Abstract
About 20% of all human cancers are caused by chronic infection or chronic inflammatory states. Recently, a new hypothesis has been proposed for prostate carcinogenesis. It proposes that exposure to environmental factors such as infectious agents and dietary carcinogens, and hormonal imbalances lead to injury of the prostate and to the development of chronic inflammation and regenerative ‘risk factor’ lesions, referred to as proliferative inflammatory atrophy (PIA). By developing new experimental animal models coupled with classical epidemiological studies, genetic epidemiological studies and molecular pathological approaches, we should be able to determine whether prostate cancer is driven by inflammation, and if so, to develop new strategies to prevent the disease.
Prostate cancer is the most common non-cutaneous malignant neoplasm in men in Western countries, responsible for the deaths of approximately 30,000 men per year in the United States1. The number of afflicted men is increasing rapidly as the population of males over the age of 50 grows worldwide. Therefore, finding strategies for the prevention of prostate cancer is a crucial medical challenge. As men in South East Asian countries have a low incidence of prostate cancer that increases rapidly after immigration to the West, this disease is not an intrinsic feature of ageing. The pathogenesis of prostate cancer reflects both hereditary and environmental components. What are the environmental factors and genetic variations that have produced such an epidemic of prostate cancer? Approximately 20% of all human cancers in adults result from chronic inflammatory states and/or chronic inflammation2–4 (BOX 1), which are triggered by infectious agents or exposure to other environmental factors, or by a combination thereof. There is also emerging evidence that inflammation is crucial for the aetiology of prostate cancer. This evidence stems from epidemiological, histopathological and molecular pathological studies. The objective of this Review is to take a multidisciplinary approach to present and analyse such studies. Because several reviews related to these topics have been published5–7, here we will focus on new findings and ideas with the purpose of sparking innovative areas of investigation that might ultimately lead to the prevention of prostate cancer.
Box 1 | Molecular mechanisms of inflammation-induced cancers.
Chronic inflammation is implicated in the development of a diverse range of human cancers, with overwhelming evidence causally linking it to cancer of the liver, stomach, large intestine, biliary tree and urinary bladder, and significant evidence to link it to cancer of the oesophagus, lung and pancreas. Many of these cancers are associated with infectious agents and/or a defined environmental exposure(s). Inflammation often collaborates with environmental exposures, such as dietary derived toxins, to increase cancer risk even further132. The molecular mechanisms that underlie the pathogenesis of inflammation-associated cancer are complex, and involve both the innate and adaptive immune systems3,133,134,135. Although viral oncogenes can contribute directly to neoplastic transformation, neither infection nor pathogen-encoded oncogenes are required for inflammatory cells to induce cancer136. Indeed, highly reactive chemical compounds, including superoxide, hydrogen peroxide, singlet oxygen and nitric oxide are released from activated phagocytic inflammatory cells of the innate immune system, and can cause oxidative or nitrosative damage to DNA in the epithelial cells, or react with other cellular components such as phospholipids, initiating a free-radical chain reaction2. The result is that many host epithelial cells are damaged and killed, and in order to preserve the barrier function of epithelia, these cells must be replaced by cell division from resident progenitor and/or stem cells. Epithelial cells that undergo DNA synthesis in the setting of these DNA-damaging agents are at an increased risk of mutation. That oxidant or nitrosative stress is important for driving prostate cancer formation is bolstered by epidemiological data, which indicate that the consumption of certain types of dietary antioxidants is associated with reduced prostate cancer risk137. Inflammatory cells also secrete cytokines that promote epithelial cell proliferation and stimulate angiogenesis138. In terms of disease progression, inflammatory cells migrate readily through the extracellular matrix as a result of the release of proteolytic enzymes and their inherent motile nature. Therefore, they might facilitate epithelial cell invasion into the stromal and vasculature compartments and, ultimately, the metastasis of tumour cells133,134. In another mechanism, the disruption of cytokine production and regulation, including cytokine deficiencies, lead to increased inflammation and cancer, whether in response to infection with a commensal organism or to chemical carcinogens139. A final mechanism is that certain immune responses can directly dampen cell-mediated anti-tumour immune surveillance mechanisms, thereby averting an immune reaction against the tumour that could potentially eliminate the cancer90.
Enigmas in the aetiology of prostate cancer
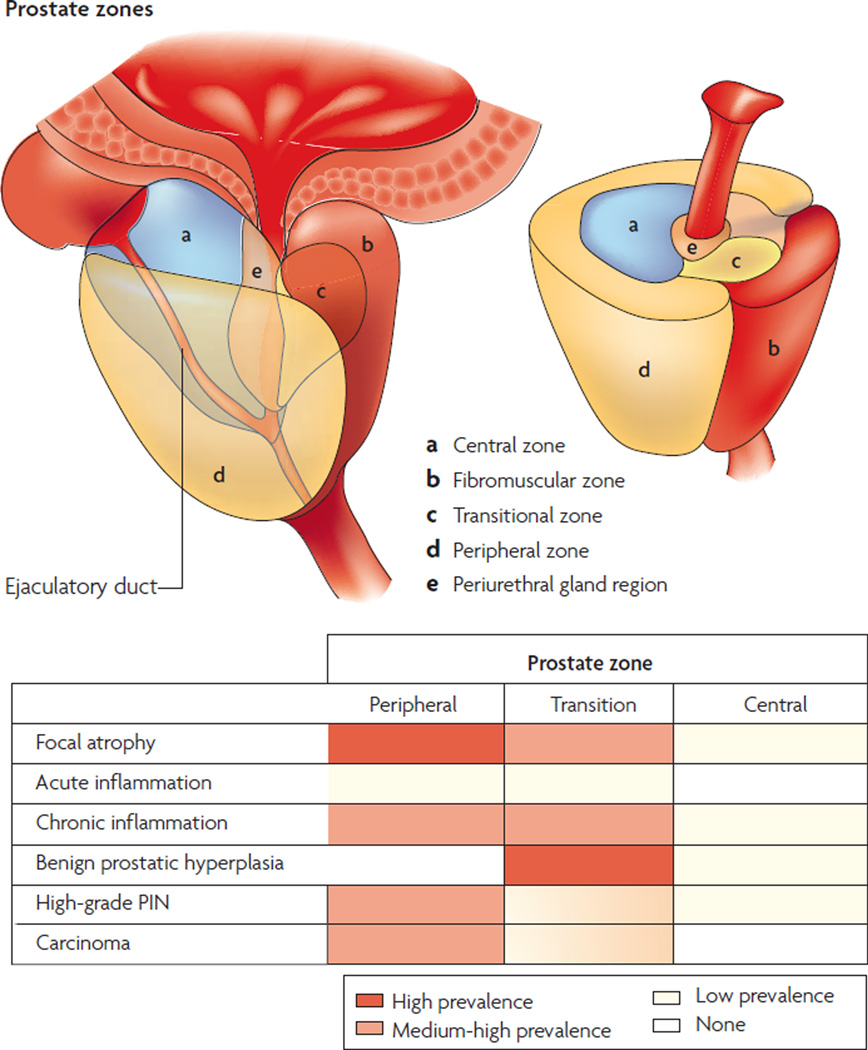
As in other cancers, prostate cancer develops through the accumulation of somatic genetic and epigenetic changes, resulting in the inactivation of tumour-suppressor genes and caretaker genes, and the activation of oncogenes8,9 (TABLE 1). There is also evidence for an underlying genetic instability that might facilitate tumour progression10,11. Although these genetic and epigenetic changes are crucial for our understanding of ‘how’ prostate cancer arises, another key remaining question is ‘why’ prostate cancer is so common. The most consistent risk factors for the development of prostate cancer are advancing age, family history and race — diet is thought to be an emerging risk factor. To answer the question of why prostate cancer is so prevalent, several puzzling facts regarding its occurrence must be explained. The first enigma is the striking organ selectivity of prostate cancer within the genitourinary system: whereas there are approximately 280,000 new cases of prostate cancer in the US each year, there have been less than 50 reported cases of primary seminal vesicle carcinoma in the English literature12. The second unexplained issue is the geographic variation in the incidence of prostate cancer: as compared with the US and Western Europe, the incidence and mortality rates for prostate cancer are much lower in Southeast and East Asia13. Chinese and Japanese men who immigrate to the west acquire higher prostate cancer risks within one generation14, supporting an effect of the environment on prostate cancer development. A third key unsolved problem is the zonal predilection of prostate cancer. Most cancer lesions occur in the peripheral zone of the gland, fewer occur in the transition zone, and almost none arise in the central zone15 (FIG. 1).
Table 1.
Common somatic genetic and epigenetic changes in prostate cancer
| Gene and gene type | Location | Notes |
|---|---|---|
| Tumour-suppressor genes | ||
| CDKN1B | 12p13.1–p12 | Encodes the cyclin-dependent kinase inhibitor p27. One allele is frequently deleted in primary tumours |
| NKX3.1 | 8p21.2 | Encodes prostate-restricted homeobox protein that can suppress the growth of prostate epithelial cells. One allele is frequently deleted in primary tumours |
| PTEN | 10q23.31 | Encodes phosphatase and tensin homologue, which suppresses cell proliferation and increases apoptosis. One allele is frequently lost in primary tumours. Some mutations are found in primary tumours and more in metastatic lesions |
| TP53 | 17p13.1 | Has many tumour-suppressor functions, including cell-cycle arrest in response to DNA damage, senescence in response to telomere dysfunction, and the induction of apoptosis. Mutations are uncommon early, but occur in about 50% of advanced or hormone-refractory prostate cancers |
| Oncogenes | ||
| MYC | 8q24 | A transcription factor that regulates many target genes involved in cell proliferation, senescence, apoptosis and cell metabolism. Overexpression can directly transform cells. mRNA levels are commonly increased in all disease stages through unknown mechanism(s). Low-level amplification of the MYC locus is common in advanced disease |
| ERG | 21q22.3 | Proposed new oncogene for prostate cancer. Fusion transcripts with the 5′ portion of androgen-regulated gene (TMPRSS22) arise from deletion or chromosomal rearrangements commonly found in all disease stages |
| ETV1–4 | 7p21.3, 19q13.12, 1q21,-q23, 17q21.31 |
Encodes ETS-like transcription factors 1–4, which are proposed to be new oncogenes for prostate cancer. Fusion transcripts with the 5′ portion of androgen-regulated gene (TMPRSS22) arise from chromosomal rearrangements commonly found in all disease stages |
| AR | Xq11–12 | Encodes the androgen receptor. Protein is expressed in most prostate cancers, and the locus is amplified or mutated in advanced disease and hormone-refractory cancers |
| Activation of the enzyme telomerase | Maintains telomere function and contributes to cell immortalization. Activated in most prostate cancers, mechanism of activation may be through MYC activation | |
| Caretaker genes | ||
| GSTP1 | 11q13 | Encodes the enzyme that catalyses the conjugation of reduced glutathione to electrophilic substrates. Functions to detoxify carcinogens. It is inactivated in more than 90% of cancers by somatic hypermethylation of the CpG island within the upstream regulatory region |
| Telomere dysfunction | Chromosome termini | Contributes to chromosomal instability. Shortened telomeres are found in more than 90% of prostatic intraepithelial neoplasia (PIN) lesions and prostate cancer lesions |
| Centrosome abnormalities | N/A | Contributes to chromosomal instability. Centrosomes are structurally and numerically abnormal in most prostate carcinomas. |
| Other somatic changes | ||
| PTGS2, APC, MDR1, EDNRB, RASSF1α, RARβ2 | Various | The hypermethylation of CpG islands within upstream regulatory regions occurrs in most primary tumours and metastatic lesions. The functional significance of these changes is not yet known |
Figure 1. Zonal predisposition to prostate disease.
Most cancer lesions occur in the peripheral zone of the gland, fewer occur in the transition zone and almost none arise in the central zone. Most benign prostate hyperplasia (BPH) lesions develop in the transition zone, which might enlarge considerably beyond what is shown. The inflammation found in the transition zone is associated with BPH nodules and atrophy, and the latter is often present in and around the BPH nodules. Acute inflammation can be prominent in both the peripheral and transition zones, but is quite variable. The inflammation in the peripheral zone occurs in association with atrophy in most cases. Although carcinoma might involve the central zone, small carcinoma lesions are virtually never found here in isolation, strongly suggesting that prostatic intraepithelial neoplasia (PIN) lesions do not readily progress to carcinoma in this zone. Both small and large carcinomas in the peripheral zone are often found in association with high-grade PIN, whereas carcinoma in the transition zone tends to be of lower grade and is more often associated with atypical adenomatous hyperplasia or adenosis, and less often associated with high-grade PIN. The various patterns of prostate atrophy, some of which frequently merge directly with PIN and at times with small carcinoma lesions, are also much more prevalent in the peripheral zone, with fewer occurring in the transition zone and very few occurring in the central zone. Upper drawings are adapted from an image on Understanding Prostate Cancer website. PIN, prostatic intraepithelial neoplasia.
