Abstract
Urochordates (ascidians) have recently supplanted cephalochordates (amphioxus) as the extant sister taxon of vertebrates. Given that urochordates possess migratory cells that have been classified as ‘neural crest-like’– and that cephalochordates lack such cells – this phylogenetic hypothesis may have significant implications with respect to the origin of the neural crest and neural crest-derived skeletal tissues in vertebrates. We present an overview of the genes and gene regulatory network associated with specification of the neural crest in vertebrates. We then use these molecular data – alongside cell behaviour, cell fate and embryonic context – to assess putative antecedents (latent homologues) of the neural crest or neural crest cells in ascidians and cephalochordates. Ascidian migratory mesenchymal cells – non-pigment-forming trunk lateral line cells and pigment-forming ‘neural crest-like cells’ (NCLC) – are unlikely latent neural crest cell homologues. Rather, Snail-expressing cells at the neural plate of border of urochordates and cephalochordates likely represent the extent of neural crest elaboration in non-vertebrate chordates. We also review evidence for the evolutionary origin of two neural crest-derived skeletal tissues – cartilage and dentine. Dentine is a bona fide vertebrate novelty, and dentine-secreting odontoblasts represent a cell type that is exclusively derived from the neural crest. Cartilage, on the other hand, likely has a much deeper origin within the Metazoa. The mesodermally derived cellular cartilages of some protostome invertebrates are much more similar to vertebrate cartilage than is the acellular ‘cartilage-like’ tissue in cephalochordate pharyngeal arches. Cartilage, therefore, is not a vertebrate novelty, and a well-developed chondrogenic program was most likely co-opted from mesoderm to the neural crest along the vertebrate stem. We conclude that the neural crest is a vertebrate novelty, but that neural crest cells and their derivatives evolved and diversified in a step-wise fashion – first by elaboration of neural plate border cells, then by the innovation or co-option of new or ancient metazoan cell fates.
Keywords: ascidians, cartilage, cephalochordates, chondrogenesis, dentine, developmental origins, evolutionary origins, gene co-option, gene regulatory networks, innovation, neural crest, neural crest cells, odontogenesis, skeleton, vertebrates
Introduction
Chordates
All chordates – cephalochordates, urochordates and vertebrates – share a dorsal notochord and a dorsal hollow nerve cord that defines the midline of the dorso-ventral axis. The three subphyla have been regarded as sharing a common chordate ancestor ever since Kowalevsky discovered that cephalochordates and larval ascidians possess a notochord (Kowalevsky, 1866, 1867, 1871, 1877; Bowler, 1996; Holland, 2000; Holland & Chen, 2001; Mikhailov & Gilbert, 2002; Hall, 2005a). Although a monophyletic clade, three divergent body plans exist among extant urochordates. These body plans have long been recognized as three classes – benthic tunicates (ascidians, sea squirts),1 pelagic appendicularians (larvaceans) and pelagic salps (thaliaceans). Only ascidians have been studied in any depth, including fate-mapping cell lineages, with most data coming from two genera, Ciona (mostly C. intestinalis) and Halocynthia roretzi. The embryology of much of tunicate diversity remains unstudied, as does the embryology of larvaceans and salps. Extant cephalochordates, on the other hand, are represented by a single body plan found in 14 species of amphioxus in two genera, Branchiostoma and Asymmetron. Much developmental work has been done on this group, but no cell-lineage fate map is available (see Holland & Holland, 2007).
The fossil record of chordates and early vertebrates shows that ascidians, hemichordates, cephalochordates and jawless vertebrates had already arisen by the Early Cambrian. =Haikouella lanceolata and =H. jianshanensis are hemichordates. =Shankouclava shankouense is an ascidian. =Cathaymyrus diadexus and =C. haikoensis are cephalochordates with a notochord and segmented body musculature, while =Myllokunmingia fengjiaoa and =Haikouichthys ercaicunensis are jawless vertebrates with notochord, myomeres, a dorsal fin, cartilaginous gills clefts, and cartilaginous olfactory and optic capsules (Shu et al. 1996; Mallatt & Chen, 2003; Fedonkin et al. 2007; Janvier, 2007; Hall, 2008b, 2009). The homologous cartilaginous skeletal elements in extant vertebrates are neural crest derivatives (Hall, 1999, 2009; Le Douarin & Kalcheim, 1999; Long et al. 2011).
The neural crest and neural crest cells
The ‘neural crest’ is a morphological term for the dorsal folds of the neural tube, seen as the neural plate rolls up to form the hollow dorsal neural tube during neurulation. Possession of a neural crest derived from dorsal neural ectoderm is a shared-derived (synapomorphic) characteristic of vertebrates. The origin of the neural crest and neural crest cells may therefore be viewed as the derivation in vertebrates of a distinct cell population within the broadly evolutionarily conserved boundary between chordate neural and non-neural ectoderm.
‘Neural crest cells’ are mesenchymal cells derived from the neural crest epithelium. Two key features of neural crest cells are migratory ability and multipotency (Donoghue et al. 2008). Neural crest cells delaminate from ectoderm, undergo an epithelial-to-mesenchymal transformation, and migrate away from the future brain and spinal cord, giving rise to a plethora of derivatives in the head and trunk. Neural crest-derived mesenchymal cells differentiate into at least 21 different cell types, including neurons (sensory, adrenergic and cholinergic); satellite, Schwann glial and chromaffin cells; melanocytes; connective tissue and skeletal cells (fibro-, chondro-, osteo- and odontoblasts); myoblasts (cardiac, striated and smooth); adipocytes and angioblasts (Le Douarin & Kalcheim, 1999; Le Douarin et al. 2004; Vickaryous & Hall, 2006; Hall, 2009). Some of these cell types also arise from mesoderm – fibroblasts, chondroblasts and osteoblasts being prime examples. Neural crest-derived cells contribute to (or form in their entirety) spinal and enteric ganglia, the parasympathetic, peripheral and sympathetic nervous systems, the thyroid and adrenal glands, the craniofacial and viscerocranial skeletons, teeth, connective and adipose tissues, smooth and striated muscles of the heart, the eye, blood vessels and endothelia. Each of these cell types, tissues and organs has its own characteristic features, the elaboration of which is beyond the scope of the review (but see the references above).
When assessed on the basis of number of differentiated cell types, the neural crest forms a greater number of cell types than does mesoderm (Vickaryous & Hall, 2006). Based on this developmental potential and diversity of cell fates, the neural crest can be classified as the fourth germ layer, and the only germ layer found exclusively in vertebrates: the embryos of diploblastic animals possess ectoderm and endoderm; the embryos of triploblastic invertebrate embryos possess ecto-, endo- and mesoderm; vertebrate embryos possess these three germ layers plus the neural crest (Hall, 1997, 2000a, b, 2008a, c, 2009; Le Douarin & Kalcheim, 1999).
Evolutionary origin(s) of the neural crest
Classically, the neural crest was thought to have originated along the vertebrate stem, coincident with the evolution of a brain, a muscular pharynx and paired sensory organs. In their ground-breaking ‘new head hypothesis,’Gans & Northcutt (1983) linked the shift from passive filter feeding to active predation with the evolutionary diversification of the neural crest and ectodermal placodes (see also Gans & Northcutt, 1985; Maisey, 1986; Gans, 1987, 1989, 1993; Maderson, 1987; Hanken & Hall, 1993; Donoghue, 2002; Mallatt & Chen, 2003; Hall, 2005c, 2008a, b, c, 2009; Schlosser, 2006, 2010; Donoghue et al. 2008).2Gans & Northcutt (1983) and Northcutt & Gans (1983) proposed that an epidermal nerve plexus or nerve net primitively controlled ciliary function during movement and filter feeding, and that as the central nervous system took over the neural control of locomotion, other derivatives of this primitive nerve net – namely, neural crest cells and ectodermal placodes – would have been freed to diversify and evolve other functions (ibid; Bowler, 1996; Northcutt, 1996; Baker & Bronner-Fraser, 1997).
Since the publication of the ‘new head hypothesis’, however, it has been recognized that ascidians possess: first, ectodermal ‘placodes’ (i.e. focal regions of neurogenic ectoderm, which may bear some relationship to vertebrate neurogenic placodes; Wada et al. 1998; Manni et al. 2004; Schlosser, 2006, 2010; Hong & Saint-Jeannet, 2007); and second, migratory NCLC (see below); cephalochordates possess neither placodes nor NCLC. Furthermore, recent detailed phylogenomic analyses place urochordates and not cephalochordates as the immediate sister group to vertebrates (Fig. 1; Bourlat et al. 2006; Delsuc et al. 2006; Dunn et al. 2010; Philippe et al. 2011). If ascidian NCLC can be homologized with vertebrate neural crest cells, then the neural crest may, in fact, have a deeper (Precambrian) origin in chordate phylogeny as a synapomorphy of Olfactores (a clade consisting of urochordates + vertebrates, ibid) rather than of vertebrates. So, are ascidian NCLC homologous with, or evolutionary precursors of, vertebrate neural crest cells? And is there any evidence for neural crest or NCLC in cephalochordates?
Fig. 1.
