Abstract
Background
HIV infection has been associated with development of prediabetes and diabetes. Optimum screening practices for these disorders in HIV-infected populations remain unclear.
Methods
We screened 377 adults, with- or at-risk for HIV infection, for incident hyperglycaemia (prediabetes or diabetes) using two oral glucose tolerance tests (OGTTs) a median of 18.6 months apart. We determined proportion of incident cases detected by fasting and 120-min plasma glucose levels. Independent predictors of incident hyperglycaemia were identified using logistic regression.
Results
The baseline OGTT was consistent with diabetes in 7% of participants and with prediabetes in 31%. Among 352 normoglycaemic and prediabetic participants at baseline, 19 (5%) developed diabetes on follow-up. Among participants normoglycaemic at baseline, an additional 38 (16%) developed prediabetes. Overall 52% of incident hyperglycaemia cases were detected by fasting plasma glucose alone, 33% by a 120-min glucose level alone and 15% by both. Factors independently associated with incident hyperglycaemia included age ≥50 years and body mass index ≥30 kg/m2. Neither HIV infection nor highly active antiretroviral therapy (HAART) use were associated with increased risk of diabetes.
Conclusions
Incident hyperglycaemia is common among older adults with or at-risk for HIV infection. HIV-infected individuals with classic diabetes risk factors should be screened for hyperglycaemia regardless of HAART use. OGTTs may be the preferred screening strategy in HIV-infected individuals at high risk for developing hyperglycaemia.
Introduction
Highly active antiretroviral therapy (HAART) has prolonged the lifespan of individuals infected with HIV [1]. This increased longevity makes HIV-infected individuals more likely to experience diseases of mid- and later life, such as diabetes mellitus [2, 3] and cardiovascular disease [1, 4]. The increased risk of diabetes among HIV-infected individuals has largely been attributed to HAART use [5, 6], although coinfection with HCV [7–10] and classic diabetes risk factors [11–14] may also play a role.
As early treatment may prevent end-organ damage [15], screening for diabetes is warranted in individuals at increased risk for this disease. Identification of individuals with prediabetes is also desirable, as interventions can prevent or delay the development of diabetes [16], as well as improve cardiovascular risk profiles [17]. Guidelines based on expert opinion recommend obtaining fasting plasma glucose levels before initiating therapy, 3–6 months after starting therapy, then annually thereafter [18]. An oral glucose tolerance test (OGTT) is recommended in patients with classic diabetes risk factors [18], such as older age, obesity and having a first-degree relative with diabetes [19]. There are data to suggest that OGTTs are more sensitive than fasting plasma glucose levels [20]; however, in clinical practice, fasting plasma glucose levels are more commonly obtained. In addition, recommendations regarding the frequency of screening these individuals at high risk for diabetes have not been rigorously tested [18].
We performed serial screening for prediabetes and diabetes mellitus among participants in two cohorts of middle-aged and older adults with or at-risk for HIV infection, analysed factors associated with incident cases, and investigated how case detection differed by measurements of fasting and 120-min glucose levels during an OGTT.
Methods
Study participants
Details about participant recruitment and study design have been published [10, 12]. Briefly, between July 2002 and January 2005, participants were recruited from two cohorts of adults with or at-risk for HIV infection, the Menopause Study (Ms) and the Cohort of HIV at-risk Aging Men’s Prospective Study (CHAMPS) in the Bronx, NY, USA. Ms and CHAMPS enrolled adults who either had documented HIV infection, or were at risk of acquiring HIV through self-reported illicit drug use or high-risk sexual behaviours [21, 22]. Ms and CHAMPS participants were followed with semi-annual research visits, during which a structured interview was administered, blood was drawn for HIV serology and T-lymphocyte studies, and weight and height were measured according to a standardized protocol. To be enrolled in the present study, participants were required to meet criteria for inclusion in one of the following groups: HIV-uninfected; HIV-infected and antiretroviral-naive (‘no HAART’); HIV-infected, currently taking HAART and protease inhibitor (PI)-naive (‘non-PI-HAART’); or HIV-infected and currently taking HAART including a PI (‘PI-HAART’). Exclusion criteria included a history of diabetes, self-reported antidiabetic medication use, pregnancy or poor venous access. The study was approved by the Institutional Review Boards for the protection of human subjects of both Montefiore Medical Center (Bronx, NY, USA) and Albert Einstein College of Medicine (Bronx, NY, USA), and all participants provided written informed consent. All procedures were in accordance with the revised Declaration of Helsinki.
Interview data
Trained research staff conducted a standardized face-to-face interview to elicit information on sociodemographic characteristics, family and medical history, and antiretroviral use with the aid of pill chart prompts. Information on physical activity and drug use behaviors was assessed using the audio computer-assisted self-interviewing technique. At each visit, participants were asked whether they had received a diagnosis of diabetes (or ‘high blood sugar’), their age at the time of diagnosis and whether they were taking diabetes medications.