Inflammation and prostate cancer: the role of PIA
Histologically, most lesions that contain either acute or chronic inflammatory infiltrates in the prostate are associated with atrophic epithelium or focal epithelial atrophy16–18. Perhaps correspondingly, focal areas of epithelial atrophy are common in the ageing prostate16,19, and often encompass a large fraction of the peripheral zone, where atrophy most often occurs19,20. Compared with normal epithelium, there is an increased fraction of epithelial cells that proliferate in focal atrophy lesions18,21,22, and we have proposed the term proliferative inflammatory atrophy (PIA) for most of these atrophic lesions18,23. Not all focal prostate atrophy lesions show increased inflammatory cells, and for these the term proliferative atrophy might be used. In morphological studies we and others have observed transitions between atrophic epithelium and adenocarcinoma16,24,25, and frequent transitions between areas of PIA and/or proliferative atrophy with high grade prostatic intraepithelial neoplasia (PIN)18,26. Although there is evidence for somatic genetic changes in PIA and proliferative atrophy, it seems from the studies published so far that most PIA and proliferative atrophy lesions do not harbour clonal genetic alterations (BOX 2). Tissue samples from patients with benign prostate hyperplasia (BPH), which occurs in the transition zone of the prostate (FIG. 1), have areas with markedly increased numbers of chronic inflammatory cells. In these areas, in almost all cases, the epithelium seems to be atrophic, indicating that these regions can be considered PIA of the transition zone.
Box 2 | Somatic genomic alterations in PIA and proliferative atrophy.
In terms of somatic DNA alterations, although normal appearing epithelium (even from cancer patients) does not contain methylated glutathione S-transferase P1 (GSTP1) alleles, approximately 6% of focal atrophy lesions contain epithelial cells with methylated GSTP1 (REF. 25). Another group found mutated p53 (REF. 140) and androgen receptor alleles141 in post atrophic hyperplasia (a form of focal atrophy), prostatic intraepithelial neoplasia (PIN) and carcinoma, but not in normal prostate tissue — albeit these mutations in post atrophic hyperplasia were apparently non-clonal.
Others have used fluorescent in situ hybridization (FISH) to show that there are increases in chromosome 8 centromere signals142,143, loss of chromosome 8p143,144 and a gain of chromosome 8q24 in focal atrophy (REF. 143), indicating that chromosomal abnormalities similar to those found in PIN and carcinoma occur in a subset of these atrophic lesions. However, there were no atrophy cases in which clonal alterations were identified. Consistent with this, we recently found no evidence for clonal alterations indicative of chromosome 8 centromeric region gain, 8p loss or 8q24 gain in focal prostate atrophy, and infrequent 8p loss and absent 8q24 gain in PIN27. Therefore, the non-clonal mutations and non-clonal chromosomal alterations are probably indicative of genomic damage and/or the emergence of genomic instability in proliferative inflammatory atrophy (PIA) and proliferative atrophy.
In addition to the molecular evidence, other evidence to indicate that PIA and proliferative atrophy represent a field effect in the prostate is that these lesions are often quite extensive in the peripheral zone, often merge directly with high-grade PIN, at times merge directly with small carcinoma lesions and can be directly induced in the rodent prostate by exposures to the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP).
Several key molecular pathways involved in prostate cancer have also been shown to be altered in PIA lesions (BOX 3). For example, the protein products of three prostate tumour-suppressor genes: NKX3.1 (REF. 27), CDKN1B, which encodes p27 (REFS 18,23), and phosphatase and tensin homologue (PTEN) (A.M.D. and D. Faith, unpublished observations) are all downregulated in focal atrophy lesions. These genes are highly expressed in normal prostate epithelium, and frequently decreased or absent in PIN and prostate cancer. In addition, one allele of their corresponding genetic loci is frequently deleted in carcinomas (TABLE 1), and forced overexpression of each of these genes causes decreased growth of prostate cancer cells in culture. Finally, animal models with targeted disruption of either one or two alleles of the corresponding mouse genes develop prostate hyperplasia, PIN and/or invasive carcinoma28.
Box 3 | Molecular pathways altered in PIA and proliferative atrophy.
In terms of molecular modes of action, p27 functions as an inhibitor of cell-cycle progression by inhibiting the activity of cyclin–cyclin dependent kinase complexes in the nucleus. Interestingly, p27 levels are generally reduced but not absent in human proliferative inflammatory atrophy (PIA), prostatic intraepithelial neoplasia (PIN) and prostate cancer23,145–147. The fact that p27 levels are not lost entirely (or biallelically inactivated by mutations) in cancer might be explained by recent findings that indicate that cytoplasmic p27 levels, which are increased by signalling through the MET receptor tyrosine kinase, are required for cell migration in response to hepatocyte growth factor signalling through MET and in response to increased cyclin D1 levels148. Therefore, although high levels of nuclear p27 can prevent cell-cycle progression, cytoplasmic p27 might be required for optimal tumour cell motility, which is a key feature of malignant transformation and tissue repair.
Phosphatase and tensin homologue (PTEN) is a dual protein and lipid phosphatase that is responsible for the dephosphorylation and inactivation of phosphatidylinositol 3,4,5-trisphosphate (PIP3), a second messenger produced after the activation of PIP3 kinase in response to the ligation of several growth factor receptors, including the insulin-like growth factor 1 receptor (IGF1R)149. PIP3 is required for the activation of the protein kinase AKT. AKT activation results in the inhibition of apoptosis and/or increased cell proliferation through several different effector mechanisms, such as the activation of mammalian target of rapamycin (mTOR) and S6 kinase.
NKX3.1 is a prostate-restricted homeodomain protein encoded within a region of chromosome 8p21 that often contains single copy deletions in prostate cancer150. In addition to suppressing the growth of prostate cells, decreased NKX3.1 protein levels result in increased oxidative DNA damage151.
PIA and proliferative atrophy also show increased BCL2 protein expression18, a gene product that is a potent suppressor of apoptosis. Other gene products that are increased in PIA and proliferative atrophy include those that are induced by oxidant and electrophilic stress, or by signals associated with cell activation and proliferation, including glutathione S-transferase P1 (GSTP1), GSTA1, cyclooxygenase 2 (PTGS2) and p16 (REFS 18, 152, 153). The fact that these stressed cells are undergoing tissue repair is supported by the finding that several proteins known to be involved in tissue repair and cell motility, such as MET23 and HAI1 (REF. 154), have increased expression in PIA and proliferative atrophy.
What is the source of prostatic inflammation?
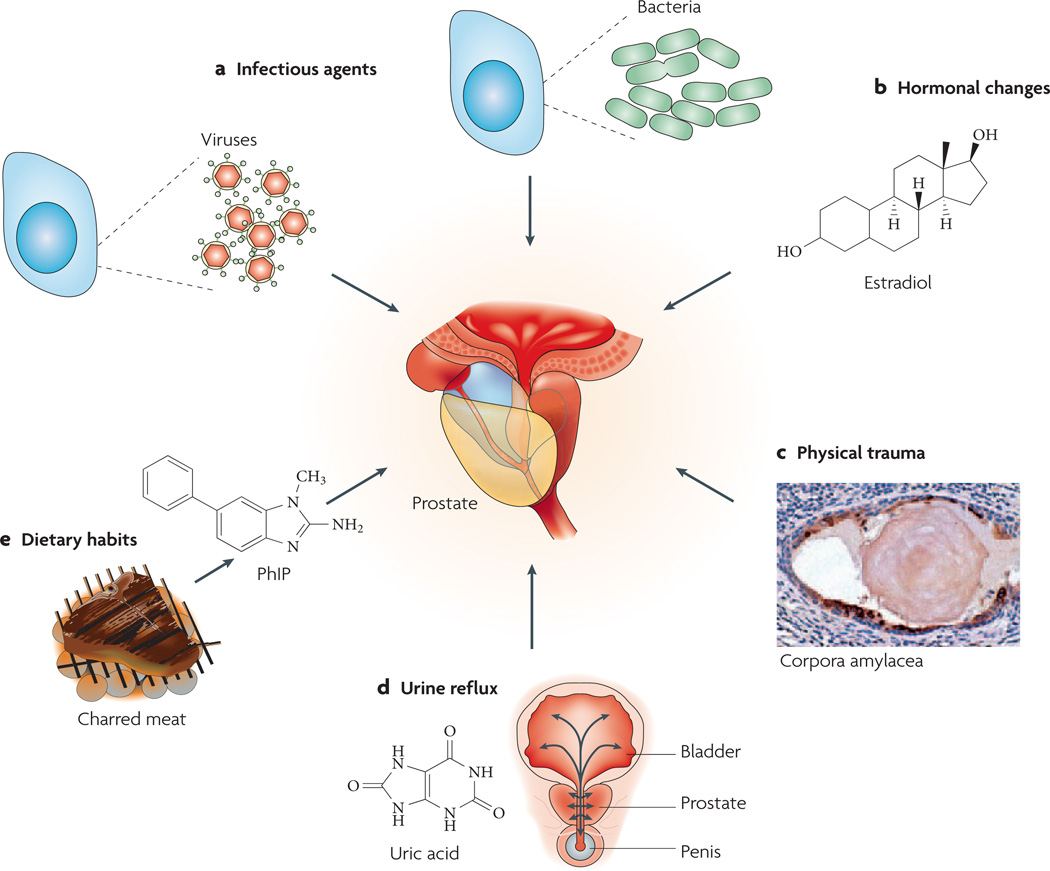
In most cases, the cause of prostatic inflammation is unclear. Various potential sources exist for the initial inciting event, including direct infection, urine reflux inducing chemical and physical trauma, dietary factors, oestrogens, or a combination of two or more of these factors (FIG. 2). Furthermore, any of these could lead to a break in immune tolerance and the development of an autoimmune reaction to the prostate.
Figure 2. Possible causes of prostate inflammation.
a | Infection. Chronic bacterial prostatitis is a rare recurring infection in which pathogenic bacteria are cultured from prostatic fluid. Viruses, fungi, mycobacteria and parasites can also infect the prostate and incite inflammation. The figure represents two prostate cells infected either by bacteria or viruses. b | Hormones. Hormonal alterations such as oestrogen exposure at crucial developmental junctures can result in architectural alterations in the prostate that produce an inflammatory response. c | Physical trauma. Corpora amylacea can traumatize the prostate on a microscopic level. The figure shows a corpora within a prostatic acinus in which its edges appear to be eroding the epithelium, resulting in an increase in expression of the stress enzyme cyclooxygenase 2 (PTGS2), represented by brown immunostaining. Prostate cell nuclei are visible in violet following haematoxylin staining. d | Urine reflux. Urine that travels up back towards the bladder (‘retrograde’ movement) can penetrate the ducts and acini of the prostate. Some compounds, such as crystalline uric acid, can directly activate innate inflammatory cells. Although these compounds would not be expected to traverse the prostate epithelium, if the epithelium was already damaged this would facilitate the leakage of these compounds into the stromal space where they would readily activate inflammatory cells. e | Dietary habits. Ingested carcinogens (for example 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), which derives from charred meat) can reach the prostate through the bloodstream or by urine reflux and cause DNA damage and mutations, and result in an influx of inflammatory cells.
Infectious agents
Many different pathogenic organisms have been observed to infect and induce an inflammatory response in the prostate. These include sexually transmitted organisms, such as Neisseria gonorrhoeae29, Chlamydia trachomatis30, Trichomonas vaginalis31 and Treponema pallidum32, and non-sexually transmitted bacteria such as Propionibacterium acnes33 and those known to cause acute and chronic bacterial prostatitis, primarily Gram-negative organisms such as Escherichia coli34. Although each of these pathogens has been identified in the prostate, the extent to which they typically infect this organ varies. For example, T. pallidum is a very rare cause of granulomatous prostatitis32, which is itself a rare pattern of prostate inflammation. In the pre-antibiotic era before 1937, a large proportion of other sexually transmitted infections (STIs, predominantly gonorrhea) resulted in severe prostatic inflammation or prostatic abscess29. However, since the introduction of antibiotics this proportion has decreased dramatically, presumably owing to treatment before progression to the prostate. Despite this decline, asymptomatic infection and inflammation of the prostate can still occur. In their study of gonorrhea, Handsfield and colleagues35 cultured N. gonorrhoeae in expressed prostate fluid after urination in 93% of men with asymptomatic gonorrhea.