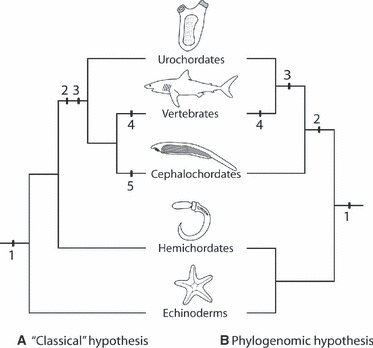
(A) The classical hypothesis of chordate interrelationships (based primarily on morphology) placed cephalochordates as the extant sister group to vertebrates. (B) The currently accepted phylogenetic hypothesis (based on extensive phylogenomic and EST sequence analysis) resolves urochordates as the sister group of vertebrates. Numbers on the phylogeny indicate key steps in the evolution of the vertebrate neural crest. 1. Dorsoventrally patterned neurectoderm is a primitive feature of deuterostomes. 2. The origin of Snail-expressing cells at the border of the neural plate. 3. The origin of migratory ‘NCLC’, as seen in ascidians (if these are deemed latent homologues of vertebrate neural crest cells – see text). 4. The origin of the neural crest. Note that if ascidian ‘NCLC’ are latent homologues of neural crest cells, then the classical hypothesis would require loss of ‘NCLC’ in cephalochordates (5).
We begin this review by briefly outlining studies that have identified genetic markers and defined a ‘gene regulatory network’ (GRN) underlying the development of the neural crest, from the early specification of neural crest precursors, to the ultimate differentiation of neural crest-derived cells. We then use this GRN, alongside cell behaviour, cell fate and embryonic context, as a basis for assessing putative latent neural crest homologues – evolutionary developmental precursors of the neural crest that may, in effect, be represented as a series of progressively more plesiomorphic ‘neural crest character states’ on a cladogram (Hall, 1994; Stone & Hall, 2004) – in cephalochordates and urochordates. Finally, we discuss the evolution of neural crest skeletal fates. With respect to the acquisition of neural crest cell fates, it is now evident that the diversification of neural crest cell fates occurred, in some instances, by the evolution of new cell types and, in other instances, by the co-option of developmental programs for cell types from other tissues. We discuss how neural crest cells have acquired skeletogenic fates, using odontoblasts (a ‘new’– i.e. neural crest-specific – cell type) and chondrocytes (primitively a mesodermally derived invertebrate cell type) as examples.
Neural crest ‘markers’, and the molecular specification of the neural crest and neural crest cells
A combination of genomic analyses and bioinformatics enabled Martinez-Morales et al. (2007) to identify 615 ‘neural crest genes’– defined as genes that are associated with the neural crest or neural crest cells. Of these, 91% are found in metazoans that lack neural crest cells. Therefore, the origination of the neural crest was not directly associated with major rounds of evolution of new gene families, and the notion of ‘neural crest genes’ is not valid. Interestingly, Martinez-Morales et al. (2007) found that half their ‘neural crest genes’ code for extracellular ligands that are expressed by other cell types. Based on this observation, they suggest that much of neural crest evolution may have been driven by the evolution of ligand regulation (with ligand-mediated signalling, in turn, functioning in the commitment of neural crest cells to individual cell lineages).
The HNK-1 carbohydrate epitope is often defined and used as a neural crest ‘marker’. HNK-1 is present on the surface of migrating avian, frog and some teleost neural crest cells, and is often used as an indicator of neural crest cell induction in experimental studies. However, HNK-1 is far from a neural crest marker; HNK-1 is expressed in a number of non-neural crest-derived vertebrate cell types and is not expressed in the neural crest cells of several vertebrate taxa (including mouse, shark and zebrafish –Holley & Yu, 1987; Kuratani & Horigome, 2000). In fact, as effectively all genes expressed in the developing neural crest or neural crest cells are also expressed in different ectodermal and/or mesodermal development contexts, one is hard-pressed to identify a single neural crest ‘marker’.
Many genes that play important inductive or regulatory roles during the development of the neural crest and neural crest cells have now been identified and characterized, including genes expressed in gastrula-stage embryos that indicate the early specification of the neural crest cell, genes expressed at the neural crest border in neurula-stage embryos, and genes expressed in pre-migratory, delaminating, migratory and differentiating neural crest cells. The interactions of these genes and their products have been sufficiently well characterized – both functionally and across phylogenetically informative taxa (e.g. chick, mouse, frog and lamprey) – to allow the construction of a putative vertebrate neural crest GRN (Meulemans & Bronner-Fraser, 2004; Sauka-Spengler et al. 2007; Sauka-Spengler & Bronner-Fraser, 2008a, b, c; Betancur et al. 2010). Among the genes in the neural crest GRN are members of the Fgf, Wnt and Bmp gene families, which are involved in induction of the neural plate; Pax3/7, Zic genes, Msx1/2 and Dlx3/5, which are involved in establishing the neural plate–epidermal ectodermal boundary (‘neural plate border specifiers’); and Snail1/2, FoxD3, Tfap2a, Sox8/9/10 and Twist, which are involved in the specification of neural crest cells and distinct neural crest cell sublineages (‘neural crest specifiers’). While an extensive review of the molecular interactions governing neural crest specification is beyond that scope of this paper – and given that these data have been eloquently reviewed elsewhere (ibid) – we simply present selected downstream effectors of early ectodermal Bmp signalling (Fig. 2) as one example of how genes at different levels of the neural crest GRN interact to regulate neural crest development.
Fig. 2.
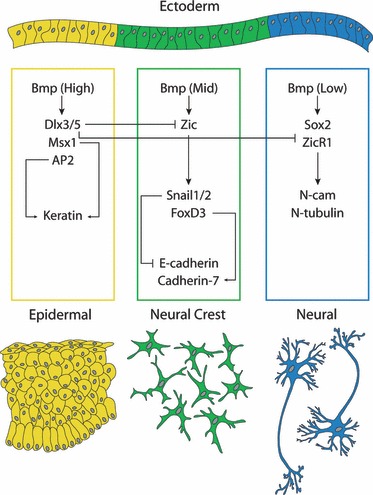
A simplified schematic illustrating how selected factors downstream of Bmp signalling interact to specify epidermal, neural and neural crest cell fates in vertebrate embryos. Molecular interactions are not necessarily direct, and are based on the putative vertebrate neural crest gene regulatory network (after Meulemans & Bronner-Fraser, 2004; Sauka-Spengler et al. 2007; Sauka-Spengler & Bronner-Fraser, 2008a,b). For details, see text.
Bmp-signalling in the ectoderm is involved in the segregation of neural from non-neural (e.g. epidermal) domains, with high levels of Bmp activating the epidermal program, and low levels of Bmp (a consequence of dorsal expression of the secreted Bmp antagonist Noggin) activating the neural program (Liem et al. 1995; Marchant et al. 1998). A high level of Bmp upregulates the expression of factors such as Msx1 (which is a direct positive transcriptional regulator of keratin), while low levels of Bmp signalling upregulate the expression of Sox2 and Zicr1 (which, in turn, activate expression of the neural genes N-cam and N-tubulin; Suzuki et al. 1997; Mizuseki et al. 1998). Attenuated levels of Bmp signalling at the neural–epidermal ectoderm boundary activate expression of neural plate border specifiers, such as the Zic genes and Pax3/7 (Nakata et al. 1997, 2000; Aruga et al. 2002). These border specifiers, in conjunction with Bmp, Fgf and Wnt signalling, in turn, regulate the expression of the neural crest specifiers Snail1, Snail2 (Slug) FoxD3 and Twist (all of which are expressed in pre-migratory neural crest cells; Nakata et al. 1997, 2000; Brewster et al. 1998; Mizuseki et al. 1998; Sasai et al. 2001).
Within the neural crest GRN, there is ample evidence for cross-regulation among neural crest specifiers. For example, in Xenopus, overexpression of FoxD3 results in the upregulation of Slug and Twist (Sasai et al. 2001), overexpression of Sox10 results in the upregulation of Slug (Aoki et al. 2003), overexpression of Snail induces expression of Slug, FoxD3 and Twist (Aybar et al. 2003), and loss of Sox10 function results in the inhibition of Slug and FoxD3 expression (Honore et al. 2003). Using a genetic approach in zebrafish, Arduini et al. (2009) have shown that the simultaneous loss of FoxD3 and Tfap2a function results in a loss of neural crest Sox9 and Sox10 expression, and a corresponding absence of all neural crest-derived structures (including neural crest-derived chromatophores, pharyngeal cartilages and neural crest-derived peripheral neurons). More recently, Wang et al. (2011) demonstrated that FoxD3/Tfap2 double mutant zebrafish also exhibit an early perturbation of Wnt and Bmp signalling during gastrulation, and suggest that, in addition to their well-established roles in neural crest cell sublineage specification, FoxD3 and Tfap2a may also play earlier roles in neural crest induction. Importantly, however, Arduini et al. (2009) observed that neural crest induction occurred normally in the absence of FoxD3 and Tfap2 function, and so the precise roles of FoxD3 and Tfap2a in neural crest induction remain unclear.
A number of ‘neural crest effector’ genes have also been identified and characterized. These genes are downstream of the neural crest specification genes, and play integral roles in the delamination, migration and differentiation of neural crest cells. Expression of the neural crest specifiers Snail and FoxD3 are negative and positive transcriptional regulators of E-cadherin and cadherin-7, respectively, and the downregulation and upregulation of E-cadherin and cadherin-7, respectively, are required for the delamination of neural crest cells during epithelial-to-mesenchymal transition (Nakagawa & Takeichi, 1998; Cano et al. 2000). Another example of a neural crest effector gene is Col2a, which encodes type II collagen – the major collagenous component of vertebrate cartilage extracellular matrix. Expression of Col2a in differentiating chondrocytes is directly regulated by the neural crest specifier Sox9 (Ng et al. 1997), though note that Sox9 also regulates the expression of Col2a in mesodermally derived cartilage (reinforcing the fact that many neural crest specifiers function similarly in non-neural crest developmental contexts).
