Abstract
The present study was performed to evaluate the significance of biologic subtype and 21-gene recurrence score relative to local recurrence and local–regional recurrence after breast conservation treatment with radiation. Eastern Cooperative Oncology Group E2197 was a prospective randomized clinical trial that compared two adjuvant systemic chemotherapy regimens for patients with operable breast carcinoma with 1–3 positive lymph nodes or negative lymph nodes with tumor size >1.0 cm. The study population was a subset of 388 patients with known 21-gene recurrence score and treated with breast conservation surgery, systemic chemotherapy, and definitive radiation treatment. Median follow-up was 9.7 years (range = 3.7–11.6 years). The 10-year rates of local recurrence and local–regional recurrence were 5.4 % and 6.6 %, respectively. Neither biologic subtype nor 21-gene Recurrence Score was associated with local recurrence or local–regional recurrence on univariate or multivariate analyses (all P ≥ 0.12). The 10-year rates of local recurrence were 4.9 % for hormone receptor positive, HER2-negative tumors, 6.0 % for triple negative tumors, and 6.4 % for HER2-positive tumors (P = 0.76), and the 10-year rates of local–regional recurrence were 6.3, 6.9, and 7.2 %, respectively (P = 0.79). For hormone receptor positive tumors, the 10-year rates of local recurrence were 3.2, 2.9, and 10.1 % for low, intermediate, and high 21-gene recurrence score, respectively (P = 0.17), and the 10-year rates of local–regional recurrence were 3.8, 5.1, and 12.0 %, respectively (P = 0.12). For hormone receptor- positive tumors, the 21-gene recurrence score evaluated as a continuous variable was significant for local–regional recurrence (hazard ratio 2.66; P = 0.03). The 10-year rates of local recurrence and local–regional recurrence were reasonably low in all subsets of patients. Neither biologic subtype nor 21-gene recurrence score should preclude breast conservation treatment with radiation.
Keywords: Early stage breast carcinoma, Breast conservation treatment, Radiation treatment, 21-Gene recurrence score, Local recurrence, Local–regional recurrence
Introduction
Gene expression profiling has become an important tool to evaluate the risk for distant recurrence and survival for patients with early stage breast carcinoma. Recent studies have demonstrated the value of gene expression profiling, in addition to conventional clinical and pathologic features of the primary tumor, for predicting the risk of distant metastatic disease and survival. In contrast, the value of gene expression profiling relative to local recurrence or local–regional recurrence is an emerging area of research, with limited published data [1–3]. Further, the value of gene expression profiling relative to local recurrence or local–regional recurrence beyond conventional clinical and pathologic factors is less well defined. In an analysis of patients enrolled in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 and B-20 studies, Mamounas et al. [1] reported that the 21-gene Oncotype DX recurrence score (hereafter referred to as the recurrence score) was associated with the 10-year rate of local–regional recurrence for patients with estrogen receptor-positive tumors treated with mastectomy or breast conservation treatment.
Similarly, recent studies have demonstrated the prognostic value of biologic subtyping relative to distant recurrence and survival for patients with early stage breast carcinoma. However, the value of biologic subtyping relative to local recurrence or local–regional recurrence is also not well defined [4–10]. Combinations of the three tumor markers of estrogen receptor status, progesterone receptor status, and HER2 status are commonly used to define biologic subtype, although recognizing the limitations of using tumor markers to approximate biologic subtype [6–16].
The present study was performed to evaluate the significance of the 21-gene recurrence score and biologic subtype relative to local recurrence and local–regional recurrence after breast conservation treatment with definitive radiation for patients enrolled in the randomized clinical trial performed by the Eastern Cooperative Oncology Group (ECOG) E2197.
Patients and methods
ECOG E2197 was a prospective randomized clinical trial that was designed to compare the relative effectiveness of two adjuvant systemic chemotherapy regimens consisting of doxorubicin plus cyclophosphamide (AC) versus doxorubicin plus docetaxel (AT) [17–20]. Eligible patients were women with operable, histologically confirmed adenocarcinoma of the breast with either: (1) pathologic involvement of 1–3 axillary lymph nodes; or (2) pathologically negative axillary lymph nodes with a primary tumor size >1.0 cm. Patients with hormone receptor-positive tumors also received adjuvant hormonal therapy. Study details and outcome results have previously been published [17–19].
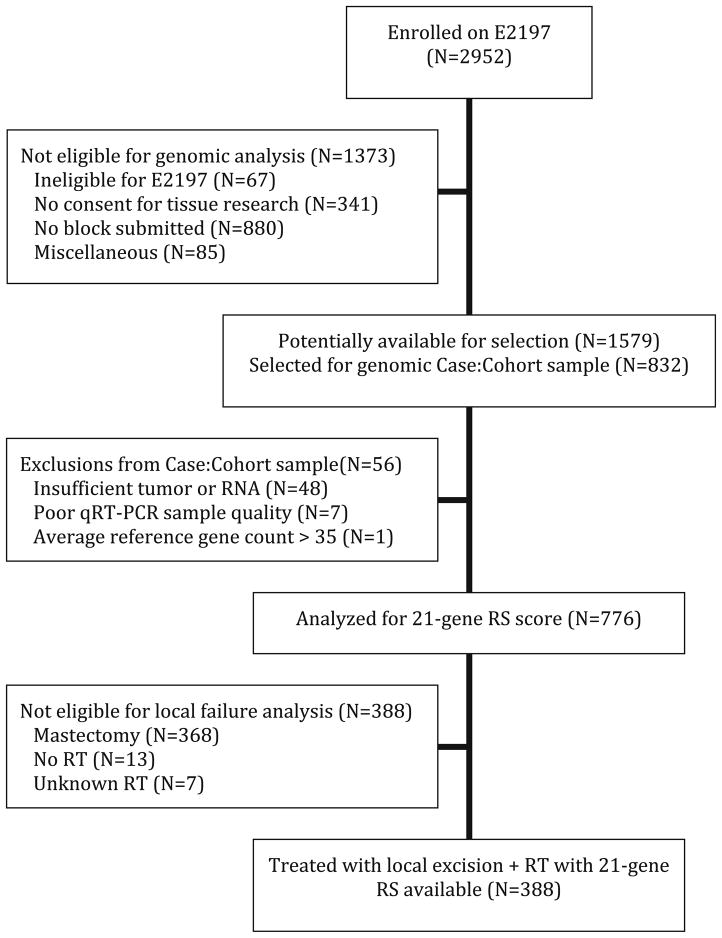
Of the overall group of women, the subset of 388 patients included in the present analysis was defined as follows: (1) enrolled in ECOG E2197; (2) known 21-gene Oncotype DX recurrence score; and (3) primary local–regional treatment using breast conservation treatment (i.e., breast conservation surgery followed by definitive radiation treatment). Figure 1 shows the details for defining the study population for the present analysis. ECOG E2197 protocol specifications included the following: (1) treatment sequence was breast conservation surgery, followed by adjuvant systemic chemotherapy, and then followed by definitive radiation treatment; (2) breast conservation surgery included local excision (lumpectomy) of the primary breast carcinoma with negative margins of resection plus axillary lymph node staging with a minimum of six lymph nodes removed; and (3) radiation treatment was delivered according to standard procedures from the treating physician’s institution. Details regarding the technical radiation treatment delivery were not included as part of the data collection for the original protocol study.
