Abstract
Human immunoglobulin G (IgG) molecules are composed of two Fab portions and one Fc portion. The glycans attached to the Fc portions of IgG are known to modulate its biological activity as they influence interaction with both complement and various cellular Fc receptors. IgG glycosylation changes significantly with pregnancy, showing a vast increase in galactosylation and sialylation and a concomitant decrease in the incidence of bisecting GlcNAc. Maternal IgGs are actively transported to the fetus by the neonatal Fc receptor (FcRn) expressed in syncytiotrophoblasts in the placenta, providing the fetus and newborn with immunological protection. Two earlier reports described significant differences in total glycosylation between fetal and maternal IgG, suggesting a possible glycosylation-selective transport via the placenta. These results might suggest an alternative maternal transport pathway, since FcRn binding to IgG does not depend on Fc-glycosylation. These early studies were performed by releasing N-glycans from total IgG. Here, we chose for an alternative approach analyzing IgG Fc glycosylation at the glycopeptide level in an Fc-specific manner, providing glycosylation profiles for IgG1 and IgG4 as well as combined Fc glycosylation profiles of IgG2 and 3. The analysis of ten pairs of fetal and maternal IgG samples revealed largely comparable Fc glycosylation for all the analyzed subclasses. Average levels of galactosylation, sialylation, bisecting GlcNAc and fucosylation were very similar for the fetal and maternal IgGs. Our data suggest that the placental IgG transport is not Fc glycosylation selective.
Keywords: Fc receptor, Galactosylation, Glycopeptide, Placenta, Sialylation
Introduction
Human immunoglobulin G (IgG) represents the most abundant class of immunoglobulins in the circulation with typical concentrations of approximately 10 mg/ml [1]. These B-cell derived immunoglobulins are soluble forms of the B cell receptor, formed after encounter between antigen-specific B cells and its cognate antigen, antigen processing and activation through helper T cells, leading to affinity maturation and class switching to one of the γ-encoding genes of the heavy-chain locus, forming IgG [1, 2]. IgGs occur in different subclasses (IgG1, IgG2, IgG3, and IgG4), named in order of decreasing abundance [1]. They are formed from two heavy chains and two lights chains which together form two fragment antigen binding (Fab) portions and one fragment crystallizable (Fc) portion, which is distinct between the subclasses, and influences their specific function through specific interaction with complement, Fc-gamma receptors (FcγR) and FcRn [3, 4]. The Fab portions contain the (hyper-)variable parts of the molecule which define its binding properties to antigens including pathogen molecular patterns, and are therefore unique for each clonally derived antibody [2]. The Fc portions of all four subclasses of IgG are known to be glycosylated at asparagine 297 [5].
The Asn297-linked glycans of IgG are biantennary complex-type structures which are predominantly core-fucosylated and are in part modified by a bisecting N-acetylglucosamine (GlcNAc) [5–8]. Antennae are partially truncated varying in their degree of galactosylation and may carry a sialic acid residue. The Fc glycans of IgG are involved in the interaction with all FcγR besides the neonatal Fc receptor (FcRn) [9, 10]. In addition to direct interaction of the Fc glycan with FcγRs [11] glycan-glycan interactions have recently been shown to modulate the affinity of IgG1 Fc portions to the FcγR, and in particular to FcγRIIIa, with the lack of core-fucose on the IgG1 Asn297 N-glycan promoting high-affinity interaction with the Asn162 N-glycan of the receptor [10, 12]. The high-affinity interaction of afucosylated IgG1 with FcγRIIIa has been shown to form the molecular basis of the enhanced efficacy of afucosylated therapeutic antibodies in killing cancer cells [13, 14], and design of the glycosylation during product development may represent an attractive way of increasing efficacy in new therapeutic IgGs [15, 16]. The reduced fucosylation of IgG1 may also be important in pathological situations, e.g. during pregnancies complicated with the formation of maternal IgG against fetal platelets, which we found to be highly skewed towards the afucosylated kind [17].
Recently, Fc sialylation of IgG has received increased attention, as it has been reported that increased sialylation makes IgGs anti-inflammatory agents [18, 19]. In murine models it has been shown that sialylated IgGs bind to DC-SIGN receptors of immune cells and leads to the upregulation of inhibitory FcγRIIb on macrophages [19–21].
Human serum IgG glycosylation is known to change with various physiological and pathological conditions. Both galactosylation and sialylation show a pronounced age and sex dependence with a higher galactosylation and sialylation of IgG in females than in males at young age, and a decrease in galactosylation and sialylation for both sexes with increasing age [22, 23]. In addition, various autoimmune and infectious diseases have been shown to result in decreased IgG galactosylation [24–26]. In contrast, pregnancy is known to be associated with an increase in galactosylation and sialylation of IgG Fc N-glycans, with a concomitant decrease in the incidence of bisecting GlcNAc [27–29]. These glycosylation changes may be typed as anti-inflammatory [18], and one may speculate that these adaptations contribute to suppressing alloimmune reactions during pregnancy [30].
Human IgG is actively transported across the placenta via FcRn into the circulation of the fetus, and this IgG provided by the mother is considered to contribute to the immunological protection of the fetus and newborn during the first months after birth [31]. The infant starts producing its own IgG in the first weeks after birth [32], but IgGs produced by the infant are still found at low levels until 8 months of age, when only IgG1 and sometimes IgG3, but not IgG2 and IgG4 can reach similar levels found for adults [33].
Two studies in 1995 [34] and 1996 [35] compared the IgG glycosylation of maternal and fetal IgG. The studies analyzed total glycosylation of IgG and described a lower level of agalactosylated structures [34, 35] and higher percentages of galactosylated N-glycan structures [35] for fetal as compared to maternal IgG. These data indicated that there might be a preferential transport of galactosylated IgG to the fetus. However, these studies analyzed total IgG glycosylation, thus including both Fc glycans and glycans of the IgG variable parts, found in approximately 30 % of all immunoglobulins [36–38]. If the reported increase was due to Fc galactosylation with a possible concomitant increase in sialylation, it may be expected to influence the effector functions of fetal IgG. We, therefore, decided to study the specific glycosylation features of fetal IgG in more detail, focusing only on the Fc glycosylation. These results would also give us insight into whether there are other receptors, besides FcRn, involved in placental transport favouring transport of certain Fc glycoforms. To this end, we chose to analyse only the IgG Fc glycosylation of paired fetal and maternal samples in a site-specific and subclass-specific manner. For this purpose, IgG was purified from plasma by protein G affinity chromatography followed by tryptic cleavage. Fc N-glycopeptides were analyzed by mass spectrometry resulting in glycosylation profiles of IgG1 and IgG4 as well as combined Fc glycosylation profiles of IgG2 and 3 [29]. The analysis of all sample pairs revealed very similar levels of galactosylation, sialylation, fucosylation, and bisecting GlcNAc for IgG between fetus and mother.
