Abstract
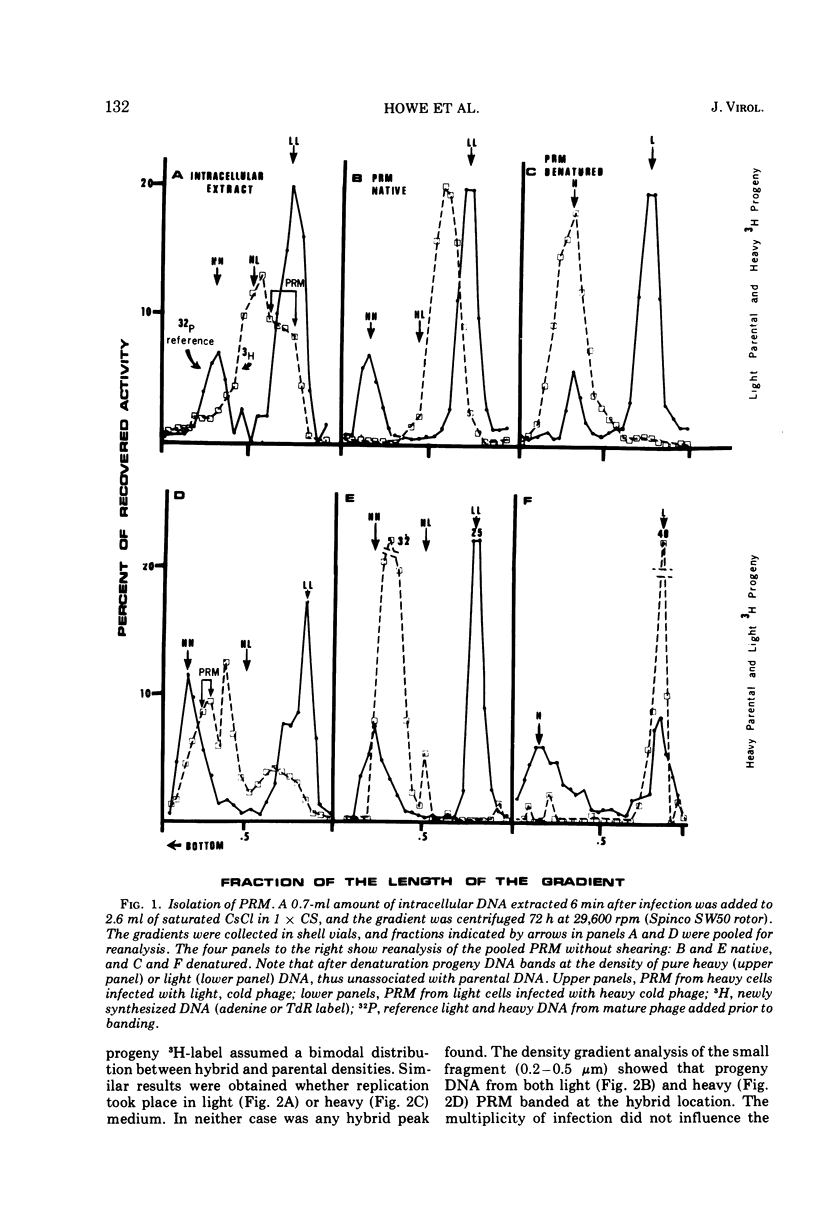
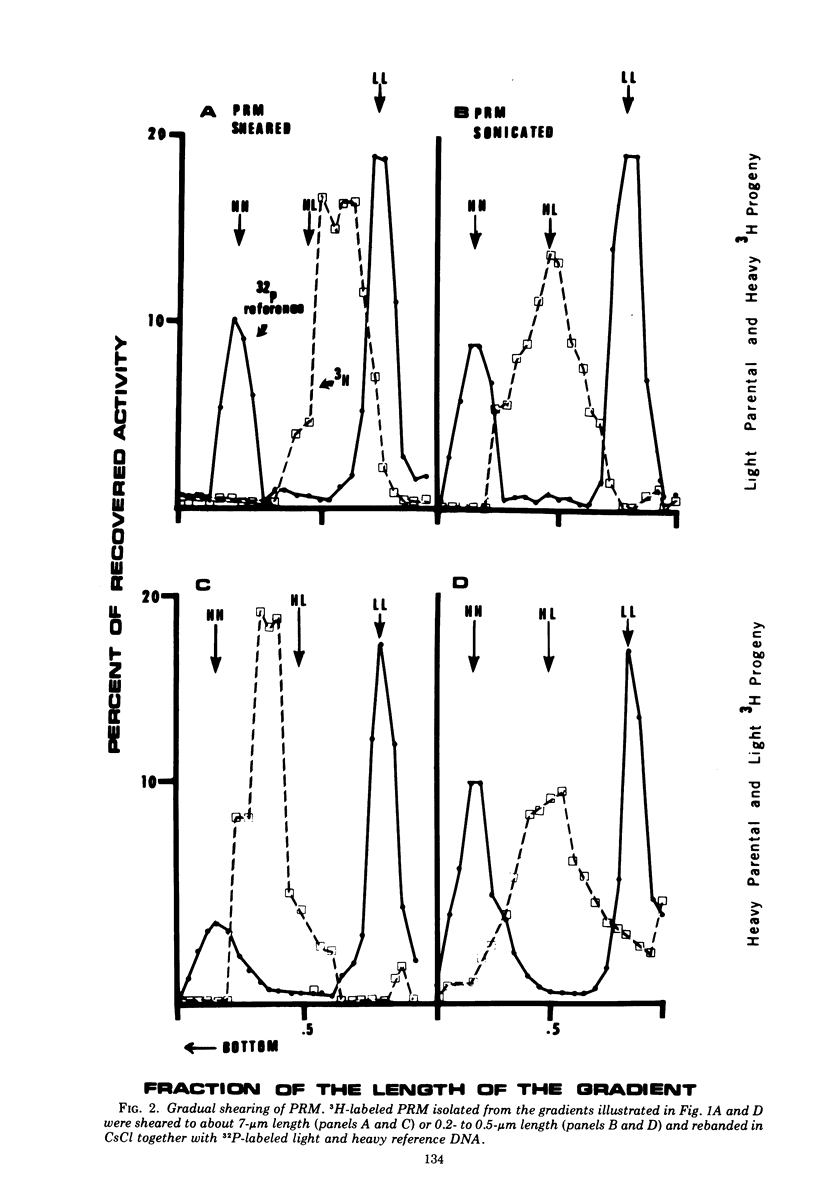
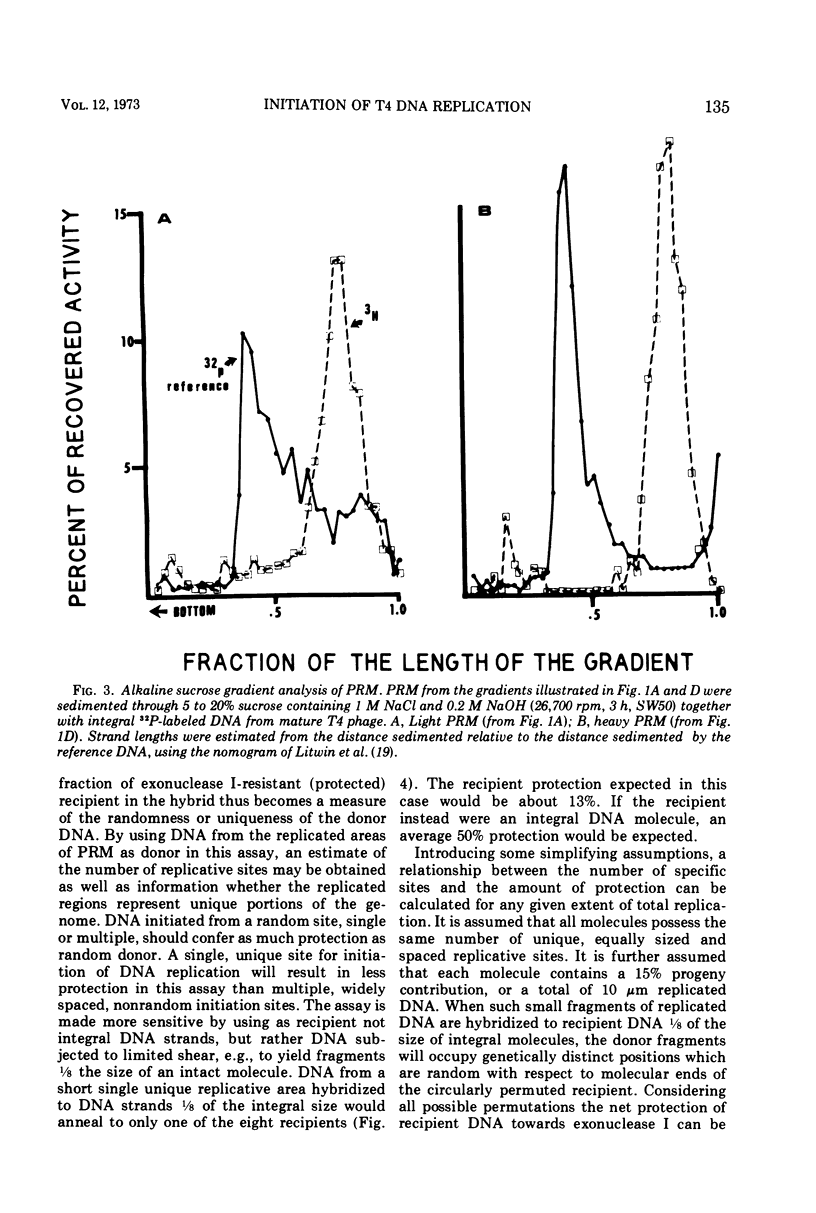
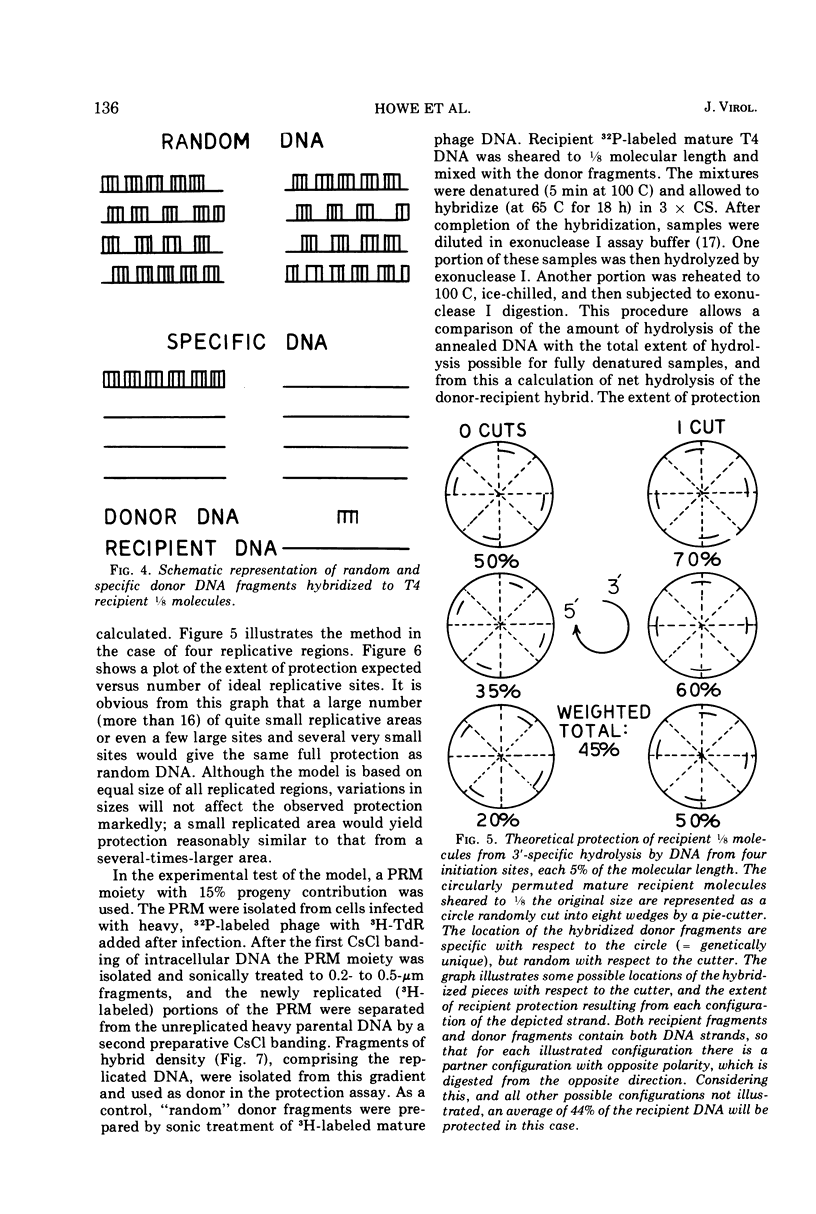
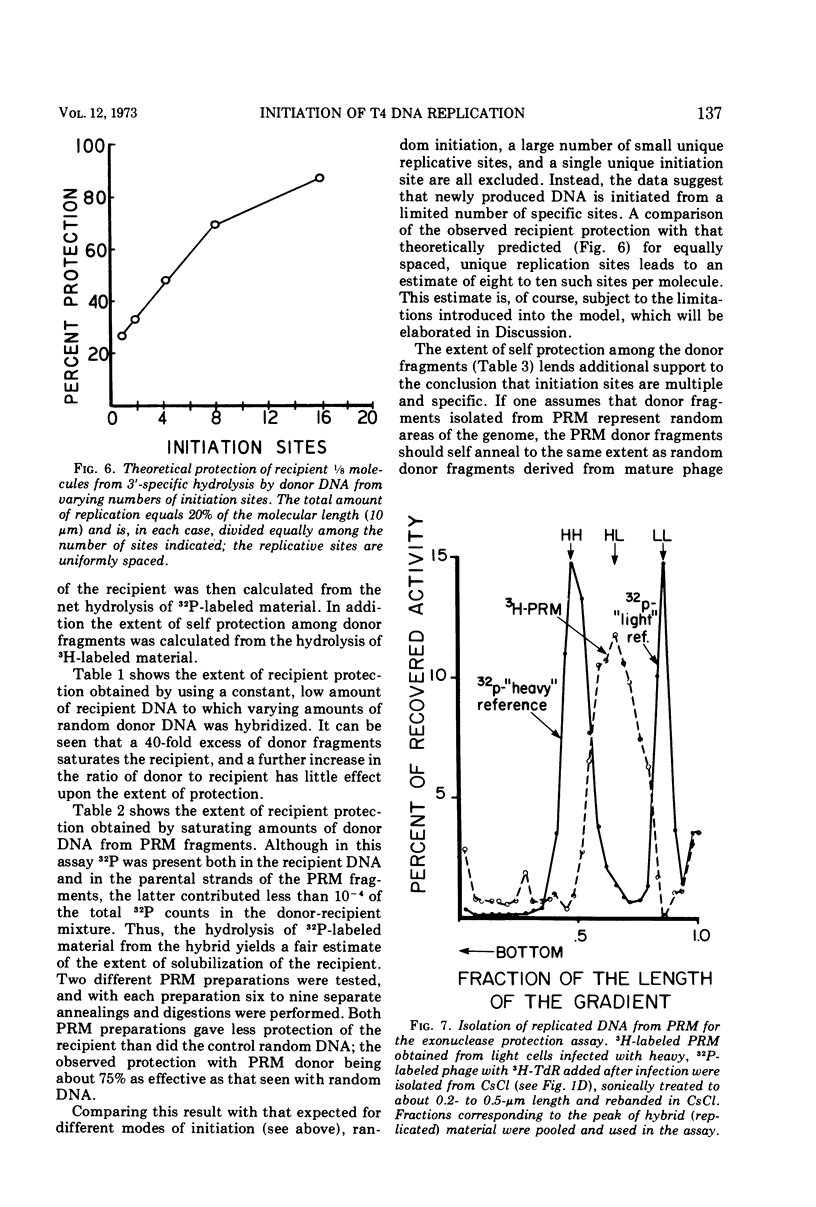
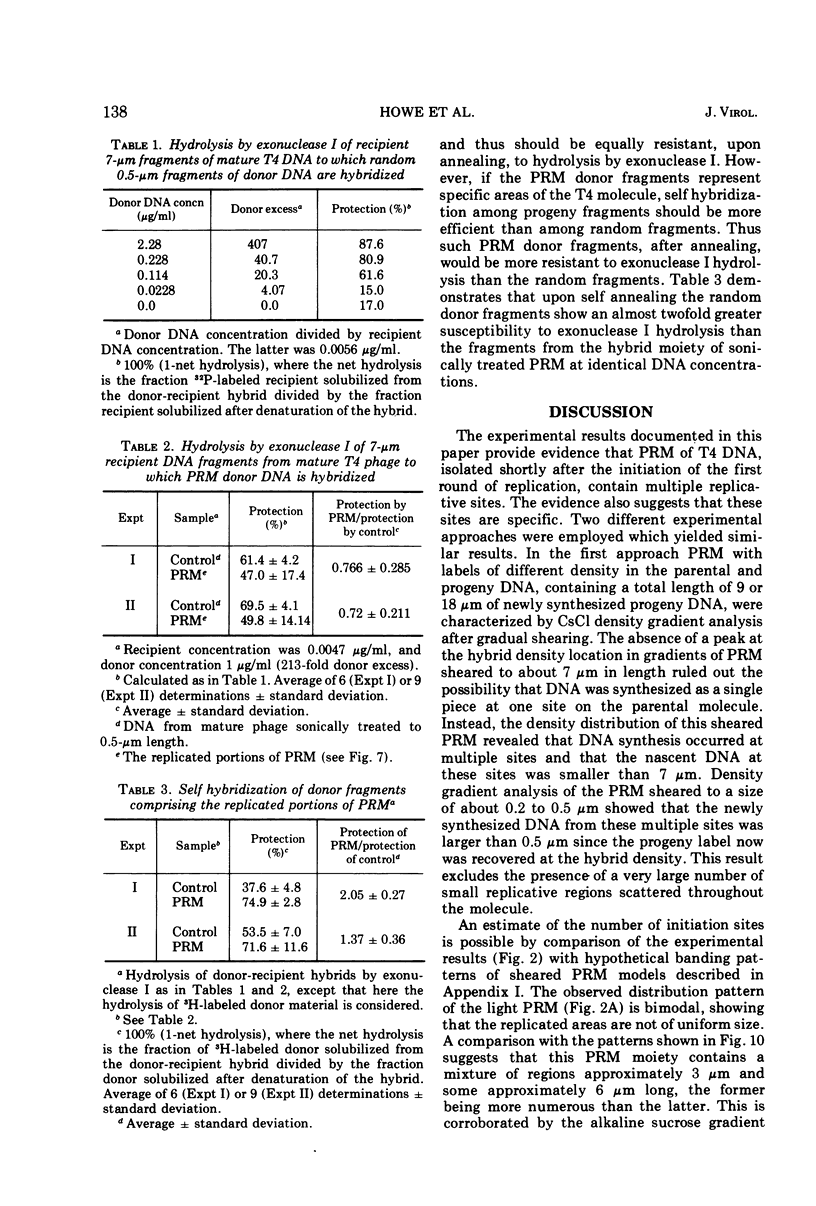
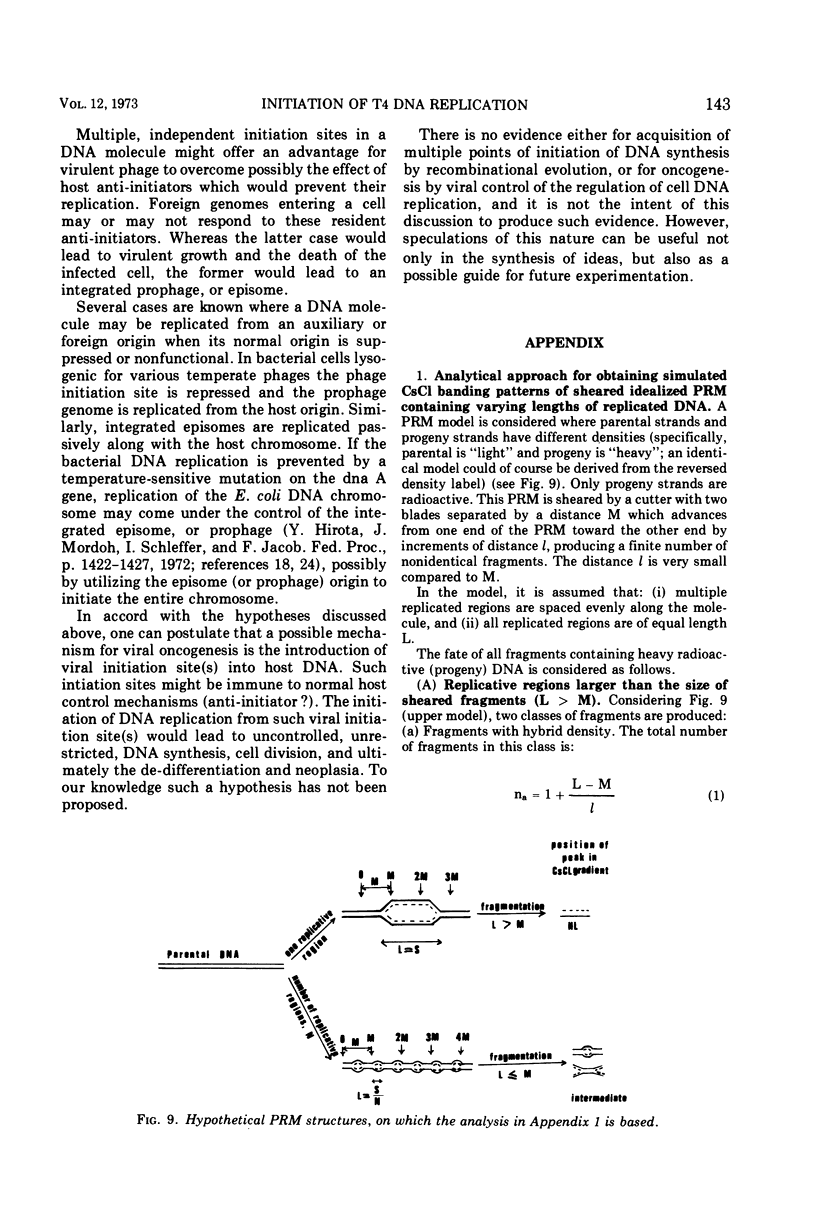
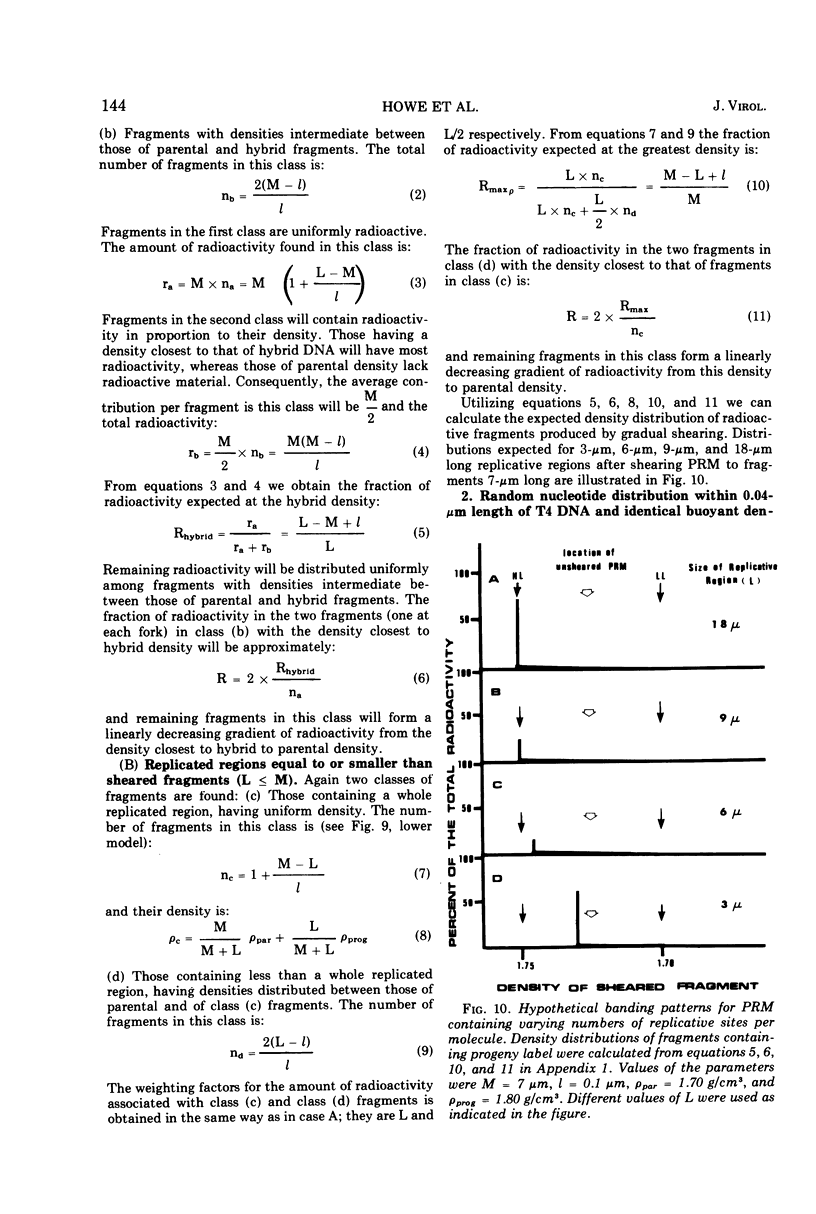
Partially replicated T4 DNA molecules (PRM) whose parental or progeny DNA was labeled with bromodeoxyuridine BUdR was analyzed by gradual shearing followed by CsCl banding of the sheared product. Analysis of PRM containing 18-μm replicated DNA showed that each replicated region was 3- to 6-μm long, indicating three to 6 replicative sites per molecule. Analysis of PRM containing 9-μm replicated DNA similarly indicated two to three replicated regions per molecule. DNA from the replicated regions of PRM containing 10-μm replicated DNA (“donor”) was hybridized to DNA from mature phage (“recipient”), and the resulting hybrid was subjected to digestion with exonuclease