Fig. 1.
CONSORT-style flow diagram for study numbers. CONSORT consolidated standards of reporting trials, RS recurrence score (i.e., 21-gene recurrence score), qRT-PCR quantitative reverse transcription polymerase chain reaction, RT radiation treatment
Local recurrence was defined as recurrence in the treated breast as the first site of recurrence, with or without simultaneous ipsilateral regional lymph node recurrence and/or distant metastases. Local–regional recurrence was defined as recurrence in the treated breast and/or ipsilateral regional lymph nodes as the first site(s) of recurrence, with or without simultaneous distant metastases. Local recurrences and local–regional recurrences were reviewed by two physicians (LJS and AR) experienced in breast conservation treatment to confirm and classify the site(s) of recurrence.
For the patients with local recurrence or local–regional recurrence, the site(s) of first recurrence were as follows: local only (n = 28); local plus regional (n = 1); local plus distant (n = 1); regional only (n = 5); or regional plus distant (n = 1). For the analysis of local recurrence, there were 30 events. For the analysis of local–regional recurrence, there were 36 events.
Tumors were classified as hormone receptor (HR) positive if estrogen receptor (ER) and/or progesterone receptor (PR) were positive based on central immunohistochemistry (IHC) [19, 20]. Tumors were classified as HR negative if both ER and PR were negative. HER2 status and histologic grade were also classified based on central IHC evaluation. Biologic subtypes of tumors were defined based on combinations of HR status and HER2 status as follows: (1) HR positive plus HER2 negative; (2) HR negative plus HER2 negative (i.e., triple negative); or (3) HER2 positive. Details of central pathology evaluation for ECOG E2197 have previously been reported [19, 20].
Consistent with prior studies, low 21-gene recurrence score was defined as <18, intermediate as 18–30, and high as ≥31 [1, 18]. Some of the cases in the present study with known 21-gene recurrence score had HR-negative disease based on prior analyses of the ECOG E2197 data. In order not to limit the statistical power for the current analysis, these cases with HR-negative disease have been retained, and the data analysis for 21-gene recurrence score are reported in two ways: (1) for the overall group of patients; and (2) for the subset of cases with HR-positive disease.
Owing to the low proportion of recurrences in ECOG E2197, a cohort sampling scheme was performed with differential sampling of recurrences and non-recurrences within strata defined by HR status, nodal status, and treatment arm. Detailed description of the cohort sampling process has previously been described [18–20]. Recurrences with analyzable tissue available were included, and approximately 3.5 times as many non-recurrences were randomly sampled within each stratum. Special weighted analysis methods were used to correct for differential sampling [21]. For comparing characteristics of subsets, raw proportions would be potentially biased if the subsets were associated with recurrence risk; therefore, weighted methods with weighting inversely proportional to sampling fractions were used.
Overall recurrence rates were separated into local recurrence versus non-local recurrence and local–regional recurrence versus non-local–regional recurrence by cumulative incidence analyses. Deaths without recurrence and new primary tumors in the opposite breast were considered to be competing events. Estimates and tests incorporating the weighted sampling were computed by a multiple imputation approach [22]. Proportional cause-specific hazard models with censoring follow-up at competing events were used for regression analysis of multiple factors. Weighted methods were used to estimate the effects test for significance in these models, with P values from the Wald test [21].
Calculation of the hazard ratio for the continuous 21-gene recurrence score was for a 50 point difference. For the 21-gene recurrence score, the median (range) value was 44 (0–99) for the overall group of patients. The respective median (range) values based on biologic subtype were 20 (0–88) for the HR positive, HER2-negative tumors, 56 (16–80) for the triple negative tumors, and 66 (12–99) for the HER2-positive tumors.
For the 275 patients alive without recurrence, the median follow-up was 9.7 years (mean = 9.5 years; range = 3.7–11.6 years).
Results
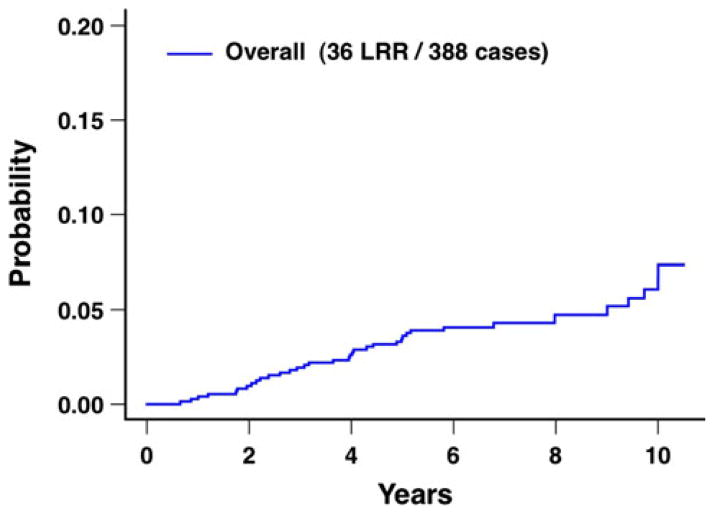
Patient, tumor, and treatment characteristics are detailed in Table 1. For the overall group of 388 patients, the 10-year rate of local recurrence was 5.4 %, and the 10-year rate of local–regional recurrence was 6.6 % (Table 2; Fig. 2).