Oral glucose tolerance test
Participants underwent two OGTTs a median of 18.6 months apart (interquartile range 18.1–19.9 months) according to World Health Organization procedures [23]. For each OGTT, participants were instructed to report to the Albert Einstein General Clinical Research Center (Bronx, NY, USA) after a 10–16 h overnight fast, and to take their morning dose of medications with water. A 75 g bolus of dextrose in water was administered orally over <5 min. Blood samples were drawn immediately before and 120 min after the dextrose ingestion. Waist circumference was measured at each OGTT visit according to a standardized protocol.
Biochemical and immunologic assays
Specimens for glucose determination were collected at 0 and 120 min during the OGTT in tubes with glycolytic inhibitors. Plasma glucose was measured using the hexokinase method. HCV antibody testing was performed by enzyme immunoassay (ELISA 3.0; Ortho-Clinical Diagnostics, Raritan, NJ, USA) using blood samples obtained at the first OGTT. For HCV-seropositive participants, HCV RNA quantification was performed on blood samples obtained at both OGTTs using the Versant HCV RNA assay (Bayer Corporation, Tarrytown, NY, USA), which has a lower limit of detection of 615 IU/ml.
Outcome variables
The primary outcome variable was hyperglycaemia, which included both prediabetes and diabetes. Prediabetes was defined as a fasting plasma glucose level ≥100 mg/dl and <126 mg/dl (impaired fasting glucose [IFG]) or a 120-min glucose level ≥140 mg/dl and <200 mg/dl (impaired glucose tolerance [IGT]) during an OGTT, according to American Diabetes Association criteria (ADA) criteria [24]. Diabetes was defined as a fasting plasma glucose level ≥126 mg/dl or a 120-min glucose level ≥200 mg/dl during an OGTT [24] or self-reported use of anti-diabetic medication.
Exposure variables
Exposure variables were based on data collected at the research visit most proximate to the second OGTT. The primary exposure variable was HIV-HAART status, classified as HIV-uninfected; HIV-infected, no HAART; HIV-infected, non-PI-HAART; and HIV-infected, PI-HAART. Abdominal obesity was defined as a waist circumference of >88 cm (35 inches) in women or >102 cm (40 inches) in men, according to American Heart Association criteria for the metabolic syndrome [25]. Physical activity was defined as moderate or strenuous exercise for ≥20 min on >1 day/week. HCV infection was defined as seropositivity and at least one detectable HCV RNA level.
Data analysis
Univariate associations of participant characteristics with incident hyperglycaemia were determined using the χ2 or Fisher’s exact test for categorical variables, and the Mann-Whitney U test for continuous variables. Variables considered included HIV-HAART status (HIV-uninfected; HIV-infected, no HAART; HIV-infected, non-PI-HAART; and HIV-infected, PI-HAART), age ≥50 years, race/ethnicity (Black, Hispanic or White/other), family history of diabetes, body mass index (BMI; <25, 25–29.9 or ≥30 kg/m2), abdominal obesity, physical activity, HCV status, methadone maintenance, heroin use and crack or cocaine use. Multivariate logistic regression analysis was performed to assess independent predictors of incident hyperglycaemia. In constructing multivariate models, we included HIV-HAART status in addition to factors with P ≤0.2 on univariate analysis. SPSS software version 18 (SPSS Inc., Chicago, IL, USA) was used for all analyses. Statistical significance was determined using two-tailed tests with α=0.05.
Results
Participant characteristics
Of the 447 Ms and CHAMPS participants who had a baseline OGTT, 377 (84%) returned for a second OGTT and are included in this analysis. Participants who returned for a second OGTT had similar sociodemographic characteristics and HIV and HCV prevalence rates compared to those who did not return, but were less likely to report crack, cocaine or heroin use in the last 6 months at enrollment (27% versus 46%; P=0.002).
Baseline participant characteristics are presented in Table 1. Of the 377 participants, 222 (59%) were HIV-infected. The majority (79%) of HIV-infected participants were antiretroviral-experienced at baseline. Compared with HIV-uninfected participants, HIV-infected participants were more often Black or Hispanic, less often employed and obese, and less likely to have used drugs in the last 5 years. There were no HIV seroconversions during follow-up.
Table 1.