Materials and methods
Patient samples and IgG isotype analysis
The pairs of plasma samples from mothers and umbilical cord of the new-born were collected right after delivery (average gestation time 37.8 weeks, range 36–39 weeks). All women had an uncomplicated pregnancy and neonatal outcomes for all children were optimal. Signed informed consent was obtained from all women, and the collection of blood samples and clinical data recieved approval by the Ethics Committee of the Leiden University Medical Center (P02-200).
The analysis of total IgG and IgG isotypes were performed using the Siemens nephelometer BNII.
Purification of IgG
IgGs were affinity captured from total human plasma as described previously [39]. Protein G Sepharose™ Fast Flow beads (GE Healthcare, Uppsala, Sweden) were washed three times with 10 volumes of PBS. 15 μL of beads in 150 μL PBS were incubated with 2 μL of serum in a 96-well filter plate (Multiscreen Solvinert, 0.45 μm pore-size low-binding hydrophilic PTFE; Millipore, Billerica, MA) on a shaker for 1 h. Beads were thoroughly washed 4 times with 200 μL of PBS and then 3 times with 200 μL of water under vacuum (pressure reduction to approximately 900 mbar). IgG was eluted into a 96-well V-bottom plate using 100 μL formic acid (100 mM). Samples were dried by vacuum centrifugation.
IgG digestion with trypsin
IgGs were digested with trypsin as described previously [8]. A 20 μg aliquot of trypsin (sequencing grade; Promega, Leiden, The Netherlands) was dissolved in 4 mL of 25 mM ammonium bicarbonate. Within 1 min after preparation, 40 μL of this mixture was added per well to the dried purified antibodies. Samples were shaken (1 min), incubated overnight at 37 °C, and stored at −20 °C until usage.
Fast nano-reverse phase-LC-ESI-MS
Nano-reverse phase-LC-ESI-MS was performed as described previously [29]. Briefly, analysis was achieved on a Ultimate 3000 HPLC system (Thermo Fisher, Waltham, MA), equipped with a Acclaim PepMap100 C18 (5 mm × 300 μm i.d.; Thermo Fisher) solid phase extraction (SPE) trap column and Ascentis Express C18 nano-LC column (50 mm × 75 μm i.d., 2.7 μm HALO fused core particles; Supelco, Bellefonte, USA). Samples were centrifuged at 4,000 rpm for 5 min and aliquots of 500 nL were applied to the trap column for 1 min at 25 μl/min. Separation was achieved with the following gradient of mobile phase A (0.1 % trifluoroacetic acid; Fluka, Steinheim, Germany) and mobile phase B (95 % acetonitrile; Biosolve BV, Valkenswaard, the Netherlands): 0 min 3 % B, 2 min 5 % B, 5 min 20 % B, 6 min 30 % B, 8 min 30 % B, 9 min 0 % B, and 14 min 0 % B. After 8 min the SPE was switched off-line and washed by three full loop injections containing 5 μL 5 % isopropanol (IPA) + 0.1 % formic acid (FA) and 5 μL 50 % IPA + 0.1 % FA. The HPLC was interfaced to a quadrupole-TOF mass spectrometer (micrOTOF-Q; Bruker Daltonics, Bremen, Germany) with a standard ESI source (Bruker Daltonics) and a sheath-flow ESI sprayer (capillary electrophoresis ESI-MS sprayer; Agilent Technologies, Santa Clara, USA) applying the UV outlet tubing (20 μm i.d., 360 μm o.d.) as sprayer needle. A sheath-flow of 50 % IPA, 20 % propionic acid and 30 % water was applied at 2 μL/min to support ESI spray formation and reduce TFA ion suppression. To improve mobile phase evaporation a nitrogen stream was applied as dry gas at 4 L/min with a nebulizer pressure of 0.4 bar. Scan spectra were recorded from 300 to 2,000 Da with 2 average scans at a frequency of 1 Hz. Quadrupole ion energy and collision energy of the MS were set at 2 and 4 eV, respectively. The total analysis time per sample was 16 min.
Data processing
Data processing was performed as described previously [29]. Briefly, LC-MS datasets were calibrated internally using a list of known glycopeptides and were exported to the open mzXML format by Bruker DataAnalysis 4.0. Each dataset was then aligned to a master dataset of a typical sample (containing many of the (glyco-)peptide species shared between multiple samples) using msalign2 [40]. Glycopeptide species which were pre-defined as peak maxima in specific mass and retention time windows, were extracted from each dataset using the in-house developed software “Xtractor2D”. The software and ancillary scripts are freely available at www.ms-utils.org/Xtractor2D. The complete sample-data matrix was finally evaluated using Microsoft Excel.
Structural assignment of the detected glycoforms was performed on the basis of literature knowledge of IgG N-glycosylation [6–8, 23, 41, 42]. Relative intensities of the glycopeptide species (Table 1) derived from IgG1 (20 glycoforms), IgG4 (10 glycoforms), and IgG2 (20 glycoforms) were obtained by integrating and summing three isotopic peaks of the triple protonated as well as the double protonated species followed by normalization to the total IgG subclass specific glycopeptide intensities.
Table 1.