Table 1.
Patient, tumor, and treatment characteristics for the overall group of 388 patients
| Characteristic | Biologic subtype
|
Total No. (%)a | ||
|---|---|---|---|---|
| HR positive, HER2 negative, No. (%)a |
HR negative, HER2 negative No. (%)a |
HER2 positive No. (%)a |
||
| Overall | 187 (100) | 143 (100) | 58 (100) | 388 (100) |
| Age (years) | ||||
| ≤39 | 12 (7) | 27 (20) | 4 (6) | 43 (11) |
| 40–49 | 50 (24) | 57 (41) | 20 (38) | 127 (32) |
| 50–59 | 83 (46) | 38 (26) | 21 (38) | 142 (39) |
| ≥60 | 42 (22) | 21 (14) | 13 (17) | 76 (19) |
| Menopausal status | ||||
| Premenopausal | 80 (41) | 85 (59) | 24 (47) | 189 (48) |
| Postmenopausal | 107 (59) | 58 (41) | 34 (53) | 199 (52) |
| T stage | ||||
| T1 | 120 (66) | 69 (52) | 29 (53) | 218 (60) |
| T2 | 66 (34) | 72 (47) | 29 (47) | 167 (40) |
| T3 | 1 (1) | 2 (1) | 0 (0) | 3 (1) |
| Pathologic axillary lymph node status | ||||
| Node negative | 93 (54) | 112 (85) | 36 (72) | 241 (66) |
| Node positive | 94 (46) | 31 (15) | 22 (28) | 147 (34) |
| 1 positive | 50 (25) | 23 (11) | 17 (22) | 90 (20) |
| 2 positive | 31 (15) | 7 (3) | 3 (4) | 41 (9) |
| 3 positive | 13 (6) | 1 (1) | 2 (2) | 16 (4) |
| Histologic grade | ||||
| Low | 41 (25) | 2 (2) | 1 (2) | 44 (14) |
| Intermediate | 86 (48) | 13 (10) | 12 (25) | 111 (32) |
| High | 60 (28) | 128 (88) | 45 (73) | 233 (54) |
| Estrogen receptor status | ||||
| Positive | 161 (89) | 0 (0) | 30 (60) | 191 (56) |
| Negative | 26 (11) | 143 (100) | 28 (40) | 197 (44) |
| Progesterone receptor status | ||||
| Positive | 178 (95) | 0 (0) | 25 (53) | 203 (59) |
| Negative | 9 (5) | 143 (100) | 33 (47) | 185 (41) |
| HER2 status | ||||
| Positive | 0 (0) | 0 (0) | 58 (100) | 58 (15) |
| Negative | 187 (100) | 143 (100) | 0 (0) | 330 (85) |
| Adjuvant systemic chemotherapy | ||||
| AC | 83 (48) | 73 (48) | 36 (67) | 192 (51) |
| AT | 104 (52) | 70 (52) | 22 (33) | 196 (49) |
| Adjuvant hormonal treatment | ||||
| Yes | 157 (88) | 27 (24) | 30 (58) | 214 (63) |
| No/unknown | 30 (12) | 116 (76) | 28 (42) | 174 (37) |
| 21-gene recurrence score | ||||
| Low (<18) | 82 (47) | 1 (1) | 4 (11) | 87 (27) |
| Intermediate (18–30) | 63 (34) | 0 (0) | 4 (11) | 67 (20) |
| High (≥31) | 42 (19) | 142 (99) | 50 (77) | 234 (53) |
| Biologic subtype | ||||
| HR positive, HER2 negative | 187 (100) | – | – | 187 (53) |
| HR negative, HER2 negative | – | 143 (100) | – | 143 (32) |
| HER2 positive | – | – | 58 (100) | 58 (15) |
Raw count and weighted distribution percent
AC doxorubicin plus cyclophosphamide, AT doxorubicin plus docetaxel, HR hormone receptor
Table 2.
Local recurrence and local–regional recurrence according to patient, tumor, and treatment characteristics
| Characteristic | No. of patients | Local recurrence
|
P value | Local–regional recurrence
|
P value | ||
|---|---|---|---|---|---|---|---|
| At 5 years % (95 % CI) |
At 10 years % (95 % CI) |
At 5 years % (95 % CI) |
At 10 years % (95 % CI) |
||||
| Overall | 388 | 2.9 (1.9–4.3) | 5.4 (3.6–8.2) | 3.5 (2.4–5.0) | 6.6 (4.6–9.6) | ||
| Age (years) | 0.50 | 0.83 | |||||
| ≤39 | 43 | 2.7 (0.8–8.6) | 7.3 (2.1–25.2) | 5.4 (2.4–12.3) | 10.1 (3.9–26.2) | ||
| 40–49 | 127 | 4.3 (2.4–7.7) | 7.5 (4.2–13.3) | 4.3 (2.4–7.7) | 7.4 (4.2–13.2) | ||
| 50–59 | 142 | 2.8 (1.5–5.4) | 4.7 (2.4–9.4) | 3.2 (1.8–5.8) | 5.0 (2.6–9.7) | ||
| ≥60 | 76 | 0.6 (0.1–4.4) | 2.8 (0.6–13.3) | 1.4 (0.5–4.4) | 6.6 (2.7–16.1) | ||
| Menopausal status | 0.26 | 0.54 | |||||
| Premenopausal | 189 | 4.0 (2.5–6.5) | 6.9 (4.2–11.1) | 4.6 (3.0–7.3) | 7.5 (4.8–11.8) | ||
| Postmenopausal | 199 | 1.8 (0.9–3.8) | 4.1 (2.0–8.6) | 2.4 (1.2–4.4) | 5.8 (3.2–10.7) | ||
| T stage | 0.02 | 0.03 | |||||