Given the extensive molecular characterization of the neural crest and neural crest cells in vertebrates, we may now use the vertebrate neural crest GRN – along with cell behaviour, cell fate and embryonic context – to assess putative evolutionary antecedents of the neural crest and neural crest cells in non-vertebrate chordates.
Putative cellular and molecular precursors of the neural crest and neural crest cells in non-vertebrate chordates
Cephalochordates
Due to their ‘simplified’ vertebrate-like adult body plan, cephalochordates have long been regarded as the sister group of the vertebrates, and have been used as a ‘proxy’ for the last common ancestor of chordates (Holland & Holland, 2001). Such an approach is fraught with problems, however, as no single extant taxon can truly represent a collection of primitive character states (such an ‘ancestor’ is necessarily hypothetical, and a product of careful character optimization on a phylogenetic tree). Cephalochordates generally lack derivatives known in vertebrates to arise, exclusively or in part, from neural crest cells, such as peripheral pigment cells, bone-, cartilage- or dentine-forming cells. Amphioxus dorsal root nerves are ensheathed by glial cells, and these have been compared with the Schwann cells of the vertebrate peripheral nervous system (Bone, 1960; Peters, 1963), though peripheral glial cells are also present in a number of marine invertebrate taxa that unequivocally lack a neural crest (Coles & Abbott, 1996).
Studies examining the expression of selected Amphioxus orthologues of candidates in the vertebrate neural crest GRN – referred to with the prefix Amphi and the orthologous vertebrate gene – have revealed that the ‘neural plate border specifiers’AmphiPax3/7, AmphiMsx and AmphiZic are all expressed at the lateral edge of the neural plate, and that AmphiDlx3/5 is expressed in ectoderm adjacent to the neural plate (Holland et al. 1999; Sharman et al. 1999; Gostling & Shimeld, 2003). As in vertebrates, these cells give rise to the dorsal neural tube, and so these gene expression patterns likely reflect a conserved mechanism for patterning the dorsal–ventral axis of the nervous system in chordates (indeed, for bilaterians; Holland & Graham, 1995; Meinertzhagen & Okamura, 2001). However, only a single ‘neural crest specifier,’AmphiSnail, is expressed in the neural plate border in amphioxus (Langeland et al. 1998). Ectodermal cells at the lateral border of the neural plate in amphioxus embryos do not appear to delaminate and migrate, and so the function/fate of AmphiSnail-expressing cells is unclear. It would be interesting to examine whether AmphiSnail functions as a transcriptional regulator of cell-adhesion molecules in amphioxus, as Snail does in vertebrates. Additionally, while the early fate map of amphioxus is reasonably well understood (Holland & Holland, 2007), there are no data (e.g. vital dye-labelling data) available on the fate of AmphiSnail-expressing cells. Thus, while evidence for cephalochordate neural crest cell antecedents and neural crest derivatives is currently scant, further molecular characterization and in vivo fate mapping of AmphiSnail-expressing neural plate border cells in amphioxus may yet reveal a latent neural crest homologue in this group. Functional analyses of regulatory interactions among factors in the putative neural crest GRN in amphioxus will be facilitated by the availability of a cephalochordate genome sequence (Putnam et al. 2008).
Ascidians
Urochordates (Urochordata) include the ascidians (tunicates, sea squirts), larvaceans (appendicularians) and thaliaceans (salps). Larvaceans and thaliaceans are virtually unstudied with respect to the presence of a neural crest or neural crest cells. Conversely, ascidians – particularly the two species Ciona intestinalis and Halocynthia roretzi, which are found in northern European and in Japanese, Chinese and Korean waters, respectively – have been the focus of intense study in the field of developmental biology (Whittaker, 1987; Satoh, 1994, 2007, 2008, 2009; Jeffery, 1997, 2006, 2007; Meinertzhagen & Okamura, 2001; Jeffery et al. 2004, 2008; Kumano & Nishida, 2007; Bishop et al. 2010). Although studied since the early 19th century (Kowalevsky, 1871), the recent description by Jeffery et al. (2004) of NCLC in ascidian embryos, the sequencing of the Ciona genome (Dehal et al. 2002) and phylogenetic analyses that place urochordates as the sister group to vertebrates (Bourlat et al. 2006; Delsuc et al. 2006) have brought ascidians to centre stage in the neural crest evolutionary play.
The colonial mangrove ascidian Ecteinascidia turbinata possesses peripheral yellow-orange pigment cells in the tunic, and these pigment cells are reminiscent of vertebrate erythrophores (a subtype of neural crest-derived pigment cell, or chromatophore). Vital dye-labelling experiments in the giant tadpole larvae of E. turbinata have shown that cells giving rise to pigment arise near (though not unequivocally from) the neural tube and developing central nervous system, and migrate either through the mesoderm, or between the mesoderm and epidermis, to populate the body wall and siphon (Jeffery et al. 2004). These migrating cells are mesenchymal in organization, though they migrate as single cells rather than in streams. These cells also express the carbohydrate epitope HNK-1, which is expressed in migrating avian neural crest cells, and they express the zinc finger transcription factor Zic3, which is expressed in the neural plate border of vertebrate embryos (though not in migrating neural crest cells). Such HNK-1-positive mesenchymal cells – termed NCLC – have been observed in 12 other species of ascidians with disparate adult organizations, developmental modes and larval sizes, and often appear to co-express tyrosinase, the rate-limiting enzyme in melanogenesis (Jeffery, 2006). If, as recent molecular phylogenies suggest, ascidians are the sister group to vertebrates, and if the NCLC reported in ascidians are forerunners of neural crest cells (‘proto-neural crest cells’), then it has been speculated that the primitive fate of these neural crest precursors may have been pigment (Jeffery, 2007).
More recently, analysis of cytochalasin-treated ‘cleavage-arrested’Ciona intestinalis embryos has suggested that the HNK-1-positive NCLC described by Jeffery et al. (2004) and Jeffery (2006) derive from the A7.6 blastomere pair. This blastomere pair gives rise to a previously characterized migratory cell population known as trunk lateral cells. Similar expression patterns of HNK-1 and the trunk lateral cell-specific marker Tlc2 (Takahashi & Satoh, 2001) led Jeffery et al. (2008) to assert that the NCLC of C. intestinalis (and other ascidians) are these trunk lateral cells. Jeffery et al. (2008) demonstrated that the C. intestinalis A7.6/trunk lateral cell lineage expresses orthologues of seven of 16 genes from the vertebrate neural crest GRN, including four patterning genes involved in neural plate induction (Bmp, Wnt, Notch, Fgf) and five ‘neural crest specifier’ genes (Twist, FoxDb, Ap2-1, Myc). Based on these expression data, Jeffery et al. (2008) speculate that ascidian NCLC/trunk lateral cells and vertebrate neural crest cells are homologous cell types,
Given these observations, and the sister group relationship of ascidians and vertebrates, are NCLC/trunk lateral cells latent neural crest cell homologues? There are a number of problems with this conjecture.
Firstly, ascidian NCLC have not been definitively shown to arise from the neural tube. An examination of DiI injection sites relative to the position of the neural tube in E. turbinata (Jeffery et al. 2004) reveals that a large population of cells adjacent to the neural tube was also labelled in these lineage-tracing experiments. While this is certainly understandable, given the size of the animal in question, caution must nevertheless be exercised when assessing the putative embryonic origin of E. turbinata NCLC.
There is also no evidence from lineage-tracing studies in other ascidians that A7.6-derived cells contribute to the border of the neural plate, or even to ectoderm more generally (Satoh, 1999). In terms of molecular marker expression, HNK-1 is not an exclusive marker for neural crest cells, and while the A7.6/trunk lateral cell lineage of C. intestinalis does express a subset of ‘neural crest specifiers’ (Twist, FoxDb, Ap2-1, Myc), as discussed above, these genes are not exclusive to the developing neural crest, and are also expressed in developing vertebrate mesodermal derivatives.
It is important to note that, in the ascidians Halocynthia roretzi and Ciona intestinalis, the A7.6 lineage of trunk lateral cells gives rise to a variety of mesendodermal fates, including blood cells, longitudinal mantle muscles, oral siphon muscles and pharyngeal epithelium (Hirano & Nishida, 1997; Tokuoka et al. 2004), and so it is difficult to disentangle roles for ‘neural crest specifiers’ in A7.6-derived NCLC vs. other mesendodermal derivatives without further functional studies.
Finally, echinoderm larvae possess pigment cells that derive from migratory mesenchymal cells of mesodermal origin (Gibson & Burke, 1985). It is therefore possible that pigment cells primitively derived from the mesendodermal lineage in deuterostomes (echinoderms, hemichordates and chordates), with a subsequent co-option of this developmental program to the neural crest along the vertebrate stem.
