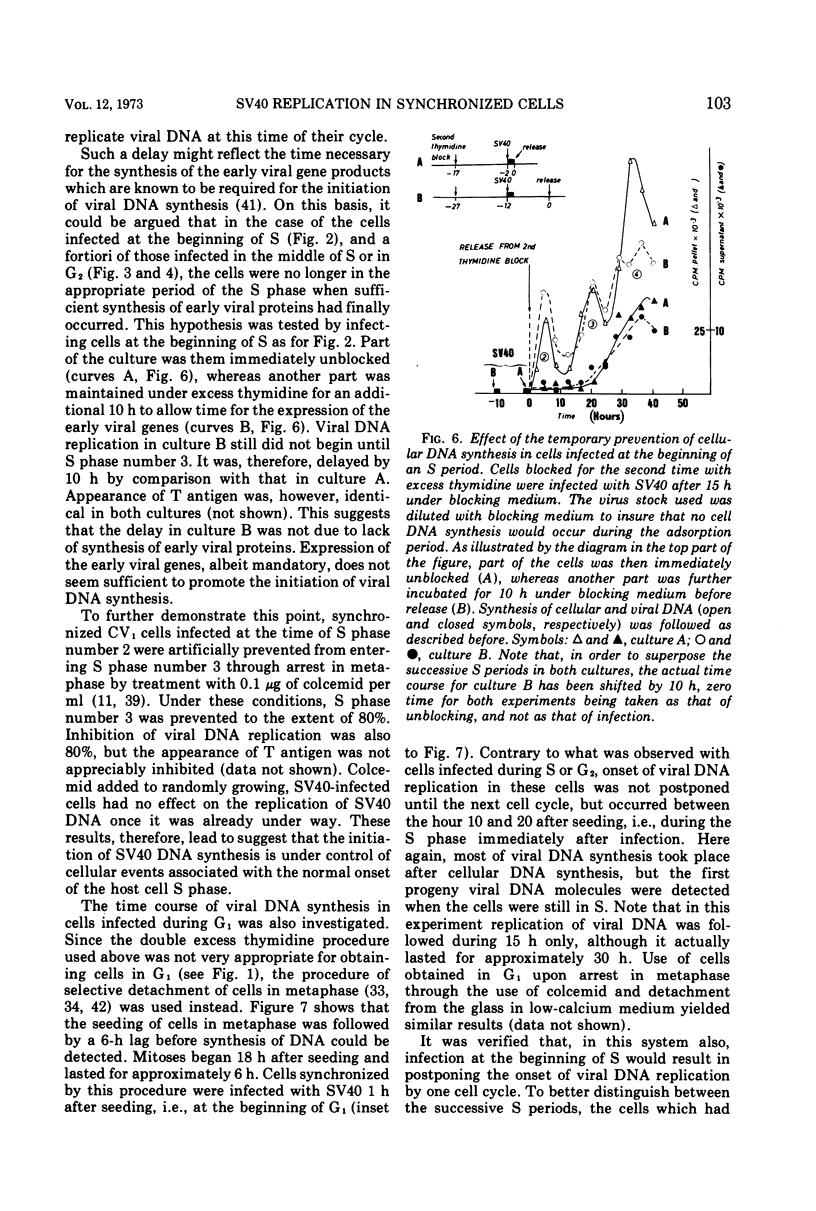
The relationship between replication of simian virus 40 (SV40) DNA and the various periods of the host-cell cycle was investigated in synchronized CV1 cells. Cells synchronized through a double excess thymidine procedure were infected with SV40 at the beginning or the middle of S, or in G2. The first viral progeny DNA molecules were in all instances detected approximately 20 h after release from the thymidine block, independent of the time of infection. The length of the early, prereplicative phase of the virus growth cycle therefore depended upon the period of the cell cycle at which the cells were infected. Infection with SV40 was also performed on cells obtained in early G1 through selective detachment of cells in metaphase. As long as the cells were in G1 at the time of infection, the first viral progeny DNA molecules were detected during the S period immediately following, whereas if infection took place once the cells had entered S, no progeny DNA molecule could be detected until the S period of the next cell cycle. These results suggest that the infected cell has to pass through a critical stage situated in late G1 or early S before SV40 DNA replication can eventually be initiated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOOTSMA D., BUDKE L., VOS O. STUDIES ON SYNCHRONOUS DIVISION OF TISSUE CULTURE CELLS INITIATED BY EXCESS THYMIDINE. Exp Cell Res. 1964 Jan;33:301–309. doi: 10.1016/s0014-4827(64)81035-1. [DOI] [PubMed] [Google Scholar]
- Bello L. J. Synthesis of DNA-like RNA in synchronized cultures of mammalian cells. Biochim Biophys Acta. 1968 Mar 18;157(1):8–15. doi: 10.1016/0005-2787(68)90258-x. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Kaplan A. S. Correlation between replication and degradation of cellular DNA in polyoma virus-infected cells. Virology. 1967 Jul;32(3):457–464. doi: 10.1016/0042-6822(67)90297-8. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Kaplan A. S., Tennant R. W. Effect of 5-fluorouracil on the multiplication of a virulent virus (pseudorabies) and an oncogenic virus (polyoma). Virology. 1967 Jul;32(3):445–456. doi: 10.1016/0042-6822(67)90296-6. [DOI] [PubMed] [Google Scholar]
- Bhorjee J. S., Pederson T. Nonhistone chromosomal proteins in synchronized HeLa cells. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3345–3349. doi: 10.1073/pnas.69.11.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borun T. W., Scharff M. D., Robbins E. Rapidly labeled, polyribosome-associated RNA having the properties of histone messenger. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1977–1983. doi: 10.1073/pnas.58.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock C. J., Prescott D. M., Kirkpatrick J. B. An evaluation of the double thymidine block for synchronizing mammalian cells at the G1-S border. Exp Cell Res. 1971 Sep;68(1):163–168. doi: 10.1016/0014-4827(71)90599-4. [DOI] [PubMed] [Google Scholar]