Baseline Participant Characteristics
| Characteristic | HIV-uninfected (n=155) | HIV-infected (n=222) |
|---|---|---|
| Median age, years (range) | 50 (37–73) | 50 (35–68) |
| Male, n (%) | 76 (49) | 104 (47) |
| Race/ethnicitya | ||
| Black, n (%) | 71 (46) | 135 (61) |
| Hispanic, n (%) | 52 (34) | 59 (27) |
| White/Other, n (%) | 32 (21) | 28 (13) |
| Employed, n (%)a | 35 (23) | 28 (13) |
| Family history of diabetes, n (%) | 69 (45) | 86 (39) |
| Body mass indexa | ||
| <25.0 kg/m2 (lean/normal), n (%) | 48 (31) | 87 (39) |
| 25–29.9 kg/m2 (overweight), n (%) | 50 (32) | 82 (37) |
| ≥30 kg/m2 (obese), n (%) | 57 (37) | 53 (24) |
| Abdominal obesity, n (%)b | 68 (45) | 79 (36) |
| Physical activity, n (%)c | 55 (36) | 59 (27) |
| Drug use in the prior 5 years | ||
| Heroin, n (%)a | 57 (37) | 47 (21) |
| Crack or cocaine, n (%) | 87 (56) | 110 (50) |
| Drug use in the prior 6 months | ||
| Heroin, n (%)a | 23 (15) | 15 (7) |
| Crack or cocaine, n (%) | 35 (23) | 51 (23) |
| Current methadone maintenance, n (%) | 22 (14) | 18 (8) |
| HCV status | ||
| HCV-seronegative, n (%) | 66 (43) | 94 (42) |
| HCV-seropositive, undetectable RNA, n (%) | 27 (17) | 31 (14) |
| HCV-seropositive, detectable RNA, n (%) | 62 (40) | 97 (44) |
| Antiretroviral use | ||
| No HAART, n (%) | 47 (21) | |
| Non-PI-HAART, n (%) | 55 (25) | |
| PI-HAART, n (%) | 120 (54) | |
| CD4+ T-cell count | ||
| ≤200 cells/μl, n (%) | 32 (15) | |
| 201–500 cells/μl, n (%) | 99 (45) | |
| >500 cells/μl, n (%) | 90 (41) | |
| Zidovudine use, n (%) | 13 (6) | |
| Stavudine use, n (%) | 67 (30) | |
| Median fasting glucose, mg/dl (range) | 93 (55–351) | 92 (53–217) |
| Median 120-min glucose, mg/dl (range) | 105 (42–477) | 109 (54–299) |
| OGTT results | ||
| Normal, n (%) | 92 (59) | 145 (65) |
| Prediabetes, n (%) | 50 (32) | 65 (29) |
| IFG, n (%) | 31 (62) | 27 (42) |
| IGT, n (%) | 12 (24) | 21 (32) |
| IFG and IGT, n (%) | 7 (14) | 17 (26) |
| Diabetes mellitus, n (%) | 13 (8) | 12 (5) |
Because of rounding, percentages may not total 100. Missing data is as follows: employed (n=4),abdominal obesity (n=6),used heroin in prior 5 years (n=1),used heroin or crack/cocaine in prior 6 months (n=4),current methadone (n=2) and CD4+ T-cell count (n=1).HAART, highly active antiretroviral therapy; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; OGTT, oral glucose tolerance test; PI, protease inhibitor
A total of 217 (58%) participants were HCV-seropositive, and of these 159 (73%) had active HCV infection determined by at least one detectable HCV RNA level during follow-up, with no difference by HIV serostatus. Among the 125 HCV seropositive participants with both a detectable HCV RNA at baseline and an injection drug use history, we estimated their median duration of HCV infection as 34 years (interquartile range 25–37) using age at first injection as a surrogate for age at HCV acquisition. A total of 14 participants reported prior treatment with interferon; of these, 1 had evidence of HCV viral clearance.
Diabetes risk factors were prevalent: 155 (41%) participants had a first-degree relative with diabetes, 242 (64%) had a BMI ≥25 kg/m2, and 147 (40%) had abdominal obesity. At baseline, 25 of 377 (7%) participants had an OGTT consistent with diabetes and 115 of 377 (31%) with prediabetes, with no difference by HIV status. Among the 115 participants with prediabetes, 58 (50%) had IFG only, 33 (29%) had IGT only and 24 (21%) had both IFG and IGT, with no difference by HIV status.