Viruses can also infect the prostate, and human papillomavirus (HPV), human herpes simplex virus type 2 (HSV2), cytomegalovirus (CMV) and human herpes virus type 8 (HHV8) have been detected in the prostate36–38. How frequently these agents infect the prostate, and whether they elicit an inflammatory response, is largely unknown. In conclusion, many different pathogens can infect the prostate. Whereas some of these are associated with inflammation, others have not been detected in association with inflammation. Because many additional bacterial sequences39, and now a new viral sequence40, can be found in prostate tissue in the absence of an ability to culture any of these organisms using traditional means, it is still possible that in analogy to H. pylori gastritis, researchers have missed a previously unidentified pathogen associated with most inflammatory lesions in the prostate.
Several epidemiological studies of STIs and prostate cancer have been undertaken (BOX 4). Adding weight to the argument for a link between inflammation and prostate cancer are data indicating that users of anti-inflammatory agents have a reduced risk of prostate cancer. Prospective and case–control studies, including a relatively small prospective analysis that we conducted, suggest a reduction of ~15–20% in the risk of prostate cancer in regular users of aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs) compared with non-users41,42,43; however, in a large study by Jacobs et al. the effect was seen only in long-term users44.
Box 4 | The epidemiology of STIs and prostate cancer.
Epidemiological studies of sexually transmited infections (STIs) and prostate cancer initially focused on gonorrhea and syphilis. Dennis and Dawson155 combined the results of these studies and estimated summary odds ratios (ORs) of 1.4 for the development of prostate cancer in patients with a history of any STI, 2.3 for a history of syphilis and 1.4 for a history of gonorrhea. Similar estimates were also calculated in a subsequent meta-analysis156. Another recent case–control study reported that a history of both gonorrhea and more than 25 previous sexual partners were associated with an increased risk of prostate cancer46. The significance of many of these studies is limited, as most were small case–control designs that may have been susceptible to selection, recall and interviewer bias. In terms of other STIs, we recently observed that men who carried antibodies against Trichomonas vaginalis had a higher risk of prostate cancer than men who did not, and the association was stronger in men who rarely used aspirin157.
In another inquiry we conducted a longitudinal study of young (median age <31) STI clinic patients by measuring serum prostate specific antigen (PSA) as a marker of prostate infection and damage. Men with an STI were more likely to have a ≥40% increase in serum PSA than men without an STI diagnosis (32% versus 2%, P <0.01)158. Increases in PSA levels were strongly suggestive of direct prostate involvement by the infectious agent, with resultant epithelial cell damage (either due to the organism itself or the inflammatory response to the organism) resulting in the release of PSA into the blood stream. As only about 32% of patients with acute STIs showed increased levels of PSA, it is apparent that either these agents do not always infect the prostate, or they do not illicit a strong inflammatory response that damages prostate tissues, or rapid antibiotic treatments prevent full-blown prostate involvement.
In terms of viral STIs and prostate cancer, Strickler and Goedert36 concluded that those studied to date are unlikely to contribute to prostate carcinogenesis, although they did suggest the possibility of a causal relationship between an as-yet unresearched and unidentified infectious agent and prostate cancer. Urisman and colleagues40 recently identified a novel γ-retrovirus in prostate tissues primarily from patients with germline RNASEL mutations. This intriguing finding is a proof of concept that specific infectious agents might persist in the prostate as a result of heritable changes in genes responsible for the clearance of these agents.
Although most studies investigating clinical prostatitis in relation to prostate cancer reported a positive association45,46, many of these studies might have been susceptible to detection bias. In our work, there was no association between clinical prostatitis and prostate cancer among men with an equal opportunity for prostate cancer screening by serum prostate specific antigen (PSA) testing, except in men diagnosed with cancer at a young age47. Although it is unclear why the effect was seen only in early-onset prostate cancer, it is possible that clinical prostatitis is associated with only a subset of prostate cancers that manifest at a relatively young age.
To determine whether inflammation is related to prostate cancer independent of clinical symptoms, it will be crucial to compare the patterns and extent of inflammation in prostate biopsy samples from men with and without carcinoma. As inflammation is so common in prostate specimens, these measurements will need to be quantitative, and will require large sample sizes. There is a US National Institutes of Health (NIH) consensus grading system48 for histological prostate inflammation, and we will be using this system in a nested case–control study to determine whether asymptomatic prostatic inflammation is associated with prostate cancer using needle biopsy specimens from the Prostate Cancer Prevention Trial (PCPT)49. This trial is a large (approximately 18,000 men) study that was carried out to determine whether the 5 α-reductase inhibitor, finasteride, could reduce the period prevalence of prostate cancer. We will measure the pattern and extent of prostate inflammation and relate these to the presence or absence of prostate cancer.
Urine reflux, chemical and physical trauma
Chemical irritation from urine reflux has been proposed as an aetiological agent for the development of chronic inflammation in the prostate50. Although urine contains many chemical compounds that might be toxic to prostate epithelium, uric acid itself might be particularly damaging51. In support of this, recent work has implicated crystalline uric acid as a ‘danger signal’ released from dying cells, and it has been shown to directly engage the caspase-1-activating NALP3 (cyropyrin) inflammasome present in cells of the innate immune system (primarily macrophages), resulting in the production of inflammatory cytokines that can increase the influx of other inflammatory cells52. In addition, urine reflux of injurious chemicals can function in conjunction with infectious agents to increase prostate inflammation. Another manner by which prostate inflammation might occur is the development of corpora amylacea53 (FIG 2). Corpora amylacea have been proposed to contribute to prostate inflammation54, persistent infection55 and prostate carcinogenesis56 because they are frequently observed adjacent to damaged epithelium and focal inflammatory infiltrates54. In terms of epidemiological research and corpora amylacea, few studies have been conducted, one of which observed a higher proportion of calculi in prostate tissue from patients with prostate cancer compared with patients with BPH56, whereas others observed no association57,58. In support of the concept that ‘flushing’ of the prostate might be beneficial, several studies have found that increased ejaculation frequency, which might determine the rate of intraluminal corpora amylacea formation and the contact time between chemical agents such as uric acid or other urinary carcinogens and prostatic epithelium59, is related to decreased prostate cancer incidence (REF. 60 and references therein). Spermatozoa have also been localized to prostate tissues, and the retrograde movement of sperm cells into the prostate has been found in association with inflammation54,61 and with PIA lesions61.
Dietary factors
Epidemiological studies have revealed a link between prostate cancer incidence and mortality and the consumption of red meat and animal fats62–64. One mechanism by which meats might stimulate cancer development could be related to the formation of hetero cyclic amines (HCAs)65. The exposure of laboratory rats to dietary 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)66 results in carcinomas of the intestine in both sexes, in the mammary gland in females and in the prostate in males65. Rodent prostates contain four different lobes that do not correspond anatomically to the zones of the human prostate, and PhIP induces cancer only in the ventral lobe of rats67. In a recent study we exposed laboratory rats to PhIP and found a similar increase in the mutation frequency in all lobes of the prostate, yet the ventral lobe selectively responded with increased cell proliferation and cell death68. Therefore, PhIP functions as both a lobe-specific classical ‘tumour initiator’ as well as a ‘tumour promoter’. We also found that only the ventral lobe showed an increase in stromal mast cells, and stromal and intraepithelial macrophages68. After 12 weeks of PhIP exposure, the ventral lobe developed widespread epithelial atrophy; later, PIN and intraductal carcinomas were observed to develop directly from the atrophic epithelium (A.M.D., Y.N. and W.G.N., unpublished observations). Others have recently reported similar findings, in that PhIP treatment was found to induce inflammation and atrophy before inducing PIN and intraductal cancers69. Although it is not yet known whether the lobe-specific increase in mast cells and macrophages has a role in the neoplastic process, mast cells have been shown to stimulate cancer formation in several animal models, probably as a result of the release of factors such as tumour necrosis factor-α (TNFα) and various proteases, which might have an important role in tumorigenesis70–72.
Oestrogens
Another line of research into the causes of prostate inflammation and prostate cancer is the study of oestrogenic exposures in the prostate. Oestrogens are strongly linked to autoimmune processes in women, who are much more predisposed to autoimmune diseases than men. Increased levels of oestrogens, whether from environmental or developmental exposures, have long been linked to the development of prostate cancer73,74. Oestrogens affect the growth and development of the prostate, and this occurs through indirect routes on the hypothalamic–pituitary–gonadal axis through prolactin, and also by direct effects mediated by oestrogen receptor-α (ERα), which is expressed primarily in the stroma, and oestrogen receptor-β(ERβ), which is expressed primarily in the epithelium73–76. Oestrogens given to neonatal rodents result in an ‘imprinted state’ or ‘developmental oestrogenization’ in which there are developmental defects, including a reduction in prostatic growth. This treatment also results in the development of lobe-specific inflammation, hyperplasia and dysplasia or PIN77,78. Virtually all of these effects are mediated through ERα79. Therefore, it is quite plausible that chronic inflammation in the adult human prostate might reflect an autoimmune reaction caused, at least in part, by oestrogens.
A break of immune tolerance to prostate antigens?
Another potential mechanism of self-perpetuating chronic inflammation in the prostate that could relate to all of the above-mentioned modes of prostate injury is that damaged prostate epithelial cells might release antigens that result in a break of the apparent immune ‘tolerance’ to the prostate. For example, many prostate antigens are not expressed until after puberty, when the gland undergoes androgen-stimulated growth and development. This is likely to result in a lack of physiological immune tolerance to these antigens. Therefore, when released during prostate injury, these antigens could prime an immune response resulting in a specific reaction to prostate-restricted antigens. Indeed, a T-cell immune response to PSA in patients with chronic prostatitis has been reported80.
In summary, many non-infectious mechanisms might lead to prostate epithelial cell and stromal damage. Injured cells are known to signal a ‘danger response’ that results in acute inflammation. Crystalline uric acid is particularly intriguing in this regard, as it directly interacts with a receptor that is part of a molecular pathway within innate immune cells that can potently stimulate inflammation. The fact that PhIP induces prostate inflammation and atrophy is also of great interest, as this might link diet to these processes in the prostate carcinogenesis pathway. Continuous exposure to the injurious agent can also set up the prostate for chronic inflammation that can lead to a sustained inflammatory response and cancer. Finally, all of these mechanisms of chronic epithelial injury might also result in a decreased barrier function, that could facilitate the growth of infectious agents that might further increase the inflammatory response, and allow toxic urinary metabolites into the prostatic interstitium, where they could further stimulate an inflammatory reaction. This is certainly an exciting area for continued research into the mechanisms of prostate carcinogenesis.
Immunobiology of prostate inflammation
The normal prostate, like all other organs, contains endogenous inflammatory cells consisting of scattered stromal and intraepithelial T and B lymphocytes81,82, macro phages and mast cells. However, most adult prostate tissues contain increased inflammatory infiltrates, albeit the extent and type of inflammation are variable (for a review, see REF. 83). In terms of the biology of the inflammatory cells and the nature of the immune response in the prostate, most of the work has focused on BPH tissues in comparison with samples from the normal transition zone, and sometimes with carcinoma samples that have occurred in this region. Steiner et al. have examined the immunophenotypic and biological properties of chronic inflammatory cells in BPH and normal prostate tissues84–86. They have shown that of the increased CD45+ cells (all leukocytes express CD45 and non-leukocytes do not), 70–80% of these are CD3+ T lymphocytes, whereas 10–15% are CD19+ or CD20+ B lymphocytes. Macrophage numbers were also increased in these inflammatory lesions. In terms of the phenotype of the T cells, there is a reversed CD8:CD4 ratio, such that most T cells present in the normal areas expressed CD8, but most T cells in the inflamed areas expressed CD4. In terms of T-cell receptors (TCRs), 90% of the cells represent ‘standard’ αβ T cells (which express TCRαβ), with less than 1% representing γδ T cells. Class II major histocompatability antigen (HLADR), which indicates whether T cells are ‘activated’ by antigen signalling, is present on approximately 40% of the CD3+ T cells, and many of these T cells expressed CD45RO, indicating that these are ‘antigen experienced’ T cells85. None of the T cells in the normal prostate epithelium showed evidence of either activation or of being antigen experienced T cells.