Theoretical m/z values of human IgG Fc glycopeptides detected by nano-LC-ESI-MS
| Glycan species | IgG1 P01857b | IgG2/3 P01859b/P01860, VAR_003892b | IgG4 P01861b | |||
|---|---|---|---|---|---|---|
| [M + 2H]2+ | [M + 3H]3+ | [M + 2H]2+ | [M + 3H]3+ | [M + 2H]2+ | [M + 3H]3+ | |
| G0Fc | 1317.527 | 878.687 | 1301.532 | 868.024 | 1309.529 | 873.356a1 |
| G1F | 1398.553 | 932.705 | 1382.558 | 922.042 | 1390.556 | 927.373a2 |
| G2F | 1479.580 | 986.722 | 1463.585 | 976.059 | 1471.582 | 981.391 |
| G0FN | 1419.067 | 946.380 | 1403.072 | 935.717 | 1411.069 | 941.049a3 |
| G1FN | 1500.093 | 1000.398 | 1484.098 | 989.735 | 1492.096 | 995.066a4 |
| G2FN | 1581.119 | 1054.416 | 1565.125 | 1043.752 | 1573.122 | 1049.084 |
| G1FS | 1544.101 | 1029.737 | 1528.106 | 1019.073 | 1536.104 | 1024.405a5 |
| G2FS | 1625.127 | 1083.754 | 1609.133 | 1073.091 | 1617.130 | 1078.423 |
| G1FNS | 1645.641 | 1097.430 | 1629.646 | 1086.767 | 1637.643 | 1092.098 |
| G2FNS | 1726.667 | 1151.447 | 1710.672 | 1140.784 | 1718.670 | 1146.116 |
| G0 | 1244.498 | 830.001 | 1228.503 | 819.338 | n.d. | n.d. |
| G1 | 1325.524 | 884.019 | 1309.529 | 873.356a1 | n.d. | n.d. |
| G2 | 1406.551 | 938.036 | 1390.556 | 927.373a2 | n.d. | n.d. |
| G0N | 1346.038 | 897.694 | 1330.043 | 887.031 | n.d. | n.d. |
| G1N | 1427.064 | 951.712 | 1411.069 | 941.049a3 | n.d. | n.d. |
| G2N | 1508.090 | 1005.730 | 1492.096 | 995.066a4 | n.d. | n.d. |
| G1S | 1471.072 | 981.051 | 1455.077 | 970.387 | n.d. | n.d. |
| G2S | 1552.098 | 1035.068 | 1536.104 | 1024.405a5 | n.d. | n.d. |
| G1NS | 1572.612 | 1048.744 | 1556.617 | 1038.081 | n.d. | n.d. |
| G2NS | 1653.638 | 1102.761 | 1637.643 | 1092.098 | n.d. | n.d. |
a1–a5 isomeric glycopeptide species of IgG4 and IgG2
b SwissProt entry number
c glycan structural features are given in terms of number of galactoses (G0, G1, G2), fucose (F), bisecting N-acetylglucosamine (N), and N-acetylneuraminic acid, sialic acid (S)
n.d. not detected
In addition, the levels of 4 major glycoforms of IgG1, IgG2/3 and IgG4 glycopeptides with one missed cleavage site were monitored as triple and quadruple charged species (Table 2) in order to judge the efficacy of the tryptic digest.
Table 2.
Theoretical m/z values of human IgG Fc glycopeptides with 1 missed cleavage site
| Glycan species | IgG1 P01857a | IgG2/3 P01859a/P01860, VAR_003892a | IgG4 P01861a | |||
|---|---|---|---|---|---|---|
| [M + 3H]3+ | [M + 4H]4+ | [M + 3H]3+ | [M + 4H]4+ | [M + 3H]3+ | [M + 4H]4+ | |
| G0Fb | 1039.453 | 779.842 | 1028.789 | 771.844 | 1034.121 | 775.843 |
| G1F | 1093.470 | 820.355 | 1082.807 | 812.357 | 1088.139 | 816.356 |
| G2F | 1147.488 | 860.868 | 1136.825 | 852.870 | 1142.156 | 856.869 |
| G2FS | 1244.520 | 933.642 | 1233.856 | 925.644 | 1239.188 | 929.643 |
a SwissProt entry number; the peptide moieties of IgG1, IgG2/3 and IgG4 are TKPREEQYNSTYR ([M + H]+ 1671.809), TKPREEQFNSTFR ([M + H]+ 1639.819), TKPREEQFNSTYR ([M + H]+ 1655.814), respectively
b Glycan structural features are given in terms of number of galactoses (G0, G1, G2), fucose (F), and N-acetylneuraminic acid, sialic acid (S)
On the basis of the normalized intensities of IgG Fc glycopeptides the level of galactosylation, sialylation, bisecting N-acetylglucosamine, and fucosylation were calculated according to the following formulas: Galactosylation = (G1F + G1FN + G1FS + G1FNS + G1 + G1N + G1S) * 0.5 + G2F + G2FN + G2FS + G2FNS + G2 + G2N + G2S. Agalactosylated structures = G0F + G0FN + G0 + G0N. Digalactosylated structures = G2F + G2FN + G2FS + G2FNS + G2 + G2N + G2S. Sialylation = G1FS + G2FS + G1FNS + G2FNS + G1S + G2S. Bisecting GlcNAc = G0FN + G1FN + G2FN + G1FNS + G2FNS + G0N + G1N + G2N. Fucosylation = G0F + G1F + G2F + G0FN + G1FN + G2FN + G1FS + G2FS. The non-fucosylated species of IgG4 remained below the limit of detection and were, therefore, not included in the IgG4 calculations.
In addition, we calculated from the isotype-specific IgG G0 levels the overall IgG G0 levels for both fetus and mother, in order to facilitate the comparison of our results with those obtained by others [34, 35]. Calculations were performed according to the following formula: Overall IgG G0 = (IgG1 G0 + IgG1 G0F + IgG1 G0FN + IgG1 G0N) × relative abundance IgG1 + (IgG2/3 G0 + IgG2/3 G0F + IgG2/3 G0FN + IgG2/3 G0N) × (relative abundance IgG2/3) + (IgG4 G0F + IgG4 G0FN) × relative abundance IgG4.
Results and discussion
IgG was purified from 20 plasma samples of maternal and umbilical vein blood (fetus) using Protein G Sepharose. IgG was subjected to tryptic cleavage, and resulting IgG Fc glycopeptides were analyzed using a recently established nano-LC-MS method employing a sheath-flow ESI sprayer [29]. IgG1 Fc glycopeptides were found to elute at approximately 7 min, IgG4 Fc glycopeptides at 7.5 min, and IgG2/3 Fc glycopeptides at 8 min (Fig. 1a, b). IgG2 and IgG3 tryptic Fc glycopeptides have the identical peptide moieties [8] and, therefore, these IgG isotypes were registered together. Glycan structures were assigned on the basis of literature knowledge of IgG glycan structures [6, 23, 41, 42] and the established elution orders of IgG Fc glycopeptides in reverse phase-LC-MS [7, 8].
Fig. 1.
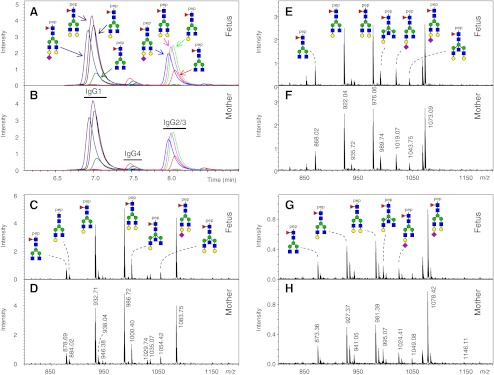
Nano-LC-ESI-MS of tryptic digests of IgG obtained from fetal and maternal blood. Extracted ion chromatograms of the triple- and double-protonated glycopeptide species G0F, G1F, G2F and G2FS of IgG1, IgG2/3, and IgG4 are displayed for fetus (a) and mother (b). Integration ranges for the MS signals are indicated by horizontal bars. The corresponding mass spectra showing the triple protonated IgG1 (c, d), IgG2/3 (e, f), and IgG4 (g, h) glycopeptide species are shown for fetus (c, e, g) and mother (d, f, h). Blue square, N-acetylglucosamine; red triangle, fucose; green circle, mannose; yellow circle, galactose; purple diamond, N-acetylneuraminic acid.