There are, nevertheless, cells at the neural plate border of ascidians that may well represent true latent neural crest homologues. Presumptive dorsal epidermal cells adjacent to the neural plate in Ciona intestinalis express orthologues of the vertebrate ‘neural plate border specifiers’Dlx3/5 and Msx, while Pax3/7 is expressed in both presumptive dorsal epidermis and dorsal neural tube (Wada et al. 1996; Mazet et al. 2003; Imai et al. 2004). Furthermore, as in amphioxus and vertebrates, the neural crest specifier Snail is expressed in neural plate border ectoderm in the ascidians Halocynthia roretzi and C. intestinalis (Corbo et al. 1997; Erives et al. 1998; Wada et al. 1998). The ascidian caudal neural tube consists of one dorsal, one ventral and two lateral rows of ependymal cells (Nicol & Meinertzhagen, 1991). In H. roretzi 76-cell embryos and C. intestinalis 110-cell embryos, Snail is expressed in the A8.15 blastomeres and the A8.15/A8.16 blastomeres, respectively. These cells give rise to the lateral ependymal cells of the neural tube, and Snail expression originating in these cells expands antero-laterally to mark the lateral border of the neural plate, and persists into the curling neural folds. Snail expression was also noted in cells that give rise to the sensory vesicle and sensory pigment cells in both H. roretzi and C. intestinalis, and in descendants of the b8.19 cells in H. roretzi, which give rise to the dorsal row of caudal neural tube ependymal cells. Given their origin from the neural plate border, it is perhaps more plausible that these Snail-expressing cells represent antecedents to the vertebrate neural crest.
Origin of neural crest-derived skeletogenic cell types
Following their migration, neural crest cells differentiate into a diversity of cell and tissue types. Among the best characterized of these are the craniofacial skeletal tissues of jawed vertebrates. Neural crest cells give rise to the chondrocytes of primary and secondary cartilage, the osteoblasts/osteocytes of dermal and endochondral bone, and the odontoblasts of tooth and scale dentine. Note, however, that both cartilage and bone may also form from mesoderm, and that only dentine derives exclusively from neural crest (Hall, 2009). When considering the evolution of neural crest-derived skeletogenic cell types – and the evolution of osteocytes and chondrocytes, in particular – it is essential to consider the phylogenetic sequence of skeletal tissue occurrence, in order to determine whether gene regulatory interactions underlying particular cell types functioned primitively in mesoderm and were co-opted to the neural crest, or vice versa. Here, we consider the evolution of neural crest-derived odontoblasts and chondrocytes, as examples of cell types whose neural crest origin likely represent cases of novelty and co-option, respectively.
Odontoblasts/dentine
Odontodes – the developmental module that gives rise to dentine-secreting odontoblasts and enamel-secreting amelobasts – are an ancient component of the vertebrate skeleton, quite possibly representing the first example of vertebrate skeletal biomineralization (Smith & Hall, 1990, 1993).
Phylogenetically, putative odontodes appear first in the fossil record at the base of the gnathostome (jawed vertebrate) stem, as the mineralized feeding elements of the conodont oral apparatus (Donoghue, 2002; Donoghue et al. 2006). Conodont elements are composed of basal tissue and crown tissue, and these tissues have been homologized with the dentine and enamel, respectively, of gnathostome teeth. Microstructurally diverse dentinous scales, denticles and tubercles are broadly distributed among various fossil taxa that sit on the gnathostome stem (reviewed by Smith & Hall, 1990; Sire et al. 2009). Among extant jawed vertebrates, odontodes are present both orally (as the oral teeth and pharyngeal denticles of bony and cartilaginous fishes) and dermally (as the dermal denticles of cartilaginous fishes and polypterid bony fishes). Given that, among extant jawed vertebrates, oral tooth odontoblasts are exclusively derived from neural crest (de Beer, 1947; Smith & Hall, 1990, 1993), it is generally assumed that all dentine-containing tissues in extant and extinct jawless vertebrates are similarly neural crest derived. However, this remains to be formally tested by neural crest lineage tracing in extant taxa with post-cranial dermal denticles (e.g. chondrichthyans; Miyake et al. 1999).
Dentine-secreting cells (odontoblasts) and bone-secreting cells (osteoblasts) are similar in many respects. Both cell types are polarized, exhibit cell processes and secrete common extracellular matrix proteins – e.g. type I collagen, bone sialoprotein (osteopontin), osteocalcin dentine matrix protein 1 and periostin (osteoblast-specific factor-2; Lu et al. 2001; Fraser et al. 2004; Kawasaki et al. 2004; Kruzynska-Frejtag et al. 2004; Pääkkönen et al. 2007). However, while osteoblasts become surrounded by their secreted matrix (then becoming osteocytes; Franz-Odendaal et al. 2006), odontoblasts retreat into a pulp cavity as they secrete their matrix, leaving only their cell processes embedded within mineralized matrix.
Odontoblasts can also be distinguished from osteoblasts/osteocytes by their secretion of certain unique matrix products (e.g. dentin sialoprotein and dentin phosphoprotein –Butler & Ritchie, 1995), and by their expression of mechanosensitive ion channels, such as TRPV1 and voltage-gated sodium channels (Okumura et al. 2005; Allard et al. 2006). Whether odontoblasts functioned primitively in a sensory capacity (Baker, 2008; Magloire et al. 2009), or co-opted their sensory function coincident with the evolution of oral teeth, remains to be determined. However, given that there are no identifiable dentine or odontoblast homologues in non-vertebrate chordates – or in any invertebrate group – we can classify odontoblasts as a vertebrate novelty, and as a novel neural crest cell fate. Indeed, consistent with the proposal for the continued evolution of neural crest-derived skeletal tissues, Kawasaki et al. (2004, 2005) have provided evidence from phylogenetic analyses of extracellular matrix proteins for the independent evolution of dentine in teleost fish and in tetrapods.
Chondrocytes/cartilage
Cartilage is an avascular, cellular supporting tissue with an extracellular matrix composed of fibrous proteins (typically, but not exclusively, type II collagen) and proteoglycans reviewed in Hall, 2005c). Regardless of germ layer origin, cartilage development is first detectable, histologically, as a condensation of preskeletal mesenchyme. Condensed mesenchymal cells proliferate, begin to secrete cartilage extracellular matrix, and subsequently undergo differentiation into chondrocytes (Hall & Miyake, 2000).
Developmental genetic and transcriptomic studies have highlighted a number of transcription factors and signalling molecules that function at different stages of cartilage condensation and differentiation. For example, members of the SoxE (Sox9) and SoxD (Sox5/6) families, as well as Twist1/2 and Its2/3 are expressed in post-migratory cranial neural crest cells, and are required, in some capacity, for subsequent chondrogenic differentiation of these neural crest cells. Another group of transcription factors, including Barx1/2, Cart1, Alx3/4, Bapx1 and Runx1/2/3, which are expressed in pre-cartilaginous condensations, appear to be upstream of genes encoding cartilage extracellular matrix proteins, such as Col2a and Aggrecan. While the regulatory interactions among these factors remain poorly understood, a preliminary GRN for vertebrate chondrogenesis has been put forward (Cameron et al. 2009; Cole, 2011).
While ascidians lack cartilage or cartilage-like tissues, cephalochordates possess an acellular collagenous skeletal tissue that supports their pharyngeal arches. This collagenous tissue is secreted by pharyngeal mesoderm, and fills both the primary gill bars and secondary (tongue) gill bars of the pharyngeal basket (Fig. 3). This tissue is most likely homologous with the endodermally secreted acellular gill bar skeleton of enteropneust hemichordates (Fig. 3); both tissues have been classified as ‘cartilage-like’ based largely on their fibrillar protein composition, which includes type II collagen. Given this shared matrix property – and the location of this secreted skeleton in cephalochordate/hemichordate pharyngeal arches – it has been variously suggested that this skeleton represents an antecedent to the neural crest-derived vertebrate pharyngeal endoskeleton. This would imply that the cartilage developmental program (or, more specifically, the type II collagen developmental program) was co-opted to the neural crest from germ layers that primitively expressed type II collagen in non-vertebrate deuterostomes – e.g. endoderm (Rychel et al. 2006; Rychel & Swalla, 2007) or mesoderm (Meulemans & Bronner-Fraser, 2007).
Fig. 3.
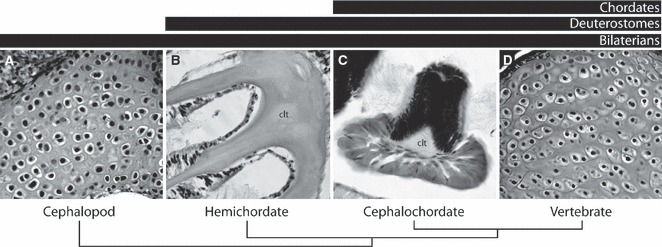
(A) The cellular funnel cartilage of the short-finned squid Illex illecebrosus resembles the cellular cartilage of vertebrates in terms of its development via a mesenchymal condensation, chondrocyte cell morphology and some shared extracellular matrix components. This tissue is distinct from the acellular collagenous ‘cartilage-like tissue’ (clt) of (B) hemichordates (represented here by Saccoglossus kowalevskii) or (C) amphioxus (represented here by Branchiostoma lanceolatum). (D) Hyaline cartilage of the dwarf African clawed toad Hymenochirus boettgeri for comparison. It is likely that the neural crest co-opted a mesodermal chondrogenic program that predates the origin of deuterostomes. Images (A) and (D) provided by Dr Alison Cole.