CD4+ T cell responses can be divided into several different types that are classified according to their cytokine profile. TH1 cells produce interferon-γ and TNFα, whereas TH2 cells produce interleukin 4 (IL4), IL5 and IL13. Regulatory T (TReg) cells, which can suppress adaptive T-cell responses and autoimmunity, are characterized by the expression of CD25 and the transcription factor FOXP3, and they secrete transforming growth factor-β (TGFβ). In BPH, Marberger’s group determined that the T-cell response is complex, in that although TH0 (T cells that do not express any of the indicated cytokines) and TH1 cells were predominant in the inflammatory lesions of BPH and in carcinoma, some features of a TH2 response were also present. Unfortunately, at this point similar experiments have not been performed in the other zones of the prostate, or in areas of focal atrophy or PIN of the peripheral zone. The need for further understanding in this area is crucial, as is illustrated by the findings that microbially-driven inflammation can lead to colon cancer in mice, and that the prior transfer of TReg cells that express CD4 and CD25 prevents the inflammatory response that leads to colon cancer in these animals87. Recently Miller et al.88, have shown that CD4+ and CD25+ T cells, with properties of TReg cells including the expression of the FOXP3 protein, are present in increased numbers in clinically localized prostate cancer tissues, compared with normal prostate tissues. Exciting new data from several groups suggest the importance of a new subset of CD4-effector T cells known as TH17 cells, which develop through distinct cytokine signals (especially IL23) with respect to those involved in TH1 and TH2 responses, and are characterized by the production of IL17 (REF. 89). These cells are required for inflammation in arthritis and encephalitis models89, and IL23 is required for skin cancer formation in response to carcinogen exposure in mice90. A potential role for TH17 cells in prostatic inflammation had already been demonstrated by Steiner et al. before the TH17 cell lineage had been recognized as being as distinct. They showed that activated T cells in BPH tissue and in prostate cancer express high levels of IL17 (REF. 85). Further work to more fully elucidate the phenotypic and biological properties of all T-cell subsets in the prostate is required before we can understand the significance of acquired cell-mediated immunity in prostate carcinogenesis. Methods such as the quantitative image analysis of immunohistochemically stained inflammatory cell subsets, as well as flow cytometry for these subsets using tissues isolated from histologically defined areas, will be crucial to obtain such data.
Inflammatory genes and prostate cancer risk
Through a variety of approaches, including family and twin studies and segregation analyses, an important role for an inherited component of prostate cancer risk has been documented (recently reviewed by Schaid91). These studies have set the stage for efforts to identify prostate cancer susceptibility genes using linkage analysis and, more recently, association-based approaches. Despite strong evidence for a genetic component to prostate cancer risk, few reliable genetic risk factors for prostate cancer have been identified. In this section we will focus on a relatively new area of investigation in this field: the possibility that allelic variants of genes involved in innate and acquired immunity play an important part in determining inherited prostate cancer risk. If chronic inflammation is indeed an important aetiological factor for prostate cancer, then allelic variants of the genes involved in inflammatory pathways are logical candidates for genetic determinants of prostate cancer risk. As a result of space limitations, we can only review what we consider the most well-studied examples to date.
RNASEL and MSR1
Following up genomic regions of interest identified by linkage studies of prostate cancer families, two genes involved in innate immunity unexpectedly emerged as candidate prostate cancer susceptibility genes. Inactivating mutations (E265X and M1I) in ribonuclease L (RNASEL) segregate with prostate cancer in two prostate cancer families: E265X with one of European descent and M1I with a family of African descent92,93. RNASEL, which is located at 1q25, is a component of the innate immune system that is required for the antiviral and antiproliferative roles of interferons94,95. Lymphoblasts from carriers of either one of the mutations mentioned above were found to be deficient in enzymatic RNase activity, although, other than prostate cancer, additional phenotypic manifestations were not obvious. Subsequent studies examining the role of RNASEL as a prostate cancer susceptibility gene have provided mixed evidence, some confirmatory91 and others not91,96–99. Although the association of this infection with prostate cancer development has yet to be shown40, carriers of a common, hypomorphic allele of RNASEL (R463Q) were found to be at risk for prostatic infection by a new γ-retrovirus. Interestingly, when RNASEL is activated in cells by its cognate interferon-inducible ligands, 2′,5′-linked oligoadenylates, mRNA species are consistently induced, one of which is encoded by the (macrophage-inhibitory cytokine 1) MIC1 gene, which is another prostate cancer susceptibility locus described below100.
The analysis of candidate genes in a different region of linkage (8p22) in prostate cancer families revealed several recurring, inactivating mutations in macrophage scavenger receptor 1 (MSR1)101. The MSR1 gene encodes a homotrimeric class A ‘scavenger receptor’, with expression largely restricted to macrophages. This receptor is capable of binding many ligands, including modified lipoproteins and both Gram-negative and Gram-positive bacteria. Mice with experimentally inactivated Msr1 are more susceptible to various types of bacterial infection102,103, although recent evidence suggests an anti-inflammatory role for this receptor, at least after exposure to certain pathogens104,105. MSR1 mutant alleles, R293X and H441R, which are found in several different prostate cancer families, code for proteins that can no longer bind bacteria or modified LDL (C.M. Ewing and W.B.I., unpublished observations). Despite the initial findings of an increased frequency of MSR1 mutations in men with prostate cancer, as with RNASEL, follow-up studies published on the possible role of MSR1 variants and prostate cancer risk have yielded inconsistent results98,101,106–111. A recent meta analysis suggests that MSR1 mutations might have a more reproducible effect on prostate cancer risk in African Americans112. Although these results do not indicate that these genes are major prostate cancer loci, they are consistent with these genes being able to modify prostate cancer risk, possibly in combination with particular environmental exposures — in this case, certain pathogens.
Toll-like receptors
The Cancer Prostate Sweden Study (CAPS) is a case–control study of prostate cancer in northern Sweden. The relative genetic homogeneity of the Swedish population and the large size of the CAPS study make it an ideal platform to identify genetic variants associated with prostate cancer risk. Studying cases and controls in CAPS over the past 3 years has led to the identification of several genes in inflammation-related pathways, including MIC1, interleukin 1 receptor antagonist (IL1RN) and members of the toll-like receptor (TLR) family, with allelic variants associated with prostate cancer risk.
As key players in innate immunity to pathogens, TLRs recognize pathogen-associated molecular patterns (PAMPs)113. The engagement of TLRs results in the production of various pro-inflammatory cytokines, chemokines and effector molecules, such as reactive oxygen and nitrogen intermediates, as well as upregulation of the expression of co-stimulatory CD86 and CD80 and major histocompatability complex II (MHC II) molecules, which facilitate adaptive immune responses. Ten members of the human TLR family have been identified, and for most of these, specific classes of ligands, typically microbial components or surrogates thereof, have been identified and characterized. Recently, sequence variants in several TLR genes have been linked to prostate cancer risk, including TLR4 and the TLR1–6–10 gene cluster114,115.
Ligands that are recognized by TLR4 include Gram-negative bacterial products, including lipopolysaccharide116, and human heat shock protein 60 (HSP60)117. In the CAPS study114, a single nucleotide polymorphism (SNP) in the 3′ UTR region of TLR4 (11381G/C) was found to be associated with prostate cancer risk. Carriers of the GC or CC genotypes of this SNP had a 26% increased risk of prostate cancer, and a 39% increased risk of early-onset prostate cancer (before the age of 65 years), compared with men with the wild-type GG genotype.
In a follow up study of a North American population, homozygosity for variant alleles of eight SNPs in TLR4 (REF. 118) was associated with a statistically significantly lower risk of prostate cancer; however, the TLR4_15844 polymorphism, which corresponds to 11381G/C implicated in the CAPS population, was not found to be associated with prostate cancer. Therefore, although both published studies of this gene indicate that genetic variants of TLR4 have a role in the development of prostate cancer, the specific variants responsible for this effect might vary across different populations.
The TLR1–6–10 cluster maps to 4p14, and encodes proteins that have a high degree of homology in their overall amino-acid sequences119. TLR6 and TLR1 recognize diacylated lipoprotein and triacylated lipoprotein as ligands, respectively120,121. However, no specific ligand has been identified for TLR10. The TLR1 and TLR6 proteins each form heterodimers with TLR2 to establish a combinational repertoire that distinguishes a large number of PAMPs120,122,123.
A study of the TLR1–6–10 cluster in prostate cancer patients in CAPS identified an association of sequence variants in TLR1–6–10 with prostate cancer risk114,115. The allele frequencies of 11 of the 17 SNPs examined in this gene cluster were significantly different between case and control subjects (P = 0.04–0.001), with odds ratios for variant allele carriers (homozygous or heterozygous) compared with wild-type allele carriers ranging from 1.20 (95% CI = 1.00–1.43) to 1.38 (95% CI = 1.12–1.70). Although further studies are necessary to understand the biological consequences of the risk variants in both TLR4 and the TLR1–6–10 cluster, the observation of prostate cancer risk associated with polymorphisms in this family of genes, which is so intimately related to innate immunity, indicates that inflammation-related processes are important in prostate cancer development.
MIC1
MIC1 is a member of the transforming growth factor-β (TGFβ) superfamily, and is thought to have an important role in inflammation by regulating macrophage activity. In a study of 1,383 patients with prostate cancer and 780 control subjects in CAPS, a significant difference (P = 0.006) in genotype frequency was observed for the non-synonymous change H6D between patients and controls124. Carriers of the GC genotype, which results in the H6D change, had a lower risk of sporadic prostate cancer (OR = 0.80, 95% CI = 0.66–0.97) and of familial prostate cancer (OR = 0.61, 95% CI = 0.42–0.89) than the CC genotype carriers. In the study population, the proportion of prostate cancer cases attributable to the CC genotype was 7.2% for sporadic cancer and 19.2% for familial cancer.
IL1RN
The protein product of the IL1RN gene belongs to the interleukin 1 cytokine family of proteins. Its primary function is as an inhibitor of the proinflammatory IL1α and IL1β. Lindmark et al. examined four haplotype-tagging SNPs (htSNPs) across the IL1RN gene in samples from patients with prostate cancer125. The most common haplotype (ATGC) was observed at a significantly higher frequency in the cases (38.7%) compared with the controls (33.5%) (P = 0.009). Carriers of the homozygous ATCG haplotype had significantly increased risk (OR = 1.6, 95% CI = 1.2–2.2). Furthermore, the association of this haplotype was even stronger among patients with advanced disease compared with controls125.
Other inflammatory-related genes
Many other genes in inflammatory pathways have been examined recently for a link to prostate cancer, generally with mixed results. For example, although McCarron et al. previously reported an association between certain alleles of IL10 and IL8 and prostate cancer126, Michaud et al. recently reported a lack of association of polymorphisms in the IL1β, IL6, IL8 and IL10 and prostate cancer in a case–control study of the Prostate, Lung, Colorectal, and Ovarian Cancer screening trial127. Further work is necessary to either confirm or refute the hypothesis that variants in genes associated with inflammation affect prostate cancer risk, and if confirmed, to understand the mechanisms that link allelic variation in inflammation genes and prostate cancer.
SNPs and the inflammatory pathway
In a more global genome-wide approach, Zheng et al.128 proposed that sequence variants in many other genes in the inflammatory pathway might be associated with prostate cancer. They evaluated 9,275 SNPs in 1,086 genes of the inflammation pathway among 200 familial cases and 200 unaffected controls selected from the CAPS study population. They found that more than the expected numbers of SNPs were significant at a nominal P value of 0.01, 0.05 and 0.1, providing overall support for the hypothesis. A small subset of significant SNPs (N = 26) were selected and genotyped in an independent sample of ~1,900 members of the CAPS population. Among the 26 SNPs, six were significantly associated with prostate cancer risk (P ≤ 0.05). These results are consistent with the idea that variation in many genes in inflammatory pathways might affect the likelihood of developing prostate cancer.