All 20 samples were checked for the completeness of the tryptic digest by monitoring the IgG1 and IgG2 Fc glycopeptides with one missed tryptic cleavage site, according to Stadlmann et al. [7] (Table 2). Miscleaved glycopeptides were found in only a minority of samples. When observed, the signal intensities of miss-cleaved glycopeptides were found to be at least 200 times lower than those of the fully cleaved glycopeptides, and the signal-noise ratio was low throughout. Due to their low abundance the signal of miss-cleaved glycopeptides were not included in the quantitative analysis.
Fetal and maternal IgG showed very similar chromatographic profiles as evidenced by extracted ion chromatograms of the major IgG1, IgG2/3, and IgG4 Fc glycopeptides (Fig. 1a, b). For all the 10 fetal and 10 maternal IgG1 samples Fc glycosylation profiles were obtained (see Fig. 1c and d for an example). Signals obtained for the triple protonated IgG1 Fc glycopeptides were observed in the range of m/z 800 to 1,200 (Fig. 1c, d), whilst the signals of double protonated species were registered in the range of m/z 1,200 to 1,800 (see Table 1) [29]. The double and triple charged signals were integrated and summed for all the 20 registered IgG1 Fc glycopeptide species. The sum of all IgG1 Fc glycopeptides species was set to 100 %, and the degree of galactosylation, sialylation, bisecting N-acetylglucosamine, and fucosylation were determined. Similarly, 20 IgG2/3 Fc glycopeptides and 10 IgG4 Fc glycopeptides were analyzed (Fig. 1e-h). While IgG2/3 Fc glycopeptides were analyzed for all 10 fetal and maternal IgG pairs, only seven of the fetal and maternal IgG4 Fc glycopeptide clusters showed sufficient intensity for glycopeptide analysis.
The level of galactosylation reflects the percentage of antennae, which are decorated with a galactose residue. Therefore, monogalactosylated and digalactosylated glycans were weighed differently to reflect their different degree of galactosylation. While the percentage of digalactosylated structures was fully included in the galactosylation term, monogalactosylated structures were only weighed half, due to the fact that they carry a galactosylated as well as a non-galactosylated antenna (see Materials and methods for the equation). Maternal IgG1 showed average levels of galactosylation of 73.8 %. This value represents elevated levels of IgG galactosylation as they have been described for advanced pregnancies. The determined galactosylation level is very much in line with the average IgG1 Fc galactosylation levels of 70.7 % recently analyzed for 26 pregnant women during the third trimester [29]. Overall, the tendency is that IgG galactosylation peaks at delivery and normalizes a few months after. IgG galactosylation levels at 3 as well as 6 months after delivery were found to be 61.2 % and 61.9 %, respectively, representing the non-pregnant levels of IgG galactosylation. Similarly, the IgG2/3 galactosylation levels of 66.4 % observed in this study are in line with previously determined values of 63.2 % for the third pregnancy trimester, and are significantly higher than the values of 52.1 % observed 6 months after delivery (non-pregnant status). For IgG4, the average galactosylation levels observed here (68.4 %) are significantly higher than those observed before for the third trimester of pregnancy (54.7 %) and 6 months after delivery (46.4 %) [29]. One may assume that this difference is linked to the slightly different sampling time points (third trimester versus at delivery). The possible physiological role of such an increased IgG4 Fc galactosylation at the end of the pregnancy is unclear.
The mean levels of Fc galactosylation of fetal and maternal IgG1 were found to be 74.6 % versus 73.8 % (Table 3; Fig. 2a). Likewise, IgG2/3 of fetus and mother showed very similar levels of galactosylation (average for the 10 analyzed pairs of 67.1 % and 66.4 %, respectively). For the seven pairs of fetal and maternal IgG4 average galactosylation values were found to be 69.5 % and 68.4 %, respectively. These results indicate no significant differences in Fc galactosylation between fetal and maternal IgG for the major isotypes (IgG1 and IgG2/3; Table 3).
Table 3.
Comparison of the Fc glycosylation features of fetal and maternal IgG. Mean values ± standard deviation are given. The standard error of the mean is given in parentheses
| Glycosylation feature | Subclass | Mean relative abundance ± standard deviation (%) | t-test | ||
|---|---|---|---|---|---|
| Fetus | Mother | Fetus–Mother | |||
| Galactosylation | IgG1 Fc | 74.6 ± 3.9 | 73.8 ± 4.7 | 0.8 ± 1.3 (0.4) | p = 0.10 |
| IgG2/3 Fc | 67.1 ± 5.8 | 66.4 ± 5.0 | 0.7 ± 2.4 (0.8) | p = 0.38 | |
| IgG4 Fc | 69.5 ± 3.2 | 68.4 ± 3.7 | 1.1 ± 1.2 (0.4) | p = 0.04 | |
| Sialylation | IgG1 Fc | 26.4 ± 2.9 | 25.4 ± 3.1 | 1.0 ± 2.0 (0.6) | p = 0.14 |
| IgG2/3 Fc | 27.4 ± 4.4 | 27.0 ± 3.4 | 0.4 ± 2.0 (0.6) | p = 0.59 | |
| IgG4 Fc | 36.7 ± 3.7 | 35.9 ± 3.4 | 0.7 ± 0.9 (0.3) | p = 0.06 | |
| Bisecting GlcNAc | IgG1 Fc | 12.9 ± 2.5 | 12.8 ± 2.6 | 0.1 ± 0.7 (0.2) | p = 0.72 |
| IgG2/3 Fc | 13.0 ± 2.3 | 13.2 ± 2.2 | −0.2 ± 0.7 (0.2) | p = 0.33 | |
| IgG4 Fc | 12.7 ± 2.8 | 12.5 ± 2.6 | 0.2 ± 0.7 (0.3) | p = 0.38 | |
| Fucosylation | IgG1 Fc | 89.7 ± 4.3 | 89.7 ± 4.5 | 0.0 ± 0.2 (0.1) | p = 0.90 |
| IgG2/3 Fc | 96.9 ± 0.8 | 96.9 ± 1.1 | −0.0 ± 0.3 (0.1) | p = 0.84 | |
Fig. 2.