I. The extent of protection of the recipient and more efficient self-annealing of progeny fragments from PRM indicated that the replicated regions represented 8 to 10 nonrandom locations of the genome. Possible significance of multiple sites for initiation of DNA replication is discussed.
Full text
PDF






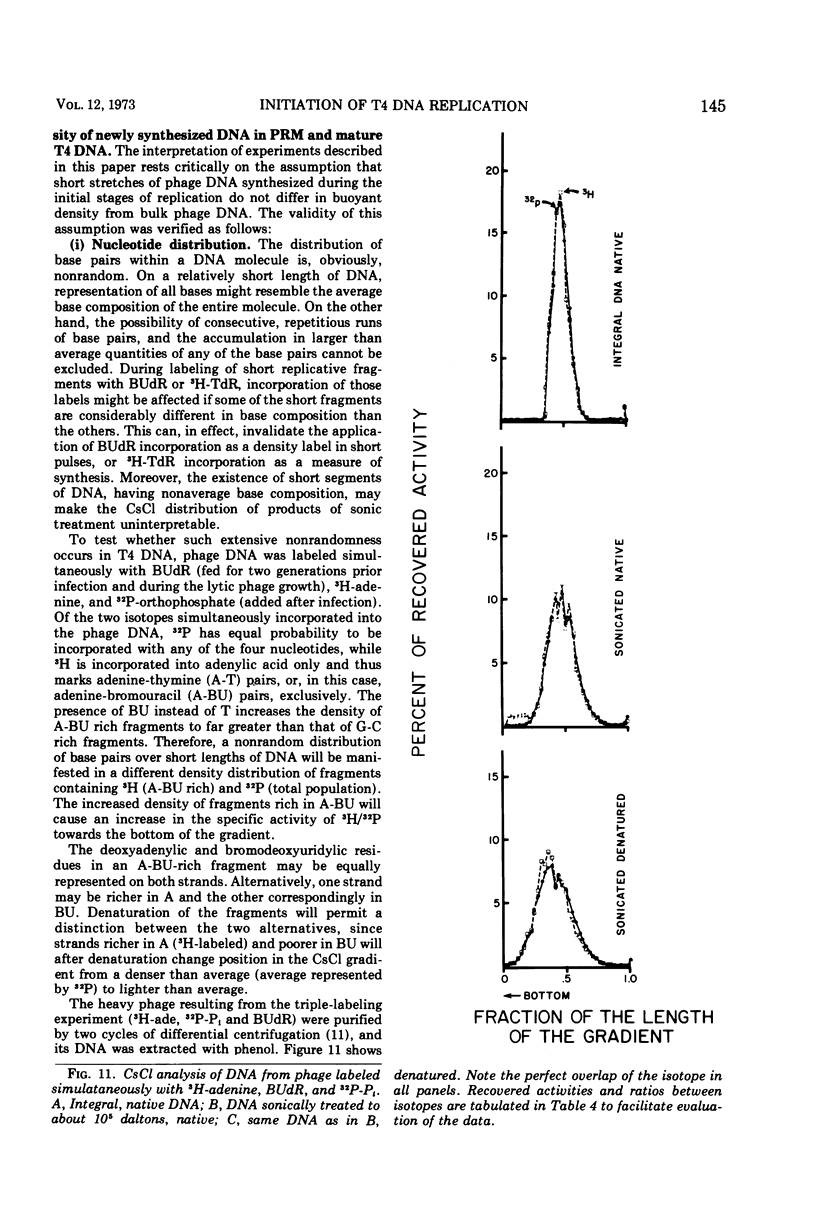
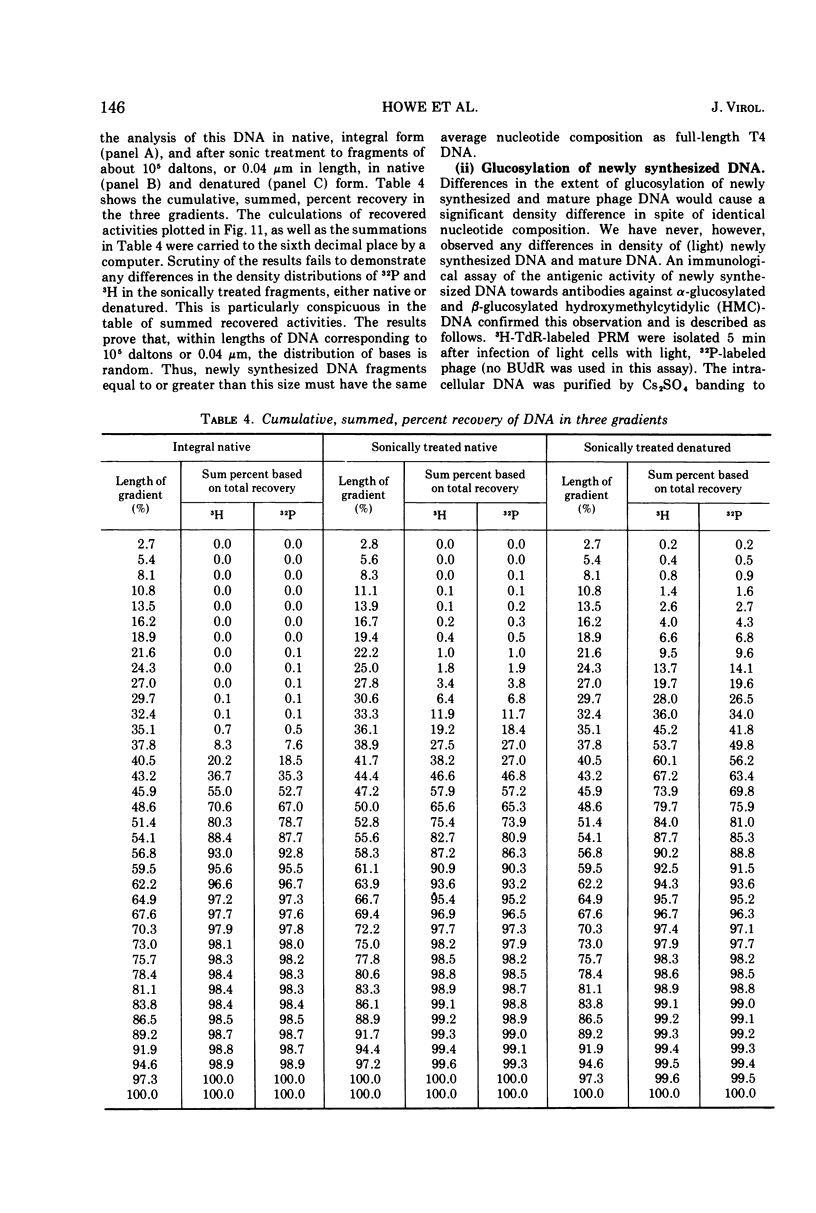


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blau S., Mordoh J. A new element in the control of DNA initiation in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2895–2898. doi: 10.1073/pnas.69.10.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius H., Howe C., Kozinski A. W. Structure of the replicating DNA from bacteriophage T4. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3049–3053. doi: 10.1073/pnas.68.12.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dressler D., Wolfson J., Magazin M. Initiation and reinitiation of DNA synthesis during replication of bacteriophage T7. Proc Natl Acad Sci U S A. 1972 Apr;69(4):998–1002. doi: 10.1073/pnas.69.