Ideally, one would prefer to correlate the presence of specific genetic polymorphisms with the pattern and extent of intraprostatic inflammation, yet in all of the studies reported above the status of the prostate in men in terms of presence, pattern and extent of inflammation is unknown. Future studies that address these issues will be crucial in evaluating the biological effects of various polymorphisms in inflammatory pathway genes.
The ‘injury and regeneration’ hypothesis
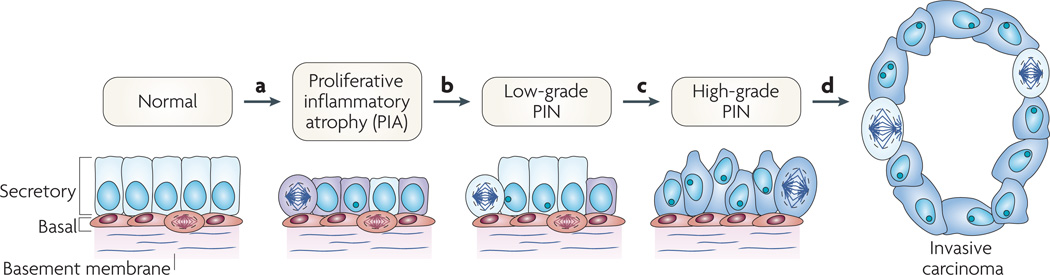
Our current working model (FIG. 3) suggests that repeated bouts of injury (and cell death) to the prostate epithelium occur, either as a result of oxidant and/or nitrosative damage from inflammatory cells in response to pathogens or autoimmune disease, from direct injury from circulating carcinogens and/or toxins derived from the diet or from urine that has refluxed into the prostate. The morphological manifestation of this injury is focal atrophy or PIA, which we postulate to be a signature of the ‘field effect’ of prostate carcinogenesis. The biological manifestations are an increase in proliferation and a massive increase in epithelial cells that possess a phenotype intermediate between basal cells and mature luminal cells5,6,23. In a small subset of cells, perhaps cells with an intermediate phenotype that contain at least some ‘stem cell’ properties, somatic genome alterations occur, such as cytosine methylation within the CpG island of the GSTP1 gene and telomere shortening. Both of these molecular changes can decrease the ‘caretaker’ phenotype and increase genetic instability that might then initiate high-grade PIN and early prostate cancer formation. In the setting of ongoing inflammatory and dietary insults in cells with compromised caretaker functions, additional changes such as gene rearrangements resulting in the activation of the ETS family of oncogenic transcription factors, the activation of MYC expression and the loss of tumour-suppressor genes such as PTEN, NKX3.1 and CDKN1B occur that drive tumour progression.
Figure 3. Cellular and molecular model of early prostate neoplasia progression.
a | This stage is characterized by the infiltration of lymphocytes, macrophages and neutrophils (caused either by repeated infections, dietary factors and/or by the onset of autoimmunity); phagocytes release reactive oxygen and nitrogen species causing DNA damage, cell injury and cell death, which trigger the onset of epithelial cell regeneration. The morphological manifestation of the cellular injury is focal prostate atrophy, which is proposed to signify the ‘field effect’ in the prostate. The downregulation of p27, NKX3.1 and phosphatase and tensin homologue (PTEN) proteins in luminal cells stimulates cell-cycle progression. Stress-response genes are induced (such as glutathione S-transferase P1 (GSTP1), GSTA1 and cyclooxygenase 2 (PTGS2)). b | The subsequent silencing of GSTP1 through promoter methylation in subsets of cells further facilitates oxidant-mediated telomere shortening. c | Cells carrying methylated GSTP1 alleles and short telomeres have dysfunctional telomeres and are more likely to bypass the senescence checkpoints. This favours the onset of genetic instability and the consequent accumulation of genetic changes (for example loss of heterozygosity on 8p21,6q or gain of function on 8q24,17q). d | The continued proliferation of genetically unstable luminal cells and the further accumulation of genomic changes, such as gene rearrangements leading to TMPRSS2–ETS family member gene fusions, lead to progression towards invasive carcinomas. PIN, prostatic intraepithelial neoplasia.
Future directions
We reviewed evidence that in men with an underlying genetic predisposition, prostate cancer might be caused by inflammation possibly coupled with dietary factors. However, additional work needs to be done to determine whether the mechanisms proposed are correct. First, we need an improved ability to diagnose and define clinical ‘prostatitis’. Second, we need studies that quantify asymptomatic inflammation in the prostate to determine the relationship between the development of prostatic inflammation and the following: age, genotype, response to specific infectious organisms, and diet. We need an improved understanding of the types of inflammatory cells and their biological properties in the normal prostate and in the various lesions such as PIA, BPH, PIN and carcinoma. Another potential avenue for future studies is to couple improvements in imaging of the prostate, including new strategies to image inflammation and atrophy, to studies aimed at quantifying various types of inflammation in prostate biopsy specimens and quantitative analyses of cytokine profiles and inflammatory cell types in prostate fluid. These studies should also be performed in conjunction with experiments designed to identify specific infectious organisms. It will be crucial in these studies to have both the genetic information and the dietary and medical history data to correlate with the immunobiological data. Improvements in our understanding of the key molecular genetic and epigenetic events that drive prostate carcinogenesis, and the identification of the precise cell types involved (that is, whether prostate epithelial stem cells or their progeny are directly transformed) need to be applied to presumed precursor lesions to define precisely the order of events in the development of early prostate cancer. As animal models of prostate cancer continue to be developed that mimic the human disease, such as those that activate MYC or inactivate PTEN, CDKN1B or NKX3.1 (REFS 28,129), strategies for determining whether infectious agents and/or specific activated inflammatory cells are required for prostate carcinogenesis also need to be developed. Examples of such studies include crossing mice that are genetically engineered to develop prostate cancer with mice that lack specific subsets of cells of the innate and adaptive immune systems, to determine the contribution of such cells to the transformation process. In other studies, one can target inflammation to the prostate by using transgenic technologies to overexpress chemokines and/or cytokines that attract inflammatory cells to the prostate or that will activate inflammatory cells that are already resident in the prostate. As rodents are quite resistant to prostate cancer development, these studies would be potentially more informative if they were carried out on genetically altered animals already prone to developing early neoplastic lesions in the prostate.
Inflammation is a very complex process, which involves hundreds of genes. Therefore, there are many genes in the inflammatory pathways that might contribute to the development of prostate cancer. Whereas many genes in the inflammatory pathway have been shown to harbour sequence variants that may or may not be associated with increased risk of prostate cancer, larger studies in different study populations are needed to confirm and more thoroughly characterize the associations discussed above. Traditional association tests that examine one gene at a time remain valuable approaches, but they are fast being supplanted by new approaches that provide an efficient and economically feasible way to study virtually all of the genes in the whole pathway. Technologies using bead-based or chip-based arrays allow for the rapid examination of thousands of SNPs among hundreds of genes, and even genome-wide searches assessing all genes. Such approaches will provide a comprehensive evaluation of genes in inflammatory pathways, and will provide an appropriate perspective of the importance of genes in these pathways in the context of all known cellular pathways. Although the data obtained so far using genome-wide scans are promising, the challenges of such studies are significant, as many associations are expected to occur by chance. Only when specific associations are validated in multiple large cohorts will the scientific community have confidence in the purported findings from such studies. Although it is currently unknown whether or not 8q24 is related to inflammatory pathways, one very recent example of a success story stems from a set of independent studies that implicated this chromosome region in prostate cancer occurring in both families and in sporadic cases130,131.
At a glance.
Prostate cancer is the most common form of non-skin cancer in men in developed countries. The cause(s) of prostate cancer have not yet been clarified. Although heritable factors are implicated, immigration studies indicate that environmental exposures are also important.
Chronic infection and inflammation cause cancer in several organs including the stomach, liver and large intestine. Data from histopathological, molecular histopathological, epidemiological and genetic epidemiological studies show that chronic inflammation might also be important in prostate carcinogenesis.
The source of intraprostatic inflammation is often unknown, but might be caused by infection (for example, with sexually transmitted agents), cell injury (owing to exposure to chemical and physical trauma from urine reflux and prostatic calculi formation), hormonal variations and/or exposures, or dietary factors such as charred meats. The resultant epithelial cellular injury might cause a loss of tolerance to normal prostatic antigens, resulting in a self-perpetuating autoimmune reaction.
-
Exposures to infectious agents and dietary carcinogens are postulated to directly injure the prostate epithelium, resulting in the histological lesions known as proliferative inflammatory atrophy (PIA), or proliferative atrophy. These lesions are postulated to be a manifestation of the ‘field effect’ caused by environmental exposures.
Despite a strong genetic component to prostate cancer risk, no highly penetrant hereditary prostate cancer genes have been uncovered to date. Although complex, genetic variation in inflammatory genes is associated with prostate cancer risk.
Several challenges remain regarding the inflammation hypothesis in prostate cancer, including the determination of the cause(s) of chronic inflammation in the prostate, an understanding of the cellular and molecular biology of the immune response in the prostate, whether inflammatory cells are truly causative in the process, and the determination of the target cell types within the proposed precursor lesions of prostate cancer.
The refinement and application of new epidemiological approaches, including high-throughput genetic epidemiology, improved rodent models of prostate inflammation and cancer, and advances in the application of molecular techniques to histopathological studies should provide insights into the cause of prostate inflammation and its relevance to prostate carcinogenesis.
Acknowledgements
The authors would like to thank Amelia K. Thomas for sketching the early concept designs for figure 2. Support was received from the Department of Defense Congressional Dir. Med. Research Program; The Public Health Services National Institutes of Health (NIH) and the National Cancer Institute, NIH and National Cancer Institute Specialized Programs of Research Excellence in Prostate Cancer, and philanthropic support from the Donald and Susan Sturm Foundation, B. L. Schwartz and R. A. Barry. A.M.D. is a Helen and Peter Bing Scholar through The Patrick C. Walsh Prostate Cancer Research Fund.
Glossary
- Prostatic intraepithelial neoplasia
A lesion characterized by cells with neoplastic features, which line pre-existing acini and ducts. PIN represents the most likely precursor to many prostate cancers.
- Benign prostatic hyperplasia
Non-cancerous enlargement consisting of excess glands and stroma affecting the transition zone of the prostate.
- Urine reflux
During urination, urine flows from the bladder through the prostatic urethra and into the penile urethra. Urine reflux occurs when urine flows inadvertently into the prostatic ducts, permeating large portions of the prostatic acini.
- Prostatitis
Technically means ‘inflammation of the prostate’. However, it is usually referred to as a clinical syndrome largely characterized by pelvic pain that has several subtypes. Some symptomatic subtypes (I and II) are associated with bacterial infections, others with inflammation but no infection (IIIa), or no inflammation and no infection (IIIb). Type IV consists of chronic inflammation without clinical symptoms.
- Expressed prostate fluid
Secretions obtained following prostate massage after digital rectal examination.
- Prostate specific antigen
A polypeptide that is expressed at very high levels prostate epithelial cells, whereas very low levels are detected in normal serum; however, several pathological conditions such as prostate cancer, prostate inflammation and benign prostatic hyperplasia can result in increased serum PSA levels.
- Inflammasome
A multiprotein intracytoplasmic complex that activates pro-inflammatory caspases, which then cleave the precursor of interleukin-1β (pro-IL1β) into the active form, leading to a potent inflammatory response.
- Corpora amylacea
Amorphous small nodules or concretions located in the lumen of benign prostate acini and ducts that accumulate with age.
- Heterocyclic amines
Molecules that are produced as a result of cooking meats at high temperatures, and which can be metabolized to biologically active, DNA-damaging agents. 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) is the most abundant HCA.
- FOXP3
A nuclear transcription factor that is expressed specifically in regulatory T cells.
- Odds ratio
The odds ratio is a way of comparing whether the probability of a certain event is the same for two groups, and is calculated using a 2×2 table. An odds ratio of one implies that an event is equally likely in both groups. An odds ratio greater than one implies that an event is more likely in the first group. An odds ratio less than one implies that the event is less likely in the first group.
- Longitudinal study
A study in which repeated observations of a set of subjects are made over time with respect to one or more study variables.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
DATABASES
The following terms in this article are linked online to:
Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene CDKN1B | CD3 | CD4 | CD8 | CD19 | CD20 | CD45 | CD80 | CD86 | ERα | ERβ | FOXP3 | GSTP1 | HSP60 | IL1RN | interferon-γ | IL4 | IL5 | IL13 | IL17 | IL23 | MIC1 | MSR1 | MYC | NKX3.1 | PTEN | RNASEL | TLR1 | TLR4 | TLR6 | TLR10 | TNFα
FURTHER INFORMATION
Angelo M. De Marzo’s homepage: http://demarzolab.pathology.jhmi.edu
Karolinska Institutet: http://ki.se/meb
Understanding Prostate Cancer: http://studentweb.usq.edu.au/home/q9210374/site/index.html
Access to this links box is available online.