Meulemans & Bronner-Fraser (2007) sought to determine whether the vertebrate cartilage developmental program was: first, co-opted to the neural crest as a conserved network; or second, assembled de novo in the neural crest by co-option of multiple factors from different germ layers or tissues. To this end, they analysed the expression patterns of 11 amphioxus orthologues of genes involved in vertebrate neural crest cell chondrogenesis. They noted that while AmphiTwist, AmphiEts, AmphiAlx and AmphiColA (an orthologue of vertebrate Col2a1) are co-expressed in amphioxus pharyngeal mesoderm, expression of AmphiSoxE and AmphiSoxD was excluded from the pharyngeal mesoderm (though was expressed in the neural tube and the pharyngeal endoderm, respectively). AmphiTwist, AmphiEts, AmphiSoxE, AmphiSoxD and AmphiColA are also expressed in the notochord. Meulemans and Bronner-Fraser conclude that cellular cartilage is a vertebrate novelty resulting from the de novo assembly of a cartilage program in neural crest cells by co-option of individual components that primitively functioned in mesoderm.
While co-option of a neural crest chondrogenic developmental program from mesoderm seems reasonable, there is evidence that the extent to which this network was assembled prior to the origin of vertebrates – and, indeed, prior to the origin of chordates – is much greater than is currently appreciated. Tissues fitting the definition of cartilage (as stated above) clearly did not originate in vertebrates, but are, in fact, well represented in several major lineages of protostome invertebrates, including brachiopods, annelids, molluscs and arthropods (Cole & Hall, 2004a, 2009; Hall, 2005c; Cole, 2011). Most studies on the origin of vertebrate cartilage suffer from a lack of consideration of the many protostome invertebrate cartilages, and so consider the acellular collagenous skeleton of the cephalochordates and/or enteropneust hemichordate pharynx as the earliest tissue potentially recognizable as cartilage (or cartilage-like; de Beer, 1937; Zhang & Cohn, 2006; Meulemans & Bronner-Fraser, 2007; Donoghue et al. 2008). As discussed previously, this conclusion is based predominantly on the presence of type II collagen. However, Col2a1 expression is not unique to cartilage; notochord, epithelial basement membranes and the vitreous humor of the eye all express Col2a1, but none of these tissues would be classified as cartilage (or even ‘cartilage-like’– with the exception, perhaps, of the notochord).
Cephalopod molluscs possess cartilages that are remarkably similar to, and share a number of histological, developmental and biochemical properties with vertebrate cartilage. For example, the developing funnel cartilage of the cuttlefish Sepia officinalis is first visible as a mesenchymal condensation underneath a cuboidal epithelium. This condensation subsequently differentiates into a cartilage that closely resembles vertebrate hyaline cartilage, with an extracellular matrix that is rich in mucopolysaccharides. Sepia officinalis cartilage matrix stains positively both for fibrillar collagen and for chondroitin sulphate (two biochemical features of vertebrate cartilage extracellular matrix), and cephalopod chondrocytes are effectively indistinguishable from the chondrocytes of vertebrate hyaline cartilage in terms of cell morphology (Fig. 3). A detailed molecular assessment of Sepia cartilage and chondrocyte differentiation is anxiously awaited, as is an assessment of the development of other protostome invertebrate cartilages (e.g. the branchial cartilage of the horseshoe crab, Limulus polyphemus, and the feeding tentacle cartilage of sabellid polychaetes; Cole & Hall, 2004b).
Based on these data, and given the striking developmental and histological similarities between certain protostome cartilages (e.g. cephalopod hyaline cartilage) and vertebrate cartilages (Fig. 3), it seems inescapable that mesodermally derived cellular cartilage has a much deeper (i.e. pre-deuterostome) origin in metazoan phylogeny than having arisen with vertebrates. Homology of invertebrate and vertebrate cartilages implies either a widespread loss of cartilage in many invertebrate lineages, or the parallel evolution of cartilage and cartilage-like tissues in brachiopods, annelids, molluscs and arthropods (Cole & Hall, 2004b). It would also follow that a well-developed chondrogenic developmental program functioned to generate mesodermally derived cellular cartilage prior to the origin of vertebrates, and that this program was co-opted to the neural crest in vertebrates. Finally, while the acellular collagenous pharyngeal skeletal tissue of amphioxus and hemichordates is likely a primitive feature of the deuterostome pharynx that has been lost in echinoderms, ascidians and vertebrates, it may best be interpreted as the independent evolution of a type II collagen containing ‘cartilage-like’ tissue that converges on vertebrate cellular cartilage (Gillis et al. in press).
Conclusion
If we define a neural crest cell as a multipotent cell that delaminates from the neural tube and migrates peripherally to differentiate into one of the many cell types discussed in the Introduction (as seems reasonable, and as has been done by countless authors before us), it is immediately apparent that no known urochordate or cephalochordate cells meet these criteria. Strictly speaking, this would render neural crest cells a bona fide novelty of the vertebrate crown group (Hall, 2005b; Hall & Kerney, 2011).
If, however, we consider cell populations in non-vertebrate chordates that meet some (though not all) criteria of the neural crest definition, then it is possible to recognize ‘latent homologues’ of the neural crest outside of vertebrates. It is important to recognize the neural crest as a continuous character that exists in multiple ordered states, and not simply a binary character that is either ‘present’ or ‘absent’. Snail-expressing cells at the border of the neural plate in cephalochordates and ascidians likely represent the extent of neural crest elaboration in extant non-vertebrate chordates, with the origin of migratory neural crest cells from this region occurring along the vertebrate stem. Recognition of latent neural crest homologues allows for a more nuanced sequence for the evolution of the neural crest and of neural crest cells, with a gradual step-wise acquisition of a distinct neural crest cell identity, migratory ability and differentiated cell fates through chordate phylogeny – though, nevertheless, with the majority of neural crest cell fates acquired, either by innovation or co-option along the vertebrate stem (Donoghue et al. 2008).
Acknowledgments
Research support from the Natural Sciences and Engineering Research Council (NSERC) of Canada (Grant A5056 to BKH) is gratefully acknowledged. JAG was supported by a Royal Society Newton International Postdoctoral Fellowship, and by a NSERC postdoctoral fellowship. Images of Illex and Hymenochirus cartilage in Fig. 3 were kindly provided by Dr Alison Cole. This is reviewed journal paper number 300 from this laboratory.
Footnotes
The terms tunicate, ascidian and sea squirt are used interchangeably in this paper.
Historical note: the ‘senior’ professor of Zoology referred to in the first paragraph of Donoghue et al. (2008) who claimed that ‘the only interesting thing about vertebrates was [is] the neural crest’, was [is] the first author of the present paper.
References
- Allard B, Magliore H, Couble ML, et al. Voltage gated sodium channels confer excitability to human odontoblasts: possible role in tooth pain transmission. J Biol Chem. 2006;281:29 002–29 010. doi: 10.1074/jbc.M601020200. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Saint-Germain N, Gyda M, et al. Sox10 regulates the development of neural crest-derived melanocytes in Xenopus. Dev Biol. 2003;259:19–33. doi: 10.1016/s0012-1606(03)00161-1. [DOI] [PubMed] [Google Scholar]
- Arduini B, Bosse KM, Henion PD. Genetic ablation of neural crest cell diversification. Development. 2009;136:1987–1994. doi: 10.1242/dev.033209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruga J, Tohmonda T, Homma S, et al. Zic1 promotes the expansion of dorsal neural progenitors in spinal cord by inhibiting neuronal differentiation. Dev Biol. 2002;244:329–341. doi: 10.1006/dbio.2002.0598. [DOI] [PubMed] [Google Scholar]
- Aybar MJ, Nieto MA, Mayor R. Snail precedes slug in the genetic cascade required for the specification and migration of the Xenopus neural crest. Development. 2003;130:483–494. doi: 10.1242/dev.00238. [DOI] [PubMed] [Google Scholar]
- Baker CVH. The evolution and elaboration of vertebrate neural crest cells. Curr Opin Genet Dev. 2008;18:536–543. doi: 10.1016/j.gde.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Baker CVH, Bronner-Fraser M. The origins of the neural crest. Part II: an evolutionary perspective. Mech Dev. 1997;69:13–29. doi: 10.1016/s0925-4773(97)00129-9. [DOI] [PubMed] [Google Scholar]