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed G. C., Garon G. F., Salzman N. P. Origin and direction of simian virus 40 deoxyribonucleic acid replication. J Virol. 1972 Sep;10(3):484–491. doi: 10.1128/jvi.10.3.484-491.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOZINSKI A. W. Fragmentary transfer of P32-labeled parental DNA to progeny phage. Virology. 1961 Jan;13:124–134. doi: 10.1016/0042-6822(61)90039-3. [DOI] [PubMed] [Google Scholar]
- KOZINSKI A. W., KOZINSKI P. B. Fragmentary transfer of P32-labeled parental DNA to progeny phage. II. The average size of the transferred parental fragment. Two-cycletransfer. Repair of the polynucleotide chain after fragmentation. Virology. 1963 Jun;20:213–229. doi: 10.1016/0042-6822(63)90109-0. [DOI] [PubMed] [Google Scholar]
- Kozinski A. W., Felgenhauer Z. Z. Molecular recombination in T4 bacteriophage deoxyribonucleic acid. II. Single-strand breaks and exposure of uncomplemented areas as a prerequisite for recombination. J Virol. 1967 Dec;1(6):1193–1202. doi: 10.1128/jvi.1.6.1193-1202.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinski A. W., Kozinski P. B. Autonomous replication of short DNA fragments in the ligase negative T4 AM H39X. Biochem Biophys Res Commun. 1968 Nov 25;33(4):670–674. doi: 10.1016/0006-291x(68)90348-3. [DOI] [PubMed] [Google Scholar]
- Kozinski A. W., Kozinski P. B. Early intracellular events in the replication T4 phage DNA. II. Partially replicated DNA. Proc Natl Acad Sci U S A. 1965 Aug;54(2):634–640. doi: 10.1073/pnas.54.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinski A. W., Kozinski P. B., James R. Molecular recombination in T4 bacteriophage deoxyribonucleic acid. I. Tertiary structure of early replicative and recombining deoxyribonucleic acid. J Virol. 1967 Aug;1(4):758–770. doi: 10.1128/jvi.1.4.758-770.