Incident hyperglycaemia
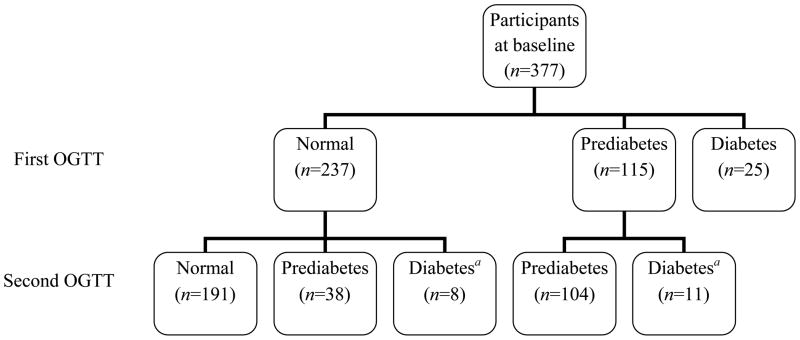
Hyperglycaemia case ascertainment is depicted in Figure 1. Among 352 participants without diabetes at baseline, 19 (5%) developed diabetes on follow-up, of whom 16 were diagnosed by OGTT and 3 self-reported new use of anti-diabetic medication. Incident cases of diabetes were more common among those with prediabetes at baseline (11/115, 10%) than among those with normoglycaemia (8/237, 3%; P=0.016). Of the 237 participants who were normoglycaemic at baseline, 46 (19%) had incident hyperglycaemia, among whom 38 (16%) developed prediabetes and 8 (3%) developed diabetes. HIV-infected participants were less likely to develop hyperglycaemia than HIV-uninfected participants (15% for HIV-infected versus 26% for HIV-uninfected; P=0.038).
Figure 1. Hyperglycaemia case ascertainment.
Hyperglycaemia case ascertainment among 377 individuals with two oral glucose tolerance tests (OGTTs) a median of 18.6 months apart. aOne case with normoglycaemia and two cases with prediabetes at baseline self-reported use of anti-diabetic medications on follow-up, for whom a second OGTT was not performed.
The proportions of incident prediabetes and diabetes cases detected by different screening tests are shown in Figure 2. Of the 16 participants with a screening test consistent with incident diabetes, 7 (44%) cases were detected only by a fasting plasma glucose level, 5 (31%) were detected only by the 120-min plasma glucose level on OGTT and 4 (25%) were detected by both tests, with no difference by HIV status. Among the 38 participants who developed prediabetes, 21 (55%) cases were detected only by a fasting plasma glucose level, 13 (34%) were detected only by the 120-min plasma glucose level on OGTT and 4 (11%) were detected by both tests, with no difference by HIV status.
Figure 2. Proportion of incident prediabetes and diabetes cases by screening test.
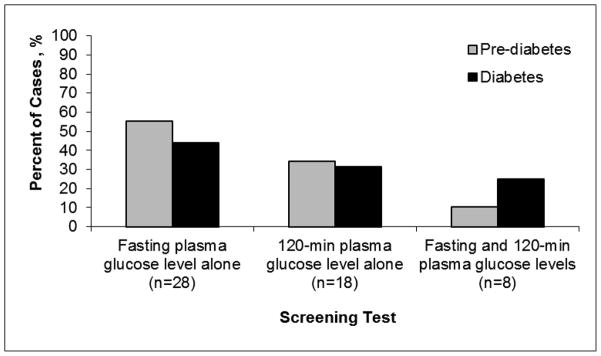
A total of 55% of prediabetes cases and 44% of diabetes cases were detected by a fasting plasma glucose level alone; 34% of prediabetes cases and 31% of diabetes cases were detected by a 120-min plasma glucose level alone; and 11% of prediabetes cases and 25% of diabetes cases were detected by both fasting and 120-min plasma glucose levels.
Of 145 HIV-infected participants who were normoglycaemic at baseline, 22 (15%) had incident hyperglycaemia, among whom 19 (13%) developed prediabetes and 3 (2%) diabetes. A total of 28 participants who were normoglycaemic at baseline were HAART-naive, and 3 of these participants started HAART (PI-containing) during follow-up. Overall, 1 of 3 of those who started HAART developed prediabetes compared to 3 of 25 (12%) of those who remained HAART-naive (P=0.38), and none developed diabetes. Among 65 HIV-infected participants who had prediabetes at baseline, 6 developed incident diabetes; those with IGT alone (3/21, 14%) or both IGT and IFG (3/17, 18%) tended to progress to diabetes more often than those with IFG alone (0/27, 0%; P=0.056).
Factors associated with incident hyperglycaemia
Among 237 participants who were normoglycaemic at baseline, factors associated with incident hyperglycaemia (prediabetes or diabetes) included age ≥50 years (odds ratio [OR] 2.2, 95% confidence interval [CI] 1.1–4.4), BMI ≥30 kg/m2 (reference <25.0; OR 3.0, 95% CI 1.3–6.5), and abdominal obesity (OR 2.9, 95% CI 1.5–5.7).