| T1 | 218 | 3.2 (2.0–5.1) | 7.4 (4.7–11.8) | 3.7 (2.4–5.8) | 8.6 (5.6–13.3) | ||
| T2 or T3 | 170 | 2.4 (1.2–4.8) | 2.4 (1.2–4.8) | 3.1 (1.7–5.7) | 3.5 (1.9–6.3) | ||
| Adjuvant systemic chemotherapy | 0.07 | 0.02 | |||||
| AC | 192 | 2.6 (1.4–4.9) | 3.4 (1.9–6.0) | 2.6 (1.4–4.9) | 3.7 (2.2–6.4) | ||
| AT | 196 | 3.1 (1.9–5.3) | 7.5 (4.4–12.9) | 4.3 (2.7–6.7) | 9.5 (6.0–15.1) | ||
| Pathologic axillary lymph node status | 0.74 | 0.65 | |||||
| Node negative | 241 | 2.5 (1.5–4.2) | 5.4 (3.2–9.1) | 3.2 (2.0–5.1) | 6.8 (4.2–10.8) | ||
| Node positive | 147 | 3.6 (2.0–6.7) | 5.5 (2.8–10.7) | 4.0 (2.3–7.1) | 6.4 (3.5–11.6) | ||
| Histologic grade | 0.09 | 0.12 | |||||
| Low or Intermediate | 155 | 1.0 (0.3–3.0) | 3.4 (1.3–8.8) | 1.0 (0.3–3.0) | 4.7 (2.1–10.2) | ||
| High | 233 | 4.5 (3.0–6.9) | 7.3 (4.7–11.3) | 5.6 (3.9–8.2) | 8.3 (5.6, 12.3) | ||
| Estrogen receptor status | 0.42 | 0.49 | |||||
| Positive | 191 | 2.2 (1.2–4.1) | 4.5 (2.4–8.4) | 2.4 (1.4–4.4) | 5.7 (3.3–10.0) | ||
| Negative | 197 | 3.7 (2.2–6.3) | 6.7 (3.9–11.6) | 4.8 (3.0–7.6) | 7.8 (4.8–12.7) | ||
| Progesterone receptor status | 0.65 | 0.92 | |||||
| Positive | 203 | 3.1 (1.9–5.1) | 4.3 (2.6–7.2) | 3.6 (2.2–5.6) | 5.8 (3.7–9.3) | ||
| Negative | 185 | 2.6 (1.3–5.0) | 7.2 (4.2–12.3) | 3.3 (1.8–6.0) | 7.8 (4.7–12.9) | ||
| Biologic subtype | 0.76 | 0.79 | |||||
| HR positive, HER2 negative | 187 | 2.6 (1.5–4.5) | 4.9 (2.7–9.0) | 2.8 (1.6–4.9) | 6.3 (3.7–10.8) | ||
| HR negative, HER2 negative | 143 | 2.5 (1.2–5.4) | 6.0 (2.8–12.6) | 3.5 (1.8–6.7) | 6.9 (3.6–13.4) | ||
| HER2 positive | 58 | 4.7 (2.1–10.8) | 6.4 (2.9–14.0) | 5.6 (2.7–11.6) | 7.2 (3.6–14.7) | ||
| 21-gene recurrence score | 0.24 | 0.21 | |||||
| Low | 87 | 1.0 (0.3–4.1) | 3.1 (1.0–9.6) | 1.0 (0.3–4.1) | 3.7 (1.4–10.0) | ||
| Intermediate | 67 | 2.9 (1.1–7.3) | 2.9 (1.1–7.3) | 2.9 (1.1–7.3) | 5.1 (1.9–13.6) | ||
| High | 234 | 3.8 (2.4–6.1) | 7.6 (4.7–12.3) | 4.9 (3.3–7.4) | 8.7 (5.6–13.5) | ||
| Subset of HR positive tumors | 0.17 | 0.12 | |||||
| Low RS | 86 | 1.0 (0.3–4.1) | 3.2 (1.0–9.7) | 1.0 (0.3–4.1) | 3.8 (1.4–10.2) | ||
| Intermediate RS | 67 | 2.9 (1.1–7.3) | 2.9 (1.1–7.3) | 2.9 (1.1–7.3) | 5.1 (1.9–13.6) | ||
| High RS | 66 | 5.9 (3.1–11.4) | 10.1 (4.9–21.0) | 7.7 (4.3–13.6) | 12.0 (6.3–22.9) | ||
| Subset of HR positive, HER2 negative tumors | 0.25 | 0.20 | |||||
| Low RS | 82 | 1.1 (0.3–4.4) | 3.4 (1.1–10.5) | 1.1 (0.3–4.4) | 4.0 (1.5–10.9) | ||
| Intermediate RS | 63 | 3.1 (1.2–8.0) | 3.1 (1.2–8.0) | 3.1 (1.2–8.0) | 5.6 (2.1–14.9) | ||
| High RS | 42 | 5.1 (2.4–10.9) | 12.1 (4.8–30.1) | 6.6 (3.2–13.5) | 13.8 (5.8–32.4) | ||
AC doxorubicin plus cyclophosphamide, AT doxorubicin plus docetaxel, HR hormone receptor, RS recurrence score (i.e., 21-gene recurrence score)
Fig. 2.
Local–regional recurrence after breast conservation treatment with radiation for the overall group of 388 patients. LRR local– regional recurrences
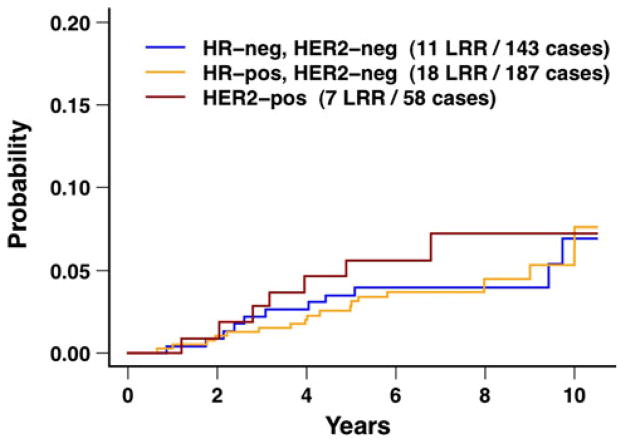
Neither biologic subtype nor the 21-gene recurrence score was associated with local recurrence or local–regional recurrence on univariate analyses (all P ≥ 0.12; Table 2; Figs. 3, 4, 5). The 10-year rates of local recurrence were 4.9 % for HR positive, HER2-negative tumors, 6.0 % for triple negative tumors, and 6.4 % for HER2-positive tumors (P = 0.76), and the 10-year rates of local–regional recurrence were 6.3, 6.9, and 7.2 %, respectively (P = 0.79). The 10-year rates of local recurrence and local–regional recurrence were higher for patients with T1 tumors compared to patients with T2/T3 tumors (both P ≤ 0.03). The 10-year rates of local recurrence and local–regional recurrence were higher for patients treated with AT compared to patients treated with AC (both P ≤ 0.07).