References
- 1.Jemal A, et al. Cancer statistics, 2005. CA Cancer J. Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2. Ames BN, Gold LS, Willett WC. The causes and prevention of cancer. Proc. Natl Acad. Sci. USA. 1995;92:5258–5265. doi: 10.1073/pnas.92.12.5258. This paper describes the main environmental causes of cancer and the molecular mechanisms by which they function.
- 3.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ACS. Cancer Facts and FIGS 2005. American Cancer Society. 2005:1–64. [Google Scholar]
- 5.De Marzo AM, et al. Pathological and molecular mechanisms of prostate carcinogenesis: implications for diagnosis, detection, prevention, and treatment. J. Cell Biochem. 2004;91:459–477. doi: 10.1002/jcb.10747. [DOI] [PubMed] [Google Scholar]
- 6.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N. Engl. J. Med. 2003;349:366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 7.Platz EA, De Marzo AM. Epidemiology of inflammation and prostate cancer. J. Urol. 2004;171:S36–S40. doi: 10.1097/01.ju.0000108131.43160.77. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalgo ML, Isaacs WB. Molecular pathways to prostate cancer. J. Urol. 2003;170:2444–2452. doi: 10.1097/01.ju.0000085381.20139.b6. [DOI] [PubMed] [Google Scholar]
- 9.Shand RL, Gelmann EP. Molecular biology of prostate-cancer pathogenesis. Curr. Opin. Urol. 2006;16:123–131. doi: 10.1097/01.mou.0000193384.39351.64. [DOI] [PubMed] [Google Scholar]
- 10.Pihan GA, Wallace J, Zhou Y, Doxsey SJ. Centrosome abnormalities and chromosome instability occur together in pre-invasive carcinomas. Cancer Res. 2003;63:1398–1404. [PubMed] [Google Scholar]
- 11.Meeker AK. Telomeres and telomerase in prostatic intraepithelial neoplasia and prostate cancer biology. Urol. Oncol. 2006;24:122–130. doi: 10.1016/j.urolonc.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Bostwick DG. In: Urologic Surgical Pathology. Bostwick DG, Eble JN, editors. Mosby: St. Louis; 1997. pp. 423–456. [Google Scholar]
- 13.Hsing AW, Tsao L, Devesa SS. International trends and patterns of prostate cancer incidence and mortality. Int. J. Cancer. 2000;85:60–67. doi: 10.1002/(sici)1097-0215(20000101)85:1<60::aid-ijc11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Peto J. Cancer epidemiology in the last century and the next decade. Nature. 2001;411:390–395. doi: 10.1038/35077256. [DOI] [PubMed] [Google Scholar]
- 15.McNeal JE, Redwine EA, Freiha FS, Stamey TA. Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread. Am. J. Surg. Pathol. 1988;12:897–906. doi: 10.1097/00000478-198812000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Franks LM. Atrophy and hyperplasia in the prostate proper. J. Pathol. Bacteriol. 1954;68:617–621. doi: 10.1002/path.1700680234. [DOI] [PubMed] [Google Scholar]
- 17. McNeal JE. In: Histology for Pathologists. Sternberg SS, editor. Philadelphia: Lippincott-Raven; 1997. pp. 997–1017. This book chapter describes in detail the now well established zonal anatomy of the prostate.
- 18.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am. J. Pathol. 1999;155:1985–1992. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rich AR. On the frequency of occurrence of occult carcinoma of the prostate. J. Urol. 1934;33:215–223. [Google Scholar]
- 20.McNeal JE. Normal histology of the prostate. Am. J. Surg. Pathol. 1988;12:619–633. doi: 10.1097/00000478-198808000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Feneley MR, Young MP, Chinyama C, Kirby RS, Parkinson MC. Ki-67 expression in early prostate cancer and associated pathological lesions. J. Clin. Pathol. 1996;49:741–748. doi: 10.1136/jcp.49.9.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruska KM, Sauvageot J, Epstein JI. Histology and cellular kinetics of prostatic atrophy. Am. J. Surg. Pathol. 1998;22:1073–1077. doi: 10.1097/00000478-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 23.van Leenders GJ, et al. Intermediate cells in human prostate epithelium are enriched in proliferative inflammatory atrophy. Am. J. Pathol. 2003;162:1529–1537. doi: 10.1016/S0002-9440(10)64286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montironi R, Mazzucchelli R, Scarpelli M. Precancerous lesions and conditions of the prostate: from morphological and biological characterization to chemoprevention. Ann. NY Acad. Sci. 2002;963:169–184. doi: 10.1111/j.1749-6632.2002.tb04108.x. [DOI] [PubMed] [Google Scholar]
- 25.Nakayama M, et al. Hypermethylation of the human GSTP1 CpG island is present in a subset of proliferative inflammatory atrophy lesions but not in normal or hyperplastic epithelium of the prostate: a detailed study using Laser-Capture Microdissection. Am. J. Pathol. 2003;163:923–933. doi: 10.1016/s0002-9440(10)63452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Putzi MJ, De Marzo AM. Morphologic transitions between proliferative inflammatory atrophy and high-grade prostatic intraepithelial neoplasia. Urology. 2000;56:828–832. doi: 10.1016/s0090-4295(00)00776-7. [DOI] [PubMed] [Google Scholar]
- 27.Bethel CR, et al. Decreased NKX3. 1 protein expression in focal prostatic atrophy, prostatic intraepithelial neoplasia and adenocarcinoma: association with Gleason score and chromosome 8p deletion. Cancer Res. 2006;66:10683–10690. doi: 10.1158/0008-5472.CAN-06-0963. [DOI] [PubMed] [Google Scholar]
- 28.Abate-Shen C, Shen MM. Mouse models of prostate carcinogenesis. Trends Genet. 2002;18:S1–S5. doi: 10.1016/s0168-9525(02)02683-5. [DOI] [PubMed] [Google Scholar]
- 29.Pelouze PS. Gonorrhea in the male and female: a book for practitioners. Philadelphia: W. B. Saunders Company; 1935. [Google Scholar]
- 30.Poletti F, et al. Isolation of Chlamydia trachomatis from the prostatic cells in patients affected by nonacute abacterial prostatitis. J. Urol. 1985;134:691–693. doi: 10.1016/s0022-5347(17)47387-3. [DOI] [PubMed] [Google Scholar]
- 31.Gardner WA, Jr, Culberson DE, Bennett BD. Trichomonas vaginalis in the prostate gland. Arch. Pathol. Lab. Med. 1986;110:430–432. [PubMed] [Google Scholar]
- 32.Thomson L. Syphilis of the prostate. Am. J. Syphilis. 1920;4:323–341. [Google Scholar]
- 33.Cohen RJ, Shannon BA, McNeal JE, Shannon T, Garrett KL. Propionibacterium acnes associated with inflammation in radical prostatectomy specimens: a possible link to cancer evolution? J. Urol. 2005;173:1969–1974. doi: 10.1097/01.ju.0000158161.15277.78. [DOI] [PubMed] [Google Scholar]
- 34.Bushman W. In: Prostatic Diseases. Lepor H, editor. Philadelphia: W. B. Saunders Company; 2000. pp. 550–557. [Google Scholar]
- 35.Handsfield HH, Lipman TO, Harnisch JP, Tronca E, Holmes KK. Asymptomatic gonorrhea in men. Diagnosis, natural course, prevalence and significance. N. Engl. J. Med. 1974;290:117–123. doi: 10.1056/NEJM197401172900301. [DOI] [PubMed] [Google Scholar]
- 36.Strickler HD, Goedert JJ. Sexual behavior and evidence for an infectious cause of prostate cancer. Epidemiol Rev. 2001;23:144–151. doi: 10.1093/oxfordjournals.epirev.a000781. [DOI] [PubMed] [Google Scholar]
- 37.Zambrano A, Kalantari M, Simoneau A, Jensen JL, Villarreal LP. Detection of human polyomaviruses and papillomaviruses in prostatic tissue reveals the prostate as a habitat for multiple viral infections. Prostate. 2002;53:263–276. doi: 10.1002/pros.10157. [DOI] [PubMed] [Google Scholar]
- 38.Samanta M, Harkins L, Klemm K, Britt WJ, Cobbs CS. High prevalence of human cytomegalovirus in prostatic intraepithelial neoplasia and prostatic carcinoma. J. Urol. 2003;170:998–1002. doi: 10.1097/01.ju.0000080263.46164.97. [DOI] [PubMed] [Google Scholar]
- 39.Riley DE, Berger RE, Miner DC, Krieger JN. Diverse and related 16S rRNA-encoding DNA sequences in prostate tissues of men with chronic prostatitis. J. Clin. Microbiol. 1998;36:1646–1652. doi: 10.1128/jcm.36.6.1646-1652.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Urisman A, et al. Identification of a novel Gammaretrovirus in prostate tumors of patients homozygous for R462Q RNASEL variant. PLoS Pathog. 2006;2:e25. doi: 10.1371/journal.ppat.0020025. This study used a new gene chip containing all known viral nucleic acids to identify a new virus in the prostate. Only men with inherited inactive RNASEL alleles were at a high risk of harbouring the virus.
- 41.Platz EA, et al. Nonsteroidal anti-inflammatory drugs and risk of prostate cancer in the Baltimore Longitudinal Study of Aging. Cancer Epidemiol. Biomarkers Prev. 2005;14:390–396. doi: 10.1158/1055-9965.EPI-04-0532. [DOI] [PubMed] [Google Scholar]
- 42.Mahmud S, Franco E, Aprikian A. Prostate cancer and use of nonsteroidal anti-inflammatory drugs: systematic review and meta-analysis. Br. J. Cancer. 2004;90:93–99. doi: 10.1038/sj.bjc.6601416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan JM, Feraco A, Shuman M, Hernandez-Diaz S. The epidemiology of prostate cancer — with a focus on nonsteroidal anti-inflammatory drugs. Hematol. Oncol. Clin. North Am. 2006;20:797–809. doi: 10.1016/j.hoc.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Jacobs EJ, et al. A large cohort study of aspirin and other nonsteroidal anti-inflammatory drugs and prostate cancer incidence. J. Natl Cancer Inst. 2005;97:975–980. doi: 10.1093/jnci/dji173. [DOI] [PubMed] [Google Scholar]
- 45.Dennis LK, Lynch CF, Torner JC. Epidemiologic association between prostatitis and prostate cancer. Urology. 2002;60:78–83. doi: 10.1016/s0090-4295(02)01637-0. [DOI] [PubMed] [Google Scholar]
- 46.Sarma AV, et al. Sexual behavior, sexually transmitted diseases and prostatitis: the risk of prostate cancer in black men. J. Urol. 2006;176:1108–1113. doi: 10.1016/j.juro.2006.04.075. [DOI] [PubMed] [Google Scholar]
- 47.Sutcliffe S, et al. Gonorrhea, syphilis, clinical prostatitis, and the risk of prostate cancer. Cancer Epidemiol. Biomarkers Prev. 2006;15:2160–2166. doi: 10.1158/1055-9965.EPI-05-0913. [DOI] [PubMed] [Google Scholar]
- 48.Nickel JC, et al. Consensus development of a histopathological classification system for chronic prostatic inflammation. BJU Int. 2001;87:797–805. doi: 10.1046/j.1464-410x.2001.02193.x. [DOI] [PubMed] [Google Scholar]
- 49.Feigl P, et al. Design of the Prostate Cancer Prevention Trial (PCPT) Control Clin. Trials. 1995;16:150–163. doi: 10.1016/0197-2456(94)00xxx-m. [DOI] [PubMed] [Google Scholar]
- 50.Kirby RS, Lowe D, Bultitude MI, Shuttleworth KE. Intra-prostatic urinary reflux: an aetiological factor in abacterial prostatitis. Br. J. Urol. 1982;54:729–731. doi: 10.1111/j.1464-410x.1982.tb13635.x. [DOI] [PubMed] [Google Scholar]
- 51.Persson BE, Ronquist G. Evidence for a mechanistic association between nonbacterial prostatitis and levels of urate and creatinine in expressed prostatic secretion. J. Urol. 1996;155:958–960. [PubMed] [Google Scholar]
- 52. Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. This paper presented genetic evidence that uric acid crystals can activate the inflammasome, and therefore produce a potent inflammatory response.