As BMI and abdominal obesity were highly correlated, two multivariate models were constructed that differed only by inclusion of one of these variables. In model 1, factors independently associated with incident hyperglycaemia included abdominal obesity (Adjusted OR [ORadj] 4.3, 95% CI 2.0–9.2) and age ≥50 years (ORadj 2.9, 95% CI 1.3–6.5). In model 2, factors associated with incident hyperglycaemia included BMI (reference <25.0; ORadj 2.0, 95% CI 0.8–4.8 for BMI 25.0–29.9 kg/m2; ORadj 4.9, 95% CI 2.0–11.9 for BMI ≥30 kg/m2), age ≥50 years (ORadj 2.7, 95% CI 1.2–5.9), and HIV-infected, no HAART (reference HIV-uninfected; ORadj 0.3, 95% CI 0.1–0.9). Use of PI-HAART and non-PI HAART were not associated with incident hyperglycaemia in either model. Adding family history of diabetes to the final models did not significantly change the results.
In analyses restricted to HIV-infected participants, neither HCV nor HAART use predicted hyperglycaemia (SP et al., data not shown).
Discussion
In this cohort of middle-aged and older adults with or at-risk for HIV infection, we found that incident diabetes occurred in 5% of at-risk participants, and incident hyperglycaemia in 19% of participants, after a median follow-up time of 18.6 months. Among the 54 new cases of hyperglycaemia diagnosed by OGTT, one-third had an isolated increased 120-min plasma glucose level. Participants with prediabetes at baseline were more likely to develop diabetes than those who were normoglycaemic. Traditional diabetes risk factors, including older age, BMI ≥30 kg/m2 and abdominal obesity, were associated with incident hyperglycaemia, whearas HAART use with or without a PI was not. Surprisingly, HIV infection in the absence of HAART was inversely associated with risk of incident hyperglycaemia in some models; a biological explanation for this finding is not obvious, and further study is necessary to assess whether such an association can be confirmed.
Although HIV-infected individuals appear to be at high risk for developing hyperglycaemia [5, 6, 12, 26], data on the optimal screening strategy in this population are lacking. Guidelines based on expert opinion have focused on obtaining fasting plasma glucose levels after initiating or switching HAART regimens, particularly in the presence of a PI [18, 27]. We did not find an association between HAART use and incident hyperglycaemia when compared to HIV-uninfected individuals, in contrast to another study which did not control for several key factors associated with diabetes including family history of diabetes and HCV status [5]. We also did not find an association between HCV infection and incident hyperglycaemia, although previously we reported that HCV infection was strongly associated with greater insulin resistance in this cohort [10]. Rather, we found that traditional diabetes risk factors, including age ≥50 years, BMI ≥30 kg/m2 and abdominal obesity were predictors of incident hyperglycaemia. The high prevalence of diabetes risk factors in our cohort may have minimized the contribution of HAART and HCV infection to incident diabetes. In the Veterans Aging Cohort Study, traditional diabetes risk factors, including older age and BMI, had a greater impact on diabetes risk in HIV-infected participants than in uninfected participants, and were associated with a greater risk for diabetes than HAART use [28]. Our findings may also be partially attributable to changing patterns of antiretroviral use, as newer PIs have less of an effect on insulin sensitivity than indinavir [29–31], which was commonly used early in the HAART era. Among HIV-infected individuals, cumulative exposure to NRTIs, particularly thymidine analogues, has been associated with risk of diabetes in some studies [3, 32], which might be attributable to NRTI-induced mitochondrial dysfunction [33].
According to guidelines from the ADA, older age and being overweight or obesity are the main risk factors to consider when targeting individuals in the general population for diabetes screening [19]. ADA recommends that all asymptomatic individuals aged 45 years and older should be screened for hyperglycaemia, as should individuals younger than 45 years who have a BMI ≥25 kg/m2 and at least one additional diabetes risk factor [19]. In this study of middle aged and older men and women, of the 24% of participants younger than 45 years without diabetes at baseline, 69% had a BMI ≥25 kg/m2 and at least one additional diabetes risk factor. Also of note, we found that 31% of incident diabetes cases and 34% of incident prediabetes cases had isolated increased 120-min plasma glucose levels, and thus would not have been detected if only a fasting plasma glucose level was measured. Others have also found that performing only a fasting glucose measurement in HIV-infected populations might miss an appreciable number of individuals with diabetes [34]. Taken together, these data suggest that similar to HIV-uninfected patients, all HIV-infected patients should be assessed for diabetes risk factors regardless of HAART use, and those with risk factors should undergo screening with an OGTT when possible.
Further studies are needed to determine the optimal interval between diabetes screening tests in HIV-infected individuals. The ADA recommends that for those with normal glucose levels on screening, the test should be repeated in 3 years, whereas for individuals with prediabetes, testing should be repeated annually [19]. These time intervals were determined by expert consensus or clinical experience. We detected an appreciable number of new hyperglycaemic cases after a median follow-up time of just 18.6 months, suggesting that perhaps an annual interval between screening tests is prudent for individuals with or at risk for HIV infection.