Fig. 3.
Local–regional recurrence after breast conservation treatment with radiation according to biologic subtype for the overall group of 388 patients. The difference between the three curves was not statistically different (P = 0.79). HR hormone receptor, LRR local– regional recurrences
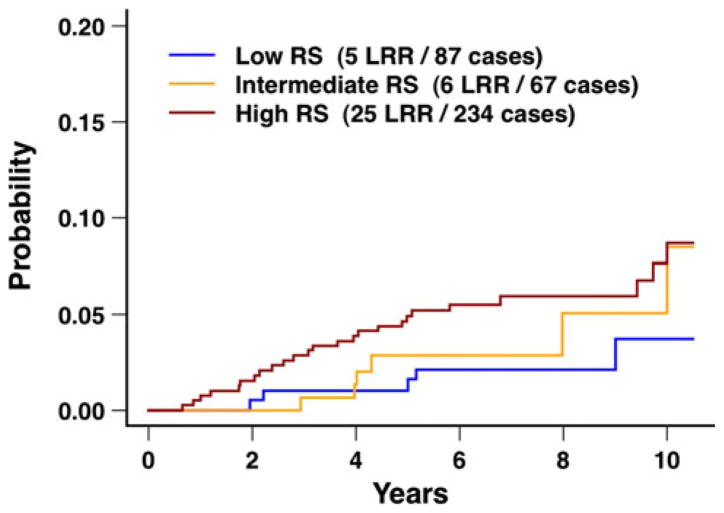
Fig. 4.
Local–regional recurrence after breast conservation treatment with radiation according to the 21-gene recurrence score for the overall group of 388 patients. The difference between the three curves was not statistically different (P = 0.21). RS recurrence score (i.e., 21-gene recurrence score), LRR local–regional recurrences
Fig. 5.
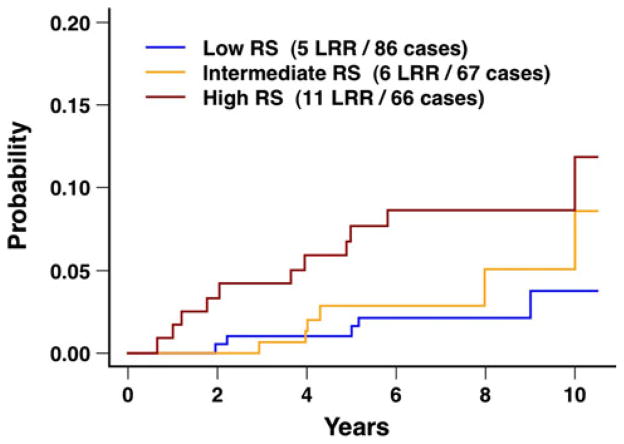
Local–regional recurrence after breast conservation treatment with radiation according to 21-gene recurrence score for the subset of 219 patients with hormone receptor positive tumors. The difference between the three curves was not statistically different (P = 0.12). RS recurrence score (i.e., 21-gene recurrence score), LRR local–regional recurrences
The 10-year rates of local recurrence were 3.1, 2.9 and 7.6 % for low, intermediate, and high 21-gene recurrence score, respectively (P = 0.24), and the 10-year rates of local–regional recurrence were 3.7, 5.1, and 8.7 %, respectively (P = 0.21; Table 2; Fig. 4). Similar results were seen for the subset of HR-positive tumors (Table 2; Fig. 5). Additional analyses were performed for the 21-gene recurrence score evaluated as a continuous variable and restricted to the subset of HR-positive tumors. In this subset, the hazard ratio was borderline statistically significant for local recurrence (hazard ratio 2.53; 95 % CI 0.94, 6.84; P = 0.07), and the hazard ratio was statistically significant for local–regional recurrence (hazard ratio 2.66; 95 % CI 1.09, 6.51; P = 0.03).
Analyses according to combinations of patient age and 21-gene recurrence score showed no statistically significant differences for local recurrence or local–regional recurrence (all P ≥ 0.09; Table 3). Similar analyses restricted to the subset of HR positive, HER2-negative tumors also showed no differences when using the three recurrence score groups (all P ≥ 0.21; data not shown). However, analyses restricted to the subset of HR positive, HER2-negative tumors evaluating the recurrence score as a continuous variable showed significant results for local recurrence and local–regional recurrence when adjusted for age (both P ≤ 0.03; data not shown).
Table 3.