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinski A. W., Lin T. H. Early intracellular events in the replication of T4 phage DNA. I. Complex formation of replicative DNA. Proc Natl Acad Sci U S A. 1965 Jul;54(1):273–278. doi: 10.1073/pnas.54.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinski A. W. Molecular recombination in the ligase negative T4 amber mutant. Cold Spring Harb Symp Quant Biol. 1968;33:375–391. doi: 10.1101/sqb.1968.033.01.044. [DOI] [PubMed] [Google Scholar]
- Kozinski A. W. Unbiased participation of T4 phage DNA strands in replication. Biochem Biophys Res Commun. 1969 Apr 29;35(2):294–299. doi: 10.1016/0006-291x(69)90281-2. [DOI] [PubMed] [Google Scholar]
- Lark K. G. Regulation of chromosome replication and segregation in bacteria. Bacteriol Rev. 1966 Mar;30(1):3–32. doi: 10.1128/br.30.1.3-32.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl G., Hirota Y., Jacob F. On the process of cellular division in Escherichia coli: replication of the bacterial chromosome under control of prophage P2. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2407–2411. doi: 10.1073/pnas.68.10.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin S., Shahn E., Kozinski A. W. Interpretation of sucrose gradient sedimentation pattern of deoxyribonucleic acid fragments resulting from random breaks. J Virol. 1969 Jul;4(1):24–30. doi: 10.1128/jvi.4.1.24-30.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters M., Broda P. Evidence for the bidirectional replications of the Escherichia coli chromosome. Nat New Biol. 1971 Aug 4;232(31):137–140. doi: 10.1038/newbio232137a0. [DOI] [PubMed] [Google Scholar]
- Mosig G. A preferred origin and direction of bacteriophage T4 DNA replication. I. A gradient of allele frequencies in crosses between normal and small T4 particles. J Mol Biol. 1970 Nov 14;53(3):503–514. doi: 10.1016/0022-2836(70)90080-x. [DOI] [PubMed] [Google Scholar]
- Mosig G., Werner R. On the replication of incomplete chromosomes of phage T4. Proc Natl Acad Sci U S A. 1969 Oct;64(2):747–754. doi: 10.1073/pnas.64.