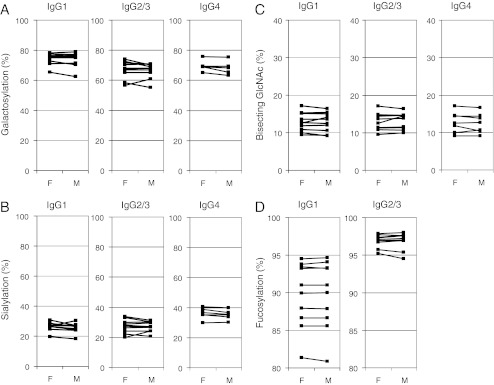
Fc glycosylation analysis of IgG from paired cord blood (fetus, F) and maternal blood (M). Galactosylation (a), sialylation (b), bisecting GlcNAc (c), and fucosylation (d) were compared for tryptic Fc glycopeptides of IgG1, IgG2/3 and IgG4
In order to facilitate the comparison of our results with those of Williams et al. [34] and Kibe et al. [35], we calculated the relative abundances of agalactosylated as well as digalactosylated structures. For the IgG1, IgG2/3 and IgG4 subclasses, we found that the levels of agalactosylated structures were very similar for fetal and maternal samples. There was a tendency of slightly lower levels of galactosylation of fetal IgG, but the mean differences in agalactosylated species between fetal and maternal IgG1, IgG2/3 and IgG4 were only 0.1 %, 0.4 %, and 0.8 %, respectively (Table 4). In contrast, Williams et al. [34] described significantly lower levels of agalactosylated structures for IgG of the fetus (mean value of 13.1 %) as compared to maternal IgG (mean value of 16.7 %; difference of 3.6 % is statistically highly significant; p = 0.000012; see Table 4). Likewise, Kibe et al. [35] analyzed 26 sets of paired samples revealing lower levels of agalactosylated structures for fetal as compared to maternal IgG (10 % versus 12 %; Table 4).
Table 4.
Comparison of the results on fetal and maternal IgG glycosylation from this study and two previous studies. Mean values ± standard deviation are given. The standard error of the mean is given in parentheses
| Sample pairs | Fetus | Mother | Fetus–Mother | t-test | Source |
|---|---|---|---|---|---|
| Agalactosylated structures (%) | |||||
| n = 10 | IgG 13.1 ± 5.6 | 16.7 ± 5.9 | −3.6 ± 1.3 | p = 0.000012 | Williams et al. 1995 [34] |
| n = 26 | IgG 10.0 ± 2 | 12 ± 2 | −2.0 | p = 0.0015 | Kibe et al. 1996 [35] |
| n = 10 | IgG1 Fc 8.0 ± 2.4 | 8.4 ± 3.1 | −0.4 ± 1.1 (0.36) | p = 0.27 | this study |
| n = 10 | IgG2/3 Fc 14.6 ± 4.4 | 14.7 ± 3.5 | −0.1 ± 2.8 (0.87) | p = 0.87 | this study |
| n = 7 | IgG4 Fc 14.4 ± 2.3 | 15.2 ± 2.6 | −0.8 ± 0.8 (0.31) | p = 0.04 | this study |
| Digalactosylated structures (%) | |||||
| n = 26 | IgG 61 ± 4 | 58 ± 4 | 3.0 | p = 0.007 | Kibe et al. 1996 [35] |
| n = 10 | IgG1 Fc 57.3 ± 5.5 | 56.3 ± 6.3 | 1.0 ± 1.5 (0.46) | p = 0.05 | this study |
| n = 10 | IgG2/3 Fc 49.4 ± 7.3 | 48.2 ± 6.7 | 1.2 ± 2.3 (0.74) | p = 0.14 | this study |
| n = 7 | IgG4 Fc 53.4 ± 4.5 | 52.0 ± 5.1 | 1.4 ± 1.5 (0.57) | p = 0.05 | this study |
Comparison of the levels of digalactosylated structures also revealed some differences with literature: Kibe et al. [35] found mean levels of digalactosylated structures to be 3 % higher on fetal as compared to maternal IgG (p = 0.007). In our study, mean differences were much lower, or around 1 %, none reaching the level of significance.
However, the conceptual, as well as methodological differences between the two previous studies and our current study should be noted. In the previous studies, the N-linked glycans were released from IgG by hydrazinolysis [34] and PNGase A treatment [35]. Glycans were radioactively labeled by Williams et al. [34], and the levels of agalactosylated structures were assessed by gel permeation chromatography after simplifying the oligosaccharide mixture by employing a cocktail of exoglycosidases (α-sialidase, α-fucosidase and β-N-acetylhexosaminidase). Kibe et al. [35] analyzed the oligosaccharides by reverse phase HPLC profiling after fluorescent labelling with 2-aminopyridine and enzymatic desialylation. In both cases, total N-glycans were registered, i.e., both Fc glycosylation, and Fab glycosylation. As our study indicates that Fc glycosylation of all the IgG subclasses is remarkably similar in fetal and maternal IgG, one may speculate about the cause of the differences observed in earlier studies. First, the changes in overall IgG glycosylation profiles as observed by Williams et al. and Kibe et al. may in part have been caused by differences in subclass ratios [34, 35]. This is a possibility because the levels of Fc galactosylation tend to be higher for IgG1, than for IgG2/3 and IgG4 (Table 3). In addition, the analysis of the IgG isotype distribution indicated that the portion of IgG1 is elevated in cord blood (72.3 %) as compared to maternal blood (64.8 %; paired t-test p = 0.0000015; Table 5). On the other hand, the relative IgG2 abundances are lower for the fetus than for the mother (22.0 % versus 28.4 %; paired t-test p = 0.0000065), and the same holds true for IgG3 (2.7 % versus 3.8 %; paired t-test p = 0.0022). In the present study, glycosylation analysis was performed in a subclass-specific manner, and therefore not influenced by the ratio of subclasses. To compare our results with the previous studies [34, 35], we used the information on the relative isotype distribution and calculated the overall levels of IgG Fc agalactosylated structures by taking the isotype ratios into account (see Materials and Methods for details). The average overall levels of IgG Fc agalactosylated species were calculated to be 9.7 % for the fetus versus 10.6 % for the mother. Hence, for the specific set of 10 paired mother/child IgG samples analyzed in this study, the observed Fc glycosylation profiles, together with the changes in relative abundances of IgG subclasses, result in a 0.9 % decrease of overall IgG Fc agalactosylated structures. Therefore, the difference in subclass ratios seems to only partly explain the pronounced differences in overall galactosylation reported previously [34, 35].
Table 5.