Local recurrence and local–regional recurrence according to combinations of patient age and the 21-gene recurrence score
| Group | No. of patients | Local recurrence
|
P value | Local–regional recurrence
|
P value | ||
|---|---|---|---|---|---|---|---|
| At 5 years % (95 % CI) |
At 10 years % (95 % CI) |
At 5 years % (95 % CI) |
At 10 years % (95 % CI) |
||||
| Overall | |||||||
| Age <50 | 0.29 | 0.23 | |||||
| Low RS | 21 | 0 | 1.9 (0.5–7.9) | 0 | 2.0 (0.5–7.9) | ||
| Intermediate RS | 26 | 8.1 (3.4–19.6) | 8.1 (3.4–19.6) | 8.1 (3.4–19.6) | 8.1 (3.4–19.6) | ||
| High RS | 123 | 3.8 (2.1–7.2) | 8.3 (4.5–15.6) | 4.9 (2.8–8.5) | 9.4 (5.3–16.5) | ||
| Age ≥ 50 | 0.49 | 0.60 | |||||
| Low RS | 66 | 1.4 (0.3–5.4) | 3.4 (1.0–12.2) | 1.4 (0.3–5.4) | 4.2 (1.4–12.5) | ||
| Intermediate RS | 41 | 0 | 0 | 0 | 3.5 (0.5–22.9) | ||
| High RS | 111 | 3.8 (1.9–7.6) | 6.8 (3.2–14.4) | 4.9 (2.7–9.0) | 7.9 (4.1–15.4) | ||
| Subset of HR positive tumors | |||||||
| Age <50 | 0.17 | 0.09 | |||||
| Low RS | 21 | 0 | 1.9 (0.5–7.9) | 0 | 2.0 (0.5–7.9) | ||
| Intermediate RS | 26 | 8.1 (3.4–19.6) | 8.1 (3.4–19.6) | 8.1 (3.4–19.6) | 8.1 (3.4–19.6) | ||
| High RS | 29 | 8.0 (3.7–17.3) | 10.3 (5.4–19.7) | 10.1 (5.2–19.4) | 12.4 (7.0–21.9) | ||
| Age ≥ 50 | 0.52 | 0.57 | |||||
| Low RS | 65 | 1.4 (0.3–5.5) | 3.5 (1.0–12.3) | 1.4 (0.3–5.5) | 4.3 (1.4–12.7) | ||
| Intermediate RS | 41 | 0 | 0 | 0 | 3.5 (0.5–22.9) | ||
| High RS | 37 | 4.3 (1.6–11.9) | 9.9 (3.2–30.7) | 5.8 (2.7–12.3) | 11.2 (4.2–29.9) | ||
RS recurrence score (i.e., 21-gene recurrence score), HR hormone receptor
Multivariate analyses for local recurrence and local–regional recurrence were performed using the variables of chemotherapy arm, patient age, HR status, pathologic axillary lymph node status, histologic grade, pathologic T stage, biologic subtype, and 21-gene recurrence score. On multivariate analyses, T stage and chemotherapy arm were statistically significant (all P ≤ 0.05). Neither biologic subtype, 21-gene recurrence score, nor other variables were statistically significant (all P ≥ 0.26).
Discussion
The present study has demonstrated reasonably low rates of local recurrence and local–regional recurrence at 10 years from ECOG E2197, which was a randomized clinical trial using doxorubicin-based and in approximately half of the patients, also taxane-based adjuvant systemic chemotherapy. For the overall group of 388 patients, local recurrence was 5.4 % and local–regional recurrence was 6.6 % at 10 years after breast conservation treatment with definitive radiation (Table 2; Fig. 2). These results for local recurrence and local–regional recurrence are consistent with other randomized clinical trials and retrospective clinical studies. Local recurrence and local–regional recurrence were reasonably low for the overall group of patients as well as for all subgroups of patients identified. Therefore, no patient subgroup was identified in the current study for which breast conservation treatment with radiation was contraindicated, including patient subgroups based on biologic subtype or 21-gene recurrence score.
The only subgroups identified in the current study with 10-year rates of local recurrence or local–regional recurrence >10 % were: (1) HR-positive tumors with a high 21-gene recurrence score; (2) HR positive, HER2-negative tumors with a high 21-gene recurrence score; and (3) patients age ≤39 years (Tables 2, 3; Fig. 5). However, the results in these subgroups are based on relatively small numbers of patients with correspondingly wide 95 % confidence intervals. Relatively few studies have examined the relationship of biologic subtype to local recurrence or local–regional recurrence after breast conservation treatment [4–10]. Many studies, including the present study, have approximated biologic subtype through the combination of the three tumor markers of ER status, PR status, and HER2 status [6–16]. The use of these three tumor markers to approximate biologic subtype has practical value in that these tumor markers are commonly available from retrospective data and guide the selection of targeted adjuvant systemic therapies (e.g., hormonal therapy, trastuzumab). Some studies have suggested an increased risk of local recurrence or local–regional recurrence after breast conservation treatment associated with the triple negative (basal-like) subtype or associated with tumors that are HER2 positive, although these associations have not been demonstrated uniformly in all studies [4–10, 23]. In the present study, no statistically significant differences in the 10-year rates of local recurrence or local– regional recurrence were observed based on biologic subtype (both P ≥ 0.76; Table 2; Fig. 3).
Mamounas et al. [1] evaluated the impact of the 21-gene recurrence score on local–regional recurrence from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 and B-20 studies. The patients in this analysis had node negative, ER-positive breast cancer, and some of the patients had received adjuvant systemic therapy consisting of tamoxifen with or without adjuvant systemic chemotherapy. Systemic chemotherapy consisted of either cyclophosphamide, methotrexate, and 5-flourouracil (CMF) or methotrexate and 5-flourouracil (MF) with leucovorin rescue. For the patients treated with chemotherapy (CMF or MF) plus tamoxifen, the 10-year rates of local– regional recurrence for low, intermediate, and high 21-gene recurrence score were 1.6, 2.7, and 7.8 %, respectively (P = 0.028). For the patients treated with tamoxifen (without chemotherapy) and either by mastectomy or breast conservation treatment, the 21-gene recurrence score was an independent predictor of local–regional recurrence on multivariate analysis (hazard ratio 2.16; P = 0.005).
For the subgroup of 390 patients treated with lumpectomy, breast radiation, and tamoxifen, Mamounas et al. reported an interaction of the 21-gene recurrence score with patient age [1]. After breast conservation treatment, the 10-year rates of local–regional recurrence for patients age <50 years were 12.5 % for low 21-gene recurrence score, 27.7 % for intermediate recurrence score, and 26.5 % for high recurrence score. However, the 10-year rates of local–regional recurrence for patients age ≥50 years were low regardless of 21-gene recurrence score (3.6, 3.7 and 4.8 %, respectively).
There are currently no data indicating that biologic markers can identify a subgroup of patients for whom breast conservation treatment is not indicated when using contemporary systemic therapies. The present study has therefore analyzed the potential role of the 21-gene recurrence score and biologic subtype in a randomized clinical trial using contemporary systemic chemotherapy and hormonal therapy.