- de Beer GR. The Vertebrate Skull. Oxford: Oxford University Press; 1937. (Reprinted 1985, University of Chicago Press, Chicago.) [Google Scholar]
- de Beer GR. The differentiation of neural crest cells into visceral cartilages and odontoblasts in Amblystoma, and a re-examination of the germ-layer theory. Proc R Soc Lond B. 1947;134:377–398. [PubMed] [Google Scholar]
- Betancur P, Bronner-Fraser M, Sauka-Spengler T. Assembling neural crest regulatory circuits into a gene regulatory network. Annu Rev Cell Dev Biol. 2010;26:581–603. doi: 10.1146/annurev.cellbio.042308.113245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop CD, Hall BK, Bates WR. Heat shock protein 90 expression in two migratory cell types of ascidian embryos and larvae: test cells deposit HSP90 on the larval tunic. Int J Dev Biol. 2010;54:1337–1346. doi: 10.1387/ijdb.082730cb. [DOI] [PubMed] [Google Scholar]
- Bone Q. The central nervous system in amphioxus. J Comp Neurol. 1960;115:27–51. [Google Scholar]
- Bourlat SJ, Juliusdottir T, Lowe CJ, et al. Deuterostome phylogeny reveals monophyletic chordates and the new phylum Xenoturbellida. Nature. 2006;444:85–88. doi: 10.1038/nature05241. [DOI] [PubMed] [Google Scholar]
- Bowler PJ. Life’s Splendid Dream. Evolutionary Biology and the Reconstruction of Life’s Ancestry. Chicago: The University of Chicago Press; 1996. [Google Scholar]
- Brewster R, Lee J, Ruiz i Altaba A. Gli/Zic factors pattern the neural plate by defining domains of cell differentiation. Nature. 1998;393:579–583. doi: 10.1038/31242. [DOI] [PubMed] [Google Scholar]
- Butler WT, Ritchie HH. The nature and functional significance of dentin extracellular matrix proteins. Int J Dev Biol. 1995;39:169–179. [PubMed] [Google Scholar]
- Cameron TL, Belluoccio D, Farlie PG, et al. Global comparative transcriptome analysis of cartilage formation in vivo. BMC Dev Biol. 2009;9:20. doi: 10.1186/1471-213X-9-20. doi: 10.1186/1471-213X-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, et al. The transcription factor Snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Cole AG. A review of diversity in the evolution and development of cartilage: the search for the origin of the chondrocyte. Eur Cells Mater. 2011;21:122–129. doi: 10.22203/ecm.v021a10. [DOI] [PubMed] [Google Scholar]
- Cole AG, Hall BK. Cartilage is a metazoan tissue; integrating data from non-vertebrate sources. Acta Zool (Stockh) 2004a;85:69–80. [Google Scholar]
- Cole AG, Hall BK. The nature and significance of invertebrate cartilages revisited: distribution and histology of cartilage and cartilage-like tissues within the Metazoa. Zoology. 2004b;107:261–274. doi: 10.1016/j.zool.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Cole AG, Hall BK. Cartilage differentiation in cephalopod molluscs. Zoology. 2009;112:2–15. doi: 10.1016/j.zool.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Coles JA, Abbott NJ. Signalling from neurones to glial cells in invertebrates. Trends Neurosci. 1996;19:358–362. doi: 10.1016/0166-2236(96)10042-4. [DOI] [PubMed] [Google Scholar]
- Corbo JC, Erives A, Di Gregorio A, et al. Dorsoventral patterning of the vertebrate neural tube is conserved in a protochordate. Development. 1997;124:2335–2344. doi: 10.1242/dev.124.12.2335. [DOI] [PubMed] [Google Scholar]
- Dehal P, Satou Y, Campbell RK, et al. The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins. Science. 2002;298:2157–2167. doi: 10.1126/science.1080049. [DOI] [PubMed] [Google Scholar]
- Delsuc F, Brinkmann H, Chourrout D, et al. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature. 2006;439:965–968. doi: 10.1038/nature04336. [DOI] [PubMed] [Google Scholar]
- Donoghue PCJ. Evolution of development of the vertebrate dermal and oral skeletons: unraveling concepts, regulatory theories, and homologies. Paleobiology. 2002;28:474–507. [Google Scholar]
- Donoghue PCJ, Sansom IJ, Downs JP. Early evolution of vertebrate skeletal tissues and cellular interactions, and the canalization of skeletal development. J Exp Zool B Mol Dev Evol. 2006;306B:278–294. doi: 10.1002/jez.b.21090. [DOI] [PubMed] [Google Scholar]
- Donoghue PCJ, Graham A, Kelsh RN. The origin and evolution of the neural crest. BioEssays. 2008;30:530–541. doi: 10.1002/bies.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn CW, Hejnol A, Matus DQ, et al. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature. 2010;452:745–749. doi: 10.1038/nature06614. [DOI] [PubMed] [Google Scholar]
- Erives A, Corbo JC, Levine M. Lineage-specific regulation of the Ciona snail gene in the embryonic mesoderm and neurectoderm. Dev Biol. 1998;194:213–225. doi: 10.1006/dbio.1997.8810. [DOI] [PubMed] [Google Scholar]
- Fedonkin MA, Gehling JG, Grey K, et al. The Rise of Animals: Evolution and Diversification of the Kingdom Animalia. Baltimore, MD: The Johns Hopkins University Press; 2007. [Google Scholar]
- Franz-Odendaal TA, Witten PE, Hall BK. Buried alive: how osteoblasts become osteocytes. Dev Dyn. 2006;235:176–190. doi: 10.1002/dvdy.20603. [DOI] [PubMed] [Google Scholar]
- Fraser GJ, Graham A, Smith MM. Conserved deployment of genes during odontogenesis across osteichthyans. Proc R Soc Lond B. 2004;271:2311–2317. doi: 10.1098/rspb.2004.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gans C. The neural crest: a spectacular invention. In: Maderson PFA, editor. Developmental and Evolutionary Aspects of the Neural Crest. New York: John Wiley; 1987. pp. 361–379. [Google Scholar]
- Gans C. Stages in the origin of vertebrates: analysis by means of scenarios. Biol Rev Camb Philos Soc. 1989;64:221–268. doi: 10.1111/j.1469-185x.1989.tb00471.x. [DOI] [PubMed] [Google Scholar]
- Gans C. Evolutionary origins of the vertebrate skull. In: Hanken J, Hall BK, editors. The Vertebrate Skull vol. II. Patterns of Structural and Systematic Diversity. Chicago: The University of Chicago Press; 1993. pp. 1–35. [Google Scholar]
- Gans C, Northcutt RG. Neural crest and the origin of vertebrates. A new head. Science. 1983;220:268–274. doi: 10.1126/science.220.4594.268. [DOI] [PubMed] [Google Scholar]
- Gans C, Northcutt RG. Neural crest: the implications for comparative anatomy. Fortschr Zool. 1985;30:507–514. [Google Scholar]
- Gibson AW, Burke RD. The origin of pigment cells in the embryos of the sea urchin Strongylocentrotus purpuratus. Dev Biol. 1985;107:414–419. doi: 10.1016/0012-1606(85)90323-9. [DOI] [PubMed] [Google Scholar]
- Gillis JA, Fritzenwanker JH, Lowe CJ. A stem-deuterostome origin of the vertebrate pharyngeal transcriptional network. Proc R Soc B Biol Sci. 2012;279:237–246. doi: 10.1098/rspb.2011.0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gostling NJ, Shimeld SM. Protochordate Zic genes define primitive somite compartments and highlight molecular changes underlying neural crest evolution. Evol Dev. 2003;5:136–144. doi: 10.1046/j.1525-142x.2003.03020.x. [DOI] [PubMed] [Google Scholar]
- Hall BK. Homology: The Hierarchical Basis of Comparative Biology. Boca Raton: Academic Press; 1994. [Google Scholar]
- Hall BK. Germ layers and the germ-layer theory revisited: primary and secondary germ layers, neural crest as a fourth germ layer, homology, demise of the germ-layer theory. Evol Biol. 1997;30:121–186. [Google Scholar]
- Hall BK. The Neural Crest in Development and Evolution. New York: Springer; 1999. [Google Scholar]
- Hall BK. The neural crest as a fourth germ layer and vertebrates as quadroblastic not triploblastic. Evol Dev. 2000a;2:3–5. doi: 10.1046/j.1525-142x.2000.00032.x. [DOI] [PubMed] [Google Scholar]
- Hall BK. The evolution of the neural crest in vertebrates. In: Jacobson C-O, Olsson L, Laurent T, editors. Regulatory Processes in Development: the Legacy of Sven Hörstadius Wenner-Gren International Series Volume 76. London: The Portland Press; 2000b. pp. 101–113. [Google Scholar]
- Hall BK. Betrayed by Balanoglossus: William Bateson’s rejection of evolutionary embryology as the basis for understanding evolution. J Exp Zool B Mol Dev Evol. 2005a;304B:1–17. doi: 10.1002/jez.b.21030. [DOI] [PubMed] [Google Scholar]
- Hall BK. Consideration of the neural crest and its skeletal derivatives in the context of novelty/innovations. J Exp Zool B Mol Dev Evol. 2005b;304B:548–557. doi: 10.1002/jez.b.21057. [DOI] [PubMed] [Google Scholar]
- Hall BK. Bone and Cartilage: Developmental and Evolutionary Skeletal Biology. London: Elsevier Academic Press; 2005c. [Google Scholar]
- Hall BK. Vertebrate origins: riding the crest of a new wave, or the wave of a new crest? Evol Dev. 2008a;10:261–263. doi: 10.1111/j.1525-142X.2008.00233.x. [DOI] [PubMed] [Google Scholar]
- Hall BK. Evolutionary origins of the neural crest and neural crest cells. Evol Biol. 2008b;35:248–266. [Google Scholar]
- Hall BK. The neural crest and neural crest cells: discovery and significance for theories of embryonic organization. J Biosci. 2008c;34:781–793. doi: 10.1007/s12038-008-0098-4. [DOI] [PubMed] [Google Scholar]