Concentrations of total IgG and IgG isotypes of fetus and mother. Concentrations are given in mg/ml. Percentages are given in parentheses
| Mother/child couple | Fetus | Mothera | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IgG1 | IgG2 | IgG3 | IgG4 | Total | IgG1 | IgG2 | IgG3 | IgG4 | Total | |
| 1 | 3.86 (54.8) | 2.44 (34.6) | 0.15 (2.1) | 0.60 (8.5) | 7.05 | 1.97 (46.2) | 1.78 (41.8) | 0.12 (2.8) | 0.39 (9.2) | 4.26 |
| 2 | 6.09 (75.8) | 1.69 (21.0) | 0.21 (2.6) | 0.04 (0.5) | 8.02 | 5.99 (68.5) | 2.43 (27.8) | 0.31 (3.5) | 0.01 (0.1) | 8.72 |
| 3 | 3.36 (73.5) | 1.00 (21.9) | 0.20 (4.4) | 0.01 (0.2) | 4.57 | 2.60 (66.2) | 1.06 (27.0) | 0.26 (6.6) | 0.01 (0.3) | 3.92 |
| 4 | 3.62 (68.8) | 1.39 (26.4) | 0.13 (2.5) | 0.12 (2.3) | 5.25 | 2.82 (58.8) | 1.71 (35.6) | 0.15 (3.1) | 0.12 (2.5) | 4.79 |
| 5 | 5.02 (60.5) | 2.48 (29.9) | 0.33 (4.0) | 0.47 (5.7) | 8.30 | 2.33 (49.3) | 1.90 (40.2) | 0.22 (4.7) | 0.28 (5.9) | 4.73 |
| 6 | 6.01 (81.0) | 1.24 (16.7) | 0.16 (2.2) | 0.01 (0.1) | 7.41 | 3.93 (73.0) | 1.30 (24.2) | 0.14 (2.6) | 0.01 (0.2) | 5.37 |
| 7 | 8.26 (82.8) | 1.13 (11.3) | 0.15 (1.5) | 0.44 (4.4) | 9.98 | 5.63 (79.0) | 1.07 (15.0) | 0.13 (1.8) | 0.30 (4.2) | 7.11 |
| 8 | 8.40 (75.5) | 2.40 (21.6) | 0.18 (1.6) | 0.14 (1.3) | 11.12 | 4.20 (70.0) | 1.60 (26.7) | 0.12 (2.0) | 0.08 (1.3) | 6.00 |
| 9 | 6.40 (76.4) | 1.60 (19.1) | 0.26 (3.1) | 0.12 (1.4) | 8.38 | 2.80 (68.6) | 1.00 (24.5) | 0.22 (5.4) | 0.06 (1.5) | 4.08 |
| 10 | 4.70 (74.0) | 1.08 (17.0) | 0.22 (3.5) | 0.35 (5.5) | 6.35 | 5.20 (68.1) | 1.60 (20.9) | 0.42 (5.5) | 0.42 (5.5) | 7.64 |
| Average | 5.57 (72.3) | 1.65 (22.0) | 0.20 (2.7) | 0.23 (3.0) | 7.65 | 3.75 (64.8) | 1.55 (28.4) | 0.21 (3.8) | 0.17 (3.1) | 5.67 |
a Reference standard values IgG1: 4.9–11.4; IgG2: 1.50–6.4; IgG3: 0.20–1.10; IgG4: 0.080–1.40
The IgG isotype concentrations of our samples are rather low compared to normal adult standard values (Table 5), which is in line with the known decrease to 60–70 % for both IgA (not transported to the fetus) and IgG in pregnant women at term [43]. Notably the average IgG concentrations found by us (7.65 mg/ml fetal IgG and 5.67 mg/ml maternal IgG) were significantly lower than those found by Kibe et al. [35] (13.15 mg/ml fetal IgG and 10.70 mg/ml maternal IgG), the reason for this discrepancy being unknown.
Lastly, differences in Fab glycosylation between fetal and maternal IgG may explain why previous studies found the markedly increased levels of overall galactosylation of fetal IgG. If true, this would indicate either a preferential transport of Fab-galactosylated IgG or retention of a Fab-agalactosylated IgG, possibly by an unknown receptor either actively involved in, or interfering with, IgG transport. This needs to be investigated in more detail. It has to be noted, however, that robust and straight-forward methods with reasonable throughput for the specific Fab glycosylation analysis of polyclonal human IgG are still lacking and, therefore, such analyses are scarce and performed on only very small numbers of samples [38, 44].
Next to IgG Fc galactosylation, the levels of sialylation of IgG1, IgG2/3 and IgG4 were assessed in our study. Similar to the observations for IgG Fc galactosylation, we found no significant changes in the levels of Fc sialylation for fetal as compared to maternal IgG (Table 3). Interestingly, sialylation levels were found to be higher for maternal IgG4 (35.9 %) as compared to IgG1 (25.4 %) and IgG2/3 (27.0 %) (Table 3). The subclass with the highest levels of galactosylation (IgG1) is not the one with the highest levels of sialylation, indicating a differential regulation of galactosylation and sialylation between IgG subclasses. Notably, in our previous study sialylation levels of IgG4 were found to be 26.4 % in the third pregnancy trimester (on average 9 weeks before delivery), whilst the values of IgG4 sialylation were down to 21.1 %, 19.9 % and 20.1 % at 6 weeks, 3 months, and 6 months after delivery, respectively [29]. Hence, the high levels of IgG4 sialylation found in the present study may point to a transient increase in IgG4 sialylation with delivery.
The comparison of the levels of bisecting GlcNAc revealed mean levels between 12.5 % and 13.2 % for fetal and maternal IgG of all analyzed subclasses (Table 3). No differences in the level of bisecting GlcNAc were found between fetal and maternal IgG (Fig. 2; Table 3).
Finally, a comparison of the levels of fucosylation was performed for both IgG1 and IgG2/3. No differences in core-fucosylation were detected between fetal and maternal IgG. IgG1 fucosylation levels were found to be in the range of 80 % to 95 %, while IgG2/3 fucosylation levels were between 94 % and 98 % for all the analyzed paired samples of fetus and mother. Interestingly, no correlation was observed between IgG1 and IgG2/3 fucosylation levels (not shown), indicating that Fc fucosylation in IgG-secreting B cells is likely to be regulated largely independently between subclasses – perhaps as a result of differential stimulation. This might be explained by the preferential class switching to IgG2 induced by T-helper independent antigens, compared to T-cell dependent antibody responses where IgG1 dominates [45].
We therefore conclude that trans-placental transport of human IgG does not favor certain Fc glycoforms. This is in line with reports demonstrating that FcRn, the receptor proposed to be solely responsible for placental transport and in vivo half life, rescues glycosylated and aglycosylated forms of IgG equally well [46]. This also fits with the structural requirements for FcRn binding to IgG, which does not involve the Fc glycans [9]. These results also exclude a significant role for FcγR isoforms that do display discriminatory binding activities to different glycoforms [10, 12, 18].