Identification of prediabetes has important clinical implications. Prediabetes increases one’s risk for diabetes [16], for other cardiovascular risk factors such as hypertension and dyslipidemia [35, 36], and for cardiovascular death [37]. Increased risk of cardiovascular events begins as early as 15 years prior to the diagnosis of diabetes [38]. There is a mounting body of evidence that an elevated 120-min plasma glucose level, and not a fasting plasma glucose level, is a more important predictor of cardiovascular morbidity and mortality among patients with IGT and diabetes [36, 39]. HIV-infected populations are at particularly high risk for cardiovascular disease as they have a high prevalence of other cardiovascular risk factors, including smoking [40, 41], obesity [3, 42, 43] and dyslipidemia [44]. Thus screening for hyperglycaemia with an OGTT, which is inexpensive and easy to perform, would allow for early interventions to prevent morbidity and mortality in this vulnerable population.
Clinical interventions in prediabetic individuals have demonstrated improvement in cardiovascular risk profiles. The Diabetes Prevention Program, a multicenter clinical trial that enrolled overweight or obese prediabetic adults, found that individuals randomized to intensive lifestyle modification or administration of metformin had significant reductions in incident diabetes compared to placebo (58% and 31%, respectively) [16]. These interventions also reduced levels of two non-traditional cardiovascular risk markers, C-reactive protein and fibrinogen [17]. Equivalent intervention strategies have not been tested in HIV-infected populations. However, diligence in screening for hyperglycaemia should prompt counseling on weight loss and increased physical activity in patients with newly diagnosed prediabetes and diabetes.
Our study had several strengths. Whereas most other studies in HIV-infected populations have defined diabetes by self-report or fasting plasma glucose levels alone, we conducted OGTTs, which increased the sensitivity of case ascertainment. We also collected specimens for glucose determination in grey top tubes with glycolytic inhibitors to decrease the false negative rate. In addition, we included a comparison group of HIV-uninfected participants with demographic and behavioral characteristics similar to those of the HIV-infected participants.
Our study also had some limitations. Similar to other epidemiologic studies, abnormal diagnostic tests were not confirmed on a different day as is recommended by the ADA [24]. We also had a small number of incident cases, which may have limited our ability to detect associations between certain risk factors and incident hyperglycaemia.
In summary, in this cohort of HIV-infected and at-risk individuals, we found that repeat screening with an OGTT after a median of 18.6 months detected incident hyperglycaemia in 19% of participants. HIV-infected individuals most likely to benefit from screening are those with classic diabetes risk factors such as age ≥50 years, BMI ≥30 kg/m2 and abdominal obesity. Screening with a 2-hour OGTT may be preferable to screening with a fasting plasma glucose level alone in high-risk patients. Early detection of hyperglycaemia provides opportunities to intervene to prevent diabetes progression and modify cardiovascular risk factors.
Acknowledgments
SP conducted and interpreted the data analyses and led the writing. AAH originated the study, supervised data analysis and interpretation, and assisted with the writing. All of the authors helped to conceptualize the idea, interpret findings and review drafts of the article.
This study was funded by National Institute on Drug Abuse grants (K23 DA015003, R01 DA13564 and R01 DA14998), a General Clinical Research Center grant from the National Institutes of Health grant (M01-RR12248), a Diabetes Research and Training Center grant from the National Institutes of Health (P60DK020541-27), a Center for AIDS Research grant (CFAR-5 P30 AI51519) from the National Institutes of Health awarded to the Albert Einstein College of Medicine of Yeshiva University and the New York State Council on Graduate Medical Education Empire Clinical Research Program.
Footnotes
P < 0.05. for χ2 test.
Abdominal obesity is defined as a waist circumference of >88 cm (35 inches) in women or >102 cm (40 inches) in men[25].
Physical activity is defined as moderate or strenuous exercise for ≥20 min on >1 day/week.
Disclosure Statement
The authors declare no competing interest.