- Burger M. M., Bombik B. M., Breckenridge B. M., Sheppard J. R. Growth control and cyclic alterations of cyclic AMP in the cell cycle. Nat New Biol. 1972 Oct 11;239(93):161–163. doi: 10.1038/newbio239161a0. [DOI] [PubMed] [Google Scholar]
- Byron J. W. Evidence for a -adrenergic receptor initiating DNA synthesis in haemopoietic stem cells. Exp Cell Res. 1972 Mar;71(1):228–232. doi: 10.1016/0014-4827(72)90283-2. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., HARTWELL L. H., VOGT M. INDUCTION OF CELLULAR DNA SYNTHESIS BY POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1965 Feb;53:403–410. doi: 10.1073/pnas.53.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGELBERG J. A method of measuring the degree of synchronization of cell populations. Exp Cell Res. 1961 Mar;23:218–227. doi: 10.1016/0014-4827(61)90032-5. [DOI] [PubMed] [Google Scholar]
- Galavazi G., Bootsma D. Synchronization of mammalian cells in vitro by inhibition of the DNA synthesis. II. Population dynamics. Exp Cell Res. 1966 Feb;41(2):438–451. doi: 10.1016/s0014-4827(66)80150-7. [DOI] [PubMed] [Google Scholar]
- Gershon D., Hausen P., Sachs L., Winocour E. On the mechanism of polyoma virus-induced synthesis of cellular DNA. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1584–1592. doi: 10.1073/pnas.54.6.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon D., Sachs L., Winocour E. The induction of cellular DNA synthesis by simian virus 40 in contact-inhibited and in x-irradiated cells. Proc Natl Acad Sci U S A. 1966 Sep;56(3):918–925. doi: 10.1073/pnas.56.3.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard M., Stehelin D., Manteuil S., Pages J. Aspects of the encapsidation of simian virus 40 deoxyribonucleic acid. J Virol. 1973 Jan;11(1):107–115. doi: 10.1128/jvi.11.1.107-115.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. Oncogenic viruses. Annu Rev Biochem. 1970;39:701–756. doi: 10.1146/annurev.bi.39.070170.003413. [DOI] [PubMed] [Google Scholar]
- Hadden J. W., Hadden E. M., Haddox M. K., Goldberg N. D. Guanosine 3':5'-cyclic monophosphate: a possible intracellular mediator of mitogenic influences in lymphocytes. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3024–3027. doi: 10.1073/pnas.69.10.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka M., Dulbecco R. Induction of DNA synthesis by SV40. Proc Natl Acad Sci U S A. 1966 Aug;56(2):736–740. doi: 10.1073/pnas.56.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Defendi V. Integration of simian virus 40 deoxyribonucleic acid into the deoxyribonucleic acid of permissive monkey kidney cells. J Virol. 1972 Apr;9(4):705–707. doi: 10.1128/jvi.9.4.705-707.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kaplan A. S., Ben-Porat T. Metabolism of animal cells infected with nuclear DNA viruses. Annu Rev Microbiol. 1968;22:427–450. doi: 10.1146/annurev.mi.22.100168.002235. [DOI] [PubMed] [Google Scholar]