Conclusion
Previous studies found a higher degree of N-glycan galactosylation of total fetal IgG as compared to maternal IgG. In contrast, when analyzing IgG Fc glycosylation in a subclass-specific manner, we did not detect skewing of glycosylation profiles in general, also not for the level of galactosylation levels. This indicates that the materno-fetal IgG transport is not Fc-glycosylation-selective in healthy pregnancies. These results lend support to the commonly held belief that FcRn is the only contributing receptor to the placental transport of antibodies to the fetus. However, results of previous studies indicate that alternative mechanisms may be in place for Fab-glycosylated IgG.
Acknowledgments
This work has been supported by funding from the European Union’s Seventh Framework Programme (FP7-Health-F5-2011) under grant agreement n°278535 (HighGlycan). M.H.J. Selman thanks Hoffmann La Roche for financial support.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Footnotes
Helga K. Einarsdottir, Maurice H. J. Selman and Rick Kapur contributed equally to this work.
References
- 1.Schur PH. IgG subclasses. A historical perspective. Monogr. Allergy. 1988;23:1–11. [PubMed] [Google Scholar]
- 2.Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302:575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- 3.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daeron M. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 4.Stapleton NM, Andersen JT, Stemerding AM, Bjarnarson SP, Verheul RC, Gerritsen J, Zhao Y, Kleijer M, Sandlie I, de Haas M, Jonsdottir I, van der Schoot CE, Vidarsson G. Competition for FcRn-mediated transport gives rise to short half-life of human IgG3 and offers therapeutic potential. Nat. Commun. 2011;2:599. doi: 10.1038/ncomms1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 6.Parekh RB, Dwek RA, Sutton BJ, Fernandes DL, Leung A, Stanworth D, Rademacher TW, Mizuochi T, Taniguchi T, Matsuta K. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316:452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- 7.Stadlmann J, Pabst M, Kolarich D, Kunert R, Altmann F. Analysis of immunoglobulin glycosylation by LC-ESI-MS of glycopeptides and oligosaccharides. Proteomics. 2008;8:2858–2871. doi: 10.1002/pmic.200700968. [DOI] [PubMed] [Google Scholar]
- 8.Wuhrer M, Stam JC, van de Geijn FE, Koeleman CAM, Verrips CT, Dolhain RJEM, Hokke CH, Deelder AM. Glycosylation profiling of immunoglobulin G (IgG) subclasses from human serum. Proteomics. 2007;7:4070–4081. doi: 10.1002/pmic.200700289. [DOI] [PubMed] [Google Scholar]
- 9.West AP, Jr, Bjorkman PJ. Crystal structure and immunoglobulin G binding properties of the human major histocompatibility complex-related Fc receptor(,) Biochemistry. 2000;39:9698–9708. doi: 10.1021/bi000749m. [DOI] [PubMed] [Google Scholar]
- 10.Shields RL, Lai J, Keck R, O'Connell LY, Hong K, Meng YG, Weikert SH, Presta LG. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 11.Sondermann P, Huber R, Oosthuizen V, Jacob U. The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex. Nature. 2000;406:267–273. doi: 10.1038/35018508. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara C, Grau S, Jager C, Sondermann P, Brunker P, Waldhauer I, Hennig M, Ruf A, Rufer AC, Stihle M, Umana P, Benz J. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc. Natl. Acad. Sci. U. S. A. 2011;108:12669–12674. doi: 10.1073/pnas.1108455108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iida S, Kuni-Kamochi R, Mori K, Misaka H, Inoue M, Okazaki A, Shitara K, Satoh M. Two mechanisms of the enhanced antibody-dependent cellular cytotoxicity (ADCC) efficacy of non-fucosylated therapeutic antibodies in human blood. BMC Cancer. 2009;9:58. doi: 10.1186/1471-2407-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niwa R, Shoji-Hosaka E, Sakurada M, Shinkawa T, Uchida K, Nakamura K, Matsushima K, Ueda R, Hanai N, Shitara K. Defucosylated chimeric anti-CC chemokine receptor 4 IgG1 with enhanced antibody-dependent cellular cytotoxicity shows potent therapeutic activity to T-cell leukemia and lymphoma. Cancer Res. 2004;64:2127–2133. doi: 10.1158/0008-5472.CAN-03-2068. [DOI] [PubMed] [Google Scholar]
- 15.Yamane-Ohnuki N, Satoh M. Production of therapeutic antibodies with controlled fucosylation. MAbs. 2009;1:230–236. doi: 10.4161/mabs.1.3.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat. Rev. Drug Discov. 2009;8:226–234. doi: 10.1038/nrd2804. [DOI] [PubMed] [Google Scholar]
- 17.Wuhrer M, Porcelijn L, Kapur R, Koeleman CA, Deelder AM, de Haas M, Vidarsson G. Regulated glycosylation patterns of IgG during alloimmune responses against human platelet antigens. J. Proteome Res. 2009;8:450–456. doi: 10.1021/pr800651j. [DOI] [PubMed] [Google Scholar]
- 18.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 19.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475:110–113. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baerenwaldt A, Lux A, Danzer H, Spriewald BM, Ullrich E, Heidkamp G, Dudziak D, Nimmerjahn F. Fcgamma receptor IIB (FcgammaRIIB) maintains humoral tolerance in the human immune system in vivo. Proc. Natl. Acad. Sci. U. S. A. 2011;108:18772–18777. doi: 10.1073/pnas.1111810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc. Natl. Acad. Sci. U. S. A. 2008;105:19571–19578. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruhaak LR, Uh HW, Beekman M, Koeleman CA, Hokke CH, Westendorp RG, Wuhrer M, Houwing-Duistermaat JJ, Slagboom PE, Deelder AM. Decreased levels of bisecting GlcNAc glycoforms of IgG are associated with human longevity. PLoS One. 2010;5:e12566. doi: 10.1371/journal.pone.0012566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pucic M, Knezevic A, Vidic J, Adamczyk B, Novokmet M, Polasek O, Gornik O, Supraha-Goreta S, Wormald MR, Redzic I, Campbell H, Wright A, Hastie ND, Wilson JF, Rudan I, Wuhrer M, Rudd PM, Josic D, Lauc G. High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol. Cell. Proteomics. 2011;10:M111. doi: 10.1074/mcp.M111.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huhn C, Selman MH, Ruhaak LR, Deelder AM, Wuhrer M. IgG glycosylation analysis. Proteomics. 2009;9:882–913. doi: 10.1002/pmic.200800715. [DOI] [PubMed] [Google Scholar]