- Hall BK. The Neural Crest and Neural Crest Cells in Vertebrate Development and Evolution. New York: Springer; 2009. [Google Scholar]
- Hall BK, Kerney R. Levels of Biological Organization and the Origin of Novelty. J Exp Zool B Mol Dev Evol. doi: 10.1002/jez.b.21425. (in press), in press. [DOI] [PubMed] [Google Scholar]
- Hall BK, Miyake T. All for one and one for all: condensations and the initiation of skeletal development. BioEssays. 2000;22:138–147. doi: 10.1002/(SICI)1521-1878(200002)22:2<138::AID-BIES5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Hanken J, Hall BK. The Vertebrate Skull. I–3. Chicago: The University of Chicago Press; 1993. [Google Scholar]
- Hirano T, Nishida H. Developmental fates of larval tissues after metamorphosis in ascidian Halocynthia roretzi I. Origin of mesodermal tissues of the juvenile. Dev Biol. 1997;192:199–210. doi: 10.1006/dbio.1997.8772. [DOI] [PubMed] [Google Scholar]
- Holland PWH. Embryonic development of heads, skeletons and amphioxus: Edwin S. Goodrich revisited. Int J Dev Biol. 2000;44:29–34. [PubMed] [Google Scholar]
- Holland PWH, Chen J. Origin and early evolution of the vertebrates: new insights from advances in molecular biology, anatomy, and paleontology. BioEssays. 2001;23:142–151. doi: 10.1002/1521-1878(200102)23:2<142::AID-BIES1021>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Holland PWH, Graham A. Evolution of regional identity in the vertebrate nervous system. Perspect Dev Neurobiol. 1995;3:17–27. [PubMed] [Google Scholar]
- Holland LZ, Holland ND. Evolution of neural crest and placodes: amphioxus as a model for the ancestral vertebrate? J Anat. 2001;199:85–98. doi: 10.1046/j.1469-7580.2001.19910085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland LZ, Holland ND. A revised fate map for amphioxus and the evolution of axial patterning in chordates. Integr Comp Biol. 2007;47:360–372. doi: 10.1093/icb/icm064. [DOI] [PubMed] [Google Scholar]
- Holland LZ, Schubert M, Kozmik Z, et al. AmphiPax3/7, an amphioxus paired box gene: insights into chordate myogenesis, neurogenesis, and the possible evolutionary precursor of definitive vertebrate neural crest. Evol Dev. 1999;1:153–165. doi: 10.1046/j.1525-142x.1999.99019.x. [DOI] [PubMed] [Google Scholar]
- Holley JA, Yu RK. Localization of glycoconjugates recognized by the HNK-1 antibody in mouse and chick embryos during early neural development. Dev Neurosci. 1987;9:105–119. doi: 10.1159/000111613. [DOI] [PubMed] [Google Scholar]
- Hong C-S, Saint-Jeannet J-P. The activity of Pax3 and Zic3 regulates three distinct cell fates at the neural plate border. Mol Biol Cell. 2007;18:2192–2202. doi: 10.1091/mbc.E06-11-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore SM, Aybar MJ, Mayor R. Sox10 is required for the early development of the prospective neural crest in Xenopus embryos. Dev Biol. 2003;260:79–96. doi: 10.1016/s0012-1606(03)00247-1. [DOI] [PubMed] [Google Scholar]
- Imai KS, Hino K, Yagi K, et al. Gene expression profiles of transcription factors and signaling molecules in the ascidian embryo: towards a comprehensive understanding of gene networks. Development. 2004;131:4047–4058. doi: 10.1242/dev.01270. [DOI] [PubMed] [Google Scholar]
- Janvier P. Homologies and evolutionary transitions in early vertebrate history. In: Anderson JS, Sues H-D, editors. Major Transitions in Vertebrate Evolution. Bloomington and Indianapolis: Indiana University Press; 2007. pp. 57–121. [Google Scholar]
- Jeffery WR. Evolution of ascidian development. Bioscience. 1997;47:417–425. [Google Scholar]
- Jeffery WR. Ascidian neural crest-like cells: phylogenetic distribution, relationship to larval complexity, and pigment cell fate. J Exp Zool B Mol Dev Evol. 2006;306B:470–480. doi: 10.1002/jez.b.21109. [DOI] [PubMed] [Google Scholar]
- Jeffery WR. Chordate ancestry of the neural crest: new insights from ascidians. Semin Dev Biol. 2007;18:481–491. doi: 10.1016/j.semcdb.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Jeffery WR, Strickler AG, Yamamoto Y. Migratory neural crest-like cells form body pigmentation in a urochordate embryo. Nature. 2004;431:696–699. doi: 10.1038/nature02975. [DOI] [PubMed] [Google Scholar]
- Jeffery WR, Chiba T, Krajka FR, et al. Trunk lateral cells are neural crest-like in the ascidian Ciona intestinalis; insights into the ancestry and evolution of the neural crest. Dev Biol. 2008;324:152–160. doi: 10.1016/j.ydbio.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Kawasaki K, Suzuki T, Weiss KM. Genetic basis for the evolution of vertebrate mineralized tissue. Proc Natl Acad Sci USA. 2004;101:11 356–11 361. doi: 10.1073/pnas.0404279101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki K, Suzuki T, Weiss KM. Phylogenetic drift in evolution: the changing genetic basis of vertebrate teeth. Proc Natl Acad Sci USA. 2005;102:18 063–18 068. doi: 10.1073/pnas.0509263102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalevsky AO. Anatomie des Balanoglossus. Mém Acad Sci St Petersburg. 1866;7:1–10. [Google Scholar]
- Kowalevsky AO. Entwickelungsgeschichte des Amphioxus lanceolatus. Mém Acad Sci St Petersburg. 1867;11:1–17. [Google Scholar]
- Kowalevsky AO. Weitere Studien über die Entwicklung der einfachen Ascidien. Arch Mikrosk Anat. 1871;7:101–130. [Google Scholar]
- Kowalevsky AO. Weitere Studien über die Entwickelungsgeschichte des Amphioxus lanceolatus. Arch Mikrosk Anat. 1877;13:181–204. [Google Scholar]
- Kruzynska-Frejtag A, Wang J, Maeda M, et al. Periostin is expressed within the developing teeth at the sites of epithelial-mesenchymal interaction. Dev Dyn. 2004;229:857–868. doi: 10.1002/dvdy.10453. [DOI] [PubMed] [Google Scholar]
- Kumano G, Nishida H. Ascidian embryonic development: an emerging model system for the study of cell fate specification in chordates. Dev Dyn. 2007;236:1732–1747. doi: 10.1002/dvdy.21108. [DOI] [PubMed] [Google Scholar]
- Kuratani S, Horigome N. Developmental morphology of branchiomeric nerves in a cat shark, Scyliorhinus torazame, with special reference to rhombomeres, cephalic mesoderm, and distribution patterns of cephalic crest cells. Zool Sci. 2000;17:893–909. [Google Scholar]
- Langeland JA, Tomsa JM, Jackman WR, Jr, et al. An amphioxus snail gene: expression in paraxial mesoderm and neural plate suggests a conserved role in patterning the chordate embryo. Dev Genes Evol. 1998;208:569–577. doi: 10.1007/s004270050216. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Kalcheim C. The Neural Crest. 2nd edn. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Le Douarin NM, Creuzet S, Couly G, et al. Neural crest cell plasticity and its limits. Development. 2004;131:4637–4650. doi: 10.1242/dev.01350. [DOI] [PubMed] [Google Scholar]
- Liem KF, Tremml G, Roelink H, et al. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell. 1995;82:969–979. doi: 10.1016/0092-8674(95)90276-7. [DOI] [PubMed] [Google Scholar]
- Long JA, Hall BK, McNamara KJ, et al. The phylogenetic origin of jaws in vertebrates: developmental plasticity and heterochrony. Kirtlandia. 2011;57:46–52. [Google Scholar]
- Lu Y, Xie Y, Zhang S, et al. DMP1-targeted Cre expression in odontoblasts and osteocytes. J Dent Res. 2001;86:320–325. doi: 10.1177/154405910708600404. [DOI] [PubMed] [Google Scholar]
- Maderson PFA, editor. Developmental and Evolutionary Aspects of the Neural Crest. New York: John Wiley; 1987. [Google Scholar]
- Magloire H, Couble M-L, Thivichon-Prince B, et al. Odontoblast: a mechano-sensory cell. J Exp Zool B Mol Dev Evol. 2009;312B:416–424. doi: 10.1002/jez.b.21264. [DOI] [PubMed] [Google Scholar]
- Maisey JG. Heads and tails: a chordate phylogeny. Cladistics. 1986;2:201–256. doi: 10.1111/j.1096-0031.1986.tb00462.x. [DOI] [PubMed] [Google Scholar]
- Mallatt J, Chen J-Y. Fossil sister group of craniates: predicted and found. J Morphol. 2003;258:1–31. doi: 10.1002/jmor.10081. [DOI] [PubMed] [Google Scholar]
- Manni L, Lane NJ, Joly J-S, et al. Neurogenic and non-neurogenic placodes in ascidians. J Exp Zool B Mol Dev Evol. 2004;302B:483–504. doi: 10.1002/jez.b.21013. [DOI] [PubMed] [Google Scholar]
- Marchant L, Linker C, Ruiz P, et al. The inductive properties of mesoderm suggest that the neural crest cells are specified by a BMP gradient. Dev Biol. 1998;198:319–329. [PubMed] [Google Scholar]
- Martinez-Morales J-R, Henrich T, Ramialison M, et al. New genes in the evolution of the neural crest differentiation program. Genome Biol. 2007;8:R36.1–R36.17. doi: 10.1186/gb-2007-8-3-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazet F, Hutt JA, Millard J, et al. Pax gene expression in the developing central nervous system of Ciona intestinalis. Gene Expr Patterns. 2003;3:743–745. doi: 10.1016/s1567-133x(03)00137-6. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen IA, Okamura Y. The larval ascidian nervous system: the chordate brain from its small beginnings. Trends Neurosci. 2001;24:401–410. doi: 10.1016/s0166-2236(00)01851-8. [DOI] [PubMed] [Google Scholar]