- Kasamaki A., Ben-Porat T., Kaplan A. S. Polyoma virus-induced release of inhibition of cellular DNA synthesis caused by iododeoxyuridine. Nature. 1968 Feb 24;217(5130):756–758. doi: 10.1038/217756a0. [DOI] [PubMed] [Google Scholar]
- Kit S., De Torres R. A., Dubbs D. R., Salvi M. L. Induction of cellular deoxyribonuleic acid synthesis by simian virus 40. J Virol. 1967 Aug;1(4):738–746. doi: 10.1128/jvi.1.4.738-746.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence W. C. Evidence for a relationship between equine abortion (herpes) virus deoxyribonucleic acid synthesis and the S phase of the KB cell mitotic cycle. J Virol. 1971 Jun;7(6):736–748. doi: 10.1128/jvi.7.6.736-748.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J. Induction of mitochondrial DNA synthesis in monkey cells infected by simian virus 40 and (or) treated with calf serum. Proc Natl Acad Sci U S A. 1971 Apr;68(4):717–720. doi: 10.1073/pnas.68.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manteuil S., Pages J., Stehelin D., Girard M. Replication of simian virus 40 deoxyribonucleic acid: analysis of the one-step growth cycle. J Virol. 1973 Jan;11(1):98–106. doi: 10.1128/jvi.11.1.98-106.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten J., Johnson G. S., Pastan I. Cyclic AMP levels in fibroblasts: relationship to growth rate and contact inhibition of growth. Biochem Biophys Res Commun. 1971 Sep;44(5):1192–1198. doi: 10.1016/s0006-291x(71)80212-7. [DOI] [PubMed] [Google Scholar]
- Puck T. T. Phasing, Mitotic Delay, and Chromosomal Aberrations in Mammalian Cells. Science. 1964 May 1;144(3618):565–566. doi: 10.1126/science.144.3618.565-c. [DOI] [PubMed] [Google Scholar]
- ROBBINS E., MARCUS P. I. MITOTICALLY SYNCHRONIZED MAMMALIAN CELLS: A SIMPLE METHOD FOR OBTAINING LARGE POPULATIONS. Science. 1964 May 29;144(3622):1152–1153. doi: 10.1126/science.144.3622.1152. [DOI] [PubMed] [Google Scholar]
- Ritzi E., Levine A. J. Deoxyribonucleic acid replication in simian virus 40-infected cells. 3. Comparison of simian virus 40 lytic infection in three different monkey kidney cell lines. J Virol. 1970 Jun;5(6):686–692. doi: 10.1128/jvi.5.6.686-692.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovera G., Baserga R., Defendi V. Early increase in nuclear acidic protein synthesis after SV40 infection. Nat New Biol. 1972 Jun 21;237(77):240–241. doi: 10.1038/newbio237240a0. [DOI] [PubMed] [Google Scholar]
- Rovera G., Baserga R. Early changes in the synthesis of acidic nuclear proteins in human diploid fibroblasts stimulated to synthesize DNA by changing the medium. J Cell Physiol. 1971 Apr;77(2):201–211. doi: 10.1002/jcp.1040770211. [DOI] [PubMed] [Google Scholar]