- 25.Gornik, O., Pavic, T., Lauc, G.: Alternative glycosylation modulates function of IgG and other proteins–Implications on evolution and disease. Biochim. Biophys. Acta, in press (2011) [DOI] [PubMed]
- 26.Dall'Olio, F., Vanhooren, V., Chen, C.C., Slagboom, P.E., Wuhrer, M., Franceschi C.: N-glycomic biomarkers of biological ageing and longevity: a link with inflammageing. Ageing Res. Rev., in press (2012) [DOI] [PubMed]
- 27.van de Geijn FE, Wuhrer M, Selman MH, Willemsen SP, de Man YA, Deelder AM, Hazes JM, Dolhain RJ. Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study. Arthritis Res Ther. 2009;11:R193. doi: 10.1186/ar2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rook GAW, Steele J, Brealey R, Whyte A, Isenberg D, Sumar N, Nelson JL, Bodman KB, Young A, Roitt IM, Williams P, Scragg I, Edge CJ, Arkwright PD, Ashford D, Wormald M, Rudd PM, Redman CWG, Dwek RA, Rademacher TW. Changes in IgG glycoform levels are associated with remission of arthritis during pregnancy. J. Autoimmun. 1991;4:779–794. doi: 10.1016/0896-8411(91)90173-A. [DOI] [PubMed] [Google Scholar]
- 29.Selman MH, Derks RJ, Bondt A, Palmblad M, Schoenmaker B, Koeleman CA, van de Geijn FE, Dolhain RJ, Deelder AM, Wuhrer M. Fc specific IgG glycosylation profiling by robust nano-reverse phase HPLC-MS using a sheath-flow ESI sprayer interface. J. Proteomics. 2012;75:1318–1329. doi: 10.1016/j.jprot.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Munoz-Suano A, Hamilton AB, Betz AG. Gimme shelter: the immune system during pregnancy. Immunol. Rev. 2011;241:20–38. doi: 10.1111/j.1600-065X.2011.01002.x. [DOI] [PubMed] [Google Scholar]
- 31.Simister NE, Story CM, Chen HL, Hunt JS. An IgG-transporting Fc receptor expressed in the syncytiotrophoblast of human placenta. Eur. J. Immunol. 1996;26:1527–1531. doi: 10.1002/eji.1830260718. [DOI] [PubMed] [Google Scholar]
- 32.Allansmith M, McClellan BH, Butterworth M, Maloney JR. The development of immunoglobulin levels in man. J. Pediatr. 1968;72:276–290. doi: 10.1016/S0022-3476(68)80324-5. [DOI] [PubMed] [Google Scholar]
- 33.Morell A, Skvaril F, Hitzig WH, Barandun S. IgG subclasses: development of the serum concentrations in “normal” infants and children. J. Pediatr. 1972;80:960–964. doi: 10.1016/S0022-3476(72)80007-6. [DOI] [PubMed] [Google Scholar]
- 34.Williams PJ, Arkwright PD, Rudd P, Scragg IG, Edge CJ, Wormald MR, Rademacher TW. Selective placental transport of maternal IgG to the fetus. Placenta. 1995;16:749–756. doi: 10.1016/0143-4004(95)90018-7. [DOI] [PubMed] [Google Scholar]
- 35.Kibe T, Fujimoto S, Ishida C, Togari H, Wada Y, Okada S, Nakagawa H, Tsukamoto Y, Takahashi N. Glycosylation and placental transport of immunoglobulin G. J. Clin. Biochem. Nutr. 1996;21:57–63. doi: 10.3164/jcbn.21.57. [DOI] [Google Scholar]
- 36.Gala FA, Morrison SL. V region carbohydrate and antibody expression. J. Immunol. 2004;172:5489–5494. doi: 10.4049/jimmunol.172.9.5489. [DOI] [PubMed] [Google Scholar]
- 37.Zhu D, Ottensmeier CH, Du MQ, McCarthy H, Stevenson FK. Incidence of potential glycosylation sites in immunoglobulin variable regions distinguishes between subsets of Burkitt’s lymphoma and mucosa-associated lymphoid tissue lymphoma. Br. J. Haematol. 2003;120:217–222. doi: 10.1046/j.1365-2141.2003.04064.x. [DOI] [PubMed] [Google Scholar]
- 38.Holland M, Yagi H, Takahashi N, Kato K, Savage CO, Goodall DM, Jefferis R. Differential glycosylation of polyclonal IgG, IgG-Fc and IgG-Fab isolated from the sera of patients with ANCA-associated systemic vasculitis. Biochim. Biophys. Acta. 2006;1760:669–677. doi: 10.1016/j.bbagen.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 39.Selman MH, McDonnell LA, Palmblad M, Ruhaak LR, Deelder AM, Wuhrer M. Immunoglobulin G glycopeptide profiling by matrix-assisted laser desorption ionization Fourier transform ion cyclotron resonance mass spectrometry. Anal. Chem. 2010;82:1073–1081. doi: 10.1021/ac9024413. [DOI] [PubMed] [Google Scholar]
- 40.Nevedomskaya E, Derks R, Deelder AM, Mayboroda OA, Palmblad M. Alignment of capillary electrophoresis-mass spectrometry datasets using accurate mass information. Anal. Bioanal. Chem. 2009;395:2527–2533. doi: 10.1007/s00216-009-3166-1. [DOI] [PubMed] [Google Scholar]
- 41.Shikata K, Yasuda T, Takeuchi F, Konishi T, Nakata M, Mizuochi T. Structural changes in the oligosaccharide moiety of human IgG with aging. Glycoconj. J. 1998;15:683–689. doi: 10.1023/A:1006936431276. [DOI] [PubMed] [Google Scholar]
- 42.Yamada E, Tsukamoto Y, Sasaki R, Yagyu K, Takahashi N. Structural changes of immunoglobulin G oligosaccharides with age in healthy human serum. Glycoconj. J. 1997;14:401–405. doi: 10.1023/A:1018582930906. [DOI] [PubMed] [Google Scholar]
- 43.Malek A, Sager R, Kuhn P, Nicolaides KH, Schneider H. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am. J. Reprod. Immunol. 1996;36:248–255. doi: 10.1111/j.1600-0897.1996.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 44.Stadlmann J, Weber A, Pabst M, Anderle H, Kunert R, Ehrlich HJ, Peter SH, Altmann F. A close look at human IgG sialylation and subclass distribution after lectin fractionation. Proteomics. 2009;9:4143–4153. doi: 10.1002/pmic.200800931. [DOI] [PubMed] [Google Scholar]
- 45.Ferrante A, Beard LJ, Feldman RG. IgG subclass distribution of antibodies to bacterial and viral antigens. Pediatr. Infect. Dis. J. 1990;9:S16–S24. [PubMed] [Google Scholar]
- 46.Tao MH, Morrison SL. Studies of aglycosylated chimeric mouse-human IgG. Role of carbohydrate in the structure and effector functions mediated by the human IgG constant region. J. Immunol. 1989;143:2595–2601. [PubMed] [Google Scholar]