- 53.Drachenberg CB, Papadimitriou JC. Prostatic corpora amylacea and crystalloids: similarities and differences on ultrastructural and histochemical studies. J. Submicrosc. Cytol. Pathol. 1996;28:141–150. [PubMed] [Google Scholar]
- 54.Gardner WA, Bennett BD. In: Pathology and pathobiology of the urinary bladder and prostate. Weinstein RS, Garnder WA, editors. Baltimore: Williams and Wilkens; 1992. pp. 129–148. [Google Scholar]
- 55.Meares EM., Jr Infection stones of prostate gland. Laboratory diagnosis and clinical management. Urology. 1974;4:560–566. doi: 10.1016/0090-4295(74)90490-7. [DOI] [PubMed] [Google Scholar]
- 56.Joachim H. La lithiase prostatique peut-elle etre consideree comme un facteur cancerogene? Urologia (Treviso) 1961;28:1–11. [Google Scholar]
- 57.Cristol DS, Emmett JL. Incidence of coincident prostatic calculi, prostatic hyperplasia and carcinoma of prostate gland. JAMA. 1944;124:646–652. [Google Scholar]
- 58.Sondergaard G, Vetner M, Christensen PO. Prostatic calculi. Acta Pathol. Microbiol. Immunol. Scand. [A] 1987;95:141–145. doi: 10.1111/j.1699-0463.1987.tb00021_95a.x. [DOI] [PubMed] [Google Scholar]
- 59.Isaacs JT. Prostatic structure and function in relation to the etiology of prostatic cancer. Prostate. 1983;4:351–366. doi: 10.1002/pros.2990040405. [DOI] [PubMed] [Google Scholar]
- 60. Leitzmann MF, Platz EA, Stampfer MJ, Willett WC, Giovannucci E. Ejaculation frequency and subsequent risk of prostate cancer. JAMA. 2004;291:1578–1586. doi: 10.1001/jama.291.13.1578. This paper presents evidence that high ejaculation frequency, especially in young men, is related to reduced prostate cancer incidence, and therefore suggests that the ‘flushing’ of the prostate of harmful chemicals or infectious agents might reduce prostate cancer risk.
- 61.Chen X, Zhao J, Salim S, Garcia FU. Intraprostatic spermatozoa: zonal distribution and association with atrophy. Hum. Pathol. 2006;37:345–351. doi: 10.1016/j.humpath.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 62.Giovannucci E, et al. A prospective study of dietary fat and risk of prostate cancer. J. Natl Cancer Inst. 1993;85:1571–1579. doi: 10.1093/jnci/85.19.1571. [DOI] [PubMed] [Google Scholar]
- 63.Norrish AE, et al. Heterocyclic amine content of cooked meat and risk of prostate cancer. J. Natl Cancer Inst. 1999;91:2038–2044. doi: 10.1093/jnci/91.23.2038. [DOI] [PubMed] [Google Scholar]
- 64.Michaud DS, et al. A prospective study on intake of animal products and risk of prostate cancer. Cancer Causes Control. 2001;12:557–567. doi: 10.1023/a:1011256201044. [DOI] [PubMed] [Google Scholar]
- 65. Sugimura T, Wakabayashi K, Nakagama H, Nagao M. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004;95:290–299. doi: 10.1111/j.1349-7006.2004.tb03205.x. This paper reviews the intriguing discovery of highly mutagenic and carcinogenic compounds formed during the high-temperature cooking of meats.
- 66. Knize MG, Felton JS. Formation and human risk of carcinogenic heterocyclic amines formed from natural precursors in meat. Nutr. Rev. 2005;63:158–165. doi: 10.1111/j.1753-4887.2005.tb00133.x. This paper reviews the discovery and significance of PhIP as the most abundant of the heterocyclic amines produced by high-temperature cooking of meats.
- 67.Inaguma S, et al. High susceptibility of the ACI and spontaneously hypertensive rat (SHR) strains to 2-amino-1-methyl-6-phenylimidazo[4, 5-b]pyridine (PhIP) prostate carcinogenesis. Cancer Sc.i. 2003;94:974–979. doi: 10.1111/j.1349-7006.2003.tb01387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakai Y, Nelson WG, De Marzo AM. The dietary charred meat carcinogen 2-amino-1-methyl-6-phenylimidazo[4, 5b]pyridine (PhIP) acts as both an initiator and tumor promoter in the rat ventral prostate. Cancer Res. 2007;67:1378–1384. doi: 10.1158/0008-5472.CAN-06-1336. [DOI] [PubMed] [Google Scholar]
- 69.Borowsky AD, et al. Inflammation and atrophy precede prostatic neoplasia in a PhIP-induced rat model. Neoplasia. 2006;8:708–715. doi: 10.1593/neo.06373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 71.Choo-Kang BS, et al. TNF-blocking therapies: an alternative mode of action? Trends Immunol. 2005;26:518–522. doi: 10.1016/j.it.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 72.Araki Y, Andoh A, Fujiyama Y, Bamba T. Development of dextran sulphate sodium-induced experimental colitis is suppressed in genetically mast cell-deficient Ws/Ws rats. Clin. Exp. Immunol. 2000;119:264–269. doi: 10.1046/j.1365-2249.2000.01094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coffey DS. Similarities of prostate and breast cancer: Evolution, diet, and estrogens. Urology. 2001;57:31–38. doi: 10.1016/s0090-4295(00)00938-9. [DOI] [PubMed] [Google Scholar]
- 74.Harkonen PL, Makela SI. Role of estrogens in development of prostate cancer. J. Steroid Biochem. Mol. Biol. 2004;92:297–305. doi: 10.1016/j.jsbmb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 75.Gilleran JP, et al. The role of prolactin in the prostatic inflammatory response to neonatal estrogen. Endocrinology. 2003;144:2046–2054. doi: 10.1210/en.2002-0038. [DOI] [PubMed] [Google Scholar]
- 76.Huang L, Pu Y, Alam S, Birch L, Prins GS. Estrogenic regulation of signaling pathways and homeobox genes during rat prostate development. J. Androl. 2004;25:330–337. doi: 10.1002/j.1939-4640.2004.tb02796.x. [DOI] [PubMed] [Google Scholar]
- 77.Naslund MJ, Strandberg JD, Coffey DS. The role of androgens and estrogens in the pathogenesis of experimental nonbacterial prostatitis. J. Urol. 1988;140:1049–1053. doi: 10.1016/s0022-5347(17)41924-0. [DOI] [PubMed] [Google Scholar]
- 78.Huang L, Pu Y, Alam S, Birch L, Prins GS. The role of Fgf10 signaling in branching morphogenesis and gene expression of the rat prostate gland: lobe-specific suppression by neonatal estrogens. Dev. Biol. 2005;278:396–414. doi: 10.1016/j.ydbio.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 79.Prins GS, et al. Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor α: studies with αERKO and βERKO mice. Cancer Res. 2001;61:6089–6097. [PubMed] [Google Scholar]
- 80.Ponniah S, Arah I, Alexander RB. PSA is a candidate self-antigen in autoimmune chronic prostatitis/chronic pelvic pain syndrome. Prostate. 2000;44:49–54. doi: 10.1002/1097-0045(20000615)44:1<49::aid-pros7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 81.Theyer G, et al. Phenotypic characterization of infiltrating leukocytes in benign prostatic hyperplasia. Lab. Invest. 1992;66:96–107. [PubMed] [Google Scholar]
- 82.Bostwick DG, de la Roza G, Dundore P, Corica FA, Iczkowski KA. Intraepithelial and stromal lymphocytes in the normal human prostate. Prostate. 2003;55:187–193. doi: 10.1002/pros.10224. [DOI] [PubMed] [Google Scholar]
- 83.De Marzo AM. In: Prostate Cancer: Biology, Genetics and the New Therapeutics. Chung LWK, Isaacs WB, Simons JW, editors. Totawa, NJ: Humana Press; in the press. [Google Scholar]
- 84.Steiner GE, et al. The picture of the prostatic lymphokine network is becoming increasingly complex. Rev. Urol. 2002;4:171–177. [PMC free article] [PubMed] [Google Scholar]
- 85.Steiner GE, et al. Expression and function of pro-inflammatory interleukin IL-17 and IL-17 receptor in normal, benign hyperplastic, and malignant prostate. Prostate. 2003;56:171–182. doi: 10.1002/pros.10238. [DOI] [PubMed] [Google Scholar]
- 86.Steiner GE, et al. Cytokine expression pattern in benign prostatic hyperplasia infiltrating T cells and impact of lymphocytic infiltration on cytokine mRNA profile in prostatic tissue. Lab. Invest. 2003;83:1131–1146. doi: 10.1097/01.lab.0000081388.40145.65. [DOI] [PubMed] [Google Scholar]
- 87.Erdman SE, et al. CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am. J. Pathol. 2003;162:691–702. doi: 10.1016/S0002-9440(10)63863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miller AM, et al. CD4+ CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J. Immunol. 2006;177:7398–7405. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- 89. Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. This paper reviews the discovery and characterization of a new class of T cells responsible for some forms of autoimmunity and perhaps cancer formation in a number of systems.
- 90. Langowski JL, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. This paper shows the requirement for IL23 in carcinogen-induced skin cancers in animals, and that it functions by inhibiting tumour immune surveillance.
- 91.Schaid DJ. The complex genetic epidemiology of prostate cancer. Hum. Mol. Genet. 2004;13(Spec No 1):R103–R121. doi: 10.1093/hmg/ddh072. [DOI] [PubMed] [Google Scholar]
- 92. Smith JR, et al. Major susceptibility locus for prostate cancer on chromosome 1 suggested by a genome-wide search. Science. 1996;274:1371–1374. doi: 10.1126/science.274.5291.1371. This is the first report in which, using a genome-wide scanning approach, a major prostate cancer-susceptibility gene was identified.