References
- 1.Palella FJ, Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43(1):27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 2.Palacios R, Santos J, Ruiz J, Gonzalez M, Marquez M. Factors associated with the development of diabetes mellitus in HIV-infected patients on antiretroviral therapy: a case-control study. AIDS. 2003;17(6):933–935. doi: 10.1097/00002030-200304110-00025. [DOI] [PubMed] [Google Scholar]
- 3.Tien PC, Schneider MF, Cole SR, et al. Antiretroviral therapy exposure and incidence of diabetes mellitus in the Women’s Interagency HIV Study. AIDS. 2007;21(13):1739–1745. doi: 10.1097/QAD.0b013e32827038d0. [DOI] [PubMed] [Google Scholar]
- 4.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165(10):1179–1184. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 6.Ledergerber B, Furrer H, Rickenbach M, et al. Factors associated with the incidence of type 2 diabetes mellitus in HIV-infected participants in the Swiss HIV Cohort Study. Clin Infect Dis. 2007;45(1):111–119. doi: 10.1086/518619. [DOI] [PubMed] [Google Scholar]
- 7.Mehta SH, Moore RD, Thomas DL, Chaisson RE, Sulkowski MS. The effect of HAART and HCV infection on the development of hyperglycemia among HIV-infected persons. J Acquir Immune Defic Syndr. 2003;33(5):577–584. doi: 10.1097/00126334-200308150-00005. [DOI] [PubMed] [Google Scholar]
- 8.Howard AA, Klein RS, Schoenbaum EE. Association of hepatitis C infection and antiretroviral use with diabetes mellitus in drug users. Clin Infect Dis. 2003;36(10):1318–1323. doi: 10.1086/374838. [DOI] [PubMed] [Google Scholar]
- 9.de Larranaga GF, Wingeyer SD, Puga LM, Alonso BS, Benetucci JA. Relationship between hepatitis C virus (HCV) and insulin resistance, endothelial perturbation, and platelet activation in HIV-HCV-coinfected patients under highly active antiretroviral treatment. Eur J Clin Microbiol Infect Dis. 2006;25(2):98–103. doi: 10.1007/s10096-006-0090-6. [DOI] [PubMed] [Google Scholar]
- 10.Howard AA, Lo Y, Floris-Moore M, et al. Hepatitis C virus infection is associated with insulin resistance among older adults with or at risk of HIV infection. AIDS. 2007;21(5):633–641. doi: 10.1097/QAD.0b013e3280464db7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoon C, Gulick RM, Hoover DR, Vaamonde CM, Glesby MJ. Case-control study of diabetes mellitus in HIV-infected patients. J Acquir Immune Defic Syndr. 2004;37(4):1464–1469. doi: 10.1097/01.qai.0000137373.26438.18. [DOI] [PubMed] [Google Scholar]
- 12.Howard AA, Floris-Moore M, Arnsten JH, et al. Disorders of glucose metabolism among HIV-infected women. Clin Infect Dis. 2005;40(10):1492–1499. doi: 10.1086/429824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howard AA, Floris-Moore M, Lo Y, et al. Abnormal glucose metabolism among older men with or at risk of HIV infection. HIV Med. 2006;7(6):389–396. doi: 10.1111/j.1468-1293.2006.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brar I, Shuter J, Thomas A, Daniels E, Absalon J. A comparison of factors associated with prevalent diabetes mellitus among HIV-Infected antiretroviral-naive individuals versus individuals in the National Health and Nutritional Examination Survey cohort. J Acquir Immune Defic Syndr. 2007;45(1):66–71. doi: 10.1097/QAI.0b013e318031d7e3. [DOI] [PubMed] [Google Scholar]
- 15.Harris MI, Klein R, Welborn TA, Knuiman MW. Onset of NIDDM occurs at least 4–7 yr before clinical diagnosis. Diabetes Care. 1992;15(7):815–819. doi: 10.2337/diacare.15.7.815. [DOI] [PubMed] [Google Scholar]
- 16.The Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Diabetes Prevention Program Research Group. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes. 2005;54(5):1566–1572. doi: 10.2337/diabetes.54.5.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schambelan M, Benson CA, Carr A, et al. Management of metabolic complications associated with antiretroviral therapy for HIV-1 infection: recommendations of an International AIDS Society-USA panel. J Acquir Immune Defic Syndr. 2002;31(3):257–275. doi: 10.1097/00126334-200211010-00001. [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association. Standards of medical care in diabetes--2008. Diabetes Care. 2008;31(Suppl 1):S12–S54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 20.The DECODE-study group on behalf of the European Diabetes Epidemiology Group. Is fasting glucose sufficient to define diabetes? Epidemiological data from 20 European studies. The DECODE-study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis of Diagnostic Criteria in Europe. Diabetologia. 1999;42(6):647–654. doi: 10.1007/s001250051211. [DOI] [PubMed] [Google Scholar]
- 21.Miller SA, Santoro N, Lo Y, et al. Menopause symptoms in HIV-infected and drug-using women. Menopause. 2005;12(3):348–356. doi: 10.1097/01.gme.0000141981.88782.38. [DOI] [PubMed] [Google Scholar]