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Caro L., Berg C. M., Hirota Y. Chromosome replication in Escherichia coli. IV. Control of chromosome replication and cell division by an integrated episome. J Mol Biol. 1971 Feb 14;55(3):441–456. doi: 10.1016/0022-2836(71)90328-7. [DOI] [PubMed] [Google Scholar]
- Prescott D. M., Kuempel P. L. Bidirectional replication of the chromosome in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2842–2845. doi: 10.1073/pnas.69.10.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnös M., Inman R. B. Position of branch points in replicating lambda DNA. J Mol Biol. 1970 Jul 14;51(1):61–73. doi: 10.1016/0022-2836(70)90270-6. [DOI] [PubMed] [Google Scholar]
- Schnös M., Inman R. B. Starting point and direction of replication in P2 DNA. J Mol Biol. 1971 Jan 14;55(1):31–38. doi: 10.1016/0022-2836(71)90278-6. [DOI] [PubMed] [Google Scholar]
- Shalitin C., Kahana S. Conversion of T4 gene 46 mutant deoxyribonucleic acid into nonviable bacteriophage particles. J Virol. 1970 Sep;6(3):353–362. doi: 10.1128/jvi.6.3.353-362.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoren M. M., Sebring E. D., Salzman N. P. Specific initiation site for simian virus 40 deoxyribonucleic acid replication. J Virol. 1972 Sep;10(3):462–468. doi: 10.1128/jvi.10.3.462-468.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H. Bi-directional initiation of DNA synthesis in developing chick erythroblasts. Nat New Biol. 1972 Apr 19;236(68):195–197. doi: 10.1038/newbio236195a0. [DOI] [PubMed] [Google Scholar]
- YOSHIKAWA H., SUEOKA N. Sequential replication of Bacillus subtilis chromosome. I. Comparison of marker frequencies in exponential and stationary growth phases. Proc Natl Acad Sci U S A. 1963 Apr;49:559–566. doi: 10.1073/pnas.49.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIKAWA H., SUEOKA N. Sequential replication of the Bacillus subtilis chromosome. II. Isotopic transfer experiments. Proc Natl Acad Sci U S A. 1963 Jun;49:806–813. doi: 10.1073/pnas.49.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]