- 93.Carpten J, et al. Germline mutations in the ribonuclease L gene in families showing linkage with HPC1. Nature Genet. 2002;30:181–184. doi: 10.1038/ng823. [DOI] [PubMed] [Google Scholar]
- 94.Silverman RH. Implications for RNase L in prostate cancer biology. Biochemistry. 2003;42:1805–1812. doi: 10.1021/bi027147i. [DOI] [PubMed] [Google Scholar]
- 95.Hassel BA, Zhou A, Sotomayor C, Maran A, Silverman RH. A dominant negative mutant of 2–5A-dependent RNase suppresses antiproliferative and antiviral effects of interferon. EMBO J. 1993;12:3297–3304. doi: 10.1002/j.1460-2075.1993.tb05999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wiklund F, et al. Genetic analysis of the RNASEL gene in hereditary, familial, and sporadic prostate cancer. Clin. Cancer Res. 2004;10:7150–7156. doi: 10.1158/1078-0432.CCR-04-0982. [DOI] [PubMed] [Google Scholar]
- 97.Maier C, et al. Mutation screening and association study of RNASEL as a prostate cancer susceptibility gene. Br. J. Cancer. 2005;92:1159–1164. doi: 10.1038/sj.bjc.6602401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rennert H, et al. Association of susceptibility alleles in ELAC2/HPC2, RNASEL/HPC1, and MSR1 with prostate cancer severity in European American and African American men. Cancer Epidemiol. Biomarkers Prev. 2005;14:949–957. doi: 10.1158/1055-9965.EPI-04-0637. [DOI] [PubMed] [Google Scholar]
- 99.Kotar K, Hamel N, Thiffault I, Foulkes WD. The RNASEL 471delAAAG allele and prostate cancer in Ashkenazi Jewish men. J. Med. Genet. 2003;40:e22. doi: 10.1136/jmg.40.3.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Malathi K, et al. A transcriptional signaling pathway in the IFN system mediated by 2’–5’-oligoadenylate activation of RNase L. Proc. Natl Acad. Sci. USA. 2005;102:14533–14538. doi: 10.1073/pnas.0507551102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu J, et al. Germline mutations and sequence variants of the macrophage scavenger receptor 1 gene are associated with prostate cancer risk. Nature Genet. 2002;32:321–325. doi: 10.1038/ng994. [DOI] [PubMed] [Google Scholar]
- 102.Gough PJ, Greaves DR, Gordon S. A naturally occurring isoform of the human macrophage scavenger receptor (SR-A) gene generated by alternative splicing blocks modified LDL uptake. J. Lipid. Res. 1998;39:531–543. [PubMed] [Google Scholar]
- 103.Peiser L, et al. The class A macrophage scavenger receptor is a major pattern recognition receptor for Neisseria meningitidis which is independent of lipopolysaccharide and not required for secretory responses. Infect. Immun. 2002;70:5346–5354. doi: 10.1128/IAI.70.10.5346-5354.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ozeki Y, et al. Macrophage scavenger receptor down-regulates mycobacterial cord factor-induced proinflammatory cytokine production by alveolar and hepatic macrophages. Microb. Pathog. 2006;40:171–176. doi: 10.1016/j.micpath.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 105.Cotena A, Gordon S, Platt N. The class A macrophage scavenger receptor attenuates CXC chemokine production and the early infiltration of neutrophils in sterile peritonitis. J. Immunol. 2004;173:6427–6432. doi: 10.4049/jimmunol.173.10.6427. [DOI] [PubMed] [Google Scholar]
- 106.Xu J, et al. Common sequence variants of the macrophage scavenger receptor 1 gene are associated with prostate cancer risk. Am. J. Hum. Genet. 2003;72:208–212. doi: 10.1086/345802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miller DC, et al. Germ-line mutations of the macrophage scavenger receptor 1 gene: association with prostate cancer risk in African-American men. Cancer Res. 2003;63:3486–3489. [PubMed] [Google Scholar]
- 108.Wang L, et al. No association of germline alteration of MSR1 with prostate cancer risk. Nature Genet. 2003;35:128–129. doi: 10.1038/ng1239. [DOI] [PubMed] [Google Scholar]
- 109.Seppala EH, et al. Germ-line alterations in MSR1 gene and prostate cancer risk. Clin. Cancer Res. 2003;9:5252–5256. [PubMed] [Google Scholar]
- 110.Hope Q, et al. Macrophage scavenger receptor 1 999C > T (R293X) mutation and risk of prostate cancer. Cancer Epidemiol. Biomarkers Prev. 2005;14:397–402. doi: 10.1158/1055-9965.EPI-04-0202. [DOI] [PubMed] [Google Scholar]
- 111.Lindmark F, et al. Analysis of the macrophage scavenger receptor 1 gene in Swedish hereditary and sporadic prostate cancer. Prostate. 2004;59:132–140. doi: 10.1002/pros.10367. [DOI] [PubMed] [Google Scholar]
- 112.Sun J, et al. Meta-analysis of association of rare mutations and common sequence variants in the MSR1 gene and prostate cancer risk. Prostate. 2006;66:728–737. doi: 10.1002/pros.20396. [DOI] [PubMed] [Google Scholar]
- 113.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 114.Zheng SL, et al. Sequence variants of toll-like receptor 4 are associated with prostate cancer risk: results from the CAncer Prostate in Sweden Study. Cancer Res. 2004;64:2918–2922. doi: 10.1158/0008-5472.can-03-3280. [DOI] [PubMed] [Google Scholar]
- 115.Sun J, et al. Sequence variants in Toll-like receptor gene cluster (TLR6-TLR1-TLR10) and prostate cancer risk. J. Natl Cancer Inst. 2005;97:525–532. doi: 10.1093/jnci/dji070. [DOI] [PubMed] [Google Scholar]
- 116.Poltorak A, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 117.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J. Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 118.Chen YC, et al. Sequence variants of Toll-like receptor 4 and susceptibility to prostate cancer. Cancer Res. 2005;65:11771–11778. doi: 10.1158/0008-5472.CAN-05-2078. [DOI] [PubMed] [Google Scholar]
- 119.Chuang T, Ulevitch RJ. Identification of hTLR10: a novel human Toll-like receptor preferentially expressed in immune cells. Biochim. Biophys. Acta. 2001;1518:157–161. doi: 10.1016/s0167-4781(00)00289-x. [DOI] [PubMed] [Google Scholar]
- 120.Takeuchi O, et al. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J. Immunol. 2002;169:10–14. doi: 10.4049/jimmunol.169.1.10. [DOI] [PubMed] [Google Scholar]
- 121.Yamamoto M, Takeda K, Akira S. TIR domain-containing adaptors define the specificity of TLR signaling. Mol. Immunol. 2004;40:861–868. doi: 10.1016/j.molimm.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 122.Takeuchi O, et al. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 2001;13:933–940. doi: 10.1093/intimm/13.7.933. [DOI] [PubMed] [Google Scholar]
- 123.Hajjar AM, et al. Cutting edge: functional interactions between toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J. Immunol. 2001;166:15–19. doi: 10.4049/jimmunol.166.1.15. [DOI] [PubMed] [Google Scholar]
- 124.Lindmark F, et al. H6D polymorphism in macrophage-inhibitory cytokine-1 gene associated with prostate cancer. J. Natl Cancer Inst. 2004;96:1248–1254. doi: 10.1093/jnci/djh227. [DOI] [PubMed] [Google Scholar]
- 125.Lindmark F, et al. Interleukin-1 receptor antagonist haplotype associated with prostate cancer risk. Br. J. Cancer. 2005;93:493–497. doi: 10.1038/sj.bjc.6602729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McCarron SL, et al. Influence of cytokine gene polymorphisms on the development of prostate cancer. Cancer Res. 2002;62:3369–3372. [PubMed] [Google Scholar]
- 127.Michaud DS, et al. Genetic polymorphisms of interleukin-1B (IL-1B), IL-6, IL-8, and IL-10 and risk of prostate cancer. Cancer Res. 2006;66:4525–4530. doi: 10.1158/0008-5472.CAN-05-3987. [DOI] [PubMed] [Google Scholar]
- 128.Zheng SL, et al. A comprehensive association study for genes in inflammation pathway provides support for their roles in prostate cancer risk in the CAPS study. Prostate. 2006;66:1556–1564. doi: 10.1002/pros.20496. [DOI] [PubMed] [Google Scholar]
- 129.Kasper S. Survey of genetically engineered mouse models for prostate cancer: analyzing the molecular basis of prostate cancer development, progression, and metastasis. J. Cell Biochem. 2005;94:279–297. doi: 10.1002/jcb.20339. [DOI] [PubMed] [Google Scholar]
- 130.Freedman ML, et al. Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. Proc. Natl Acad. Sci. USA. 2006;103:14068–14073. doi: 10.1073/pnas.0605832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Amundadottir LT, et al. A common variant associated with prostate cancer in European and African populations. Nature Genet. 2006;38:652–658. doi: 10.1038/ng1808. [DOI] [PubMed] [Google Scholar]
- 132.Groopman JD, Kensler TW. Role of metabolism and viruses in aflatoxin-induced liver cancer. Toxicol. Appl. Pharmacol. 2005;206:131–137. doi: 10.1016/j.taap.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 133.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 134.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 135.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nature Rev. Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 136. Chisari FV. Rous-Whipple Award Lecture. Viruses, immunity, and cancer: lessons from hepatitis B. Am. J. Pathol. 2000;156:1117–1132. doi: 10.1016/s0002-9440(10)64980-2. This paper reviews the key discovery that liver cancer can be induced simply by the transfer of activated T cells that recognize virally encoded antigens.
- 137.Neill MG, Fleshner NE. An update on chemoprevention strategies in prostate cancer for 2006. Curr. Opin. Urol. 2006;16:132–137. doi: 10.1097/01.mou.0000193388.31727.d2. [DOI] [PubMed] [Google Scholar]
- 138.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nature Rev. Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 140.Tsujimoto Y, Takayama H, Nonomura N, Okuyama A, Aozasa K. Postatrophic hyperplasia of the prostate in Japan: histologic and immunohistochemical features and p53 gene mutation analysis. Prostate. 2002;52:279–287. doi: 10.1002/pros.10116. [DOI] [PubMed] [Google Scholar]
- 141.Tsujimoto Y, et al. In situ shortening of CAG repeat length within the androgen receptor gene in prostatic cancer and its possible precursors. Prostate. 2004;58:283–290. doi: 10.1002/pros.10333. [DOI] [PubMed] [Google Scholar]
- 142.Shah R, Mucci NR, Amin A, Macoska JA, Rubin MA. Postatrophic hyperplasia of the prostate gland: neoplastic precursor or innocent bystander? Am. J. Pathol. 2001;158:1767–1773. doi: 10.1016/S0002-9440(10)64132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yildiz-Sezer S, et al. Assessment of aberrations on chromosome 8 in prostatic atrophy. BJU Int. 2006;98:184–188. doi: 10.1111/j.1464-410X.2006.06233.x. [DOI] [PubMed] [Google Scholar]
- 144.Macoska JA, Trybus TM, Wojno KJ. 8p22 loss concurrent with 8c gain is associated with poor outcome in prostate cancer. Urology. 2000;55:776–782. doi: 10.1016/s0090-4295(00)00468-4. [DOI] [PubMed] [Google Scholar]
- 145.Guo YP, Sklar GN, Borkowski A, Kyprianou N. Loss of the cyclin-dependent kinase inhibitor P27(Kip1) protein in human prostate cancer correlates with tumor grade. Clin. Cancer Res. 1997;3:2269–2274. [PubMed] [Google Scholar]
- 146.De Marzo AM, Meeker AK, Epstein JI, Coffey DS. Prostate stem cell compartments: expression of the cell cycle inhibitor p27Kip1 in normal, hyperplastic, and neoplastic cells. Am. J. Pathol. 1998;153:911–919. doi: 10.1016/S0002-9440(10)65632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yang RM, et al. Low p27 expression predicts poor disease-free survival in patients with prostate cancer. J. Urol. 1998;159:941–945. [PubMed] [Google Scholar]
- 148.Denicourt C, Dowdy SF. Cip/Kip proteins: more than just CDKs inhibitors. Genes Dev. 2004;18:851–855. doi: 10.1101/gad.1205304. [DOI] [PubMed] [Google Scholar]
- 149.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nature Rev. Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 150.Shen MM, Abate-Shen C. Roles of the Nkx3.1 homeobox gene in prostate organogenesis and carcinogenesis. Dev. Dyn. 2003;228:767–778. doi: 10.1002/dvdy.10397. [DOI] [PubMed] [Google Scholar]
- 151.Ouyang X, DeWeese TL, Nelson WG, Abate-Shen C. Loss-of-function of Nkx3.1 promotes increased oxidative damage in prostate carcinogenesis. Cancer Res. 2005;65:6773–6779. doi: 10.1158/0008-5472.CAN-05-1948. [DOI] [PubMed] [Google Scholar]
- 152.Parsons JK, et al. GSTA1 expression in normal, preneoplastic, and neoplastic human prostate tissue. Prostate. 2001;49:30–37. doi: 10.1002/pros.1115. [DOI] [PubMed] [Google Scholar]
- 153.Zha S, et al. Cyclooxygenase-2 is up-regulated in proliferative inflammatory atrophy of the prostate, but not in prostate carcinoma. Cancer Res. 2001;61:8617–8623. [PubMed] [Google Scholar]
- 154.Knudsen BS, et al. Regulation of hepatocyte activator inhibitor-1 expression by androgen and oncogenic transformation in the prostate. Am. J. Pathol. 2005;167:255–266. doi: 10.1016/S0002-9440(10)62970-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dennis LK, Dawson DV. Meta-analysis of measures of sexual activity and prostate cancer. Epidemiology. 2002;13:72–79. doi: 10.1097/00001648-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 156.Taylor ML, Mainous AG, 3rd, Wells BJ. Prostate cancer and sexually transmitted diseases: a meta-analysis. Fam. Med. 2005;37:506–512. [PubMed] [Google Scholar]
- 157. Sutcliffe S, et al. Plasma antibodies against Trichomonas vaginalis and subsequent risk of prostate cancer. Cancer Epidemiol. Biomarkers Prev. 2006;15:939–945. doi: 10.1158/1055-9965.EPI-05-0781. This is the first study linking objective evidence of exposure to Trichomonas vaginalis with prostate cancer risk.
- 158.Sutcliffe S, et al. Sexually transmitted infections and prostatic inflammation/cell damage as measured by serum prostate specific antigen concentration. J. Urol. 2006;175:1937–1942. doi: 10.1016/S0022-5347(05)00892-X. [DOI] [PubMed] [Google Scholar]