- Meulemans D, Bronner-Fraser M. Gene-regulatory interactions in neural crest evolution and development. Dev Cell. 2004;7:291–299. doi: 10.1016/j.devcel.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Meulemans D, Bronner-Fraser M. Insights from amphioxus into the evolution of vertebrate cartilage. PLoS ONE. 2007;2(8):e787. doi: 10.1371/journal.pone.0000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailov AT, Gilbert SF. From development to evolution: the re-establishment of the “Alexander Kowalevsky Medal”. Int J Dev Biol. 2002;46:693–698. [PubMed] [Google Scholar]
- Miyake T, Vaglia JL, Taylor LH, et al. Development of dermal denticles in skates (Chondrichthyes, Batoidea): patterning and cellular differentiation. J Morphol. 1999;241:61–81. doi: 10.1002/(SICI)1097-4687(199907)241:1<61::AID-JMOR4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Mizuseki K, Kishi M, Matsui M, et al. Xenopus Zic-related-1 and Sox-2, two factors induced by chordin, have distinct activities in the initiation of neural induction. Development. 1998;125:579–587. doi: 10.1242/dev.125.4.579. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Takeichi M. Neural crest emigration from the neural tube depends on regulated cadherin expression. Development. 1998;125:2963–2971. doi: 10.1242/dev.125.15.2963. [DOI] [PubMed] [Google Scholar]
- Nakata K, Nagai T, Aruga J, et al. Xenopus Zic3, a primary regulator both in neural and neural crest development. Proc Natl Acad Sci USA. 1997;94:11 980–11 985. doi: 10.1073/pnas.94.22.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K, Koyabu Y, Aruga J, et al. A novel member of the Xenopus Zic family, Zic5, mediates neural crest development. Mech Dev. 2000;99:83–91. doi: 10.1016/s0925-4773(00)00480-9. [DOI] [PubMed] [Google Scholar]
- Ng LJ, Wheatley S, Muscat GE, et al. SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev Biol. 1997;183:108–121. doi: 10.1006/dbio.1996.8487. [DOI] [PubMed] [Google Scholar]
- Nicol D, Meinertzhagen IA. Cell counts and maps in the larval central nervous system of the ascidian Ciona intestinalis (L.) J Comp Neurol. 1991;309:415–429. doi: 10.1002/cne.903090402. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. The origin of craniates – neural crest, neurogenic placodes, and homeobox genes. Isr J Zool. 1996;42:S273–S313. [Google Scholar]
- Northcutt RG, Gans C. The genesis of neural crest and epidermal placodes: a reinterpretation of vertebrate origins. Q Rev Biol. 1983;58:1–28. doi: 10.1086/413055. [DOI] [PubMed] [Google Scholar]
- Okumura R, Shima K, Muramatsu T, et al. The odontoblast is a sensory receptive cell? The expression of TRPV1 (VR-1) channels. Arch Histol Cytol. 2005;68:251–257. doi: 10.1679/aohc.68.251. [DOI] [PubMed] [Google Scholar]
- Pääkkönen V, Vuoristo JT, Salo T, et al. Comparative gene expression profile analysis between native human odontoblasts and pulp tissue. Int Endod J. 2007;41:117–127. doi: 10.1111/j.1365-2591.2007.01327.x. [DOI] [PubMed] [Google Scholar]
- Peters A. The structure of the dorsal root nerves of amphioxus. An electron microscope study. J Comp Neurol. 1963;121:287–295. doi: 10.1002/cne.901210210. [DOI] [PubMed] [Google Scholar]
- Philippe H, Brinkmann H, Copley RR, et al. Acoelomorph flatworms are deuterostomes related to Xenoturbella. Nature. 2011;470:255–258. doi: 10.1038/nature09676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam NH, Butts T, Ferrier DE, et al. The amphioxus genome and the evolution of the chordate karyotype. Nature. 2008;453:1064–1071. doi: 10.1038/nature06967. [DOI] [PubMed] [Google Scholar]
- Rychel AL, Swalla BJ. Development and evolution of chordate cartilage. J Exp Zool B Mol Dev Evol. 2007;308B:325–335. doi: 10.1002/jez.b.21157. [DOI] [PubMed] [Google Scholar]
- Rychel AL, Smith SE, Shimamoto HT, et al. Evolution and development of the chordates: collagen and pharyngeal cartilage. Mol Biol Evol. 2006;23:541–549. doi: 10.1093/molbev/msj055. [DOI] [PubMed] [Google Scholar]
- Sasai N, Mizuseki K, Sasai Y. Requirement of FoxD3-class signaling for neural crest determination in Xenopus. Development. 2001;128:2525–2536. doi: 10.1242/dev.128.13.2525. [DOI] [PubMed] [Google Scholar]
- Satoh N. Developmental Biology of Ascidians. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Satoh N. Cell fate determination in the ascidian embryo. In: Moody SA, editor. Cell Lineage and Fate Determination. London: Academic Press; 1999. pp. 59–74. [Google Scholar]
- Satoh N. Toward a new paradigm for studying ascidian developmental dynamics. Dev Dyn. 2007;236:1695–1697. [Google Scholar]
- Satoh N. An aboral-dorsalization hypothesis for chordate origin. Genesis. 2008;46:614–622. doi: 10.1002/dvg.20416. [DOI] [PubMed] [Google Scholar]
- Satoh N. An advanced filter-feeder hypothesis for urochordate evolution. Zool Sci. 2009;26:97 111. doi: 10.2108/zsj.26.97. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008a;9:557–568. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M. Evolution of the neural crest viewed from a gene regulatory perspective. Genesis. 2008b;46:673–682. doi: 10.1002/dvg.20436. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M. Insights from a sea lamprey into the evolution of neural crest gene regulatory network. Biol Bull. 2008c;214:303–314. doi: 10.2307/25470671. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Meulemans D, Jones M, et al. Ancient evolutionary origin of the neural crest gene regulatory network. Dev Cell. 2007;13:405–420. doi: 10.1016/j.devcel.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Induction and specification of cranial placodes. Dev Biol. 2006;294:303–351. doi: 10.1016/j.ydbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Making senses: development of vertebrate cranial placodes. Int Rev Cell Mol Biol. 2010;283C:129–234. doi: 10.1016/S1937-6448(10)83004-7. [DOI] [PubMed] [Google Scholar]
- Sharman AC, Shimeld SM, Holland PWH. An amphioxus Msx gene expressed predominantly in the dorsal neural tube. Dev Genes Evol. 1999;209:260–263. doi: 10.1007/s004270050251. [DOI] [PubMed] [Google Scholar]
- Shu D-G, Conway Morris S, Zhang X-L. A Pikaia-like chordate from the Lower Cambrian of China. Nature. 1996;384:157–158. [Google Scholar]
- Sire J-V, Donoghue PCJ, Vickaryous MK. Origin and evolution of the integumentary skeleton in non-tetrapod vertebrates. J Anat. 2009;214:409–440. doi: 10.1111/j.1469-7580.2009.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MM, Hall BK. Developmental and evolutionary origins of vertebrate skeletogenic and odontogenic tissues. Biol Rev Camb Philos Soc. 1990;65:277–374. doi: 10.1111/j.1469-185x.1990.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Smith MM, Hall BK. A developmental model for evolution of the vertebrate exoskeleton and teeth: the role of cranial and trunk neural crest. Evol Biol. 1993;27:387–448. [Google Scholar]
- Stone JR, Hall BK. Latent homologues for the neural crest as an evolutionary novelty. Evol Dev. 2004;6:123–129. doi: 10.1111/j.1525-142x.2004.04014.x. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Ueno N, Hemmati-Brivanlou A. Xenopus msx1 mediates epidermal induction and neural inhibition by BMP4. Development. 1997;124:3037–3044. doi: 10.1242/dev.124.16.3037. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Satoh N. Trunk lateral cell-specific genes of the ascidian Halocynthia roretzi. Zool Sci. 2001;18:361–366. [Google Scholar]
- Tokuoka M, Imai KS, Satou Y, et al. Three distinct lineages of mesenchyme cells in Ciona intestinalis embryos demonstrated by specific gene expression. Dev Biol. 2004;274:211–224. doi: 10.1016/j.ydbio.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Vickaryous M, Hall BK. Human cell type diversity, evolution development classification with special reference to cells derived from the neural crest. Biol Rev Camb Philos Soc. 2006;81:425–455. doi: 10.1017/S1464793106007068. [DOI] [PubMed] [Google Scholar]
- Wada H, Holland PW, Satoh N. Origin of patterning in neural tubes. Nature. 1996;384:123. doi: 10.1038/384123a0. [DOI] [PubMed] [Google Scholar]
- Wada H, Saiga H, Satoh N, et al. Tripartite organization of the ancestral chordate brain and the antiquity of placodes: insights from ascidian Pax-2/5/8Hox and Otx genes. Development. 1998;125:1113–1122. doi: 10.1242/dev.125.6.1113. [DOI] [PubMed] [Google Scholar]
- Wang W-D, Melville DB, Montero-Balaguer M, et al. Tfap2a and Foxd3 regulate early steps in the development of the neural crest progenitor population. Dev Biol. 2011;360:173–185. doi: 10.1016/j.ydbio.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker JR. Cell lineages and determinants of cell fate in development. Am Zool. 1987;27:607–622. [Google Scholar]
- Zhang G, Cohn MJ. Hagfish and lancelet fibrillar collagens reveal that type II collagen-based cartilage evolved in stem vertebrates. Proc Natl Acad Sci USA. 2006;103:16 829–16 833. doi: 10.1073/pnas.0605630103. [DOI] [PMC free article] [PubMed] [Google Scholar]