- 22.Klein RS, Lo Y, Santoro N, Dobs AS. Androgen levels in older men who have or who are at risk of acquiring HIV infection. Clin Infect Dis. 2005;41(12):1794–1803. doi: 10.1086/498311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO Study Group. WHO technical report series 727. Vol. 1985. Geneva: WHO; 1985. Diabetes Mellitus. Report of a WHO study group; p. 99. [PubMed] [Google Scholar]
- 24.American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2008;31(Supplement_1):S55–S60. doi: 10.2337/dc08-S055. [DOI] [PubMed] [Google Scholar]
- 25.Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 26.Hadigan C, Meigs JB, Corcoran C, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32(1):130–139. doi: 10.1086/317541. [DOI] [PubMed] [Google Scholar]
- 27.Panel on Antiretroviral Guidelines for Adult and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. Department of Health and Human Services; 2009. p. 120. [Google Scholar]
- 28.Butt AA, McGinnis K, Rodriguez-Barradas MC, et al. HIV infection and the risk of diabetes mellitus. AIDS. 2009;23(10):1227–1234. doi: 10.1097/QAD.0b013e32832bd7af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noor MA, Lo JC, Mulligan K, et al. Metabolic effects of indinavir in healthy HIV-seronegative men. AIDS. 2001;15(7):F11–F18. doi: 10.1097/00002030-200105040-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noor MA, Parker RA, O’Mara E, et al. The effects of HIV protease inhibitors atazanavir and lopinavir/ritonavir on insulin sensitivity in HIV-seronegative healthy adults. AIDS. 2004;18(16):2137–2144. doi: 10.1097/00002030-200411050-00005. [DOI] [PubMed] [Google Scholar]
- 31.Lee GA, Seneviratne T, Noor MA, et al. The metabolic effects of lopinavir/ritonavir in HIV-negative men. AIDS. 2004;18(4):641–649. doi: 10.1097/00002030-200403050-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Wit S, Sabin CA, Weber R, et al. Incidence and risk factors for new onset diabetes mellitus in HIV infected patients: the D:A:D study. Diabetes Care. 2008 doi: 10.2337/dc07-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shikuma CM, Day LJ, Gerschenson M. Insulin resistance in the HIV-infected population: the potential role of mitochondrial dysfunction. Curr Drug Targets Infect Disord. 2005;5(3):255–262. doi: 10.2174/1568005054880163. [DOI] [PubMed] [Google Scholar]
- 34.Samaras K. Prevalence and pathogenesis of diabetes mellitus in HIV-1 infection treated with combined antiretroviral therapy. J Acquir Immune Defic Syndr. 2009;50(5):499–505. doi: 10.1097/QAI.0b013e31819c291b. [DOI] [PubMed] [Google Scholar]
- 35.Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA. 1990;263(21):2893–2898. doi: 10.1001/jama.263.21.2893. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez BL, Lau N, Burchfiel CM, et al. Glucose intolerance and 23-year risk of coronary heart disease and total mortality: the Honolulu Heart Program. Diabetes Care. 1999;22(8):1262–1265. doi: 10.2337/diacare.22.8.1262. [DOI] [PubMed] [Google Scholar]
- 37.Tominaga M, Eguchi H, Manaka H, et al. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care. 1999;22(6):920–924. doi: 10.2337/diacare.22.6.920. [DOI] [PubMed] [Google Scholar]
- 38.Hu FB, Stampfer MJ, Haffner SM, et al. Elevated risk of cardiovascular disease prior to clinical diagnosis of type 2 diabetes. Diabetes Care. 2002;25(7):1129–1134. doi: 10.2337/diacare.25.7.1129. [DOI] [PubMed] [Google Scholar]
- 39.The DECODE Study Group. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161(3):397–405. doi: 10.1001/archinte.161.3.397. [DOI] [PubMed] [Google Scholar]
- 40.Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349(21):1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 41.Feldman JG, Minkoff H, Schneider MF, et al. Association of cigarette smoking with HIV prognosis among women in the HAART era: a report from the women’s interagency HIV study. Am J Public Health. 2006;96(6):1060–1065. doi: 10.2105/AJPH.2005.062745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kruzich LA, Marquis GS, Wilson CM, Stephensen CB. HIV-infected US youth are at high risk of obesity and poor diet quality: a challenge for improving short- and long-term health outcomes. J Am Diet Assoc. 2004;104(10):1554–1560. doi: 10.1016/j.jada.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 43.Karmon SL, Moore RD, Dobs AS, et al. Body shape and composition in HIV-infected women: an urban cohort. HIV Med. 2005;6(4):245–252. doi: 10.1111/j.1468-1293.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- 44.Fontas E, van Leth F, Sabin CA, et al. Lipid profiles in HIV-infected patients receiving combination antiretroviral therapy: are different antiretroviral drugs associated with different lipid profiles? J Infect Dis. 2004;189(6):1056–1074. doi: 10.1086/381783. [DOI] [PubMed] [Google Scholar]