In the present study, the rates of local recurrence and local–regional recurrence were higher with increasing 21-gene recurrence score, although these did not achieve statistical significance on univariate and multivariate analyses (Table 2; Figs. 4, 5). Similarly, for the subset of patients with HR-positive tumors, a trend was seen for higher 10-year rates of local recurrence and local–regional recurrence with increasing 21-gene recurrence score, although also not statistically significant. For the subset of HR-positive tumors, evaluation of the 21-gene recurrence score as a continuous variable showed a statistically significant hazard ratio for local–regional recurrence (hazard ratio 2.66; P = 0.03).
The results from the present study are consistent with a possible effect of biologic subtype based on 21-gene recurrence score, given that the 21-gene recurrence score was designed and validated in the setting of patients with HR-positive tumors who were treated with hormonal therapy. However, the magnitude of the differences between the recurrence score groups may be reduced in patients receiving contemporary adjuvant systemic treatment, including doxorubicin-based chemotherapy, taxane-based chemotherapy, and for HR-positive tumors, hormonal therapy. In addition, the magnitude of these differences may be further reduced due to a greater sensitivity of tumors with high 21-gene recurrence score to cytotoxic chemotherapy [24, 25].
Adjuvant systemic chemotherapy and adjuvant hormonal treatment have both been shown to reduce local recurrence and local–regional recurrence in numerous studies. Retrospective studies have documented improvements in local recurrence and local–regional recurrence associated with improvements in patient selection and treatment techniques over time [26, 27]. In the study by Mamounas et al. [1], adjuvant tamoxifen and chemotherapy reduced the risk of local–regional recurrence within each recurrence score subgroup (low, intermediate, and high) in comparison to placebo-treated patients.
The rate of local–regional recurrence was 10.1 % for patients age ≤39 years (Table 2). However, patient age did not achieve statistical significance for local recurrence or local–regional recurrence (both P ≥ 0.50). Nonetheless, there is a trend, which is not statistically significant, toward increasing local recurrence and local–regional recurrence in the younger age population, consistent with other studies. The lack of statistical significance may be secondary to the relatively small number of patients in the youngest age group (n = 43 for age ≤39 years).
The factors identified in the present study as statistically significantly associated with local recurrence and local– regional recurrence on both univariate and multivariate analyses were T stage and adjuvant systemic chemotherapy arm (Table 2). In the current study, the local recurrence and local–regional recurrence rates were higher for T1 tumors in comparison with T2/T3 tumors, which is in contrast to other reported studies. There is no clear explanation for the finding that the local recurrence and local–regional recurrence rates were higher for T1 tumors in comparison to T2/T3 tumors and for patients treated with AT in comparison to AC. No imbalance or other factors were found to explain these differences. One possible explanation is that these results are false positive findings associated with the play of chance.
There are several limitations of the present study. First, the numbers of patients in some of the subsets were relatively small with correspondingly wide 95 % confidence intervals. Second, the patients treated in the present study predated the era of adjuvant trastuzumab for HER2-positive tumors, although only 15 % of the tumors in the present study were HER2 positive (Table 1). The addition of trastuzumab for HER2-positive tumors has been associated with a reduction in local recurrence [28, 29]. Third, biologic subtype was approximated based on the combination of three tumor markers of ER status, PR status, and HER2 status, although recognizing the limitations of this approximation. Finally, patients in the present study represent a subset of the overall population of patients included in the original E2197 study (Fig. 1).
In summary, the present study has demonstrated reasonably low 10-year rates of local recurrence and local– regional recurrence for patients in a randomized clinical trial using doxorubicin-based and for approximately half of the patients, also taxane-based adjuvant systemic chemotherapy. The current analysis identified no subgroup of patients for which breast conservation treatment was contraindicated, including subgroups based on biologic subtype and the 21-gene recurrence score. On the basis of the current analysis, neither biologic subtype nor the 21-gene recurrence score should preclude breast conservation treatment with radiation.
Acknowledgments
This study was coordinated by the Eastern Cooperative Oncology Group (Robert L. Comis, M.D., Chair), and supported in part by the Public Health Service Grants CA23318, CA66636, CA21115, CA14958, CA80775, CA49883, CA39229, CA27525, CA25224, CA32102, CA45389, the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services. Other supporting grants include Sanofi-Aventis and The Breast Cancer Research Foundation. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute. Biospecimens were provided by the ECOG Pathology Coordinating Office and Reference Laboratory.
Footnotes
Conflict of interest Compensated Robert Gray: Research Funding, Genomic Health, Inc.; Lori J. Goldstein: Member, Advisory Board, Genomic Health, Inc.; Frederick L. Baehner: Employee, Genomic Health, Inc., also with stock ownership; Steven Shak: Employee, Genomic Health, Inc., also with stock ownership; Sunil Badve: Other remuneration, Genomic Health, Inc.
Not Compensated Lawrence J. Solin: (a) Member, Advisory Board, Genomic Health, Inc.; (b) Principal Investigator, Biomarkers in Tissue Samples from Patients with Ductal Breast Carcinoma in situ NCT01132560 at ClinicalTrials.gov
Contributor Information
Lawrence J. Solin, Email: solin@einstein.edu, Department of Radiation Oncology, Albert Einstein Medical Center, 5501 Old York Road, Philadelphia, PA 19141, USA
Robert Gray, The Dana-Farber Cancer Institute, Boston, MA, USA. Eastern Cooperative Oncology Group Coordinating Center, Boston, MA, USA.
Lori J. Goldstein, Department of Medical Oncology, Fox Chase Cancer Center, Philadelphia, PA, USA
Abram Recht, Department of Radiation Oncology, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Frederick L. Baehner, Genomic Health, Inc., Redwood City, CA, USA
Steven Shak, Genomic Health, Inc., Redwood City, CA, USA.
Sunil Badve, Department of Pathology, Indiana University, Indianapolis, IN, USA.
Edith A. Perez, Mayo Clinic, Jacksonville, FL, USA
Lawrence N. Shulman, The Dana-Farber Cancer Institute, Boston, MA, USA
Silvana Martino, The Angeles Clinic and Research Institute, Santa Monica, CA, USA.