- Stein G. S., Borun T. W. The synthesis of acidic chromosomal proteins during the cell cycle of HeLa S-3 cells. I. The accelerated accumulation of acidic residual nuclear protein before the initiation of DNA replication. J Cell Biol. 1972 Feb;52(2):292–307. doi: 10.1083/jcb.52.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G., Baserga R. Nuclear proteins and the cell cycle. Adv Cancer Res. 1972;15:287–330. doi: 10.1016/s0065-230x(08)60378-4. [DOI] [PubMed] [Google Scholar]
- Studzinski G. P., Lambert W. C. Thymidine as a synchronizing agent. I. Nucleic acid and protein formation in synchronous HeLa cultures treated with excess thymidine. J Cell Physiol. 1969 Apr;73(2):109–117. doi: 10.1002/jcp.1040730204. [DOI] [PubMed] [Google Scholar]
- TERASIMA T., TOLMACH L. J. Growth and nucleic acid synthesis in synchronously dividing populations of HeLa cells. Exp Cell Res. 1963 Apr;30:344–362. doi: 10.1016/0014-4827(63)90306-9. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne H. V. An investigation of factors influencing the susceptibility of Balb-c 3T3 cells to polyoma virus. J Gen Virol. 1973 Feb;18(2):153–162. doi: 10.1099/0022-1317-18-2-153. [DOI] [PubMed] [Google Scholar]
- Thorne H. V. Cyclic variation in susceptibility of Balb-c 3T3 cells to polyoma virus. J Gen Virol. 1973 Feb;18(2):163–169. doi: 10.1099/0022-1317-18-2-163. [DOI] [PubMed] [Google Scholar]
- Vogt M., Dulbecco R., Smith B. Induction of cellular DNA synthesis by polyoma virus. 3. Induction in productively infected cells. Proc Natl Acad Sci U S A. 1966 Apr;55(4):956–960. doi: 10.1073/pnas.55.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. D. The regulation of DNA synthesis in eukaryotes. Adv Cell Biol. 1971;2:1–46. doi: 10.1007/978-1-4615-9588-5_1. [DOI] [PubMed] [Google Scholar]
- Weil R., Michel M. R., Ruschmann G. K. Induction of cellular DNA synthesis by polyoma virus. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1468–1475. doi: 10.1073/pnas.53.6.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werchau H., Maass G., Westphal H., Haas R. Untersuchungen über den Nucleinsäurestoffwechsel von Affennierengewebe-Kulturzellen nach Infektion mit SV-40. 3. UNTERSUCHUNGEN ZUM Mechanismus der virusbedingten DNS-Synthesesteigerung. Arch Gesamte Virusforsch. 1967;21(2):265–275. [PubMed] [Google Scholar]
- XEROS N. Deoxyriboside control and synchronization of mitosis. Nature. 1962 May 19;194:682–683. doi: 10.1038/194682a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman J. E., Jr, Raska K., Jr Inhibition of adenovirus type 12 induced DNA synthesis in G1-arrested BHK21 cells by dibutyryl adenosine cyclic 3':5'-monophosphate. Nat New Biol. 1972 Oct 4;239(92):145–147. doi: 10.1038/newbio239145a0. [DOI] [PubMed] [Google Scholar]