Nancy E. Davidson, University of Pittsburgh Cancer Institute, Pittsburgh, PA, USA
George W. Sledge, Jr., Department of Medical Oncology, Indiana University, Indianapolis, IN, USA
Joseph A. Sparano, Department of Medical Oncology, Montefiore Medical Center, Albert Einstein College of Medicine, New York, NY, USA
References
- 1.Mamounas EP, Tang G, Fisher B, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol. 2010;28:1677–1683. doi: 10.1200/JCO.2009.23.7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kreike B, Halfwerk H, Armstrong N, et al. Local recurrence after breast-conserving therapy in relation to gene expression patterns in a large series of patients. Clin Cancer Res. 2009;15:4181–4190. doi: 10.1158/1078-0432.CCR-08-2644. [DOI] [PubMed] [Google Scholar]
- 3.Nuyten DS, Kreike B, Hart AA, et al. Predicting a local recurrence after breast-conserving therapy by gene expression profiling. Breast Cancer Res. 2006;8:R62. doi: 10.1186/bcr1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voduc KD, Cheang M, Tyldesley S, et al. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 5.Millar EK, Graham PH, O’Toole SA, et al. Prediction of local recurrence, distant metastasis, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J Clin Oncol. 2009;27:4701–4708. doi: 10.1200/JCO.2008.21.7075. [DOI] [PubMed] [Google Scholar]
- 6.Solin LJ, Hwang W-T, Vapiwala N. Outcome after breast conservation treatment with radiation for women with triple-negative early-stage invasive breast carcinoma. Clin Breast Cancer. 2009;9:96–100. doi: 10.3816/CBC.2009.n.018. [DOI] [PubMed] [Google Scholar]
- 7.Freedman GM, Anderson PR, Li T, et al. Locoregional recurrence of triple-negative breast cancer after breast-conserving surgery and radiation. Cancer. 2009;115:946–951. doi: 10.1002/cncr.24094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haffty BG, Yang Q, Reiss M, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24:5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 9.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26:2373–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 11.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 12.Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007;8:235–244. doi: 10.1016/S1470-2045(07)70074-8. [DOI] [PubMed] [Google Scholar]
- 13.Rakha EA, El-Sayed ME, Green AR, et al. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109:25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- 14.Rakha EA, Reis-Filho JS, Ellis IO. Basal-like breast cancer: a critical review. J Clin Oncol. 2008;26:2568–2581. doi: 10.1200/JCO.2007.13.1748. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez-Aya LF, Chavez-MacGregor M, Lei X, et al. Nodal status and clinical outcomes in a large cohort of patients with triple-negative breast cancer. J Clin Oncol. 2011;29:2628–2634. doi: 10.1200/JCO.2010.32.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdulkarim BS, Cuartero J, Hanson J, et al. Increased risk of locoregional recurrence for women with T1-2N0 triple-negative breast cancer treated with modified radical mastectomy without adjuvant radiation therapy compared with breast-conserving therapy. J Clin Oncol. 2011;29:2852–2857. doi: 10.1200/JCO.2010.33.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstein LJ, O’Neill A, Sparano JA, et al. Concurrent doxorubicin plus docetaxel is not more effective than concurrent doxorubicin plus cyclophosphamide in operable breast cancer with 0 to 3 positive axillary nodes: North American Breast Cancer Intergroup Trial E2197. J Clin Oncol. 2008;26:4092–4099. doi: 10.1200/JCO.2008.16.7841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein LJ, Gray R, Badve S, et al. Prognostic utility of the 21-gene assay in hormone receptor-positive operable breast cancer compared with classical clinicopathologic features. J Clin Oncol. 2008;26:4063–4071. doi: 10.1200/JCO.2007.14.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sparano JA, Goldstein LJ, Childs BH, et al. Relationship between topoisomerase 2A RNA expression and recurrence after adjuvant chemotherapy for breast cancer. Clin Cancer Res. 2009;15:7693–7700. doi: 10.1158/1078-0432.CCR-09-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badve SS, Baehner FL, Gray RP, et al. Estrogen- and progesterone-receptor status in ECOG 2197: comparison of immunohistochemistry by local and central laboratories and quantitative reverse transcription polymerase chain reaction by central laboratory. J Clin Oncol. 2008;26:2473–2481. doi: 10.1200/JCO.2007.13.6424. [DOI] [PubMed] [Google Scholar]
- 21.Gray RJ. Weighted analysis for cohort sampling designs. Lifetime Data Anal. 2009;15:24–40. doi: 10.1007/s10985-008-9095-z. [DOI] [PubMed] [Google Scholar]
- 22.Ruan PK, Gray RJ. Analyses of cumulative incidence functions via non-parametric multiple imputation. Stat Med. 2008;27:5709–5724. doi: 10.1002/sim.3402. [DOI] [PubMed] [Google Scholar]
- 23.Harris EE, Hwang W-T, Lee EA, et al. The impact of HER-2 status on local recurrence in women with stage I–II breast cancer treated with breast-conserving therapy. Breast J. 2006;12:431–436. doi: 10.1111/j.1075-122X.2006.00297.x. [DOI] [PubMed] [Google Scholar]
- 24.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang G, Shak S, Paik S, et al. Comparison of the prognostic and predictive utilities of the 21-gene recurrence score assay and Adjuvant! for women with node-negative, ER-positive breast cancer: results from NSABP-B-14 and NSABP B-20. Breast Cancer Res Treat. 2011;127:133–142. doi: 10.1007/s10549-010-1331-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cabioglu N, Hunt KK, Buchholz TA, et al. Improving local control with breast-conserving therapy: a 27-year single-institution experience. Cancer. 2005;104:20–29. doi: 10.1002/cncr.21121. [DOI] [PubMed] [Google Scholar]
- 27.Pass H, Vicini FA, Kestin LL, et al. Changes in management techniques and patterns of disease recurrence over time in patients with breast carcinoma treated with breast-conserving therapy at a single institution. Cancer. 2004;101:713–720. doi: 10.1002/cncr.20410. [DOI] [PubMed] [Google Scholar]
- 28.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 29.Halyard MY, Dueck AC, Pisansky TM, et al. Impact of adjuvant trastuzumab on local regional recurrence: Data from the North Central Cancer Treatment Group (NCCTG) N9831 study (Abstract). Presented at the 33rd Annual San Antonio Breast Cancer Symposium (SABCS); San Antonio, Texas. 8–12 December 2010.2010. [Google Scholar]